Open Access Article
Open Access ArticleComparative evaluations on phenolic antioxidants of nine adulterants and anti-inflammation of four alternatives with their original herb Erycibe schmidtii†
Qiang Xue,
Hang Fan,
Ke Li,
Lingguang Yang,
Liwei Sun* and
Yujun Liu *
*
National Engineering Laboratory for Tree Breeding, College of Biological Sciences and Biotechnology, Beijing Forestry University, Beijing 100083, China. E-mail: xuetian20130607@163.com; fwqh1990@163.com; like17931@163.com; yanglingguangxdjqz@163.com; lsun2013@bjfu.edu.cn; yjliubio@bjfu.edu.cn
First published on 3rd November 2017
Abstract
Erycibe schmidtii is widely used as folk medicine in China for treatments of various inflammations. Recently, with reduction of its wild sources, various adulterants have been misused as substitutes. To distinguish reliable alternatives from various adulterants, total phenolics and antioxidant activities of E. schmidtii and its nine adulterants, as well as three marker compounds of E. schmidtii defined by Chinese Pharmacopoeia were simultaneously quantified. And HPLC fingerprints of these ten herbs were established. Compared with E. schmidtii (S1), Porana sinensis (S2), Porana sinensis var. delavayi (S3), Celastrus hindsii (S4), and Morinda umbellata (S5) exhibited similar or higher total phenols, flavonoids and tannins along with similar or greater capacities scavenging DPPH˙, ABTS+˙ and AAPH˙, contained similar or higher total amounts of the three marker compounds, and possessed higher similarities in HPLC profiles. The other five adulterants (S6–S10, i.e., Illigera parviflora, Morinda parvifolia, Piper puberulum, Piper kadsura and Iodes seguini in sequence) were identified as absolute fakes, thus were excluded from alternatives of S1. Further anti-inflammatory experiments with LPS-induced RAW264.7 macrophages cells on NO release and transcriptions of inflammatory factors iNOS, COX-2, IL-6 and IL-1β showed that S2 and S3 possessed higher anti-inflammation activities than and similar mechanisms to those of S1. Taken together, S2 and S3 could be the best potentially alternatives of E. schmidtii among the nine adulterants. S4 and S5 might be also considered as alternatives for they contained similar fundamental compounds and equally impressive anti-inflammatory potential with E. schmidtii concerning iNOS and COX-2.
1. Introduction
Erycibe schmidtii Craib (Convolvulaceae) is a traditional Chinese herb, which is mainly distributed in Southeast Asia and Australia. In China, it is found in Southern provinces such as Guangdong and Guangxi, and its roots and stems are widely used as folk medicines for treatments of various inflammations. Recently, with reduction of wild E. schmidtii sources,1 at least nine adulterants as listed below in Materials and methods have emerged in herbal markets. However, few studies have carried out on comparison of E. schmidtii with its adulterants. Therefore, comparing and finding out its potentially effective alternatives are of great significance as well as urgent.Phenolics, a large group of plant secondary metabolites, including simple phenols, flavonoids, anthocyanins and tannins, have attracted more and more attention in recent years due to their antioxidant, antimicrobial, anticancer, antifungal, and anti-inflammatory activities.2–6 Previous phytochemical investigations revealed that main constituents of E. schmidtii are coumarins, chlorogenic acid derivatives, and flavonoids.7–9 Among them, chlorogenic acid and two coumarins, i.e., scopoletin and scopolin, are chemical markers that are determined as standards by the Chinese Pharmacopoeia for discriminating E. schmidtii from its various adulterants.
Moderate oxidative stress is necessary for aerobic life. However, under certain conditions, it can also be toxic thus must be responsible for causing a variety of diseases, including arthritis, cancer, cardiovascular diseases, cataracts, atherosclerosis, diabetes, immune deficiency diseases and ageing.10–15 Antioxidants such as various phenolics exhibit abilities of scavenging free radicals produced by oxidative stresses and might be important tools for prevention or postponement of these diseases. Hsu et al.16 reported that E. schmidtii possess considerable antioxidant capacities, indicating that antioxidant activity might also be employed for discriminating E. schmidtii from its adulterants. Recently, there are various methods available for evaluating antioxidant capacities based on different principles and mechanisms of action. Among these methods, DPPH, ABTS and ORAC assays are used widely,17,18 in which the former two are based on electron transfer reaction and the latter follows the principle of hydrogen atom transfer.
Chromatographic fingerprinting can be obtained with various analytical techniques, such as GC, HPLC, high performance thin layer chromatography, and capillary electrophoresis,19 and it has also been applied to screen herbs such as adulterated and authentic commodities of Pericarpium Citri Reticulatae and Pericarpium Citri Reticulatae Viride,20 plant origins of Ganoderma lucidum,21,22 as well as cultivation areas of Angelica acutiloba.23 In addition, several chemometric methods applied to fingerprinting such as similarity evaluation, hierarchical clustering analysis and principal component analysis make it easier to get more comprehensive and intuitive information.
To achieve potentially effective alternatives to E. schmidtii, it is the most important to compare their anti-inflammatory activities. Inflammation, a fundamental and complex biological process in response to tissue injuries and infections, is indispensable for homeostasis, thus is finely regulated. Inflammation plays a vital role in pathological processes of several diseases such as arthritis,24 diabetes,25 Alzheimer's disease26 and cancer.27 As the first line providing defence against invaders, macrophages can protect host from damaging triggered by inflammatory inducing factors such as lipopolysaccharides (LPS) and secrete pro-inflammatory cytokines and mediators such as NO, prostaglandin E2 (PGE2), interleukin-6 (IL-6) and interleukin-1β (IL-1β)28 which are regarded as important inducers and enhancers in the development of inflammation. Hence, LPS-induced macrophages are the most frequently used cellular model to assess the activities of anti-inflammatory drugs in vitro.
In the present study, total phenolic components and antioxidant activities of E. schmidtii and its nine adulterants, as well as the three marker compounds of E. schmidtii were simultaneously quantified. A concise and efficient HPLC fingerprint for each of these ten vine herbs was also developed. On this basis, anti-inflammatory activities of E. schmidtii and its four alternatives were further evaluated, their mechanisms were deliberated, and Porana sinensis and P. sinensis. var. delavayi were finally determined as two of the best potentially effective alternatives to E. schmidtii. To our knowledge, this is the first report to comprehensively analyze and compare E. schmidtii of so many indexes with its nine adulterants.
2. Materials and methods
2.1. Chemicals and preparation of herb extracts
Methanol and formic acid of chromatographic grade were purchased from Thermo Fisher Scientific Inc. (Tedia, USA). Standard chlorogenic acid, scopoletin, scopolin, gallic acid and rutin were bought from National Institutes for Food and Drug Control (Beijing, China). 1,1-Diphenyl-2-picrylhydrazyl (DPPH), 2,2′-azinobis-(3-ethylbenzthiazoline-6-sulphonate) (ABTS), 6-hydroxy-2,5,7,8-tetramethyl-chroman-2-carboxylic acid (trolox), Folin-Ciocalteu reagent, 2,2′-azobis(2-methylpropionamidine) dihydrochloride (AAPH), lipopolysaccharide (LPS) from Escherichia coli (0111:B4), Griess reagent and dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich Chemical (St. Louis, USA). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), phosphate-buffered saline (PBS), Dulbecco's modified Eagle medium (DMEM), fetal bovine serum (FBS) and non-essential amino-acids were provided by Beijing BioDee Biotechnology Co.Ltd (Beijing, China). Other chemicals used were all of analytical grade.Liana stems of ten woody herbs, i.e., E. schmidtii Craib, Porana sinensis Hemsl., Porana sinensis Hemsl. var. delavayi (Gagn. et Courch.) Rehd., Celastrus hindsii Benth., Morinda umbellata L., Illigera parviflora Dunn, Morinda parvifolia Bartl. ex DC., Piper puberulum (Benth.) Maxim., Piper kadsura (Choisy.) Ohwi, and Iodes seguini (Levl.) Rehd.29–32 marked as samples S1 through S10 in sequence were collected from herb markets of Bozhou, Anhui Province and Anguo, Hebei Province, and authenticated by Associate Prof. Dr Zhonghua Liu, Beijing Forestry University, China.
All collected herbs (S1–S10) were dried further for 24 h at 50 °C, ground using a mill, and sieved through a no. 50-mesh to ensure homogeneous size. Each ground herb was accurately weighed (1.5000 g) and transferred to a 50 mL centrifuge tube, and 15 mL methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (80
water (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 v/v) was immediately added into the tube, which was sealed and extracted in an ultrasonic bath for 30 min. The tube was then centrifuged at 800 rpm for 5 min, and the supernatant was collected. These extraction procedures were repeated twice more. The three supernatants of each herb were merged together, filtered through a 0.22 μm membrane, and stored at 4 °C to obtain extract solutions for measurement of phenolics, determination of antioxidant activities, and HPLC quantification and fingerprints analyses. For further assessment of anti-inflammatory activities, extract solutions of S1–S5 were also evaporated using a rotary evaporator (Labconco, Kansas city, MO, USA) until dryness, then the dried extracts were dissolved in DMEM at different concentrations and filtered by sterile syringe filter with a 0.22 μm pore size.
20 v/v) was immediately added into the tube, which was sealed and extracted in an ultrasonic bath for 30 min. The tube was then centrifuged at 800 rpm for 5 min, and the supernatant was collected. These extraction procedures were repeated twice more. The three supernatants of each herb were merged together, filtered through a 0.22 μm membrane, and stored at 4 °C to obtain extract solutions for measurement of phenolics, determination of antioxidant activities, and HPLC quantification and fingerprints analyses. For further assessment of anti-inflammatory activities, extract solutions of S1–S5 were also evaporated using a rotary evaporator (Labconco, Kansas city, MO, USA) until dryness, then the dried extracts were dissolved in DMEM at different concentrations and filtered by sterile syringe filter with a 0.22 μm pore size.
2.2. Measurement of phenolics
2.3. Determination of antioxidant activities
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 48 to an absorbance of 0.70 ± 0.02 at 734 nm to produce an ABTS+˙ working solution. After the working solution was prepared, 5 μL standard solution (20–100 mg L−1 trolox, final concentration), each of S1–S10 or blank (distilled water) was quickly added to corresponding wells in a 96-well microplate, followed by adding 200 μL working solution to each well. After incubation for 5 min at 30 °C in darkness, absorbance was read again at 734 nm using the microplate reader. The RSA for ABTS was also calculated as RSA (%) = (A0 − As)/A0 × 100, where As is the absorbance of the sample solution and A0 is the absorbance of the blank. All examinations were performed in triplicate and results were expressed as mg trolox equivalents per 100 g.
48 to an absorbance of 0.70 ± 0.02 at 734 nm to produce an ABTS+˙ working solution. After the working solution was prepared, 5 μL standard solution (20–100 mg L−1 trolox, final concentration), each of S1–S10 or blank (distilled water) was quickly added to corresponding wells in a 96-well microplate, followed by adding 200 μL working solution to each well. After incubation for 5 min at 30 °C in darkness, absorbance was read again at 734 nm using the microplate reader. The RSA for ABTS was also calculated as RSA (%) = (A0 − As)/A0 × 100, where As is the absorbance of the sample solution and A0 is the absorbance of the blank. All examinations were performed in triplicate and results were expressed as mg trolox equivalents per 100 g.2.4. HPLC quantification and fingerprints analyses
Accurately weighed standard scopolin, chlorogenic acid or scopoletin was dissolved into methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (80
water (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 v/v) to yield corresponding stock solution at 1.0 mg mL−1, and its standard solution was prepared by serial dilution of the stock solution to the working range of mobile phase for each standard. All standard solutions at 1.0, 0.5 and 0.25 mg mL−1 were stored at 4 °C in darkness, kept at room temperature for 10 min, and filtered through a 0.22 μm filter prior to HPLC analyses.
20 v/v) to yield corresponding stock solution at 1.0 mg mL−1, and its standard solution was prepared by serial dilution of the stock solution to the working range of mobile phase for each standard. All standard solutions at 1.0, 0.5 and 0.25 mg mL−1 were stored at 4 °C in darkness, kept at room temperature for 10 min, and filtered through a 0.22 μm filter prior to HPLC analyses.
HPLC analyses were carried out with a Shimadzu HPLC system (Shimadzu, Kyoto, Japan) equipped with a SPDM20A ultraviolet detector, an Eclipse XDB-C18 column (Agilent, 250 mm × 4.6 mm i.d., 5 μm), and a SIL-20AC TH autosampler controlled by an analytical software (LC Solution-Release 1.23SP1). Mobile phase was: (A) formic acid (0.1%, v/v) and (B) methanol (100%, v/v), and its gradient was: 0–10 min, 15–34% B; 10–20 min, 34% B; 20–35 min, 34–38% B; 35–45 min, 38–50% B; 45–55 min, 50–70% B; 55–75 min, 70–100% B. Injection volume, column temperature, and mobile phase flow rate were 10 μl, 25 °C, and 1 mL min−1, respectively, and monitor wavelength was set at 310 nm.
2.5. Assessment of anti-inflammatory activities
| Gene | Sense primer sequences | Antisense primer sequences |
|---|---|---|
| iNOS | 5′-AATGGCAACATCAGGTCGGCCATCACT-3′ | 3′-GCTGTGTGTCACAGAAGTCTCGAACTC-5′ |
| IL-6 | 5′-GAGGATACCACTCCCAACAGACC-3′ | 3′-AAGTGCATCATCGTTGTTCATACA-5′ |
| COX-2 | 5′-TGAAGCCGTACACATCATTTGAA-3′ | 3′-TGGTCTCCCCAAAGATAGCATCT-5′ |
| IL-1β | 5′-TGCAGAGTTCCCCAACTGGTACATC-3′ | 3′-GTGCTGCCTAATGTCCCCTTGAATC-5′ |
| β-Actin | 5′-GTGCTATGTTGCTCTAGACTTCG-3′ | 3′-ATGCCACAGGATTCCATACC-5′ |
2.6 Statistical analysis
Data were presented as mean ± SD (n = 3) and analyzed by IBM SPSS statistical software 20.0 (SPSS Inc., Chicago, IL) with p < 0.05 as significant. Evaluation of chromatographic fingerprints were conducted by Similarity Evaluation System for Chromatographic fingerprint of Traditional Chinese Medicine (version 2004A, National Committee of Pharmacopoeia, China).3. Results and discussion
3.1. Differences in contents of total phenolics between E. schmidtii and its nine adulterants
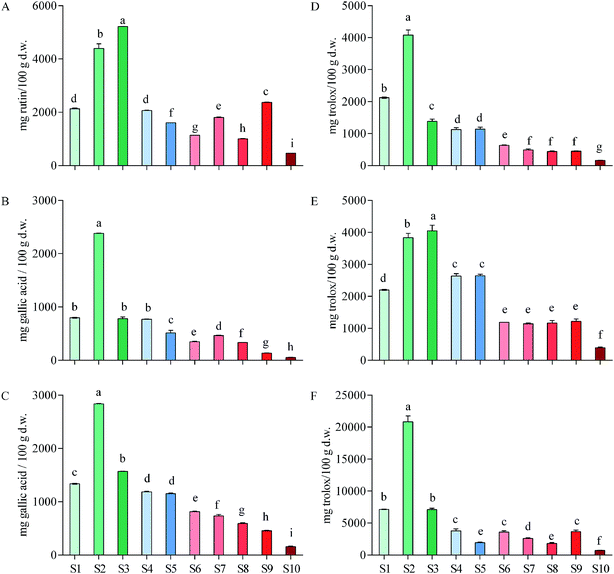
By calculation based on gallic acid equivalent, total tannins contributed to a considerable proportion of total phenols in the ten herbs (29.23–84.00%), with the largest proportion being found in S2. Total tannins accounted for more than half (59.93%) of total phenols in S1 (Fig. 1B and C). It can be concluded that tannins were one of the major components in these medicinal vines. In addition, as total flavonoids in rutin equivalent accounted for 0.5–5.2% of these vines in dry weight, and total flavonoids in S1 accounted for 2.1%, it can be conferred that they contained also considerable flavonoids (Fig. 1A). Overall, S1, as well as S2–S4, contained considerable phenols, tannins and flavonoids comparing with other herbs that were associated with anti-inflammatory activity.
Pharmacists usually target an herb with high total phenols including tannins and flavonoids for treating different diseases.45 High total phenols indicate a high ability of the herb to dealing inflammatory diseases and are implicated in wound healing.46 Therefore, it can be concluded that total phenols in E. schmidtii (S1) were considerable high among the main medicinal plans used for anti-inflammatory thus itself can be confirmed as a good Chinese medicine to treat inflammation that have in fact been employed in the long-term practice by veteran doctors of Traditional Chinese Medicine. Compared with the contents in S1, total phenols, tannins and flavonoids in S2–S4 are also considerably high (Fig. 1A–C) and they should be considered as potentially effective alternatives to rather than ‘adulterants’ of S1 (i.e., E. schmidtii).
3.2. Differences in antioxidant activities between E. schmidtii and its nine adulterants
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 343.57 mg trolox per 100 g) and the lowest in Amaranthus caudatus (30.03). Our S2 exceeded 15 and S1, S3, S4 and S5 exceeded 10 of their 27 plant species. Among the 10 plants, Oxalis tuberosa (210.24) was used against rheumatism and arthritis.
343.57 mg trolox per 100 g) and the lowest in Amaranthus caudatus (30.03). Our S2 exceeded 15 and S1, S3, S4 and S5 exceeded 10 of their 27 plant species. Among the 10 plants, Oxalis tuberosa (210.24) was used against rheumatism and arthritis.Floegel et al.48 reported antioxidant capacity of 50 most popular foods in USA by ABTS/DPPH assays, and the results showed that the DPPH data of each food were similar to the ABTS data of itself. Similar results were found in Acer truncatum leaves49 and oak cup crude extract.50 However, it was found by the present study that the ABTS values of each vine herb (Fig. 1E) were significant higher than its DPPH values (Fig. 1D). This is probably an important finding, since it raises a possibility that those compositions that were more sensitive to ABTS+˙ than to DPPH˙ in these herbs likely belong to alkaloids or other nitrogen-containing components that might also be responsible for the inflammatory activities.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 818.75 mg trolox per 100 g), which was almost three times of the second S1 (7136.39) and the third S3 (7094.16), followed by S4, S9, S6, S7, S5, S8, and S10 (3776.31–713.38). No significant differences observed between S1 and S3, S5 and S8, as well as among S4, S6 and S9. ORAC values of these ten herbs are higher than their corresponding DPPH and ABTS values as indicated by the y-axis values and this difference might be due to the different chemical properties of bioactive compounds and the distinct mechanisms of these three antioxidant assays.
818.75 mg trolox per 100 g), which was almost three times of the second S1 (7136.39) and the third S3 (7094.16), followed by S4, S9, S6, S7, S5, S8, and S10 (3776.31–713.38). No significant differences observed between S1 and S3, S5 and S8, as well as among S4, S6 and S9. ORAC values of these ten herbs are higher than their corresponding DPPH and ABTS values as indicated by the y-axis values and this difference might be due to the different chemical properties of bioactive compounds and the distinct mechanisms of these three antioxidant assays.Leandroj et al.41 also reported ORAC values of their 19 medicinal plants used for treatment of inflammatory and rheumatic in Amazonia. By comparing our data with theirs obtained using the same AAPH radical generator, it is clear that S1–S3 showed an even higher ORAC value than all those 19 medicinal plants, with S2 was about 3-folds higher than that of their highest species. Samaradivakara et al.52 analyzed antioxidant capacities by ORAC assay of 16 medicinal plants distributed in SriLankan. Among these medicinal plants, S2 possessed similar ORAC value with Caesalpinia bonducella (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 169 mg trolox per 100 g extract), Elephantopus scaber (20
169 mg trolox per 100 g extract), Elephantopus scaber (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 844) and other six medicinal plants. It must be pointed out again that our data are on a dry weight plant material rather than extract base. Considering this aspect, our data might be even higher than most of, if not all, their 16 species.
844) and other six medicinal plants. It must be pointed out again that our data are on a dry weight plant material rather than extract base. Considering this aspect, our data might be even higher than most of, if not all, their 16 species.
In general, it is obviously that E. schmidtii (S1) used for treating a variety of inflammations possesses a good antioxidant activity. Once again, compared with three antioxidant activities of S1, S2–S4 also exhibited a high level of antioxidant activities and thus should be taken into account as potentially effective alternatives of E. schmidtii. This conclusion was consistent with the previous conclusion based on measurement of phenolic contents.
3.3. Differences in HPLC fingerprints between and quantification of the three marker compounds in E. schmidtii and its nine adulterants
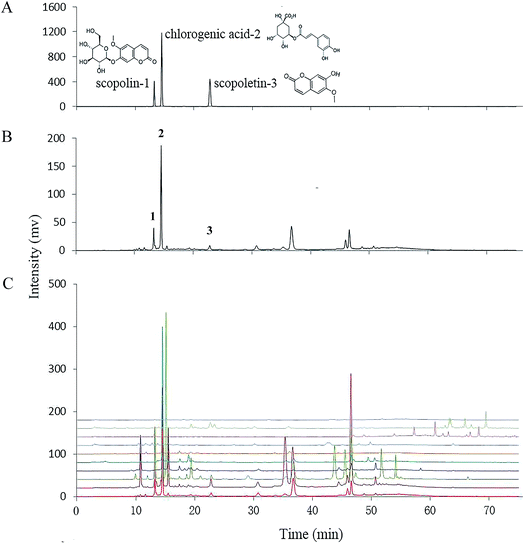
A HPLC-UV method was used for fingerprinting phytochemicals of E. schmidtii and its nine adulterants. Chromatographic and detection conditions was selected based on a previously report53 to maximize the number and relative intensity of detected signals. As shown in Fig. 2C, complex phytochemical extracts of the ten herbs were all properly resolved and a wide range of different compounds (discernible peaks) could be recognized. The chromatogram of S1 (Fig. 2B) was taken as the reference fingerprint for the nine adulterants. Similarities calculated by Similarity Evaluation System for Chromatographic Fingerprint of Traditional Chinese Medicine (Version 2004A) between the reference fingerprint and individual adulterants were listed in Table 2. Generally, the closer the values similar to the value 1, the more similarity the two chromatograms. Obviously, the nine adulterants could be divided into two groups based on their similarities to S1, with one group consisting of S7–S10 for their similarities with S1 far below 0.5 and the other group thus including S2–S6. The higher similarity usually means the more similar compositions. Our results show that S1 possessed good composition similarities with S5, S6, S4, S2 and S3 in sequence.| Similarity | S-lin* | CA | S-letin | Total | |
|---|---|---|---|---|---|
| a *S-lin: scopolin; CA: chlorogenic acid; S-letin: scopoletin. Each sample was analyzed three times (n = 3); all values are mean ± SD (mg/100 g). a–iIn each column different letters mean significant differences between two groups (P < 0.05). | |||||
| S1 | 1 | 62.28 ± 0.07c | 333.21 ± 0.36b | 9.87 ± 0.11d | 405.36 ± 0.5c |
| S2 | 0.617 | 195.21 ± 0.04a | 254.76 ± 0.12c | 25.10 ± 0.05a | 475.07 ± 0.2a |
| S3 | 0.512 | 182.15 ± 0.10b | 219.48 ± 0.23d | 12.70 ± 0.07c | 414.33 ± 0.3b |
| S4 | 0.691 | 6.10 ± 0.07d | 200.23 ± 0.33e | 0g | 206.33 ± 0.4e |
| S5 | 0.876 | 1.61 ± 0.09f | 348.28 ± 0.28a | 0g | 349.89 ± 0.4d |
| S6 | 0.824 | 0g | 76.51 ± 0.15f | 1.98 ± 0.08e | 78.49 ± 0.2f |
| S7 | 0.150 | 0g | 5.21 ± 0.08g | 0.15 ± 0.02g | 5.36 ± 0.1h |
| S8 | 0.079 | 0g | 3.58 ± 0.05h | 0g | 3.58 ± 0.05i |
| S9 | 0.099 | 0g | 5.55 ± 0.06g | 22.70 ± 0.12b | 28.25 ± 0.2g |
| S10 | 0.195 | 2.60 ± 0.02e | 2.65 ± 0.03i | 0.60 ± 0.03f | 3.25 ± 0.1i |
It is obvious from HPLC profiles that peaks 1–3 in Fig. 2B are matched precisely to those of the three authentic standards in Fig. 2A, i.e., scopolin, chlorogenic acid, and scopoletin, respectively, which are defined as markers of the herb E. schmidtii by the Chinese Pharmacopoeia. And all these compounds present good anti-nociceptive and anti-inflammatory activities.54–56 The right four columns of data in Table 2 show individual and total contents of the three markers in S1–S10. It can be found that S1, S3 and S2, being increased significantly in sequence from 405.36 to 475.07 mg per 100 g, exhibited outstandingly higher total contents of scopolin, chlorogenic acid and scopoletin than those of the other adulterants (S4–S10). Moreover, rank orders of both scopolin and scopoletin in S1–S3 was the same as that of the total contents of the three markers. For chlorogenic acid, it was contained in E. schmidtii (S1) and all the adulterants (S2–S10), with an order from the highest to the lowest as S5, S1–S4, S6, S9, S7, S8, and S10. The contents of scopolin, chlorogenic acid and scopoletin in E. schmidtii shows a little difference with results reported by Chen et al.53 probably due to different methods of sample preparation. From results shown in Table 2, it can be concluded that among the nine adulterants S2 and S3 could be recognized as alternatives of S1 as they not only contain but have high total contents of the three compounds. The total contents in S4 and S5 are also relatively higher because they possess high levels of chlorogenic acid. They might be selected as alternatives from both phenolic content and antioxidant ability bases (see Sections 3.1 and 3.2); however, they are lack of scopoletin that is recognized as one of the two active ingredients, scopolin and scopoletin, which were responsible for anti-rheumatic in E. schmidtii. Others (S6–S10) should be determined as quack medicines in that they contained only two of the three markers in an extremely low total amount.
Hierarchical clustering analyses (ESI Fig. S1†) show that S1 was close to S4 and S5 in phenolic components (total phenols, flavonoids and tannins, as well as the three identified phenolic compounds), antioxidant capacities (DPPH, ABTS and ORAC) and HPLC fingerprint similarities, while S2 and S3, being much higher with the above various indexes (Fig. 1 and Table 2), were clustered into another group. Correlations among the various indexes analyzed by SPSS regression were listed in ESI Table S1.† It is worth emphasizing that significant correlations between phenolics components and antioxidant activities indicate that total flavonoids contributed to mainly antioxidant activity of ABTS, but total tannins, to those of both DPPH and ORAC. As to the three marker compounds, scopolin possessed antioxidant capacity much higher than scopoletin and chlorogenic acid did.
In summary, all the above results indicate that S2–S5 (i.e., Porana sinensis, Porana sinensis var. delavayi, Celastrus hindsii and Morinda umbellata in sequence) could be preliminarily determined as alternatives to S1 (i.e., E. schmidtii), which required further confirmation by comparison of their anti-inflammatory activities described below. The other five woody vine plants, namely, S6–S10 (i.e. Illigera parviflora, Morinda parvifolia, Piper puberulum, Piper kadsura and Iodes seguini in sequence), were identified as absolute fakes or adulterants, thus were excluded from alternatives of E. schmidtii.
3.4. Confirmation of the alternatives to E. schmidtii via comparison of anti-inflammatory activities
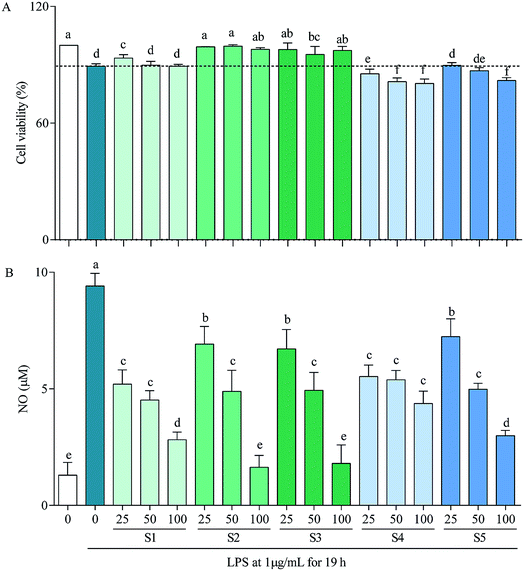
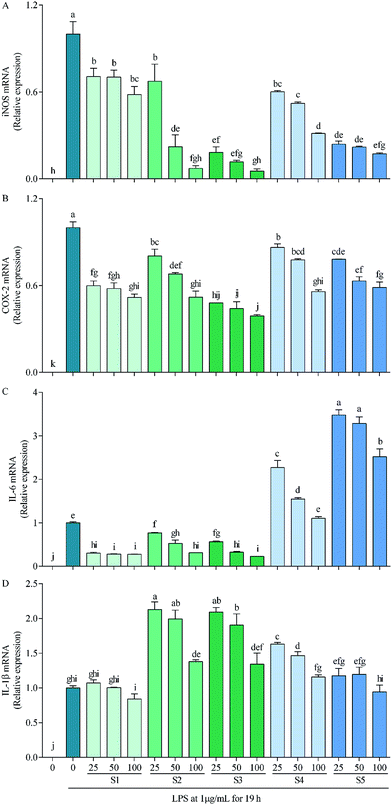
In RAW264.7 cells, LPS can induce iNOS, COX-2, IL-6 and IL-1β transcriptions, which consequently lead to overproductions of NO, PEG2, IL-6 and IL-1β,59 with that of NO being demonstrated in Fig. 3B as a representative. Thereinto, high concentration of NO causes oxidative damage, promotes release of inflammatory cytokines, then results in inflammation and regulates cell growth and differentiation;60 COX-2 is inducible, at least partly via activation of NF-κB by many factors such as cytokines, mitogens, growth factors and tumor promoters, and is over-expressed in inflammation and cancer;61 IL-6 participates in regulation of vast majority of acute-phase proteins (APPs) produced by non-specific reactions triggered by inflammation; and IL-1β is produced during the initial stage of inflammation and is present in many types of inflammatory diseases.62 Inhibitions of these factors have been taken as a strategy to treat inflammation-related diseases. The present study reveals that the mechanism of anti-inflammatory by E. schmidtii (S1) was to suppress mRNA expressions of iNOS, COX-2 and IL-6. S2 and S3 possessed the same and more effective mechanism to that of S1, and S4 and S5 suppressed mRNA expressions of only iNOS and COX-2 but not IL-6 and IL-1β. Therefore, it can be concluded that S2 and S3 would be the best potentially effective alternatives for S1 at least at the in vitro cellular level.
4. Conclusion
In this work, total phenols, flavonoids and tannins, and antioxidant activities of E. schmidtii and its nine adulterants (i.e., S2–S10) were compared thoroughly, the three marked compounds (scopolin, chlorogenic acid and scopoletin) of E. schmidtii defined by Chinese Pharmacopoeia were simultaneously quantified, and a concise and efficient HPLC fingerprints methodology for these ten liana herbs were subsequently developed. Compared with S1, there existed relatively higher phenolics in S2 and S3, with S4 and S5 containing similar or slightly lower phenolics. In in vitro antioxidant assays, S2 exhibited significant potent for scavenging free radicals than S1 did, and the antioxidant activities of S3–S5 were close to or slightly higher than that of S1. S1–S3 showed similarly large contents of scopoletin, scopolin and chlorogenic acid. Furthermore, S2–S5 also possessed better similarities with S1 than S6–S10 did. Based on the above results, anti-inflammatory activities and mechanisms of E. schmidtii (S1) and its four alternatives (i.e., S2–S5) were then explored. It is clear that S2 and S3 exhibited higher potenial in anti-inflammatory activities and similar but even more effective anti-inflammatory mechanism to that of S1. Overall, it is concluded that S2 and S3 could be two of the best potentially effective alternatives among the four alternatives of E. schmidtii, and S4 and S5 might also be considered as alternatives of E. schmidtii for they contained similar fundamental compounds and equally impressive anti-inflammatory potential concerning the two inflammatory factors iNOS and COX-2. However, further animal studies and more detailed clinical research are needed to provide greater support for their use as alternatives.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This research was supported by the China Special Fund for Forestry Research in the Public Interest (Grant No. 201504606)References
- L. H. Wu, E. Y. Zhu, Z. J. Zhang and Z. T. Wang, Chin. Tradit. Herb. Drugs, 2005, 36, 1398–1400 Search PubMed.
- V. Breinholt, Desirable versus harmful levels of intake of flavonoids and phenolic acids, in Natural antioxidants and anticarcinogens in nutrition, health and disease, ed. J. Kumpulainen and J. E. Salonen, The Royal Society of Chemistry, Cambridge, 1999, pp. 93–105 Search PubMed.
- G. G. Duthie, S. J. Duthie and J. A. M. Kyle, Nutr. Res. Rev., 2000, 13, 79–106 CrossRef CAS PubMed.
- F. Shahidi and M. Naczk, Food phenolics: sources, chemistry, effects, applications, Technomic Publishing Co. Inc., USA, 1995 Search PubMed.
- F. Shahidi and M. Naczk, Phenolics in Food and Nutraceuticals, 2004, vol. 13, pp. 12–15 Search PubMed.
- Y. Yi, J. Sun, J. Xie, T. Min, L. M. Wang and H. X. Wang, Molecules, 2016, 21, 863 CrossRef PubMed.
- W. Song, R. L. Jin and J. H. Liu, China J. Chin. Mater. Med., 1997, 22, 359–360 CAS.
- T. Morikawa, F. Xu, H. Matsuda and M. Yoshikawa, Chem. Pharm. Bull., 2006, 54, 1530–1534 CrossRef CAS PubMed.
- J. Liu, Z. M. Feng, J. F. Xu, Y. H. Wang and P. C. Zhang, Phytochemistry, 2007, 68, 1775–1780 CrossRef CAS PubMed.
- B. N. Ames, Science, 1983, 221, 1256–1264 CAS.
- Y. Aniya, C. Miyagi, A. Nakandakari, S. Kamiya, N. Imaizumi and T. Ichiba, Phytomedicine, 2002, 9, 239–244 CrossRef CAS PubMed.
- L. Leong and G. Shui, Food Chem., 2002, 76, 69–75 CrossRef CAS.
- Y. Lim, T. Lim and J. Tee, Food Chem., 2007, 103, 1003–1008 CrossRef CAS.
- S. Lopes, A. Jurisicova, J. G. Sun and R. F. Casper, Hum. Reprod., 1998, 13, 896–900 CrossRef CAS PubMed.
- I. L. C. Chapple, J. Clin. Periodontol., 1997, 24, 287–296 CrossRef CAS PubMed.
- H. Y. Hsu, J. Y. Chen and J. J. Yang, Am. J. Chin. Med., 1999, 27, 117–122 CrossRef CAS PubMed.
- C. Grajeda-Iglesias, E. Salas and N. Barouh, Food Chem., 2016, 194, 749–757 CrossRef CAS PubMed.
- J. A. John and F. Shahidi, J. Funct. Foods, 2010, 2, 196–209 CrossRef CAS.
- J. Mazina, M. Vaher, M. Kuhtinskaja, L. Poryvkina and M. Kaljurand, Talanta, 2015, 139, 233–246 CrossRef CAS PubMed.
- L. Z. Yi, D. L. Yuan, Y. Z. Liang, P. S. Xie and Y. Zhao, Anal. Chim. Acta, 2007, 588, 207–215 CrossRef CAS PubMed.
- Y. Chen, S. B. Zhu, M. Y. Xie, S. P. Nie, W. Liu, C. Li, X. F. Gong and Y. X. Wang, Anal. Chim. Acta, 2008, 623, 146–156 CrossRef CAS PubMed.
- D. Xin, K. C. C. Kelvin, W. L. Hei and W. H. Carmen, J. Chromatogr. A, 2003, 1018, 85–95 CrossRef.
- S. Tianniam, T. Bamba and E. Fukusaki, J. Sep. Sci., 2009, 32, 2233–2244 CrossRef CAS PubMed.
- S. P. Young, S. R. Kapoor and M. R. Viant, Arthritis Rheumatol., 2014, 65, 2015–2023 CrossRef PubMed.
- M. Haemmerle, Diabetes, 2013, 7, 2509–2529 CrossRef PubMed.
- T. Wyss Coray and J. Rogers, Inflammation in Alzheimer disease-a brief review of the basic science and clinical literature, Cold Spring Harbor Perspect. Med., 2012, 2, a006346 Search PubMed.
- M. Locatelli, J. Neuroimmunol., 2013, 1, 99–106 CrossRef PubMed.
- J. S. Shin, Y. M. Park, J. H. Choi, H. J. Park, M. C. Shin, Y. S. Lee and K. T. Lee, Int. Immunopharmacol., 2010, 10, 943–950 CrossRef CAS PubMed.
- J. F. Wei and G. Xi, J. Tradit. Chin. Med., 1986, 9, 40–41 Search PubMed.
- Z. W. Xie, Collection of National Chinese Traditional Herbal Medicine, People's Medical Publishing House, Beijing, 1996 Search PubMed.
- C. M. Zhang, Y. J. Zhang and W. Song, Primary J. Chin. Mater Med., 2000, 14, 25 Search PubMed.
- J. N. Tan and Z. X. Gao, Journal of Guangxi Academy of Sciences, 2008, 24, 49–52 Search PubMed.
- U. Xie, L. Huang, C. Zhang and Y. Zhang, J. Funct. Foods, 2015, 16, 460–471 CrossRef.
- F. V. Dulf, D. C. Vodnar, E. H. Dulf and M. I. Tosa, J. Agric. Food Chem., 2015, 63, 3489–3500 CrossRef CAS PubMed.
- V. L. Singleton, R. Orthofer and R. M. Lamuela Raventos, Methods Enzymol., 1999, 299, 152–178 CAS.
- S. Zhao, J. Y. Liu, S. Y. Chen, L. L. Shi, Y. J. Liu and C. Ma, Molecules, 2011, 16, 8590–8600 CrossRef CAS PubMed.
- M. E. Alañón, L. Castro Vázquez, M. C. Díaz Maroto, M. H. Gordon and M. S. Pérez Coello, Food Chem., 2011, 128, 997–1002 CrossRef.
- L. Sun, C. K. Isaak, Y. Zhou, J. C. Petkau, K. O and Y. L. Liu, Life Sci., 2012, 91, 151–158 CrossRef CAS PubMed.
- T. A. S. Araújo, N. L. Alencar, E. L. C. De Amorim and U. P. De Albuquerque, J. Ethnopharmacol., 2008, 120, 72–80 CrossRef PubMed.
- F. M. Awah, P. N. Uzoegwu, P. Ifeonu, J. O. Oyugi, J. Rutherford, X. J. Yao, F. Fehrmann, K. R. Fowke and M. O. Eze, Food Chem., 2012, 131, 1279–1286 CrossRef CAS.
- L. Leandroj, B. Fadil, R. M. Begoña and R. S. José, Food Chem., 2010, 119, 1566–1570 CrossRef.
- T. Okuda, Phytochemistry, 2005, 66, 2012–2031 CrossRef CAS PubMed.
- M. Qasim, Z. Abideen and M. Y. Adnan, S. Afr. J. Bot., 2016, 110, 240–250 CrossRef.
- S. Li, S. K. Li, R. Y. Gan, F. L. Song, L. Kuang and H. B. Li, Ind. Crops Prod., 2013, 51, 289–298 CrossRef CAS.
- S. Petti and C. Scully, J. Dent., 2009, 37, 413–423 CrossRef CAS PubMed.
- N. Akhtara, Ihsan-ul-Haq and B. Mirza, Arabian J. Chem., 2015, 4 DOI:10.1016/j.arabjc.2015.01.013.
- R. Chirinos, R. Pedreschi and H. Rogez, Ind. Crops Prod., 2013, 47, 145–152 CrossRef CAS.
- A. Floegel, D. O. Kim and S. J. Chung, J. Food Compos. Anal., 2011, 24, 1043–1048 CrossRef CAS.
- L. Yang, P. Yin and K. Li, Arabian J. Chem., 2017 DOI:10.1016/j.arabjc.2017.01.009.
- P. Yin, L. Yang and K. Li, Arabian J. Chem., 2016 DOI:10.1016/j.arabjc.2016.09.018.
- K. M. Schaich, X. Tian and J. Xie, J. Funct. Foods, 2015, 14, 111–125 CrossRef CAS.
- S. P. Samaradivakara, R. Samarasekera and S. M. Handunnetti, Ind. Crops Prod., 2016, 83, 227–234 CrossRef CAS.
- Z. Chen, L. Liao, Z. Zhang, L. Wu and Z. T. Wang, J. Ethnopharmacol., 2013, 150, 501–506 CrossRef CAS PubMed.
- H. J. Kim, S. I. Jang, Y. J. Kim, H. T. Chung, Y. J. Yun, T. H. Kang, O. S. Jeong and Y. C. Kim, Fitoterapia, 2004, 75, 261–266 CrossRef CAS PubMed.
- M. D. Dos Santos, M. C. Almeida, N. P. Lopes and G. E. P. Souza, Biol. Pharm. Bull., 2006, 29, 2236–2240 CAS.
- R. Pan, Y. Dai, X. Gao and Y. Xia, Int. Immunopharmacol., 2009, 9, 859–869 CrossRef CAS PubMed.
- G. Nagy, J. M. Clark, E. I. Buzas, C. L. Gorman and A. P. Cope, Immunol. Lett., 2007, 111, 1–5 CrossRef CAS PubMed.
- J. E. Yuste, E. Tarragon, C. M. Campuzano and F. Ros-Bernal, Front Cell Neurosci., 2015, 9, 322 Search PubMed.
- M. Fujihara, M. Muroi, K. Tanamoto, T. Suzuki, H. Azuma and H. Ikeda, Pharmacol. Ther., 2003, 100, 171 CrossRef CAS PubMed.
- D. J. Stuehr and M. A. Marletta, J. Immunol., 1987, 139, 518 CAS.
- H. Ohshima, H. Tazawa, B. S. Sylla and T. Sawa, Mutat. Res., Fundam. Mol. Mech. Mutagen., 2005, 591(1), 110–122 CrossRef CAS PubMed.
- M. Mihara, M. Hashizume and H. Yoshida, Clin. Sci., 2012, 122, 143 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra10767f |
| This journal is © The Royal Society of Chemistry 2017 |