Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Facile synthesis of a dopant-free hole transporting material with a phenothiazine core for planar perovskite solar cells†
Xiaoyuan Liuab,
Xiao Tana,
Qian Chenb,
Haiquan Shanb,
Changmei Liua,
Jiaju Xub,
Zhi-Kuan Chen*a,
Wei Huang*a and
Zong-Xiang Xu *b
*b
aKey Laboratory of Flexible Electronics (KLOFE), Institute of Advanced Materials (IAM), National Jiangsu Synergetic Innovation Center for Advanced Materials, Nanjing Tech University, 30 South Puzhu Road, Nanjing 211816, P. R. China. E-mail: iamzkchen@njtech.edu.cn; iamwhuang@njtech.edu.cn
bDepartment of Chemistry, South University of Science and Technology of China, Shenzhen, P. R. China. E-mail: xu.zx@sustc.edu.cn
First published on 22nd November 2017
Abstract
A novel electron-rich small-molecule, 4,4′-(10-(4-octylphenyl)-10H-phenothiazine-3,7-diyl)bis(N,N-(4-methoxyphenyl)anilene) (PTZ-TPA), containing phenothiazine as the core with triphenylamine side groups, was synthesized via a Suzuki–Miyaura cross-coupling reaction. When PTZ-TPA was incorporated into a CH3NH3PbI3 perovskite solar cell as a dopant-free hole transporting material (HTM), a short circuit photocurrent density of 21.5 mA cm−2, an open circuit voltage of 0.982 V, and a fill factor of 0.679 were obtained, giving rise to an overall power conversion efficiency of 14.3%, which is comparable to the power conversion efficiency obtained using the current state-of-the-art HTM 2,20,7,70-tetrakis(N,N′-di-p-methoxyphenylamine)-9,90-spirobifluorene with dopant (Spiro-MeOTAD, power conversion efficiency of 17.1%). PTZ-TPA is thus a promising HTM with the potential to replace the expensive Spiro-MeOTAD owing to its comparable performance and much simpler synthesis route; it also presented a better stability during a one week aging test compared with Spiro-MeOTAD.
1. Introduction
In the past few years, organic–inorganic metal halide perovskite solar cells (PSCs) have emerged as a competitive alternative to conventional inorganic solar cells, owing to the ease of their fabrication processes, relatively cheap materials, and very high power conversion efficiencies.1–4 Perovskite light absorbers possess several appealing optoelectronic properties, such as a broad spectral absorption range, high absorption coefficient, low exciton binding energy, high charge carrier mobility, and long carrier diffusion length. Significant progress has been made in PSCs over the past few years and the power conversion efficiency (PCE) of PSCs has increased dramatically from an initial value of 3.8% (ref. 5) to a certified value of 22.1% in 2016.6In the most efficient PSC devices with a regular n–i–p cell configuration, hole-transporting materials (HTMs) are indispensable for achieving high PCEs.7,8 They play an important role in extracting photo-generated holes from the perovskite layer and transporting them to the cathode, thereby circumventing undesired recombination losses at the interfaces. Numerous small organic molecules have been investigated as HTMs in PSCs. To date, the highest reported PCEs have been reached with molecules such as 2,20,7,70-tetrakis(N,N′-di-p-methoxyphenylamine)-9,90-spirobifluorene (Spiro-MeOTAD) that have the spiro functionality as a common structural feature. However, Spiro-MeOTAD suffers from a low hole mobility and low conductivity in its pristine form because of its amorphous nature. Thus p-type dopants or additives such as lithium bis(trifluoromethanesulfonyl)imide (Li-TFSI) are ubiquitously adopted to generate free carriers and to increase conductivity for higher efficiencies.9,10 However, the introduction of p-type dopants not only complicates the optimization process, but also induces additional production costs. Moreover, lithium salts are hydroscopic, which may accelerate the degradation of PSC devices.9 In this regard, a great deal of research efforts have been devoted to developing dopant-free HTMs for PSCs; alternative building blocks, such as fluorene,11,12 dithienosilole,13 diketopyrrolopyrrole,14 triphenylamine,11,15,16 benzodithiophene,17 tetraphenylethene,18 C3h truxene,19 bithiophene,20 indacenodithienothiophene,21 and copper(II) phthalocyanine,22 have been used for the molecular cores to reach state-of-the-art photovoltaic figures of merit in PSCs. To further improve the stability of PSCs and enrich our understanding of the relationship between HTM structures and PSC performance, we must extend the scope of our search for HTMs.
Phenothiazine derivatives have a well-conjugated heterocyclic ring system and they are widely used in dye sensitized solar cells and organic solar cells;23,24 phenothiazine derivatives possess unique electronic and optical properties. Further, there has also been a report on a doped HTM incorporating phenoxazine;25 this system has been proven to be effective as a HTM in PSCs. These reports demonstrate that the minor structural difference between PTZ1 and PTZ2 dramatically influence the molecular geometry and relevant optoelectronic parameters for HTMs in perovskite solar cells. These studies did, however, reveal the potential versatility of the phenothiazine building block for the development of inexpensive and efficient molecular HTMs for perovskite-based solar cells. Our previous work showed that the use of an alkyl chain results in a higher hydrophobicity, which can prevent the penetration of atmospheric moisture into the perovskite layer. This may improve device stability.26
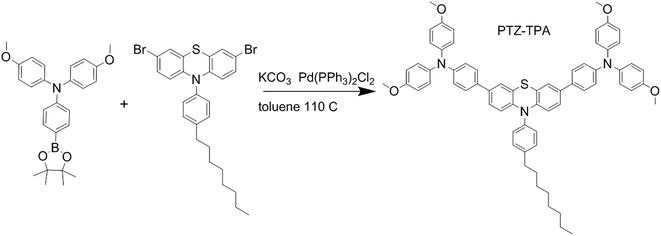
The present study reports a novel 4,4′-(10-(4-octylphenyl)-10H-phenothiazine-3,7-diyl)bis(N,N-(4-methoxyphenyl)anilene) (PTZ-TPA) material (Scheme 1) based on an electron-rich phenothiazine core. Phenothiazine is a low-cost and flexible electron-rich heteroaromatic unit, which provides access to a wide library of HTMs with relatively cheap synthetic procedures.27 In this work, an electron rich phenothiazine was used as the core to which hole accepting triphenylamine moieties were linked, while hexyl chains were attached to the core's carbon atom to increase the molecule's solubility and hydrophobicity.28 The phenothiazine core has a planar structure relative to triphenylamine, which is desirable for promoting intramolecular π-delocalization and intermolecular π–π stacking, which increases both the charge carrier mobility and the lifetime, thus boosting device efficiency.29 PTZ-TPA was synthesized from a relatively inexpensive commercially available phenothiazine derivative in a single step with an acceptable yield (79%). In addition to an extensive investigation of its material properties, we have also investigated the performance of PTZ-TPA in planar perovskite solar cells in terms of their J–V characteristics and charge carrier dynamics. The devices fabricated using dopant free PTZ-TPA demonstrated a PCE of 14.3%, which is comparable to the values obtained using state-of-the-art Spiro-MeOTAD with a dopant; additionally, our fabricated devices showed a better device stability.
2. Experimental sections
2.1 Materials and instruments
All materials were used as purchased without further purification unless specified otherwise. Organic solvents were purchased from Sigma Aldrich. Spiro-MeOTAD, CH3NH3I, and PbI2 were purchased from TCI.PTZ-TPA was synthesized using a one-step Suzuki–Miyaura cross-coupling reaction. A mixture of 4-methoxy-N-(4-methoxyphenyl)-N-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)aniline (0.560 g, 2.20 mmol), 3,7-dibromo-10-(4-octylphenyl)-10H-phenothiazine (0.545 g, 1.00 mmol), and Pd(PPh3)4 (0.028 g, 0.025 mmol) in toluene (10 mL) and a 2 M K2CO3 aqueous solution (5 mL) was stirred at 100 °C for 24 h. After cooling down the reaction mixture to room temperature, the mixture was diluted with dichloromethane and washed with water. The organic layer was dried over Na2SO4 and the remaining solvent was evaporated. The crude product was purified by column chromatography (SiO2, petroleum ether/CH2Cl2 = 1/4 vol/vol) to obtain PTZ-TPA (0.789 g, 79.3% yield) as a yellow solid (1H NMR (500 MHz, CD2Cl2) δ 7.95–6.10 (34H, m), 3.78 (12H, s), 2.73 (2H, s), 1.71 (2H, s), 1.30 (11H, s), 0.89 (3H, s); MS: m/z (M+) 992.544).
The ultraviolet-visible (UV-vis) spectra of the solutions and of the solid thin films were obtained on a PerkinElmer Lambda750S spectrophotometer. Thermogravimetric analysis (TGA) was performed using a Discovery thermogravimetric analyzer. 1H NMR spectroscopy was performed using a Bruker DPX 400 MHz spectrometer. Matrix assisted laser desorption/ionization time-of-flight mass spectra were obtained on a Bruker Daltonics flexAnalysis. The highest occupied molecular orbital (HOMO) energy level of PTZ-TPA was measured using photoelectron yield spectroscopy under N2 (Model IPS-4). Steady-state photoluminescence spectra were measured using a FLS980 Spectrometer (Edinburgh Instruments). The samples were excited through the perovskite or PTZ-TPA layer with an excitation wavelength of 475 nm. Room-temperature photoluminescence (PL) decay curves were acquired for the perovskite films on fluorine doped tin oxide (FTO), for the perovskite films on PCBM/SnO2/FTO, of the Spiro-MeOTAD device, and of the PTZ-TPA/perovskite/PCBM/SnO2/FTO stack (excitation using a 405 nm-wavelength pulsed laser). The hole mobilities of PTZ-TPA and Spiro-MeOTAD were estimated using the space-charge limited current method with devices with a structure consisting of ITO/PEDOT:PSS/PTZ-TPA or Spiro-MeOTAD/Au. The current J–V curves of the devices were recorded using a Keithley 2400 source. Hole mobilities were calculated using the Mott–Gurney law by fitting eqn (1), where J is the current density, ε0 is the permittivity of free space (8.85 × 10−12 F m−1), ε is the relative permittivity of the material (approaching 3 for organic semiconductors), μ is the hole mobility, V is the applied voltage, and d is the thickness of the active layer, respectively.
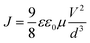 | (1) |
2.2 Device fabrication
PSCs were fabricated with the following structure: FTO/SnO2/PCBM/perovskite/PTZ-TPA/Au on patterned FTO glass. The FTO glass (with a sheet resistance of 20 Ω □−1, PV Tech, China) substrates were pre-cleaned using an ultrasonic bath of chlorobenzene and acetone followed by a treatment in an ultraviolet-ozone chamber (Novascan Company, USA) for 15 min. The SnO2 electron transport layer was applied following a previously reported procedure.30 SnCl2·2H2O in ethanol was used as a precursor solution (0.1 M). The precursor solution was spin-coated onto the substrate at a speed of 3000 rpm for 30 s and then the films were annealed under an ambient atmosphere at 180 °C for 1 h. A thin layer of PCBM (10 nm) was prepared on the FTO/SnO2 surface at a speed of 2000 rpm and annealed at 100 °C for 10 min. The PCBM solutions were prepared by dissolving 15 mg PCBM in 1 mL chlorobenzene. The perovskite (CH3NH3PbI3) layer (∼320 nm) was then fabricated on the SnO2 film. The film was annealed at 90 °C for 15 min. The hole-transporting materials PTZ-TPA (41 nm) were deposited by spin coating at 4000 rpm for 30 s from a chlorobenzene solution. The thickness of the photosensitive layer was measured using an Ambios Technology (USA) XP-2 profilometer. Finally, a 100 nm-thick Au film was deposited by thermal evaporation (Mbraun MB200) as the cathode. The active area (0.11 cm2) of the devices was determined by the overlap of FTO and the gold electrode. The current density–voltage (J–V) characteristics of the devices were measured with a computer-controlled Keithley (Zolix ss150 Solar Simulator) 236 source meter. The light source was a xenon lamp coupled with an AM1.5 solar spectrum filter; the optical power at the sample was 100 mW cm−2. The incident photon-to-current conversion efficiency (IPCE) spectra were recorded using a solar cell quantum efficiency/external quantum efficiency measurement system (Zolix Solar cell scan 100) model SR830 DSP lock-in amplifier coupled with a WDG3 monochromator and a 500 W xenon lamp.3. Results and discussion
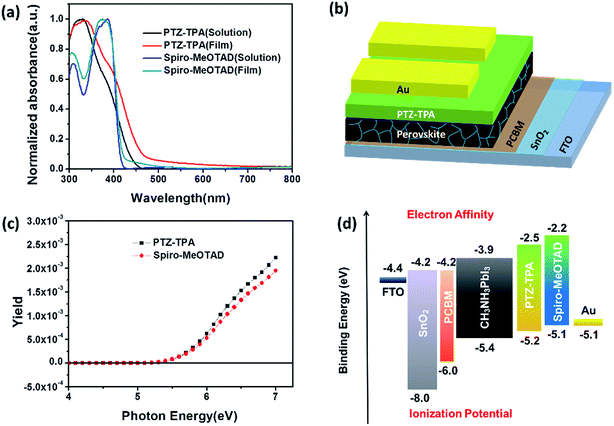
All materials were purchased from Sigma Aldrich and used as received. The synthesis route of PTZ-TPA (Scheme 1) is shorter and less expensive compared with that of Spiro-MeOTAD. The commercially available relatively inexpensive material 3,7-dibromo-10-(4-octylphenyl)-10H-phenothiazine was Suzuki coupled with a triphenylamine derivative31 to give PTZ-TPA in a 79% yield from an unoptimised reaction. NMR spectroscopy and matrix assisted laser desorption/ionization time-of-flight mass spectrometry confirmed the molecular structure of PTZ-TPA (Fig. S1 and S2 ESI†). The thermal gravimetric analysis of PTZ-TPA is shown in Fig. S3 (ESI†). A decomposition temperature (5% weight loss) of 464 °C was observed for PTZ-TPA. The material exhibited an excellent thermal stability. Fig. S4 (ESI†) shows the chemical structure of the PTZ-TPA and Spiro-MeOTAD together with the electronic density distribution of their frontier orbitals as calculated by density functional theory. For PTZ-TPA, the HOMO level shows a π-bonding character, spreading over the whole molecule, while the lowest unoccupied molecular orbital (LUMO) shows a π*-antibonding character, as it is predominately localized on the central phenothiazine unit and on the two benzene units. The calculated HOMO energy of PTZ-TPA (−4.45 eV) is relatively close to that of Spiro-MeOTAD (−4.31 eV), which should promote effective hole transfer from the perovskite layer to PTZ-TPA.32Table 1 summarizes the optical and electrochemical properties of the two HTMs. The optoelectronic properties of PTZ-TPA have been systematically investigated and compared with those of Spiro-MeOTAD. In the UV-vis absorption spectra (shown in Fig. 1a), the absorption peaks of PTZ-TPA and Spiro-MeOTAD can be observed at 338 and 374 nm, respectively. The absorption onset wavelengths of PTZ-TPA and Spiro-MeOTAD are at 459 and 420 nm, respectively, which corresponds to band gaps of 2.70 and 2.95 eV, respectively. The onset of absorption in PTZ-TPA was slightly red shifted with respect to that in Spiro-MeOTAD, which can be attributed to the more extended conjugation in the backbone of PTZ-TPA because of the presence of two extra phenyl rings between the nitrogen atoms. Obtained from the ionization energy measurement system of PTZ-TPA and Spiro-MeOTAD, the HOMO levels of PTZ-TPA and Spiro-MeOTAD are 5.21 and 5.11 eV, respectively, while the reported HOMO energy level of CH3NH3PbI3 is 5.40 eV, which indicates that PTZ-TPA has favourable energetics for hole transfer (Fig. 1c). And the results are given in Table 1 and shown in Fig. 1c. The energetics of the system employing PTZ-TPA/Spiro-MeOTAD are presented in Fig. 1d.
| Compounds | λmaxsol (nm) | λmaxfilm (nm) | λonset (nm) | HOMOa (eV) | LUMOb (eV) | Band gapc (eV) |
|---|---|---|---|---|---|---|
| a HOMO energy level was measured by photoelectron yield spectroscopy.b LUMO calculated by LUMO = HOMO + band gap.c Optical band gap obtained from the onset value of absorption (λonset). | ||||||
| PTZ-TPA | 329 | 338 | 459 | −5.21 | −2.51 | 2.70 |
| Spiro-MeOTAD | 385 | 374 | 420 | −5.11 | −2.16 | 2.95 |
Hole mobility is an important parameter for HTMs. The hole mobilities of PTZ-TPA and Spiro-MeOTAD were estimated using the space-charge limited current method with devices with a structure consisting of indium tin oxide/PEDOT:PSS/PTZ-TPA or Spiro-MeOTAD/Au (Fig. S5†). The extracted hole mobility of the undoped PTZ-TPA was 6.75 × 10−4 cm2 V−1 s−1, which is considerably higher than that of Spiro-MeOTAD (1.06 × 10−5 cm2 V−1 s−1). Therefore, it is a realistic hypothesis that a better photovoltaic performance should be obtained using PTZ-TPA as a HTM in a PSC; such a PSC was thus fabricated and the results discussed in detail later in this report.
We investigated the photovoltaic properties of PTZ-TPA and Spiro-MeOTAD as HTMs in PSCs. The optimized device structure (FTO/SnO2/PC61BM/CH3NH3PbI3/HTMs/Au) is shown in Fig. 1b. The devices were prepared according to protocols reported in the literature.22
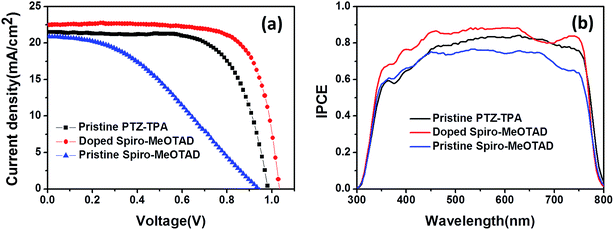
The photovoltaic performances of PSCs employing dopant-free PTZ-TPA as a HTM were measured under AM 1.5G (100 mW cm−2) simulated light illumination. For comparison, a reference cell with Spiro-MeOTAD with and without a Li-TFSI dopant and a 4-tert-butylpyridine (t-BP) additive was also fabricated under similar conditions. The current density–voltage (J–V) characteristics of the perovskite solar cells are shown in Fig. 2 and summarized in Table 2. The PCE of the PSC with the dopant-free PTZ-TPA was 14.3%, with a short circuit photocurrent density (Jsc) of 21.5 mA cm−2, an open circuit voltage (Voc) of 0.982 V, and a fill factor (FF) of 0.679. As shown in Fig. S6,† the stabilized power outputs were 14.00% for the PTZ-TPA-based devices, which is consistent with the obtained PCEs. Under the same fabrication conditions, the best devices based on pristine Spiro-MeOTAD showed a dramatic decrease of the Jsc and FF, resulted in a considerably lower PCE of merely 7.39%, which could be caused largely by the lower hole mobility of pristine Spiro-MeOTAD compared with that of PTZ-TPA. The cell based on doped Spiro-MeOTAD achieved a PCE of 17.1% with a Jsc of 22.5 mA cm−2, a Voc of 1.03 V, and a FF of 0.736. The slightly higher current using Spiro-MeOTAD can be attributed to its higher HOMO level compared with that of PTZ-TPA, which gives Spiro-MeOTAD a higher driving force for charge transfer from the perovskite to the HTM layer. The lower Jsc values obtained for devices based on PTZ-TPA compared with for doped Spiro-MeOTAD is in agreement with the IPCE spectra shown in Fig. 2b. The device that contained Spiro-MeOTAD exhibited a much higher FF, resulting in a significantly increased PCE. The devices with PTZ-TPA showed a slightly lower open circuit voltage (Voc = 0.982 V) than the devices employing Spiro-MeOTAD (Voc = 1.03 V). However, our ionization energy measurement system data show that the HOMO energy level of PTZ-TPA is deeper than that of Spiro-MeOTAD, which should result in a higher Voc. As the Voc depends on (1) the splitting of the Fermi levels for the photogenerated charges, (2) the recombination dynamics, and (3) the energetics of the devices,33–35 the slightly lower voltage in the devices fabricated with PTZ-TPA as the HTM might be caused by a higher recombination rate. This would suggest that PTZ-TPA has slightly inferior charge extraction capabilities compared with Spiro-MeOTAD. The statistics for the device characteristics (Voc, Jsc, FF, and PCE) collected from the same batch are presented in Fig. 3; the data demonstrate the good reproducibility of PSCs employing PTZ-TPA as a HTM.
 | ||
| Fig. 2 (a) J–V characteristics of the PSC devices studied. (b) IPCE spectra of PSC devices with pristine PTZ-TPA, pristine Spiro-MeOTAD, and doped Spiro-MeOTAD as a HTM. | ||
| HTMs | Jsc (mA cm−2) | Voc (V) | FF (%) | PCE (%) |
|---|---|---|---|---|
| a Ten devices fabricated from the same batch and the average performance is shown in parentheses. | ||||
| Doped Spiro-MeOTAD | 22.5 | 1.03 | 73.7 | 17.1 |
| Pristine Spiro-MeOTAD | 20.9 | 0.939 | 37.6 | 7.39 |
| Pristine PTZ-TPAa | 21.5 (21.5 ± 0.57) | 0.982 (0.959 ± 0.022) | 67.9 (65.2 ± 1.9) | 14.3 (13.5 ± 0.48) |
 | ||
| Fig. 3 Statistical data of the photovoltaic parameters for devices containing PTZ-TPA; all data points were collected from the same batch of devices. | ||
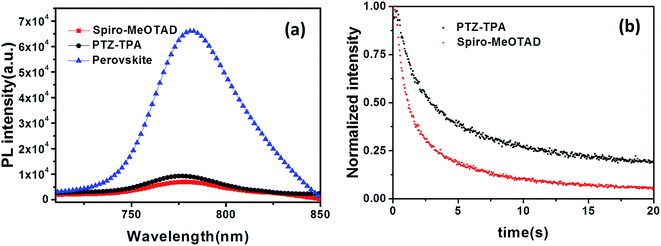
To further understand the origin of the low Voc obtained of the PTZ-TPA devices, the charge-carrier dynamics at the perovskite/HTM interface and hole extraction ability were investigated using steady-state and time-resolved PL (TRPL) measurements; in such measurements efficient quenching of the steady-state PL and a reduction of the PL lifetime would indicate efficient charge extraction at the perovskite/HTM interface. From the PL spectra (Fig. 4a), it is evident that both HTMs significantly quench the perovskite emission signal, with HTM Spiro-MeOTAD having a slightly better PL quenching efficiency compared with PTZ-TPA. The time-resolved PL measurements were conducted with an excitation wavelength of 405 nm and the response was monitored across the entire emission spectral range (Fig. 4b). The CH3NH3PbI3/Spiro-MeOTAD samples showed a decay time of 0.7655 ns, whereas the CH3NH3PbI3/PTZ-TPA samples showed a longer decay lifetime of 1.250 ns. This indicates that Spiro-MeOTAD has a slightly better hole extraction ability than PTZ-TPA. As a result, it is unlikely that further device optimization will enable the device performance of PTZ-TPA to exceed that of Spiro-MeOTAD-based devices. Thus, the much lower cost of PTZ-TPA, owing to its simpler synthesis methods, in combination with its comparable device performance might offer a potentially lower cost/power ratio, which is the ultimate economic criterion for photovoltaic systems.
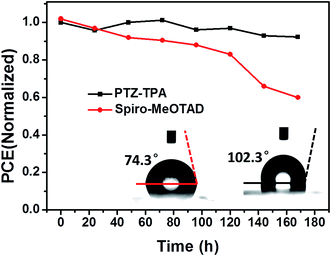
To further demonstrate the improvements using PTZ-TPA yielded for PSCs, we investigated the long-term stability of devices using these HTMs; we performed stability tests of PSCs without encapsulation and under identical storage conditions (storage time = 168 h). As seen in Fig. 5, the PCEs of PSCs with Spiro-MeOTAD (doped) as the HTM retained only 59% of their initial efficiency. However, the PTZ-TPA-based devices exhibited a PCE retention of 92%. This result clearly demonstrates the excellent device stability of PSCs with PTZ-TPA HTMs over the course of a week. As reported by many research groups, higher stabilities are recorded for PSCs with a hydrophobic HTM; the greater the hydrophobicity, the higher the stability.36 The PTZ-TPA film (Fig. 5) shows a larger water contact angle of 102.3° than Spiro-MeOTAD (74.3°), which effectively prevents moisture penetration into the perovskite layer. PTZ-TPA has a sufficient number of alkyl chains to be solution processable. Furthermore, alkyl chains improve hydrophobicity, which in turn improves the stability of the resulting PSCs.26,36
4. Conclusions
In conclusion, a HTM PTZ-TPA-based phenothiazine core was designed and prepared using a simple one-step synthesis procedure using inexpensive commercial precursors with a high yield. We employed the new material as a hole transporting layer in CH3NH3PbI3-based perovskite solar cells. With the PTZ-TPA HTM without dopants a PCE of 14.3% was achieved, which is comparable to that of devices based on the well-known doped HTM Spiro-MeOTAD (17.1%). Long-term stability studies showed that the PTZ-TPA-based devices were more stable when exposed to air than cells employing Spiro-MeOTAD. The low-cost, facile one-step synthetic method as well as the excellent hole mobility and appropriate energy level makes PTZ-TPA a promising candidate to substitute Spiro-MeOTAD in the large scale production of high performance, stable perovskite solar cells.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The work at South University of Science and Technology of China was supported by Special Funds for the Development of Strategic Emerging Industries in Shenzhen (JCYJ20170412154240645). This research was also supported by the National Basic Research Program of China (Fundamental Studies of Perovskite Solar Cells 2015CB932200), and National Natural Science Foundation of China (Nos. 51373076, 91433118, 61605075).References
- M. M. Lee, J. Teuscher, T. Miyasaka, T. N. Murakami and H. J. Snaith, Science, 2012, 338, 643–647 CrossRef CAS PubMed.
- H.-S. Kim, C.-R. Lee, J.-H. Im, K.-B. Lee, T. Moehl, A. Marchioro, S.-J. Moon, R. Humphry-Baker, J.-H. Yum, J. E. Moser, M. Grätzel and N.-G. Park, Sci. Rep., 2012, 2, 591 CrossRef PubMed.
- W. Chen, Y. Wu, Y. Yue, J. Liu, W. Zhang, X. Yang, H. Chen, E. Bi, I. Ashraful, M. Grätzel and L. Han, Science, 2015, 350, 944–948 CrossRef CAS PubMed.
- F. Bella, G. Griffini, J.-P. Correa-Baena, G. Saracco, M. Grätzel, A. Hagfeldt, S. Turri and C. Gerbaldi, Science, 2016, aah4046 Search PubMed.
- A. Kojima, K. Teshima, Y. Shirai and T. Miyasaka, J. Am. Chem. Soc., 2009, 131, 6050–6051 CrossRef CAS PubMed.
- W. S. Yang, B.-W. Park, E. H. Jung, N. J. Jeon, Y. C. Kim, D. U. Lee, S. S. Shin, J. Seo, E. K. Kim, J. H. Noh and S. I. Seok, Science, 2017, 356, 1376–1379 CrossRef CAS PubMed.
- Z. Yu and L. Sun, Adv. Energy Mater., 2015, 5, 1500213 CrossRef.
- S. Ameen, M. A. Rub, S. A. Kosa, K. A. Alamry, M. S. Akhtar, H.-S. Shin, H.-K. Seo, A. M. Asiri and M. K. Nazeeruddin, ChemSusChem, 2016, 9, 10–27 CrossRef CAS PubMed.
- J. Liu, Y. Wu, C. Qin, X. Yang, T. Yasuda, A. Islam, K. Zhang, W. Peng, W. Chen and L. Han, Energy Environ. Sci., 2014, 7, 2963 CAS.
- Y. Liu, Q. Chen, H.-S. Duan, H. Zhou, Y. Yang, H. Chen, S. Luo, T.-B. Song, L. Dou, Z. Hong and Y. Yang, J. Mater. Chem. A, 2015, 3, 11940–11947 CAS.
- Y.-K. Wang, Z.-C. Yuan, G.-Z. Shi, Y.-X. Li, Q. Li, F. Hui, B.-Q. Sun, Z.-Q. Jiang and L.-S. Liao, Adv. Funct. Mater., 2016, 26, 1375–1381 CrossRef CAS.
- J. Zhang, B. Xu, L. Yang, A. Mingorance, C. Ruan, Y. Hua, L. Wang, N. Vlachopoulos, M. Lira-Cantú, G. Boschloo, A. Hagfeldt, L. Sun and E. M. J. Johansson, Adv. Eng. Mater., 2017, 7, 1602736 Search PubMed.
- Y. Liu, Z. Hong, Q. Chen, H. Chen, W.-H. Chang, Y. Yang, T.-B. Song and Y. Yang, Adv. Mater., 2016, 28, 440–446 CrossRef CAS PubMed.
- S. Jeon, U. K. Thakur, D. Lee, W. Yin, D. Kim, S. Lee, T. K. Ahn, H. J. Park and B. G. Kim, Org. Electron., 2016, 37, 134–140 CrossRef CAS.
- F. Zhang, C. Yi, P. Wei, X. Bi, J. Luo, G. Jacopin, S. Wang, X. Li, Y. Xiao, S. M. Zakeeruddin and M. Grätzel, Adv. Eng. Mater., 2016, 6, 1600401 Search PubMed.
- F. Zhang, X. Zhao, C. Yi, D. Bi, X. Bi, P. Wei, X. Liu, S. Wang, X. Li, S. M. Zakeeruddin and M. Grätzel, Dyes Pigm., 2017, 136, 273–277 CrossRef CAS.
- G.-W. Kim, G. Kang, J. Kim, G.-Y. Lee, H. I. Kim, L. Pyeon, J. Lee and T. Park, Energy Environ. Sci., 2016, 9, 2326–2333 CAS.
- F. Wu, B. Wang, R. Wang, Y. Shan, D. Liu, K. Y. Wong, T. Chen and L. Zhu, RSC Adv., 2016, 6, 69365–69369 RSC.
- C. Huang, W. Fu, C.-Z. Li, Z. Zhang, W. Qiu, M. Shi, P. Heremans, A. K. Y. Jen and H. Chen, J. Am. Chem. Soc., 2016, 138, 2528–2531 CrossRef CAS PubMed.
- G. Gong, N. Zhao, D. Ni, J. Chen, Y. Shen, M. Wang and G. Tu, J. Mater. Chem. A, 2016, 4, 3661–3666 CAS.
- X. Liu, X. Zheng, Y. Wang, Z. Chen, F. Yao, Q. Zhang, G. Fang, Z.-K. Chen, W. Huang and Z.-X. Xu, ChemSusChem, 2017, 10, 2833 CrossRef CAS PubMed.
- G. Yang, Y.-L. Wang, J.-J. Xu, H.-W. Lei, C. Chen, H.-Q. Shan, X.-Y. Liu, Z.-X. Xu and G.-J. Fang, Nano Energy, 2017, 31, 322–330 CrossRef CAS.
- M. Cheng, X. Yang, C. Chen, Q. Tan and L. Sun, J. Mater. Chem. A, 2014, 2, 10465–10469 CAS.
- M. Cheng, B. Xu, C. Chen, X. Yang, F. Zhang, Q. Tan, Y. Hua, L. Kloo and L. Sun, Adv. Energy Mater., 2015, 5, 1401720 CrossRef.
- M. Cheng, C. Chen, X. Yang, J. Huang, F. Zhang, B. Xu and L. Sun, Chem. Mater., 2015, 27, 1808–1814 CrossRef CAS.
- Y. Wang, X. Liu, H. Shan, Q. Chen, T. Liu, X. Sun, D. Ma, Z. Zhang, J. Xu and Z.-X. Xu, Dyes Pigm., 2017, 139, 619–626 CrossRef.
- R. Grisorio, B. Roose, S. Colella, A. Listorti, G. P. Suranna and A. Abate, ACS Energy Lett., 2017, 2, 1029–1034 CrossRef.
- Y. Hou, H. Zhang, W. Chen, S. Chen, C. O. R. Quiroz, H. Azimi, A. Osvet, G. J. Matt, E. Zeira, J. Seuring, N. Kausch-Busies, W. Lövenich and C. J. Brabec, Adv. Energy Mater., 2015, 5, 1500543 CrossRef.
- Y. Sun, G. C. Welch, W. L. Leong, C. J. Takacs, G. C. Bazan and A. J. Heeger, Nat. Mater., 2012, 11, 44 CrossRef CAS PubMed.
- W. Ke, G. Fang, Q. Liu, L. Xiong, P. Qin, H. Tao, J. Wang, H. Lei, B. Li, J. Wan, G. Yang and Y. Yan, J. Am. Chem. Soc., 2015, 137, 6730–6733 CrossRef CAS PubMed.
- C. Teng, X. Yang, C. Yang, S. Li, M. Cheng, A. Hagfeldt and L. Sun, J. Phys. Chem. C, 2010, 114, 9101–9110 CAS.
- A. Krishna, D. Sabba, J. Yin, A. Bruno, L. J. Antila, C. Soci, S. Mhaisalkar and A. C. Grimsdale, J. Mater. Chem. A, 2016, 4, 8750–8754 CAS.
- N. J. Jeon, J. Lee, J. H. Noh, M. K. Nazeeruddin, M. Grätzel and S. I. Seok, J. Am. Chem. Soc., 2013, 135, 19087–19090 CrossRef CAS PubMed.
- A. Krishna, D. Sabba, H. Li, J. Yin, P. P. Boix, C. Soci, S. G. Mhaisalkar and A. C. Grimsdale, Chem. Sci., 2014, 5, 2702–2709 RSC.
- A. Krishna, D. Sabba, J. Yin, A. Bruno, P. P. Boix, Y. Gao, H. A. Dewi, G. G. Gurzadyan, C. Soci, S. G. Mhaisalkar and A. C. Grimsdale, Chem.–Eur. J., 2015, 21, 15113–15117 CrossRef CAS PubMed.
- S. S. Reddy, K. Gunasekar, J. H. Heo, S. H. Im, C. S. Kim, D.-H. Kim, J. H. Moon, J. Y. Lee, M. Song and S.-H. Jin, Adv. Mater., 2016, 28, 686–693 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra10677g |
| This journal is © The Royal Society of Chemistry 2017 |