Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Palladium/silver reagent-promoted aryl phosphorylation: flexible synthesis of substituted-3-benzylidene-2-(2-(diphenylphosphoryl)-aryl)-isoindolin-1-one†
Peng Shia,
Qing Wanga,
Xiao Zengb,
Yingsheng Zhao *a and
Runsheng Zeng
*a and
Runsheng Zeng *a
*a
aKey Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry Chemical Engineering and Materials Science, Soochow University, Suzhou, Jiangsu 215123, China. E-mail: yszhao@suda.edu.cn; zengrunsheng@suda.edu.cn
bDepartment of Chemistry, National University of Singapore, Singapore
First published on 27th November 2017
Abstract
A novel Pd(OAc)2/Ag2CO3-catalyzed coupling reaction was investigated. Substituted 3-benzylidene-2-arylisoindolin-1-ones were reacted with diphenylphosphine oxide to afford 3-arylidene-2-(2-(diphenylphosphoryl)aryl)isoindolin-1-ones. The reaction proceeded at 25 °C in an air atmosphere in the absence of base and ligands. Our results indicate that the diphenylphosphine oxide free radical tends to attack the aryl rather than the double bond in this reaction.
Introduction
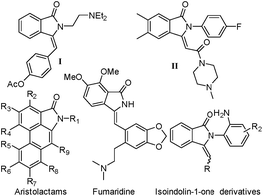
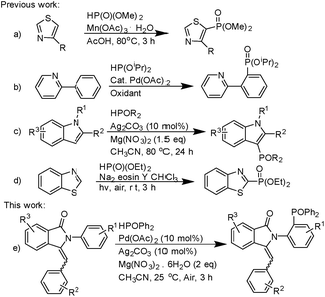
Nitrogen-containing heterocycles are a series of notable compounds known for their bioactivity in nature.1 In particular, isoindolin-1-ones such as I and II are the core structural motifs of several compounds of medicinal value (Fig. 1). 3-Methyleneisoindolin-1-ones are known for their use as anaesthetic and sedative drugs.2 As these compounds have a double bond and phenyl cycle we have tried to find a catalytic system for direct phosphorylation of substituted 3-benzylidene-2-arylisoindolin-1-one.Diphenylphosphine oxide has become a research focus in the field of organic synthesis due to its unique biological activities.3 In 1982, Hirao and co-workers reported the first palladium-catalyzed phosphorylation of aryl iodides.4 Since then, a series of formations of Csp2–P bonds by cross-coupling has been published in the last thirty years. The coupling partners, included aryl triflates, tosylate, diaryliodonium salts, diazonium salts, boronic acids, triarylbismuths, phenylhydrazine and so on.5 Moreover, direct radical phosphorylation of benzene derivatives6 and heterocycle7 with diethyl phosphate has been successfully developed by using Mn(OAc)3 as an oxidant (Scheme 1a). There were also studies of the manganese-catalyzed reactions of H-phosphinates.8 Recently, palladium-catalyzed direct phosphorylation reaction of arene and heteroarenes has been established (Scheme 1b).9,10 On the other hand, Ag system has been used for the synthesis of direct phosphorylation reaction (Scheme 1c).11 Visible light promoted C–P functionalization has been rapidly developed (Scheme 1d).12 As part of our research on the transition metal-catalyzed C–P bond formation, this communication reports the first example of a coupling reaction of 3-benzylidene-2-arylisoindolin-1-one with diphenylphosphine oxide catalyzed by Pd(OAc)2/Ag2CO3 (Scheme 1e).
Results and discussion
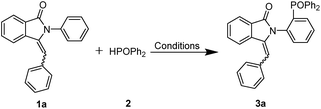
When the model reaction of 3-benzylidene-2-phenylisoindolin-1-one (1a) with diphenylphosphine oxide (2) was performed in CH3CN in the presence of oxidants such as TBHP, DTBP, Mn(OAc)3 and AgNO3, no desired products were obtained (Table 1, entries 1, 2, 3 and 5). After the addition of 10 mol% Ag2CO3 and Mg(NO3)2·6H2O, the reaction proceeded smoothly to afford the desired product, 3-benzylidene-2-(2-(diphenylphosphoryl)phenyl) isoindolin-1-one (3a) in 60% yield (Table 1, entry 4).| Entry | Catalyst (%) | Oxidant (%) | Temperature | Solvent | Yeildb |
|---|---|---|---|---|---|
| a Reaction condition: 1a (0.2 mmol), HPOPh2 (0.4 mmol), Pd(OAc)2, (0.02 mmol), Ag2CO3 (0.02 mmol), Mg(NO3)2·6H2O (0.4 mmol), solvent (2 ml), at 25 °C in air atmosphere, 3 h.b Yields are given for isolated products.c Without Mg(NO3)2·6H2O.d HPOPh2 (0.2 mmol) was added.e HPOPh2 (0.6 mmol) was added.f Molecular sieves (0.1 g) was added.g 1 h.h 2 h.i 4 h. | |||||
| 1 | TBHP(10) | 25 °C | CH3CN | N.D | |
| 2 | DTBP(10) | 25 °C | CH3CN | N.D | |
| 3 | Mn(OAc)3 | 25 °C | CH3CN | N.D | |
| 4 | Ag2CO3(10) | 25 °C | CH3CN | 60% | |
| 5 | AgNO3(10) | 25 °C | CH3CN | N.D | |
| 6 | Pd(PPh3)4(10) | Ag2CO3(10) | 25 °C | CH3CN | 38% |
| 7 | Pd(OAc)2(10) | Ag2CO3(10) | 25 °C | CH3CN | 78% |
| 8 | PdCl2(10) | Ag2CO3(10) | 25 °C | CH3CN | 72% |
| 9 | Pd(OAc)2(10) | Ag(OAc)10 | 25 °C | CH3CN | 48% |
| 10 | Pd(OAc)2(10) | Ag2CO3(10) | 25 °C | THF | 32% |
| 11 | Pd(OAc)2(10) | Ag2CO3(10) | 25 °C | DMF | 36% |
| 12 | Pd(OAc)2(10) | Ag2CO3(10) | 25 °C | i-PrOH | 40% |
| 13 | Pd(OAc)2(10) | Ag2CO3(10) | 25 °C | Toluene | 30% |
| 14 | Pd(OAc)2(5) | Ag2CO3(10) | 25 °C | CH3CN | 48% |
| 15 | Pd(OAc)2(20) | Ag2CO3(10) | 25 °C | CH3CN | 65% |
| 16c | Pd(OAc)2(10) | Ag2CO3(10) | 25 °C | CH3CN | 20% |
| 17 | Pd(OAc)2(10) | Ag2CO3(10) | 0 °C | CH3CN | 10% |
| 18 | Pd(OAc)2(10) | Ag2CO3(10) | 40 °C | CH3CN | 72% |
| 19 | Pd(OAc)2(10) | Ag2CO3(10) | 60 °C | CH3CN | 20% |
| 20d | Pd(OAc)2(10) | Ag2CO3(10) | 25 °C | CH3CN | 30% |
| 21e | Pd(OAc)2(10) | Ag2CO3(10) | 25 °C | CH3CN | 58% |
| 22f | Pd(OAc)2(10) | Ag2CO3(10) | 25 °C | CH3CN | 76% |
| 23g | Pd(OAc)2(10) | Ag2CO3(10) | 25 °C | CH3CN | 35% |
| 23h | Pd(OAc)2(10) | Ag2CO3(10) | 25 °C | CH3CN | 66% |
| 23i | Pd(OAc)2(10) | Ag2CO3(10) | 25 °C | CH3CN | 75% |
Further investigations of palladium catalysts, the yield of 3a was improved to 78% when we used Pd(OAc)2 as the catalyst (Table 1, entries 7). On the other hand, when we use Ag(OAc) in place of Ag2CO3, the reaction afforded the desired product in a lower yield (Table 1, entry 9). By screening polar solvents such as DMF, THF, i-PrOH, and a representative nonpolar solvent, toluene (Table 1, entries 10–13), we found that CH3CN works best for the reaction. Apart from the above-mentioned factors, the effects of catalyst loading, reaction temperature, time and molecular sieves were also investigated, and the optimal reaction conditions were determined to be room temperature reaction for 3 h in air atmosphere, with the addition of 10 mol% Pd(OAc)2 and 10 mol% Ag2CO3 as catalyst, Mg(NO3)2·6H2O as promoter and CH3CN as solvent (Table 1, entries 14–23).
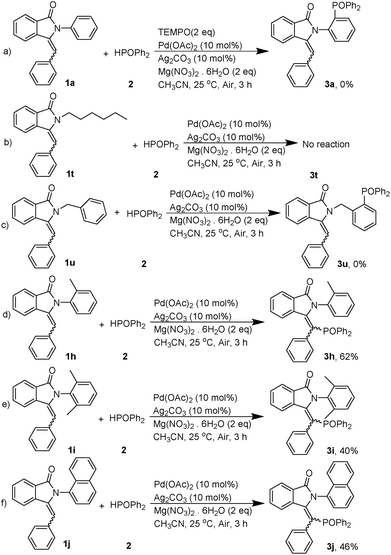
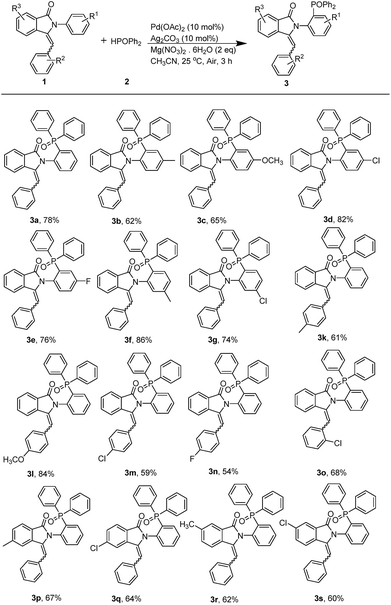
With the promising results obtained in the model reaction, we subsequently examined the substrate scope of 3-benzylidene-2-arylisoindolin-1-one under the optimized reaction conditions (10 mol% Pd(OAc)2 and 10 mol% Ag2CO3 as catalyst, and Mg(NO3)2·6H2O as promoter in CH3CN at 25 °C, for 3 h in air atmosphere). As shown in Table 2, electron-withdrawing substituent such as F and Cl groups on the para-position of arylamine ring of substituted 3-benzylidene-2-arylisoindolin-1-one (1) facilitated the reaction to afford the arylphosphonates (3) in good yields (Table 2, 3d and 3e). On the contrary, election-donating groups such as alkyl and methoxy were unfavorable for the reaction and led to lower yields (Table 2, 3b and 3c). Substituted groups such as CH3 and Cl on the meta-position of arlyamine ring are both given good yields (Table 2, 3f and 3g). We also investigated the substituent R2 on the benzylidene and the substituent R3 on the isoindolin-1-one, the results indicate that the reaction affords the arylphosphonates (3) in moderate to good yields (Table 2, 3k, 3l, 3m, 3n, 3o, 3p, 3q, 3r and 3s). In order to understand the reaction mechanism, following control experiments were carried out. We repeated the reaction in the presence of radical quencher 2,2,6,6-tetramethylpiperidin-1-oxyl (TEMPO) and none of 3a was obtained (Scheme 2a). The result suggested that the diphenylphosphine oxide free radical was probably generated during the reaction. Furthermore, neither aliphatic amine nor benzylamine substrate produced the corresponding products (Scheme 2b and c). These results indicated that the reaction is only suitable for arylamine substrates which have enough electron cloud density.
| a Reaction condition: 1 (0.2 mmol), HPOPh2 (0.4 mmol), Pd(OAc)2, (0.02 mmol), Ag2CO3 (0.02 mmol), Mg(NO3)2·6H2O (0.4 mmol), CH3CN (2 ml), at 25 °C in air atmosphere, 3 h. Yield of isolated products are given. |
|---|
 |
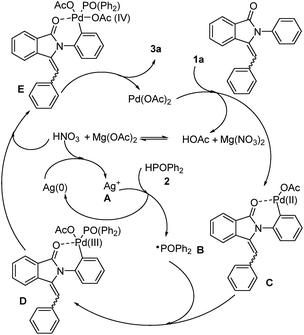
As the phosphorylation always took place on the ortho-position of aniline in the above experiments, we wondered whether the reaction would proceed if the aniline had substituent groups at the ortho-position. Hence, we did further reactions (Scheme 2d–f). Surprisingly, the diphenylphosphine oxide free radical will attack the double bond instead of aniline with a moderate yield. These results further indicated that this reaction has a high regioselectivity that the diphenylphosphine oxide free radical would prioritize its attack on the ortho-position of aniline rather than the para-position of aniline or the double bond. On the basis of the mechanistic studies and experimental results, a plausible mechanism is proposed in Scheme 3.
Initially, Pd(OAc)2 reacted with the substrate (1a) to form a six-membered palladacycle (C) in presence of Mg(NO3)2, and generated HNO3 through C–H activation. The diphenylphosphine oxide (2) was excited by Ag(I) (A) to generate the key intermediate P-centered radical (B), which then underwent addition with palladacycle (C) to form PdIII intermediate D.13 Thereafter, the radical intermediate D was oxidized by HNO3 to produce the PdIV intermediate E. E underwent reductive elimination to afford the product (3a) together with the regeneration of Pd(OAc)2 which could complete the palladium catalytic cycle. Finally, Ag (0) was also oxidized to Ag(I) by HNO3 to complete the silver catalytic cycle.
Conclusions
In summary, we have developed a novel catalytic system for direct phosphorylation of substituted 3-benzylidene-2-arylisoindolin-1-one via a radical pathway. The reaction has a high regioselectivity that diphenylphosphine oxide free radical is prone to attacking aryl rather than a double bond. The method has a broad scope and offers a good yield. The corresponding products are potentially useful in drug discovery.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge financial support from the Prospective Study Program of Jiangsu (BY2015039-08), the National Natural Science Foundation of China (No. 21572149), and the Priority Academic Program Development of Jiangsu Higher Education Institutions.Notes and references
- A. K. Mailyan, J. A. Eickhoff, A. S. Minakova, Z. Gu, P. Lu and A. Zakarian, Chem. Rev., 2016, 116, 4441 CrossRef CAS PubMed.
- (a) L. Li, M. Wang, X. J. Zhang, Y. W. Jiang and D. W. Ma, Org. Lett., 2009, 11, 1309 CrossRef CAS PubMed; (b) V. Rys, A. Couture, E. Deniau and P. Grandclaudon, Tetrahedron, 2003, 59, 6615 CrossRef CAS; (c) H. Fuwa and M. Sasaki, Org. Biomol. Chem., 2007, 5, 1849 RSC; (d) Y. L. Choi, J. K. Kim, S. U. Choi, Y. K. Min, M. A. Bae, B. T. Kim and J. N. Heo, Bioorg. Med. Chem. Lett., 2009, 19, 3036 CrossRef CAS PubMed.
- (a) P. Schultz and R. A. Lerner, Acc. Chem. Res., 1993, 26, 391 CrossRef CAS; (b) J. D. Stewart, L. J. Liotta and S. J. Benkovic, Acc. Chem. Res., 1993, 26, 396 CrossRef CAS; (c) R. J. Cox, M. B. Mayo Martín and A. T. Hadfield, Chem. Commun., 2001, 18, 1710 RSC; (d) R. R. Breaker, G. R. Gough and P. T. Gilham, Biochemistry, 1993, 32, 9125 CrossRef CAS PubMed.
- T. Hirao, T. Masunaga and N. Yamada, Bull. Chem. Soc. Jpn., 1982, 55, 909 CrossRef CAS.
- (a) J. Yang, J. Xiao, T. Chen and L. B. Han, J. Organomet. Chem., 2016, 820, 120 CrossRef CAS; (b) J. Xu, P. Zhang, Y. Gao, Y. Chen, G. Tang and Y. Zhao, J. Org. Chem., 2013, 78, 8176 CrossRef CAS PubMed; (c) R. Berrino, S. Cacchi, G. Fabrizi, A. Goggiamani and P. Stabile, Org. Biomol. Chem., 2010, 8, 4518 RSC; (d) R. Q. Zhuang, Z. S. Cai, G. Tang, M. J. Fang and Y. F. Zhao, Org. Lett., 2011, 13, 2110 CrossRef CAS PubMed; (e) T. Fu, H. Qiao, Z. Peng, G. Hu, X. Wu, Y. Gao and Y. Zhao, Org. Biomol. Chem., 2014, 12, 2895 RSC; (f) M. Andaloussi, J. Lindh, J. Savmarker, P. J. Sjoberg and M. Larhed, Chem.–Eur. J., 2009, 15, 13069 CrossRef CAS PubMed; (g) T. Wang, S. Sang, L. Liu, H. Qiao, Y. Gao and Y. Zhao, J. Org. Chem., 2014, 79, 608 CrossRef CAS PubMed; (h) X. H. Zhang, H. Z. Liu, X. M. Hu, G. Tang, J. Zhu and Y. F. Zhao, Org. Lett., 2011, 13, 3478 CrossRef CAS PubMed; (i) T. Satoh and M. Miura, Chem. Lett., 2007, 36, 200 CrossRef CAS; (j) H. Y. Zhang, M. Sun, Y. N. Ma, Q. P. Tian and S. D. Yang, Org. Biomol. Chem., 2012, 10, 9627 RSC; (k) C. S. Demmer, N. Krogsgaard-Larsen and L. Bunch, Chem. Rev., 2011, 111, 7981 CrossRef CAS PubMed; (l) Y. L. Zhao, G. J. Wu, Y. Li, L. X. Gao and F. S. Han, Chem.–Eur. J., 2012, 18, 9622 CrossRef CAS PubMed; (m) S. Y. Chen, R. S. Zeng, J. P. Zou and O. T. Asekun, J. Org. Chem., 2014, 79, 1449 CrossRef CAS PubMed; (n) N. Iranpoor, H. Firouzabadi, K. R. Moghadam and S. Motavalli, RSC Adv., 2014, 4, 55732 RSC; (o) J. Li, X. Bi, H. Wang and J. Xiao, RSC Adv., 2014, 4, 19214 RSC; (p) H. Luo, H. Liu, X. Chen, K. Wang, X. Luo and K. Wang, Chem. Commun., 2017, 53, 956 RSC; (q) Y. Luo and J. Wu, Organometallics, 2009, 28, 6823 CrossRef CAS; (r) R. S. Shaikh, S. J. S. Düsel and B. König, ACS Catal., 2016, 6, 8410 CrossRef CAS; (s) K. Xu, F. Yang, G. Zhang and Y. Wu, Green Chem., 2013, 15, 1055 RSC.
- T. Kagayama, A. Nakano, S. Sakaguchi and Y. Ishii, Org. Lett., 2006, 8, 407 CrossRef CAS PubMed.
- X. J. Mu, J. P. Zou, Q. F. Qian and W. Zhang, Org. Lett., 2006, 8, 5291 CrossRef CAS PubMed.
- (a) O. Berger and J. L. Montchamp, Chem.–Eur. J., 2014, 20, 12385 CrossRef CAS PubMed; (b) S. H. Kim, S. H. Kim, C. H. Lim and J. N. Kim, Tetrahedron Lett., 2013, 54, 1697 CrossRef CAS; (c) S. O. Strekalova, K. M. N. Khrizanforov, T. V. Gryaznova, V. V. Khrizanforova and Y. H. Budnikova, Russ. Chem. Bull., 2016, 65, 1295 CrossRef CAS.
- C. G. Feng, M. Ye, K. J. Xiao, S. Li and J. Q. Yu, J. Am. Chem. Soc., 2013, 135, 9322 CrossRef CAS PubMed.
- (a) Y. Kuninobu, T. Yoshida and K. Takai, J. Org. Chem., 2011, 76, 7370 CrossRef CAS PubMed; (b) M. Xia, M. M. Huang, J. Y. Zhang, C. Y. Wang and Y. J. Wu, Org. Lett., 2013, 15, 6266 CrossRef PubMed; (c) C. B. Xiang, Y. J. Bian, X. R. Mao and Z. Z. Huang, J. Org. Chem., 2012, 77, 7706 CrossRef CAS PubMed; (d) C. Hou, Y. Ren, R. Lang, X. Hu, C. Xia and F. Li, Chem. Commun., 2012, 48, 5181 RSC; (e) C. Li, T. Yano, N. Ishida and M. Murakami, Angew. Chem., Int. Ed., 2013, 52, 9801 CrossRef CAS PubMed.
- (a) C. B. Xiang, Y. J. Bian, X. R. Mao and Z. Z. Huang, J. Org. Chem., 2012, 77, 7706 CrossRef CAS PubMed; (b) B. Wan, H. Wang, X. Li and F. Wu, Synthesis, 2012, 44, 941 CrossRef; (c) H. J. Zhang, W. Lin, Z. Wu, W. Ruan and T. B. Wen, Chem. Commun., 2015, 51, 3450 RSC; (d) S. H. Kim, K. H. Kim, J. W. Lim and J. N. Kim, Tetrahedron Lett., 2014, 55, 531 CrossRef CAS; (e) W. B. Sun, J. F. Xue, G. Y. Zhang, R. S. Zeng, L. T. An, P. Z. Zhang and J. P. Zou, Adv. Synth. Catal., 2016, 358, 1753 CrossRef CAS.
- P. Peng, L. Peng, G. Wang, F. Wang, Y. Luo and A. Lei, Org. Chem. Front., 2016, 3, 749 RSC.
- (a) D. Kalyani, K. B. McMurtrey, S. R. Neufeldt and M. S. Sanford, J. Am. Chem. Soc., 2011, 133, 18566 CrossRef CAS PubMed; (b) C. Qian, D. Lin, Y. Deng, X. Q. Zhang, H. Jiang, G. Miao, X. Tang and W. Zeng, Org. Biomol. Chem., 2014, 12, 5866 RSC; (c) H. Song, D. Chen, C. Pi, X. Cui and Y. Wu, J. Org. Chem., 2014, 79, 2955 CrossRef CAS PubMed; (d) P. Das, D. Saha, D. Saha and J. Guin, ACS Catal., 2016, 6, 6050 CrossRef CAS; (e) C. Premi, A. Dixit and N. Jain, Org. Lett., 2015, 17, 2598 CrossRef CAS PubMed; (f) Y.-F. Liang, X. Li, X. Wang, Y. Yan, P. Feng and N. Jiao, ACS Catal., 2015, 5, 1956 CrossRef CAS; (g) D. Zhang, X. Cui, Q. Zhang and Y. Wu, J. Org. Chem., 2015, 80, 1517 CrossRef CAS PubMed; (h) P. Y. Lee, P. Liang and W. Y. Yu, Org. Lett., 2017, 19, 2082 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra10902d |
| This journal is © The Royal Society of Chemistry 2017 |