Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Comparison of phytolith-occluded carbon in 51 main cultivated rice (Oryzasativa) cultivars of China
Xing Sun a,
Qin Liu†
a,
Qin Liu†
 *b,
Gengmao Zhao
*b,
Gengmao Zhao c,
Xiang Chen†
c,
Xiang Chen† b,
Tongtong Tang†
b,
Tongtong Tang† b and
Yuyong Xiang
b and
Yuyong Xiang a
a
aSchool of Biological Science and Food Engineering, Chu Zhou University, 239000, Chuzhou, China. E-mail: xsun@chzu.edu.cn; xyy10657@sohu.com
bInstitute of Soil Science, Chinese Academy of Sciences, 210008, Nanjing, Jiangsu Province, China. E-mail: qliu@issas.ac.cn; xchen@issas.ac.cn; tttang@issas.ac.cn; Fax: +86 25 86881263; Tel: +86 25 86881388
cCollege of Resources and Environmental Sciences, Nanjing Agricultural University, 210095, Nanjing, China. E-mail: seawater@njau.edu.cn
First published on 29th November 2017
Abstract
In this study, the carbon (i.e., C) bio-sequestration within phytoliths (PhytOC) in 51 rice cultivars was evaluated to breed cultivars with a high efficiency of carbon sequestration in phytoliths and high productivity. The phytolith extraction from rice plants was achieved through wet digestion procedures, and the C content of phytoliths was determined using an Elemental Analyzer 3000. The phytolith contents in the rice organs ranged from 9.69 to 175.52 mg g−1, with significant differences in the phytolith contents in the different organs of each rice cultivar. The estimated PhytOC fluxes of rice plants in 51 rice cultivars were approximately 0.006–0.035 Mg-e-CO2 per ha per year. High variation coefficients of phytoliths and contents of phytoliths of plant in indica and japonica rice cultivars implied considerable variation among these rice cultivars. Additional results showed no correlation between the phytolith contents and the C content of phytoliths (R = 0.170, p > 0.05), and the C content of phytoliths was significantly correlated with the PhytOC content in dry plant weight (R = 0.804, p < 0.01). However, the estimated PhytOC flux was significantly correlated with the phytolith content (R = 0.651, p < 0.01), with the C content of phytoliths (R = 0.512, p < 0.01) and with the PhytOC content in dry plant weight (R = 0.727, p < 0.01). Selected rice cultivars herein with a high efficiency of C sequestration in phytoliths and high productivity, therefore, played important roles in controlling the C sink and Si biogeochemical cycle in soil-rice systems.
1. Introduction
During growth, many crops, such as rice, bamboo, sugarcane, wheat, millet, wetland plants etc.,1–8 form phytoliths in plant tissues through the absorption of monosilicic acid (Si(OH)4) from soils, during which time the C, nitrogen (i.e., N), and sulphur (i.e., S) elements are occluded in the phytolith.9 During the formation of phytoliths, C can be occluded (PhytOC).10 Compared with other C fractions, PhytOC is relatively stable.6 The formation of phytoliths is complex, and different plant organs might form phytoliths in different ways;11–13 the shapes of phytoliths in different organs are different (e.g., double-peaked, bulliform and paralleled dumbbell phytoliths).6,14 Different plant species organs might also produce different contents of phytoliths.6,8,14–17 Li et al.6 and Prajapati et al.14 reported that the phytolith content in different rice organs (stem, sheath, leaf and grains) ranged from 15.47–143.95 mg g−1 and 12.46–26.39 mg g−1, respectively. Similar results and trends were reported by other researchers.8Some studies have considered that PhytOC is derived through photosynthesis,3,8,18–20 of which 1–6% is typically occluded within phytolith.19 The phytolith content in plant matter varied greatly among different crop types (0.37–8.38%, average 3.68%) and even among crop species of the same crop types.20 Recent studies have shown that the global CO2 sequestration in rice phytoliths is 1.97 × 107 Mg-e-CO2 per year,6 and this value is slightly lower compared with wetland plants (4.39 × 107 Mg-e-CO2 per year)7 and higher compared with bamboo leaf litter (1.56 × 107 Mg-e-CO2 per year),21 sugarcane leaves (0.72 × 107 Mg-e-CO2 per year),22 and millet (2.37 × 106 Mg-e-CO2 per year).4 After plants return to the soil, decomposition through microorganisms is initiated, and phytoliths in straws are released directly into the soil.23–25 Phytoliths can tolerate extreme environments, such as earthquakes, dust storms, floods, forest fire erosion, etc., and are often retained in the in situ environment.10,26–30 The phytolith decomposition rate depends on the solubility of these molecules, which is similar to amorphous Si (10−2.74) and falls between Si glass (10−2.71) and quartz (10−4.00).31 Thus, because of the stability of phytolith, the C occluded within phytoliths is relatively stable and persists in the soil for a millennium, reflecting a strong resistance to decomposition, compared with other organic matter fractions. Indeed, PhytOC can be retained for a thousand years,19,27,28,32,33 and phytoliths can contribute 15–37% of long-term biogeochemical C sequestration.19 Thus, PhytOC plays a major role in the soil C cycle,19 and the importance of this molecule in relation to climate change has been emphasized.3,8,18,34–36
Rice is a staple crop grown on nearly every continent worldwide, with a global rice-planting area of approximately 1.64 × 108 ha in 2014.14 As a Si accumulator, contains several phytoliths and can occlude more organic C in its organs compared with other plants.3 China, as one of the largest crop-producing countries worldwide, has approximately 1.60 × 108 ha of crop lands,37 of which 3.03 × 107 ha is rice cropland.38 Thus, the PhytOC of rice croplands may represent the magnitude of the C sink in cropland ecosystems.3,19 According to the correlations between the phytolith content and the PhytOC content in crops, these results showed that the PhytOC content of plants can be increased by crop species or cultivar optimization.6,20,39 However, the variability of phytolith and PhytOC accumulation within more cultivars of rice has not previously been examined. In the present study, we evaluated PhytOC produced in the rice plants of 51 rice cultivars. The aim of the present study was to select rice cultivars with a high efficiency of C sequestration in phytoliths and high productivity, to increase the C long-term sequestration in phytoliths, reduce CO2 emissions and relieve the environmental stress resulting from greenhouse gas emissions.
2. Methods
2.1 Rice cultivars
The samples of 51 rice cultivars (Oryza sp.), belonging to six varieties, were randomly obtained from six provinces (Jiangxi, Jiangsu, Henan, Heilongjiang, Anhui and Zhejiang) of China in June 2013. The cultivars were derived from the conventional cultivars in the locality, and were used to examine the phytolith accumulation, determine the PhytOC yield, and estimate the PhytOC fluxes in different rice cultivars every year.2.2 Experimental site
In the present study, to avoid the influence of some factors, the root, stem, leaf, sheath and grain of 51 cultivars of rice were grown on the same permeable paddy soil under the same environmental conditions at Changshu Agroecological Experimental Station, Chinese Academy of Sciences. The base is located in the county of Xinzhuang, South Changshu, Suzhou, Jiangsu Province, P. R. China (31°32′88′′N/120°41′88′′E). The geology of the study site comprised the incubation and development of paddy soils in part of Taihu Lake in the Yangtze Delta plain. The climate in this region is a subtropical humid climate of the Central District, with an annual average temperature of 16.6 °C and an annual average rainfall of 1321 mm, and a rice–wheat rotation system prevailed in this region. The initial soil had a pH of 7.54, 39.89 g kg−1 of organic matter, 2.40 g kg−1 of total N, 0.73 g kg−1 of total P, 20.16 g kg−1 of total K, 34.27 mg kg−1 of Na2CO3-extractable P, 101.7 mg kg−1 of NH4OAc-extractable K and 252.3 mg kg−1 of NH4OAc-extractable Si.2.3 Sample preparation
After rice cultivars harvest, each rice plant was separated into five different organs: root, stem, leaf, sheath and grain. All rice samples were rinsed twice in distilled water and placed in an ultrasonic bath for 20 min and subsequently dried at 70 °C for 24 h. After hulling, the rice samples were preserved for phytolith extraction and PhytOC analysis.2.4 Phytolith extraction from rice organs and PhytOC measurements
The phytolith was extracted using a revised wet digestion procedure described in detail by Sun et al.4,40 Phytolith residue assemblages were mounted onto glass slides in Balsam Canada mounting medium. Slides were viewed by scanning electron microscope (SEM) to ensure that no organic material exists according to Parr and Sullivan (Fig. 1).41 The PhytOC was determined using an Elemental Analyzer 3000 (GmbH Company, Germany). | ||
| Fig. 1 SEM images of phytoliths extracted from the rice samples using the wet ashing method according to Zuo (2011) and Sun (2016). | ||
2.5 Statistical analyses
The mean values of all parameters were determined from the measurements of three replicates, and the standard error of the means was calculated. One-way ANOVA was applied to determine the significance of the results between different varieties, and subsequently, Turkey's multiple range tests (p < 0.05) were performed. All of the statistical analyses were performed using SPSS v.17 for Windows.3. Results and discussion
3.1 Phytolith and PhytOC contents of different organs in 51 rice cultivars
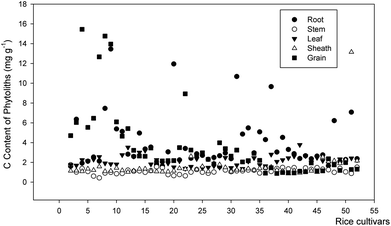
Phytoliths content of plant is affected by many factors like location, soil type, climate, management practices.8,21,42,43 To avoid the influence of these factors, in the present study, the collected rice seeds were planted in a multi-year paddy (the same basic soil nutrient status, the same climate condition and the same water and fertilizer management, etc.) and final estimates of the PhytOC flux artificially unified the biomass. The samples of 51 rice cultivars (Oryza sp.), the conventional cultivars in the locality, were randomly obtained from six provinces of China and were grown on the same permeable paddy soil under the same environmental conditions at Changshu Agroecological Experimental Station, Chinese Academy of Sciences. After harvest, the phytoliths extracted from the rice samples using the wet ashing method and the SEM image of phytolith was showed at Fig. 1. The phytolith contents of rice organs (root, stem, sheath, leaf and grains) were different. The phytolith contents ranged from 9.69 to 175.52 mg g−1 (Fig. 2). The phytoliths content of root, sheath and leaf were higher than these of stem and grains. The phytolith content of grains was lowest of the rice organs. The shape of phytoliths in different organs were different (e.g., double-peaked, bulliform and paralleled dumbbell phytoliths).6,14 Li et al.6 and Prajapati et al.14 reported that the phytoliths content in different rice organs (stem, sheath, leaf and grains) ranged from 15.47–143.95 mg g−1 and 12.46–26.39 mg g−1, respectively. The similar results and trends were reported by other researcher.8In addition, the C contents of phytoliths substantially varied in different rice cultivars. The C contents of phytoliths ranged from 0.43 to 15.46 mg g−1 (Fig. 3), and the PhytOC content in dry organs weight ranged from 0.017 to 0.97 mg g−1 (Fig. 4). The phytolith content in plant matter greatly varied among different crop types (0.37–8.38%, average 3.68%) and even among crop species of the same crop types.20 The similar results and trends were reported by many researchers.6,8,14 Many studies analysing the physical and chemical properties of phytoliths have shown that the phytoliths can occlude C to levels ranging from 2 to 58 mg g−1.34 The occlusion of C within phytoliths has been retained in soils for more than a millennium, generating an important long-term terrestrial C fraction.28,38–40 Thus, appropriate measures can improve the phytolith content of crops through enhancing soil available Si contents,39 aboveground net primary productivity,44 cultivar selection,4,21,22 nitrogen application,43 and basalt powder amendment,8 etc. Its mechanism will be considered in future work.
The production of PhytOC fluxes in rice organs are depends primarily on the dry weight, phytolith content, C content of phytolith of various plant parts and the total dry biomass.6,14 According to the before data, we estimated PhytOC fluxes in rice, and these results showed different fluxes of PhytOC in 51 rice cultivars (from 0.12 to 8.95 kg-e-CO2 per ha per year) (Fig. 5). The estimated PhytOC fluxes of different organs (root, stem, sheath, leaf and grains) ranged from 0.12 to 8.95 kg-e-CO2 per ha per year, 0.43 to 1.55 kg-e-CO2 per ha per year, 1.28 to 6.24 kg-e-CO2 per ha per year, 1.20 to 5.69 kg-e-CO2 per ha per year, 0.44 to 6.57 kg-e-CO2 per ha per year, respectively. The estimated PhytOC fluxes of stem were lowest of the rice organs.
3.2 Phytolith and PhytOC contents in 51 rice cultivars
The phytolith and C contents of phytoliths in 51 rice cultivars were significantly different (Tables 1 and 2). The phytolith contents ranged from 53.83 to 77.15 mg g−1 in indica rice cultivars and 45.64 to 78.89 mg g−1 in japonica rice cultivars. The ranges of C contents of phytoliths in indica and japonica rice cultivars were 1.21 to 7.21 mg g−1 and 1.56 to 5.24 mg g−1, respectively, and the PhytOC content in dry plant weight ranged from 0.09 to 0.51 mg g−1 and 0.08 to 0.38 mg g−1. According to the before data and biomass of single rice crops, we estimated PhytOC fluxes in rice, and these results showed different PhytOC fluxes in 19 indica rice cultivars (from 0.007 to 0.035 Mg-e-CO2 per ha per year) (Table 1) and 32 japonica rice cultivars (from 0.006 Mg-e-CO2 per ha per year to 0.035 Mg-e-CO2 per ha per year); these values were significantly different among the 51 rice cultivars. Many workers reported that the PhytOC sequestration rate can be obtained by selecting high PhytOC cultivars.2,6,8,21 This study also showed that high PhytOC sequestration rate rice cultivars may be selected in indica rice and japonica rice cultivars.| Cultivars | Phytolith content (mg g−1) | C content of phytoliths (mg g−1) | PhytOC content in dry plant weight (mg g−1) | Estimated PhytOC fluxes (Mg–CO2 per ha per year) |
|---|---|---|---|---|
| a Data obtained from the Changshu Agroecological Experimental Station, Chinese Academy of Sciences. | ||||
| Zhonghan 35 | 59.47 ± 6.45ab | 5.09 ± 3.96ab | 0.31 ± 0.24abc | 0.011–0.023 |
| Guichao 2 | 53.83 ± 14.76b | 4.06 ± 2.35abc | 0.21 ± 0.09bc | 0.012–0.025 |
| Xinliangyou 6 | 60.31 ± 5.13ab | 1.21 ± 0.02c | 0.09 ± 0.01c | 0.007–0.015 |
| Rongyou 463 | 67.04 ± 1.56ab | 4.11 ± 0.31abc | 0.29 ± 0.03abc | 0.013–0.027 |
| Tianyou 998 | 77.15 ± 17.96a | 7.21 ± 5.28a | 0.50 ± 0.30a | 0.018–0.035 |
| Yueyou 9114 | 74.63 ± 18.71 ab | 3.13 ± 0.72bc | 0.24 ± 0.05bc | 0.011–0.022 |
| Gangyou 188 | 59.48 ± 2.17ab | 1.80 ± 0.17bc | 0.11 ± 0.01c | 0.009–0.019 |
| IIyou 1259 | 60.32 ± 5.23ab | 2.05 ± 0.40bc | 0.13 ± 0.01c | 0.012–0.025 |
| IIyou 501 | 72.45 ± 11.83 ab | 1.94 ± 0.31bc | 0.15 ± 0.04b | 0.012–0.024 |
| Fuyou 21 | 64.89 ± 14.85 ab | 3.43 ± 1.47bc | 0.24 ± 0.15bc | 0.018–0.035 |
| Chuannong 1 | 60.21 ± 2.86ab | 1.54 ± 0.30bc | 0.10 ± 0.01c | 0.009–0.018 |
| Zhongyou 7 | 61.86 ± 6.44ab | 1.40 ± 0.40bc | 0.10 ± 0.03c | 0.009–0.019 |
| Yixiang 2079 | 68.96 ± 4.43ab | 1.46 ± 0.11bc | 0.11 ± 0.00c | 0.010–0.021 |
| IIyou 1313 | 60.06 ± 3.87ab | 1.29 ± 0.11bc | 0.09 ± 0.01c | 0.008–0.017 |
| Yixiang 725 | 74.17 ± 18.94 ab | 1.36 ± 0.40bc | 0.12 ± 0.06c | 0.010–0.020 |
| Nenyou 8015 | 64.18 ± 7.53ab | 1.85 ± 0.40bc | 0.14 ± 0.03c | 0.011–0.022 |
| Qianyou 0508 | 82.00 ± 21.83a | 2.28 ± 0.27bc | 0.17 ± 0.04ab | 0.014–0.027 |
| Tenuo 2072 | 60.41 ± 9.32ab | 1.64 ± 0.46bc | 0.11 ± 0.04c | 0.009–0.018 |
| Zhenzhunuo | 64.73 ± 4.75ab | 2.32 ± 1.03bc | 0.16 ± 0.08bc | 0.012–0.025 |
| Cultivars | Phytolith content (mg g−1) | C content of phytoliths (mg g−1) | PhytOC content in dry plant weight (mg g−1) | Estimated PhytOC fluxes (Mg–CO2 per ha per year) |
|---|---|---|---|---|
| a Data obtained from the Changshu Agroecological Experimental station, Chinese Academy of Sciences. | ||||
| Huaidao 5 | 62.96 ± 6.02abc | 4.17 ± 1.38ab | 0.27 ± 0.07abc | 0.010–0.021 |
| Xindao 18 | 46.80 ± 13.99bc | 5.02 ± 2.89a | 0.23 ± 0.10bcde | 0.006–0.013 |
| Wuyunjeng 7 | 65.55 ± 6.53abc | 4.04 ± 1.68abc | 0.27 ± 0.10abcd | 0.012–0.024 |
| Xindao 20 | 53.37 ± 2.83abc | 3.55 ± 1.36abcde | 0.20 ± 0.06bcdef | 0.010–0.019 |
| Shengdao 16 | 64.54 ± 1.23abc | 2.11 ± 0.44def | 0.15 ± 0.03bc | 0.008–0.015 |
| Huaidao 11 | 78.06 ± 9.66ab | 2.10 ± 0.34def | 0.18 ± 0.05bcdef | 0.009–0.018 |
| Zhendao 99 | 54.24 ± 3.42abc | 1.96 ± 0.33def | 0.12 ± 0.02def | 0.006–0.012 |
| Lianjing 11 | 62.86 ± 9.66abc | 2.17 ± 0.43cdef | 0.15 ± 0.03bc | 0.009–0.018 |
| Yanjing 47-12 | 65.55 ± 3.87 abc | 2.26 ± 0.50cdef | 0.16 ± 0.05cdef | 0.010–0.019 |
| Wuyunjing 21 | 69.43 ± 8.45 abc | 1.91 ± 0.32def | 0.15 ± 0.04cdef | 0.008–0.016 |
| Zhengdao 18 | 45.64 ± 1.01c | 2.19 ± 0.70cdef | 0.11 ± 0.03ef | 0.006–0.012 |
| Jin G2 | 78.89 ± 21.24a | 3.65 ± 1.50abcd | 0.31 ± 0.20 ab | 0.018–0.035 |
| Zhonghua 11 | 58.80 ± 17.19 abc | 2.39 ± 0.15bcdef | 0.15 ± 0.04cdef | 0.012–0.024 |
| Ribenqing | 53.82 ± 11.19 abc | 2.41 ± 1.21bcdef | 0.15 ± 0.10cdef | 0.006–0.013 |
| Fengguan 16 | 53.90 ± 9.69 abc | 2.09 ± 0.17def | 0.13 ± 0.02def | 0.007–0.015 |
| Gangyouguba | 78.40 ± 22.26 ab | 2.21 ± 0.97cdef | 0.21 ± 0.14bcdef | 0.011–0.022 |
| Longjing 38 | 63.56 ± 6.31 abc | 2.09 ± 0.24def | 0.14 ± 0.03cdef | 0.011–0.021 |
| Longdao 12 | 57.63 ± 6.65 abc | 1.62 ± 0.25ef | 0.11 ± 0.01ef | 0.007–0.014 |
| Longjing 21 | 48.45 ± 2.80 abc | 1.76 ± 0.11def | 0.10 ± 0.01ef | 0.006–0.012 |
| Longjing 36 | 56.10 ± 10.32abc | 1.80 ± 0.04def | 0.11 ± 0.02ef | 0.008–0.016 |
| Suidao 3 | 48.38 ± 5.18abc | 1.54 ± 0.35f | 0.09 ± 0.02ef | 0.007–0.013 |
| Jixidao 1 | 54.67 ± 17.18abc | 1.44 ± 0.13f | 0.09 ± 0.03ef | 0.006–0.012 |
| Nanjing 5055 | 52.41 ± 9.31abc | 1.29 ± 0.11f | 0.08 ± 0.01f | 0.006–0.012 |
| Nanjing 46 | 53.44 ± 8.36abc | 1.51 ± 0.19f | 0.09 ± 0.01ef | 0.006–0.012 |
| Xiushui 09 | 52.75 ± 5.98abc | 1.68 ± 0.14ef | 0.10 ± 0.01ef | 0.008–0.016 |
| Zhejing 88 | 64.21 ± 5.42abc | 2.02 ± 0.26def | 0.14 ± 0.01cdef | 0.09–0.019 |
| Xiushui 134 | 65.50 ± 3.65abc | 1.51 ± 0.68f | 0.11 ± 0.04ef | 0.008–0.017 |
| 8you 682 | 63.84 ± 9.29abc | 2.61 ± 0.58bcdef | 0.18 ± 0.06bcdef | 0.011–0.022 |
| Zheyou 12 | 68.42 ± 4.61abc | 1.62 ± 0.19ef | 0.12 ± 0.01def | 0.010–0.019 |
| Wuxiangnuo 8333 | 72.17 ± 3.99abc | 5.05 ± 2.88a | 0.38 ± 0.22a | 0.011–0.023 |
| Sujingnuo 1 | 66.11 ± 9.83abc | 1.92 ± 0.52def | 0.14 ± 0.05cdef | 0.008–0.017 |
| Zhenuo 65 | 51.84 ± 12.49abc | 1.78 ± 0.32def | 0.10 ± 0.01ef | 0.007–0.014 |
In the present study, we estimated that the C fluxes of the rice plants were 0.006 and 0.035 Mg-e-CO2 per ha per year in terms of the PhytOC in rice plant material on a dry weight basis.45 However, Li, Z. et al.6 and Prajapati et al.14 reported that the flux of the rice PhytOC is 0.03–0.13 Mg-e-CO2 per ha per year, and 0.05–0.12 Mg-e-CO2 per ha per year, which was higher than the results obtained in the present study.6 The differences between these results might reflect (i) the number of tested cultivars (i.e., this manuscript tested 51 rice cultivars, and Li et al.6 tested only 5 rice cultivars); (ii) the Si content of the paddy field, for example, previous studies have demonstrated that soil with high Si could enhance the phytolith content in crops;46–48 and (iii) the tested measure (i.e., this manuscript examined a revision to the wet digestion method, and Li et al. examined the microwave digestion method6). But still it is likely that breeding for high PhytOC rice cultivars would result in more amount of securely bio-sequestered C in rice crops.
3.3 Phytolith and PhytOC contents in 6 rice varieties
The phytolith and C contents of phytoliths in 6 rice varieties were not significantly different. The phytolith contents ranged from 57.87 to 67.52 mg g−1 in 6 rice varieties. The C contents of phytoliths ranged from 2.10 to 3.05 mg g−1, and subsequently, the PhytOC content in dry plant weight ranged from 0.13 to 0.21 mg g−1. According to the before data and biomass of single rice crops, we estimated PhytOC fluxes in rice, and these results showed no difference in the PhytOC fluxes in 6 rice cultivars (from 0.009 to 0.012 Mg-e-CO2 per ha per year) (Table 3), thus, there were no significant differences among the 6 rice varieties.| Varieties | n | Phytolith content (mg g−1) | C content of phytoliths (mg g−1) | PhytOC content in dry plant weight (mg g−1) | Estimated PhytOC fluxes (Mg–CO2 per ha per year) |
|---|---|---|---|---|---|
| a Data obtained from the Changshu Agroecological Experimental Station, Chinese Academy of Sciences. | |||||
| Indica rice | 3 | 57.87 ± 3.52a | 2.90 ± 1.39a | 0.21 ± 0.11a | 0.010–0.021 |
| Indica three line hybrid rice | 14 | 67.21 ± 6.00a | 2.46 ± 1.10a | 0.19 ± 0.12a | 0.012–0.024 |
| Indica glutinous rice | 2 | 62.57 ± 3.06a | 2.10 ± 0.46a | 0.13 ± 0.03a | 0.011–0.022 |
| Japonica rice | 27 | 59.63 ± 9.35a | 2.54 ± 0.91a | 0.15 ± 0.06a | 0.009–0.017 |
| Japonica three line hybrid rice | 2 | 67.52 ± 5.20a | 2.32 ± 0.68a | 0.15 ± 0.02a | 0.010–0.021 |
| Japonica glutinous rice | 3 | 63.38 ± 10.44a | 3.09 ± 1.86a | 0.21 ± 0.15a | 0.009–0.018 |
3.4 The potential of securely bio-sequestered C in rice cultivars
As shown in Table 4 (Panels A and B), the coefficient of the variation in the phytolith and C contents of phytoliths of plants of indica and japonica rice cultivars was high, illustrating considerable variation among these rice cultivars. Compared with japonica rice cultivars, the coefficient of variation for the phytolith content and C contents of phytoliths of plants of the indica rice cultivars was higher, indicating that the variation among indica rice cultivars was greater (Table 4). The results demonstrated that there was no relationship (R = 0.170, p > 0.05) between the C contents of phytoliths and the phytolith content of these materials (Table 5). It has previously been shown that the C contents of phytoliths in bamboo, wheat, sugarcane, and millet is not directly correlated with the phytolith content absorbed by the plant.4,21,22,42 These data indicate that for rice, wheat, bamboo and sugarcane, it is the efficiency by which C is encapsulated by Si within the epidermal cell walls (phytoliths), rather than the actual quantity of Si taken up by the plant, that is most important to determining the relative PhytOC yield in plant materials.| Parameters | Phytolith content (mg g−1) | C content of phytoliths (mg g−1) | PhytOC content in dry plant weight (mg g−1) | Estimated PhytOC fluxes (Mg–CO2 per ha per year) |
|---|---|---|---|---|
| Panel A: indica rice cultivars | ||||
| Minimum value | 53.38 | 1.44 | 0.09 | 0.007 |
| Maximum value | 77.15 | 7.21 | 0.51 | 0.018 |
| Range | 23.32 | 5.77 | 0.42 | 0.010 |
| Mean | 65.25 | 2.62 | 0.19 | 0.011 |
| Standard deviation | 6.38 | 1.44 | 0.11 | 0.003 |
| Coefficient of variation (%) | 9.78 | 55.02 | 60.57 | 24.01 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Panel B: japonica rice cultivars | ||||
| Minimum value | 45.64 | 1.56 | 0.08 | 0.006 |
| Maximum value | 78.89 | 5.24 | 0.38 | 0.018 |
| Range | 33.25 | 3.68 | 0.30 | 0.012 |
| Mean | 60.47 | 2.58 | 0.16 | 0.009 |
| Standard deviation | 9.26 | 0.98 | 0.07 | 0.003 |
| Coefficient of variation (%) | 15.32 | 38.04 | 44.50 | 29.16 |
| Variables | Phytolith content | C content of phytoliths | PhytOC content in dry plant weight | Estimated PhytOC fluxes |
|---|---|---|---|---|
| a Correlation is significant at the 0.01 level (2-tailed). | ||||
| Phytolith content | 1 | |||
| C content of phytoliths | 0.170 | 1 | ||
| PhytOC content in dry plant weight | 0.505a | 0.804a | 1 | |
| Estimated PhytOC fluxes | 0.651a | 0.512a | 0.727a | 1 |
In fact, the C contents of phytoliths primarily depended on the efficiency of the C occluded within phytoliths during plant growth. Genetic and physiological differences within rice cultivars could also affect the formation and efficiency of the C occluded within phytoliths.48 However, the C contents of phytoliths was significantly correlated with the PhytOC content in dry plant weight in the 51 rice cultivars (R = 0.804, p < 0.01) (Table 5) and with the PhytOC content in dry plant weight in 6 rice varieties (R = 0.864, p < 0.05) (Table 6). Similarly Guo et al., Li et al. and Prajapati et al. reported that the PhytOC content in dry plant weight was the significantly correlated with the content of phytoliths and the C content of phytoliths, implying that the PhytOC content in dry plant weight depends not only on the content of phytolith but also on the C content of phytolith.6,8,14 Thus, how to increase phytolith content and the C content of phytoliths will require further in-depth study.
| Variables | Phytolith content | C content of phytoliths | PhytOC content in dry plant weight | Estimated PhytOC fluxes |
|---|---|---|---|---|
| a Correlation is significant at the 0.01 level (2-tailed). | ||||
| Phytolith content | 1 | |||
| C content of phytoliths | −0.392 | 1 | ||
| PhytOC content in dry plant weight | −0.076 | 0.864a | 1 | |
| Estimated PhytOC fluxes | −0.042 | −0.630 | −0.670 | 1 |
The global rice-planting area was approximately 1.64 × 108 ha in 2014.14 Considering that the largest PhytOC flux of rice plants was 0.035 Mg-e-CO2 per ha per year, globally, 5.74 × 106 Mg-e-CO2 would have been occluded within the phytolith of rice plants per year,6 although the annual CO2 occlusion within the rice phytoliths of the unit area is likely lower than that of other plants, such as bamboo leaf litter (1.56 × 107 Mg-e-CO2 per year)30 and wetland plants (4.39 × 107 Mg-e-CO2 per year),8 and grasslands (4.14 × 107 Mg-e-CO2 per year),3 and higher than millet (2.37 × 106 Mg-e-CO2 per year)5 and sugarcane leaf (0.72 × 107 Mg-e-CO2 per year).31 In this study we had showed that estimated PhytOC fluxes in rice cultivars varied considerably between different rice cultivars. So the selection of high phytOC rice cultivars became one of effective measure to improve securely bio-sequestered C in rice crops.
4. Conclusion
The C contents of phytoliths was significantly different among the 51 rice cultivars. The C contents of phytoliths ranged from 1.21 to 7.21 mg g−1, showing high coefficient of variation among rice cultivars. In addition, high variation coefficients of phytolith and contents of phytoliths of plant in indica and japonica rice cultivars implied considerable variation among these rice cultivars. This provides us a feasible way to select rice varieties with high PhytOC content. In this study, we estimated that the PhytOC flux of rice cultivars ranges from 0.006 to 0.035 Mg-e-CO2 per ha per year. Totally, rice plants might sequester 0.18 × 106 to 1.06 × 106 t CO2 equivalents per year in China. Given the global rice area of 1.64 × 108 ha and the largest flux (0.035 Mg-e-CO2 per ha per year) of the estimated PhytOC flux of rice plants, that approximately 5.43 × 106 t CO2 equivalents per year would have been sequestrated in the phytoliths of rice plants all over the world. Therefore, selection/breeding of rice cultivars with high PhytOC contents might provide a new approach for increasing atmospheric CO2 bio-sequestration through phytoliths, offering a potential opportunity.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work was partially supported through the National Natural Science Foundation of China (No. 41271208), the National Natural Science Foundation of China (No. 31400464), the National Natural Science Foundation of China (No. 31370422), and Anhui Province University Natural Science Research Foundation (KJ2017A423), and Chuzhou University scientific research Foundation (2016qd11).The authors would like to thank Ms Wen Feng and Ms Yilan Liu for assistance with sampling.References
- C. C. Perry, R. J. P. Williams and S. C. Fry, J. Plant Physiol., 1987, 126, 437–448 CrossRef CAS.
- S. Rajendiran, M. V. Coumar, S. Kundu, M. L. Dotaniya and A. S. Rao, Curr. Sci., 2012, 103, 911–920 Search PubMed.
- Z. Song, H. Liu, Y. Si and Y. Yin, GCB Bioenergy, 2012, 18, 3647–3653 Search PubMed.
- X. X. Zuo and H. Y. Lü, Chin. Sci. Bull., 2011, 56, 3451–3456 CrossRef CAS.
- W. Pan, Z. Song, H. Liu, K. Müeller, X. Yang, X. Zhang, Z. Li, X. Liu, S. Qiu and Q. Hao, Geoderma, 2017, 308, 86–92 CrossRef CAS.
- Z. Li, Z. Song, J. F. Parr and H. Wang, Plant Soil, 2013, 370, 615–623 CrossRef CAS.
- Z. Li, Z. Song and P. Jiang, Chin. Sci. Bull., 2013, 58, 2480–2487 CrossRef CAS.
- F. Guo, Z. Song, L. Sullivan, H. Wang, X. Liu, X. Wang, Z. Li and Y. Zhao, Sci. Bull., 2015, 60, 591–597 CrossRef CAS.
- Y. J. Wang, J. Oceanogr. Huanghai Bohai Seas, 1998, 16, 33–38 Search PubMed.
- L. H. P. Jones and A. A. Milne, Plant Soil, 1963, 18, 358–371 CrossRef CAS.
- N. Ru, Z. Song, H. Liu, X. Liu, F. Guo, X. Zhang and X. Wu, Silicon, 2016, 1–10 Search PubMed.
- I. E. Pamirsky, A. G. Klykov, G. A. Murugova and K. S. Golokhvast, IOP Conf. Ser.: Mater. Sci. Eng., 2017, 225 DOI:10.1088/1757-1899X/1225/1081/012238.
- T. K. Trinh, T. T. H. Nguyen, N. N. Tu, T. Y. Wu, A. A. Meharg and M. N. Nguyen, Soil Tillage Res., 2017, 171, 19–24 CrossRef.
- K. Prajapati, S. Rajendiran, M. Vassanda Coumar, M. L. Dotaniya, Ajay, S. Kundu, J. K. Saha and A. K. Patra, Appl. Ecol. Environ. Res., 2016, 14, 265–281 CrossRef.
- L. Qi, F. Y. Li, Z. Huang, P. Jiang, T. Baoyin and H. Wang, Sci. Total Environ., 2017, 577, 413–417 CrossRef CAS PubMed.
- W. Pan, Z. Song, H. Liu, L. V. Zwieten, Y. Li, X. Yang, Y. Han, X. Liu, X. Zhang and Z. Xu, J. Soils Sediments, 2017, 17, 2420–2427 CrossRef CAS.
- Z. Song, K. Mcgrouther and H. Wang, Earth-Sci. Rev., 2017 DOI:10.1016/j.earscirev.2016.1011.1001.
- B. Li, Z. Song, H. Wang, Z. Li, P. Jiang and G. Zhou, Sci. Rep., 2014, 4, 52–62 Search PubMed.
- J. F. Parr and L. A. Sullivan, Soil Biol. Biochem., 2005, 37, 117–124 CrossRef CAS.
- Z. Song, H. Wang, P. J. Strong and F. Guo, Eur. J. Agron., 2014b, 53, 10–15 Search PubMed.
- J. F. Parr, L. Sullivan, B. Chen, Y. E. Gongfu and W. Zheng, GCB Bioenergy, 2010, 16, 2661–2667 Search PubMed.
- J. Parr, L. Sullivan and R. Quirk, Sugar Tech, 2009, 11, 17–21 CrossRef CAS.
- Z. Song, H. Liu, S. Cae, X. Yang and X. Zhang, Sci. Total Environ., 2017, 603–604, 502–509 CrossRef CAS PubMed.
- Z. Song, K. Mcgrouther and H. Wang, Earth-Sci. Rev., 2016, 158, 19–30 CrossRef CAS.
- Z. Ji, X. Yang, Z. Song, H. Liu, X. Liu, S. Qiu, J. Li, F. Guo, Y. Wu and X. Zhang, Grass Forage Sci., 2017 DOI:10.1111/gfs.12316.
- L. H. P. Jones, K. A. Handreck and A. G. Norman, Adv. Agron., 1967, 19, 107–149 CAS.
- L. P. Wilding, R. E. Brown and N. Holowaychuk, Soil Sci., 1967, 103, 56–61 CrossRef CAS.
- L. P. Wilding, Clay Clay Miner., 1974, 22, 295–306 CAS.
- D. M. Hart and G. S. Humphreys, Quaternary Australia, 1997, 15, 17–25 Search PubMed.
- J. F. Parr, Geoarchaeology, 2006, 21, 171–185 CrossRef.
- W. L. Lindsay, Clay Clay Miner., 1979, 28, 319 Search PubMed.
- C. A. E. Strömberg, Palaeogeogr., Palaeoclimatol., Palaeoecol., 2004, 207, 239–275 CrossRef.
- V. Prasad, C. A. Strömberg, H. Alimohammadian and A. Sahni, Science, 2005, 310, 1177–1180 CrossRef CAS PubMed.
- R. M. Gifford, Aust. J. Plant Physiol., 1994, 21, 1–15 CrossRef.
- P. Falkowski, R. J. Scholes, E. Boyle, J. Canadell, D. Canfield, J. Elser, N. Gruber, K. Hibbard, P. Högberg and S. Linder, Science, 2000, 290, 291–296 CrossRef CAS PubMed.
- S. Kosten, F. Roland, D. M. L. D. M. Marques, E. H. V. Nes, N. Mazzeo, L. D. S. L. Sternberg, M. Scheffer and J. J. Cole, Global Biogeochem. Cycles, 2010, 24, 1063 CrossRef.
- S. Piao, J. Fang, P. Ciais, P. Peylin, Y. Huang, S. Sitch and T. Wang, Nature, 2009, 458, 1009–1013 CrossRef CAS PubMed.
- D. F. Zhu, Y. P. Zhang, H. Z. Chen, J. Xiang and Y. K. Zhang, Sci. Agric. Sin., 2015, 48, 3404–3414 Search PubMed.
- Z. Song, K. Müller and H. Wang, Earth-Sci. Rev., 2014a, 139, 268–278 Search PubMed.
- X. Sun, Q. Liu, J. Gu, X. Chen and K. Zhu, Front. Earth Sci., 2016, 10, 683–690 CrossRef CAS.
- J. F. Parr and L. A. Sullivan, Plant Soil, 2014, 374, 45–53 CrossRef CAS.
- J. F. Parr and L. A. Sullivan, Plant Soil, 2011, 342, 165–171 CrossRef CAS.
- Y. Zhao, Z. Song, X. Xu, H. Liu, X. Wu, Z. Li, F. Guo and W. Pan, Ecol. Res., 2016, 31, 117–123 CrossRef CAS.
- S. W. Blecker, R. L. Mcculley, O. A. Chadwick and E. F. Kelly, Global Biogeochem. Cycles, 2006, 20, 4253–4274 CrossRef.
- S. Wang, C. Zhang, F. Hu, K. Zeng, W. Zhang and W. Wang, Chinese Agr. Sci. Bull., 2008, 24, 201–205 Search PubMed , (in Chinese).
- J. Alvarez and L. E. Datnoff, Crop Prot., 2001, 20, 43–48 CrossRef CAS.
- Y. Liang, H. Hua, Y. G. Zhu, J. Zhang, C. Cheng and V. Römheld, New Phytol., 2006, 172, 63–72 CrossRef CAS PubMed.
- J. Mecfel, S. Hinke, W. A. Goedel, G. Marx, R. Fehlhaber, E. Bäucker and O. Wienhaus, J. Plant Nutr. Soil Sci., 2010, 170, 769–772 CrossRef.
Footnote |
| † Present/permanent address: Institute of Soil Science, Chinese Academy of Sciences, 71 East Beijing Road, Nanjing, Jiangsu Province, 210008, P. R. China. |
| This journal is © The Royal Society of Chemistry 2017 |