Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Lithium triethylborohydride-promoted generation of α,α-difluoroenolates from 2-iodo-2,2-difluoroacetophenones: an unprecedented utilization of lithium triethylborohydride†
Peng Penga,
Jing-jing Wu *ab,
Jun-qing Lianga,
Tian-yu Zhanga,
Jin-wen Huangab and
Fan-hong Wu*a
*ab,
Jun-qing Lianga,
Tian-yu Zhanga,
Jin-wen Huangab and
Fan-hong Wu*a
aSchool of Chemical and Environmental Engineering, Shanghai Institute of Technology, Shanghai 201418, China. E-mail: wfh@sit.edu.cn
bKey Laboratory of Organofluorine Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, Shanghai 200032, China
First published on 12th December 2017
Abstract
Lithium triethylborohydride was found to promote the generation of α,α-difluoroenolates from 2-iodo-2,2-difluoroacetophenones, and applied to the synthesis of polyfluorinated β-hydroxy ketones via self-condensation or aldol reaction. The reaction indicates an unprecedented utilization of lithium triethylborohydride and provides novel access to the generation of α,α-difluoroenolates.
Difluoroenolates are useful fluorinated synthons for preparing difluoromethylene compounds,1 with significant application in medicinal chemistry.2 Accordingly, the protocols to generate difluoroenolates have attracted much attention. Colby firstly reported that compounds trifluoromethyl-α,α-difluoro-β-keto gem-diols could be used to give difluoroenolates by the release of trifluoroacetate.3 Additionally, more difluorinated compounds have been developed as valuable precursors of difluoroenolates, such as α,α-difluoroketones,4 α,α-difluoro-α-(trimethylsilylacet)amides,5 α,α-difluoro-β-ketoesters,6 α,α,α-trifluoroketones,7 and 2,2-difluoro-1,3-diketones.8 Furthermore, difluoroenoxysilanes or difluoroenol O-Boc esters were often served as difluoroenolates in different difluoromethylenation reactions,9 as well as transition-metal difluoroenolates.10
2-Iodo-2,2-difluoroacetophenones are one of the important building blocks to introduce α,α-difluoromethyl ketone fragment into molecules.11 Our group has reported several reactions with 2-iodo-2,2-difluoroacetophenones to construct structurally diverse difluoromethylene compounds.11d–f As part of our continued research, further structure modification of 2-iodo-2,2-difluoroacetophenones was investigated. Lithium triethylborohydride (LiEt3BH, a superhydride, 1 M in THF) is a powerful reducing agent and can efficiently reduce a wide range of functional groups, especially halogen atoms.12 However, the reaction with 2-iodo-2,2-difluoroacetophenones and LiEt3BH result in the generation of difluoroenolates instead of reduction products. Herein, we disclosed the LiEt3BH-promoted in situ generation of difluoroenolates from 2-iodo-2,2-difluoroacetophenones and their application in aldol-type self-condensation and aldol reactions to give various α,α-difluoro-β-hydroxy ketones (Scheme 1).
 | ||
| Scheme 1 The novel utilization of LiEt3BH for the generation of α,α-difluoroenolates from 2-iodo-2,2-difluoroacetophenones. | ||
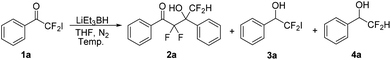
Initially, we chose LiEt3BH as one of the reducing agents to selectively reduce the carbonyl group or iodine atom of 2-iodo-2,2-difluoroacetophenones. Surprisingly, our initial attempt to perform the reaction between 2,2-difluoro-2-iodo-1-phenylethanone 1a and LiEt3BH at −78 °C did not furnish any reduction product, instead leading to a self-adduct α,α,γ,γ-tetrafluoro-β-hydroxy ketone 2a with high yield (Table 1, entry 1). Raising the temperature of LiEt3BH only led to a little increasing of the yield of reduction product 3a and 4a (entries 2 and 3). It was speculated that a difluoroenolate 5 was generated from 2,2-difluoro-2-iodo-1-phenylethanone with LiEt3BH, then the difluoroenolate 5 went through protonation process to form α,α-difluoroacetophenone 6. The aldol reaction between 5 and 6 gave the α,α,γ,γ-tetrafluoro-β-hydroxy ketone 2a (Scheme 2).
We wonder that whether the fluorine atom in 2-iodo-2,2-difluoroacetophenone play an important role in the fortuitous reaction. Therefore, the reaction of 2-halo-acetophenone or 2-chloro(bromo, fluoro)-2,2-difluoroacetophenone or 2,2-difluoroacetophenone with LiEt3BH were also performed in THF at −78 °C. The results showed that all these reduction reactions proceeded well leading to the formation of different reduction products (see Scheme 1 in ESI†), which demonstrates the specific characteristic of substrate 2-iodo-2,2-difluoroacetophenone owing to the adjacent fluorine atoms of carbonyl group.
Then, other reducing agents were also investigated using 2,2-difluoro-2-iodo-1-phenylethanone 1a as substrate, only led to the formation of different reduction products (Scheme 3). It was noteworthy that the reducing agents sodium borohydride and Zn power show high selectivity, affording 2,2-difluoro-2-iodo-1-phenylethan-1-ol 3a and 2,2-difluoro-1-phenylethanone 6 as major products separately.
Next, the scope of 2,2-difluoro-2-iodo-1-phenylethanone 1 was explored through the LiEt3BH promoted self-condensation (Table 2). The 2-iodo-2,2-difluoroacetophenones 1b–f bearing electron-donating groups, electron-withdrawing group or halogen atoms, 2,2-difluoro-2-iodo-1-(thiophen-2-yl)ethanone 1g and 2,2-difluoro-2-iodo-1-(naphthalen-2-yl)ethanone 1h were subjected to the self-condensation reaction and all were found applicable to the reaction, giving the corresponding α,α,γ,γ-tetrafluoro-β-hydroxy ketones 2a–h in good yields. Few of the reduction products 4d, 4f, 4g were still observed in low yields. The results indicated that 2-iodo-2,2-difluoroacetophenone derivatives bearing different aryl or heterocyclic ring could be served as difluoroenolates precursors through the reaction with LiEt3BH, which provided a novel and efficient access to difluoromethylenation reactions.
α,α-Difluoro-β-hydroxy ketones are an important class of substructure in medicinal chemistry,13 which could be obtained via aldol reaction with difluoroenolates.14 Accordingly, to further demonstrate the utility of LiEt3BH, aldol reaction of 2-iodo-2,2-difluoroacetophenones with aldehydes promoted by LiEt3BH were conducted. 2-Iodo-2,2-difluoro-1-phenylethanone 1a and benzaldehyde 7 was selected as model reaction substrates to optimize the reaction condition (Table 3). The corresponding aldol produce 8 was produced in the presence of LiEt3BH (1.2 equiv. to compound 1a) at −78 °C in THF (entry 1). The results showed that raising the temperature led to an increased yield of reduction product 4a (entries 2–4) and the conversation decreased with the decreasing of the amount of LiEt3BH (entry 5).
Hence, more aldol reactions were carried out under the optimized condition (Scheme 4). To our delight, the aldol products polyfluorinated β-hydroxy ketones were obtained in high yields by employing the fluorinated aldehydes such as 2,2-difluoro-1-phenylethanone or 2,2,2-trifluoro-1-phenylethanone (Scheme 4a and b). The substrate 1-(4-bromophenyl)-2,2-difluoro-2-iodoethan-1-one 1c was also suitable for the aldol reaction (Scheme 4c). Furthermore, 2,2,4,4,4-pentafluoro-3,3-dihydroxy-1-phenylbutan-1-one 12 was also used to react with benzaldehyde in the present of LiEt3BH. The reaction gave the aldol product 2,2-difluoro-3-hydroxy-1,3-diphenylpropan-1-one 8 and self-condensation product 2,2,4,4-tetrafluoro-3-hydroxy-1,3-diphenylbutan-1-one 2a with 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio (Scheme 4d), which indicated that difluoroenolate can be generated from 2,2,4,4,4-pentafluoro-3,3-dihydroxy-1-phenylbutan-1-one with LiEt3BH either.
1 ratio (Scheme 4d), which indicated that difluoroenolate can be generated from 2,2,4,4,4-pentafluoro-3,3-dihydroxy-1-phenylbutan-1-one with LiEt3BH either.
In summary, we have demonstrated an unprecedented utilization of LiEt3BH for the generation of α,α-difluoroenolates from 2-iodo-2,2-difluoroacetophenones in THF. Applications of the protocol led to the synthesis of polyfluorinated β-hydroxy ketones via self-condensation reaction and aldol reaction. The effectiveness of LiEt3BH was discussed by the variation of α-halogen acetophenones and reducing agents in the reduction reaction. It turned out that LiEt3BH was served as a reducing agent with most α-halogen acetophenones, resulting in the formation of different reduction products. The result disclosed the specific property of 2-iodo-2,2-difluoroacetophenones, which might due to that the fluorine atoms of 2-iodo-2,2-difluoroacetophenones have significant impact and change the property of the adjacent C–I bond. Further study to apply the protocol for the preparation of diverse difluoromethylene compounds are in progress in our laboratory.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful for financial supports from the National Natural Science Foundation of China (No. 21472126, 21672151).References
- (a) D. Stolz and U. Kazmaier, in Chemistry of Metal Enolates, ed. J. Zabicky, Wiley, London, 2009, p. 355 Search PubMed; (b) B. M. Stoltz, N. B. Bennett, D. C. Duquette, A. F. G. Goldberg, Y. Liu, M. B. Loewinger and C. M. Reeve, in Comprehensive Organic Synthesis II, ed. P. Knochel and G. A. Molander, Elsevier, Oxford, 2014, vol. 3, p. 1 Search PubMed.
- (a) J. Wang, M. Sánchezroselló, J. L. Aceña, P. C. Del, A. E. Sorochinsky, S. Fustero, V. A. Soloshonok and H. Liu, Chem. Rev., 2014, 114, 2432 CrossRef CAS PubMed; (b) J. O. Link, J. G. Taylor, L. Xu, M. Mitchell, H. Guo, H. Liu, D. Kato, T. Kirschberg, J. Sun, N. Squires, J. Parrish, T. Keller, Z.-Y. Yang, C. Yang, M. Matles, Y. Wang, K. Wang, G. Cheng, Y. Tian, E. Mogalian, E. Mondou, M. Cornpropst, J. Perry and M. C. Desai, J. Med. Chem., 2014, 57, 2033 CrossRef CAS PubMed; (c) F. Xue, H. Li, S. L. Delker, J. Fang, P. Martásek, L. J. Roman, T. L. Poulos and R. B. Silverman, J. Am. Chem. Soc., 2010, 132, 14229 CrossRef CAS PubMed; (d) M. O. Anderson, J. Zhang, Y. Liu, C. Yao, P.-W. Phuan and A. S. Verkman, J. Med. Chem., 2012, 55, 5942 CrossRef CAS PubMed.
- (a) C.-H. Han, E. H. Kim and D. A. Colby, J. Am. Chem. Soc., 2011, 133, 5802 CrossRef CAS PubMed; (b) C.-H. Han, A. E. Salyer, E. H. Kim, X.-Y. Jiang, R. E. Jarrard, M. S. Powers, A. M. Kirchhoff, T. K. Salvador, J. A. Chester, G. H. Hockerman and D. A. Colby, J. Med. Chem., 2013, 56, 2456 CrossRef CAS PubMed; (c) R. A. Hazlitt, Q.-L. Tran, M. F. Sowaileh and D. A. Colby, J. Org. Chem., 2017, 82, 2231 CrossRef CAS PubMed; (d) R. A. Hazlitt, J. P. John, Q.-L. Tran and D. A. Colby, Tetrahedron Lett., 2016, 57, 1906 CrossRef CAS PubMed.
- S. Ge, W. Chaladaj and J. F. Hartwig, J. Am. Chem. Soc., 2014, 136, 4149 CrossRef CAS PubMed.
- Q. Chen, J. Zhou, Y. Wang, C. Wang, X. Liu, Z. Xu, L. Lin and R. Wang, Org. Lett., 2015, 17, 4212 CrossRef CAS PubMed.
- M. Yang, D. L. Orsi and R. A. Altman, Angew. Chem., Int. Ed., 2015, 54, 2361 CrossRef CAS PubMed.
- R. Doi, M. Ohashi and S. Ogoshi, Angew. Chem., Int. Ed., 2016, 55, 341 CrossRef CAS PubMed.
- J.-L. Qian, W.-B. Yi, X. Huang, J. P. Jasinski and W. Zhang, Adv. Synth. Catal., 2016, 358, 2811 CrossRef CAS.
- (a) Y.-L. Liu and J. Zhou, Chem. Commun., 2012, 48, 1919 RSC; (b) Y.-L. Liu, X.-P. Zeng and J. Zhou, Chem.–Asian J., 2012, 7, 1759 CrossRef CAS PubMed; (c) J.-S. Yu, Y.-L. Liu, J. Tang, X. Wang and J. Zhou, Angew. Chem., Int. Ed., 2014, 53, 9512 CrossRef CAS PubMed; (d) S. Sasaki, T. Suzuki, T. Uchiya, S. Toyota, A. Hirano, M. Tanemura, H. Teramoto, T. Yamauchi and K. Higashiyama, J. Fluorine Chem., 2016, 192, 78 CrossRef CAS.
- (a) R. Doi, M. Ohashi and S. Ogoshi, Angew. Chem., Int. Ed., 2016, 55, 341 CrossRef CAS PubMed; (b) R. Doi, K. Kikushima, M. Ohashi and S. Ogoshi, J. Am. Chem. Soc., 2015, 137, 3276 CrossRef CAS PubMed; (c) P. V. Ramachandran and A. Chatterjee, Org. Lett., 2008, 10, 1195 CrossRef CAS PubMed.
- (a) Z.-M. Qiu and D. J. Burton, Tetrahedron Lett., 1993, 34, 3239 CrossRef CAS; (b) K. C. Kwak, H. Oh, Y. G. Yun, B. H. Kim, Y. H. Lee and K. Y. Chai, Bull. Korean Chem. Soc., 2002, 23, 157 CrossRef CAS; (c) Z.-M. Qiu and D. J. Burton, J. Org. Chem., 1995, 60, 6798 CrossRef CAS; (d) J.-X. Wang, J.-J. Wu, H. Chen, S.-W. Zhang and F.-H. Wu, Chin. Chem. Lett., 2015, 26, 1381 CrossRef CAS; (e) D.-F. Wang, J.-J. Wu, J.-W. Huang, J.-Q. Liang, P. Peng, H. Chen and F.-H. Wu, Tetrahedron, 2017, 73, 3478 CrossRef CAS; (f) H. Chen, J.-X. Wang, J.-J. Wu, Y.-J. Kuang and F.-H. Wu, J. Fluorine Chem., 2017, 200, 41 CrossRef CAS.
- (a) S. Krishnamurthy, R. M. Schubert and H. C. Brown, J. Am. Chem. Soc., 1973, 75, 8486 CrossRef; (b) S. Krishnamurthy and H. C. Brown, J. Am. Chem. Soc., 1973, 95, 1669 CrossRef; (c) F.-P. Liang, H. W. Schmalle and H. Berke, Eur. J. Inorg. Chem., 2006, 5081 CrossRef CAS; (d) J.-J. Wu and S. Cao, ChemCatChem, 2011, 3, 1582 CrossRef CAS; (e) S. Chowdhury and R. F. Standaert, J. Org. Chem., 2016, 81, 9957 CrossRef CAS PubMed; (f) L. Silva, R. F. Affeldt and D. S. Lüdtke, J. Org. Chem., 2016, 81, 5464 CrossRef CAS PubMed; (g) B. Denolf, E. Leemans and N. De Kimpe, J. Org. Chem., 2007, 72, 3211 CrossRef CAS PubMed; (h) H. Tanaka and K. Ogasawara, Tetrahedron Lett., 2002, 43, 4417 CrossRef CAS.
- (a) C.-H. Han, A. E. Salyer, E. H. Kim, X.-Y. Jiang, R. E. Jarrard, M. S. Powers, A. M. Kirchhoff, T. K. Salvador, J. A. Chester, G. H. Hockerman and D. A. Colby, J. Med. Chem., 2013, 56, 2456 CrossRef CAS PubMed; (b) M.-H. Yang, J. R. Hunt, N. Sharifi and R. A. Altman, Angew. Chem., Int. Ed., 2016, 55, 9080 CrossRef CAS PubMed; (c) A. M. Doherty, I. Sircar, B. E. Kornberg, J. Quinlll, R. Thomas Winters, J. S. Kaltenbronn, M. D. Taylor, B. L. Batley and S. R. Rapundalo, J. Med. Chem., 1992, 35, 2 CrossRef CAS PubMed.
- (a) P. Zhang and C. Wolf, J. Org. Chem., 2012, 77, 8840 CrossRef CAS PubMed; (b) P. Zhang and C. Wolf, Angew. Chem., Int. Ed., 2013, 52, 7869 CrossRef CAS PubMed; (c) C. Xie, L.-M. Wu, H.-B. Mei, V. A. Soloshonok, J.-L. Han and Y. Pan, Tetrahedron Lett., 2014, 55, 5908 CrossRef CAS; (d) H.-B. Mei, C. Xie, J. L. Aceña, V. A. Soloshonok, G.-L. Röschenthaler and J.-L. Han, Eur. J. Org. Chem., 2015, 6401 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra12130j |
| This journal is © The Royal Society of Chemistry 2017 |