Telescoped continuous flow generation of a library of highly substituted 3-thio-1,2,4-triazoles†
Mariana C. F. C. B.
Damião
a,
Renan
Galaverna
a,
Alan P.
Kozikowski
b,
James
Eubanks
c and
Julio C.
Pastre
 *a
*a
aInstitute of Chemistry, University of Campinas - UNICAMP, PO Box 6154, 13083-970, Campinas, SP, Brazil. E-mail: juliopastre@iqm.unicamp.br; Tel: +55 (19) 3521 3143
bStarWise Therapeutics LLC, University Research Park, 53719-1235, Madison, Wisconsin, USA
cDivision of Genetics and Development, Krembil Research Institute, M5T 2S8, Toronto, Ontario, Canada
First published on 4th October 2017
Abstract
We report herein the successful application of continuous flow micro reactors to prepare important building blocks based on the 3-thio-1,2,4-triazole core. A telescoped continuous flow process was developed based on the condensation of hydrazides and isothiocyanates to deliver an in situ stream of a thiosemicarbazide, which subsequently was cyclized under basic conditions. The obtained 1,2,4-triazole-3-thiol was further alkylated with benzyl/alkyl halides. In addition, we evaluated the scope of heterocycle formation and alkylation using different hydrazides, isothiocyanates, and aryl/alkyl chlorides, bromides and iodides. We were able to synthesize a small library of 18 compounds in 48 minutes of residence time for each synthesis, and in moderate to excellent yields, in a telescoped fashion. The fully integrated synthesis flow platform enables the fast generation of compound libraries, reducing the time consumed in preliminary stages of a drug discovery process.
Introduction
Lead compound identification and optimization, synthesis of chemical/compound libraries, and supply of materials in sufficient quantity for clinical investigation can be time-consuming and a laborious task for medicinal chemists.1 Consequently, further advances are needed to reduce the time taken to synthesize libraries of compounds, identify potential leads, optimize synthetic routes to afford final compounds, and put drug candidates into production.Moreover, recent studies reveal that the production of active pharmaceutical ingredients (APIs) by the pharmaceutical industry generates up to 1000% more waste than other chemical manufacturing such as oil refining, bulk chemicals, and fine chemicals production.2 Solvent-related waste represents 80% of this amount, as reported by GlaxoSmithKline (GSK),3 and several studies have emphasized the need for novel technologies in organic synthesis in order to circumvent and alleviate these problems.4
In this context, continuous flow chemistry is finding increasing use as an enabling technology,5 providing a number of advantages over batch processes for organic synthesis, and leading to a variety of interesting and exciting opportunities.6 For example, the use of small diameter tubes enables higher mass/heat transfer and can increase the overall reaction rate. In addition, continuous flow processing enables simple scale up of reactions, enhanced mixing, temperature and pressure control, decrease of energy consumption, and integration of several reaction steps (telescoped synthesis).7
Regarding telescoped synthesis, a considerable decrease of waste production can be achieved since several steps are combined in a single process without the need of exhaustive workup procedures, the latter involving extensive manual labour for each step and more waste generation.4–7 Furthermore, the volume of reagents/solvents is reduced, which facilitates the screening of reaction conditions, and consequently, the rapid generation of focused compound libraries. These aspects are extremely appealing to pharmaceutical companies, which could benefit from cost reduction and rapid drug manufacturing.
In addition, bringing these features into a MedChem program, the discovery of drug candidates, synthesis of chemical libraries, and scale up of reactions for the preparation of enough material for clinical trials would become faster and simpler, shortening the time needed to deliver new drug candidates.5b,c,8
Indeed, in view of the aforementioned advantages, the application of continuous flow technology to produce APIs and fine chemicals has become very popular,9 especially in academia. Moreover, although the pharmaceutical industry still relies on multipurpose batch reactors, it is clear that attention is arising toward continuous flow manufacturing of APIs, as can be seen by the recent synthesis of the chemotherapy drug candidate prexasertib at Eli Lilly & Co., using current good manufacturing practices (cGMPs) and linking each step in the continuous process to quality-control systems.10 The continuous process afforded superior performance and safety relative to batch and also allowed containment of a potentially hazardous material.
Heterocyclic compounds define an important class of chemical entities in drug discovery, and are present in a wide variety of hit/lead compounds and marketed drugs.11 As a result, new methodologies and technologies that facilitate the generation of heterocycle libraries are valuable tools to a drug discovery program, and have a great impact in the pharmaceutical industry. The successful development of practical and relevant continuous methods to give different heterocyclic building blocks has been recently reported in the literature.12
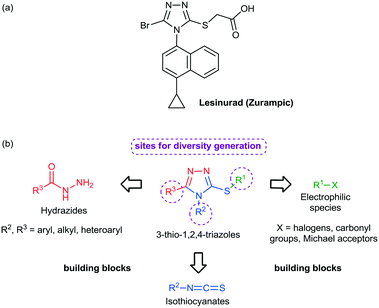
Among the heterocycles relevant for drug discovery, sulfur containing compounds represent an interesting class and have received considerable attention due to their feasible synthesis and biological profile.13 For example, mercapto- and thio-substituted 1,2,4-triazole ring systems have been incorporated into a diversity of drug candidates including anti-inflammatory,14 antimicrobial,15 antitubercular,16 and antitumor agents.17 In 2015, the American Food and Drug Administration approved lesinurad (Zurampic) for the treatment of gout since it acts as an urate anion exchange trasporter 1 (URAT1) inhibitor (Fig. 1a).18
 | ||
| Fig. 1 (a) Chemical structure of lesinurad. (b) Building blocks for the construction of the 3-thio-1,2,4-triazole core and its sites for modification/diversity generation. | ||
Some research groups have previously investigated the synthesis of the 1,2,4-triazole core using continuous flow chemistry.7c For example, Baxendale et al. reported the synthesis of 1,2,4-triazole and pyrrolo[1,2-c]pyrimidine in continuous flow regime. For the telescoped synthesis, ethyl isocyanoacetate was prepared in situ by the dehydration reaction of N-formylglycine using reactive triphosgene. In addition to 1,2,4-triazoles, the continuous flow syntheses of other important heterocycles, such as thiazoles,19 1,2,4-thiadiazoles,12k aziridines,12g hydantoins,12h 1,2,3-triazoles,12c,20 imidazol[1,2-a]pyridines21 pyrazoles and pyrazolines,22 imidazoles,23 pyrrolidines and pyrroles,24 oxazolines and oxazoles25 have been successfully reported in the literature. As such, the development of efficient continuous methods to produce libraries of heterocycles is with no doubt a valuable alternative to conventional batch methods that adopt classical organic synthesis conditions.26
Preparation of novel heterocyclic building blocks containing suitable derivatization sites is of great importance to the successful discovery of new bioactive molecules and drug candidates. Bearing this in mind, sulfur-containing heterocycles, such as 3-thio-1,2,4-triazoles, represent interesting building blocks to develop thio/mercapto derivatives due to the wide range of modifications that could take place at positions 3, 4 and 5, according to the selection of the corresponding starting materials (Fig. 1b) and to several organic reactions that could take place at the sulfur atom. Taking this into account, we report herein our results toward the continuous flow generation of 3-thio-1,2,4-triazoles from various hydrazides, isothiocyanates and halides using a unified continuous flow platform.
Results and discussion
Synthesis of 3-thio-1,2,4-triazoles in batch
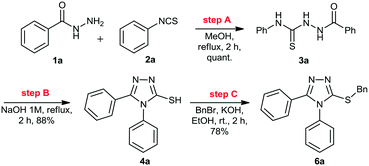
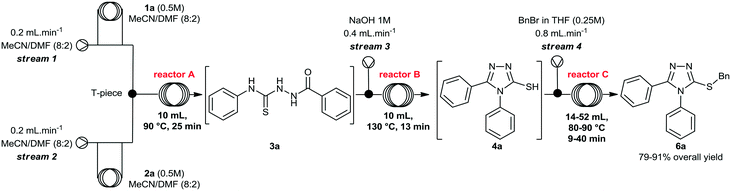
Initially, we focused on the batch synthesis of the 3-thio-1,2,4-triazoles. For this purpose, we performed the synthesis of compound 6a using classical batch methods previously reported.27 Typically, the synthesis of the 1,2,4-triazole-3-thiol core is achieved by a condensation reaction between a hydrazide and isothiocyanate, followed by cyclization in basic conditions. Under these conditions, 1,2,4-triazole-3-thiols are usually obtained in 4 to 7 hours, and in good to excellent yields, after neutralization of the reaction medium and filtration.23Firstly, condensation of the commercially available hydrazide 1a and phenylisothiocyanate (2a) gave thiosemicarbazide 3a in quantitative yield. Subsequently, thiol 4a was generated in 88% yield by a cyclization reaction using a 1M NaOH solution. Lastly, 4a was alkylated with benzyl bromide (5a) to afford 6a in 69% overall yield in 3 steps after 6 hours (Scheme 1). These yields are in accordance with the literature, however, we observed the formation of a solid under these conditions, which would not be suitable for a direct translation into a continuous flow process.
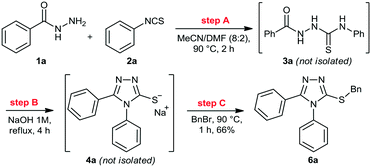
We then performed a quick screening of solvents to keep all reagents and products soluble inside the flow system, to avoid solid formation, which in turn would lead to blockages and shutdown of the flow system. From this study, a mixture of MeCN/DMF (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) was selected and it was applied as solvent to perform the one-pot synthesis of 6a (Scheme 2). Thus, condensation of hydrazide 1a and isothiocyanate 2a proceeded smoothly to give thiosemicarbazide 3a. Sequentially, addition of 1M NaOH solution in the reaction vessel gave the cyclic product 4a, which was further alkylated by the addition of benzyl bromide (5a). The desired 3-thio-1,2,4-triazole 6a was obtained in 66% overall yield after 7 hours, without the need of isolation and purification of the intermediates 3a and 4a, thereby saving time and reducing waste generation.
2) was selected and it was applied as solvent to perform the one-pot synthesis of 6a (Scheme 2). Thus, condensation of hydrazide 1a and isothiocyanate 2a proceeded smoothly to give thiosemicarbazide 3a. Sequentially, addition of 1M NaOH solution in the reaction vessel gave the cyclic product 4a, which was further alkylated by the addition of benzyl bromide (5a). The desired 3-thio-1,2,4-triazole 6a was obtained in 66% overall yield after 7 hours, without the need of isolation and purification of the intermediates 3a and 4a, thereby saving time and reducing waste generation.
At this stage, we decided to develop a convenient integrated flow synthesis for rapid generation of molecules bearing the 3-thio-1,2,4-triazole heterocycle. To this end, continuous flow conditions were evaluated aiming at reducing reaction time, improving yields, and avoiding exhaustive steps such as isolation, purification and characterization of intermediates.
Evaluation of the condensation step (step A) under segmented flow conditions
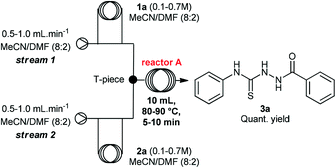
Initially, we evaluated and optimized each step separately using a continuous flow system. We decided to start using the commercially available substrates phenylhydrazide (1a) and phenylisothiocyanate (2a). The flow reactor was set up according to the conditions described in Table 1. Hydrazide 1a was taken up in MeCN/DMF (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and filled into loop 1, and isothiocyanate 2a was taken up in MeCN/DMF (8
2) and filled into loop 1, and isothiocyanate 2a was taken up in MeCN/DMF (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and filled into loop 2. Both loops were simultaneously injected into streams of MeCN/DMF (8
2) and filled into loop 2. Both loops were simultaneously injected into streams of MeCN/DMF (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and the plugs met at a T-piece (simple junction, PTFE) before passing through a 10 mL coil reactor.
2) and the plugs met at a T-piece (simple junction, PTFE) before passing through a 10 mL coil reactor.
A rapid screening of reaction parameters was performed, including reaction temperature, flow rate, and reagent concentration. We observed full conversion in all experiments and it was established that the desired thiosemicarbazide 3a could be obtained in quantitative yield at 90 °C within 5 min residence time. Instead of pushing this simple condensation reaction to its limits, we decided to move on and tackle the more challenging cyclization reaction, which took 4 hours to go to completion in batch.
After briefly evaluating the conditions for the preparation of the thiosemicarbazide 3a in continuous flow regime, we turned our attention to its cyclization under basic conditions. To perform the multi-step flow synthesis, an integrated system was configured as shown in Table 2, consisting of a Uniqsis FlowSyn system, and a Vapourtec R2+R4 unit. Using this flow setup, the thiosemicarbazide 3a was generated in reactor A at 90 °C. The outcome stream was mixed with a 1M NaOH aqueous solution (stream 3) via a T-piece, and the resulting stream was pumped through reactor B, without isolating intermediate 3a.
| Entry | Res. time (min) | Total flow ratea (mL min−1) | Flow rate stream 3 (mL min−1) | Temp. reactor B (°C) | Yieldb (%) |
|---|---|---|---|---|---|
| a Values represent the sum of streams 1, 2 and 3. b Isolated yield. c 75 psi back-pressure regulator. d 100 psi back-pressure regulator to avoid solvent evaporation. | |||||
| 1 | 33 | 0.75 | 0.25 | 120c | 50 |
| 2 | 33 | 0.75 | 0.25 | 130d | 60 |
| 3 | 42 | 0.60 | 0.20 | 130d | 83 |
| 4 | 38 | 0.80 | 0.40 | 130 | 85 |
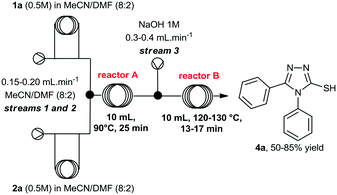
At this point, we also performed a quick evaluation of the flow rate of streams 1, 2 and 3, and the temperature of reactor B to achieve complete conversion of intermediate 3a (Table 2), whereas the temperature of reactor A, and concentration of reagents 1a and 2a were kept the same.
Evaluation of the cyclization step (step B) under segmented flow conditions
From this study, we could confirm that the residence time and temperature of reactor B strongly influenced the reaction outcome. Indeed, a 10 °C rise from 120 to 130 °C (entries 2 and 3) increased reaction yield from 50 to 60%. In addition, reduction of flow rates further improved the yield to 83%. Also, variation of the flow rate at stream 3 (entry 4, from 0.2 to 0.4 mL min−1), in order to increase the amount of base in the mixture, slightly affected the yield.Under ideal conditions, compound 4a was generated in 85% isolated yield in 38 min total residence time. It is important to mention that we previously used a flow rate of 2 mL min−1 for streams 1 and 2, however, we reduced it to 0.4 mL min−1 to accommodate the total residence time and achieve complete consumption of intermediate 3a, rather than increasing the volume of reactor B.
Evaluation of the alkylation step (step C) under segmented flow conditions
Having optimized the cyclization step, we evaluated the final reaction, and a multi-step flow sequence was configured as shown in Fig. 2. Alkylation of compound 4a showcases the set of reactions that could be explored using the thiolate intermediate as nucleophile. Moreover, this sequence, which involves condensation, cyclization and alkylation, is used for the batch synthesis of the drug lesinurad (Zurampic) on the industrial scale.28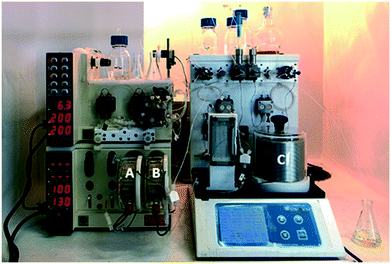 | ||
| Fig. 2 Picture of the full setup used for the continuous flow synthesis of the library of 3-thio-1,2,4-triazoles. Reactors A, B and C, respectively.29 | ||
Thus, reaction conditions for steps 1 and 2 were maintained and a new stream (stream 4) containing a solution of benzyl bromide (5a) in THF was added to the system. The resulting mixture was pumped through a reaction coil, and the product was collected and worked up by evaporation, followed by extraction and further purification by flash chromatography to remove minor impurities.
We briefly evaluated selected reaction parameters such as concentration of benzyl bromide, temperature, volume of reactor C, and the flow rate of stream 4 (Table 3). Firstly, changing the volume of reactor C from 52 mL to 14 mL afforded a slight decrease in the chemical yield from 85% to 81% and we decided to carry on the study using the smaller reactor since the residence time was considerably shorter. Next, increasing the flow rate of stream 4, i.e. the amount of the electrophile, resulted in a slight increase in the yield to 85% (entry 3), and variation of temperature at reactor C from 80 °C to 90 °C afforded compound 6a in 91% isolated yield.
| Entry | Res. time (min) | Flow rate stream 4 (mL min−1) | Vol. reactor C (mL) | Conc. BnBr (M) | Temp. reactor Ca (°C) | Yieldb (%) |
|---|---|---|---|---|---|---|
| a 100 psi back-pressure regulator to avoid solvent evaporation. b Isolated yield. | ||||||
| 1 | 78 | 0.5 | 52 | 0.25 | 80 | 85 |
| 2 | 48 | 0.5 | 14 | 0.25 | 80 | 81 |
| 3 | 48 | 0.8 | 14 | 0.25 | 80 | 85 |
| 4 | 48 | 0.8 | 14 | 0.25 | 90 | 91 |
| 5 | 48 | 0.8 | 14 | 0.125 | 90 | 79 |
Finally, the concentration of benzyl bromide was lowered to 0.125 M, and the yield of compound 6a dropped to 79%, confirming that an excess of the alkylating agent was necessary. Therefore, we decided to carry out the subsequent reactions using the conditions described in Table 3 (entry 4).
These outcomes demonstrate that the final compound 6a was generated rapidly (48 min) and in high yield (91% overall yield) using a continuous flow setup. Furthermore, no isolation of intermediates 3a and 4a was required, and reaction time and yield were significantly better when compared to the batch process (7 hours, and 66% overall yield).
Segmented flow synthesis of 1,2,4-triazole-3-thiols and telescoped gram scale synthesis of compound 6a
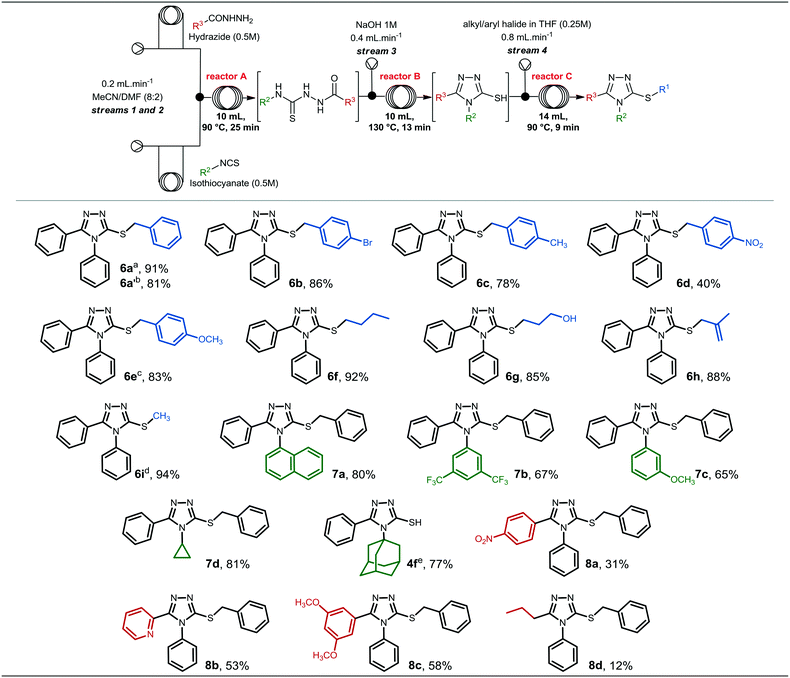
After accomplishing the synthesis of compound 6a in segmented flow regime, we were interested in investigating the scope of heterocycle formation and alkylation.Reactions with different alkyl/aryl hydrazides, isothiocyanates and alkyl/benzyl halides were assessed. The reaction conditions used to generate these compounds were similar to those used for the synthesis of compound 6a in the previous section, and the results are presented in Fig. 3.
Alkyl/benzyl halides could be reacted with the 4,5-diphenyl-4H-1,2,4-triazole-3-thiolate 4a to obtain compounds 6a–i in good to excellent yields. In general, modifications at the R1 position did not affect reaction yields significantly; however, compound 6d, which contains a nitro group at position 4 of the aromatic ring, presented the lowest yield (40%) among compounds 6a–i.
Additionally, compounds 6a′ and 6e were generated from the respective benzyl chlorides, revealing that less reactive halides are able to give the final alkylated product in good yields. Compound 6i presented the highest yield (94%), and it was synthesized from thiol 4a and methyl iodide.
The synthesis of 3-thio-1,2,4-triazoles 7a–d from different isothiocyanates required similar conditions to form and to alkylate the 1,2,4-triazole-3-thiolate intermediate. To synthesize compounds 7a and 7d, specifically, we used the previously described parameters, and they were obtained in 80 and 81% yield, respectively. On the other hand, compound 7b required longer residence time (185 min) to ensure complete conversion of the thiosemicarbazide intermediate to the 1,2,4-triazole-3-thiol. The presence of electron-withdrawing groups at positions 3 and 5 (3,5-CF3 groups) of the isothiocyanate might affect the intramolecular attack during the cyclization step, and consequently, a longer reaction time was required to generate the 1,2,4-triazole-3-thiol heterocycle and to give compound 7b.
On the other hand, compound 7c, which contains an electron-donating group attached to the aromatic ring, was obtained in a moderate yield (46%) using the standard conditions. To achieve a better yield, increasing the temperature of reactor C to 100 °C led to the formation of compound 7c in a superior yield (65%). The last isothiocyanate to be evaluated was 1-adamantyl isothiocyanate, however, we could not observe conversion of the 1,2,4-triazole-3-thiol into the corresponding alkylated product in either batch or continuous flow regime. The thiol intermediate 4f was obtained in 77% yield and over 75 min residence time under continuous flow conditions, however, no alkylation took place under the optimized conditions or increasing temperature and residence time for the alkylation step. Thus, we concluded that the alkylation was probably hampered due to steric hindrance of the bulky adamantyl group.
Lastly, we investigated the generation of 3-thio-1,2,4-triazoles starting from other alkyl and aryl hydrazides. 4-Nitrobenzohydrazide, 2-pyridinecarbohydrazide and 3,5-dimethoxybenzohydrazide were previously prepared in batch (experimental procedures are described in the ESI†), while butylhydrazide was commercially available. As can be seen, the substitution pattern of the hydrazides affected the overall yields and compounds 8a–d were obtained in low to moderate yields, in sharp contrast to the result obtained with butylhydrazide.
Compound 8a, which contains a nitro group attached to the aromatic ring presented a reduced yield in comparison to 8b and 8c, prepared from 2-pyridinecarbohydrazide and 3,5-dimethoxybenzohydrazide, respectively. Therefore, different substituents connected to the benzohydrazide ring, either electron-withdrawing or electron-donating groups, might affect its reactivity, and consequently, the chemical yield for the whole process. Compound 8d was obtained in only 12% yield and, thus, we decided to investigate its batch synthesis, however, it was obtained in 10% yield under the conditions described in Scheme 2. This result indicates that alkyl hydrazides might not be appropriate to generate the desired 3-thio-1,2,4-triazole core under the developed conditions; however, further experiments should be conducted for these specific substrates.
It is noteworthy to mention that some compounds were obtained in moderate or low yields, however we demonstrated the applicability of the continuous process developed to rapidly generate 3-thio-1,2,4-triazoles from various hydrazides, isothiocyanates, and halides in sufficient quantities (19–180 mg) for initial biological studies, especially in vitro assays that require small amounts of the compounds.
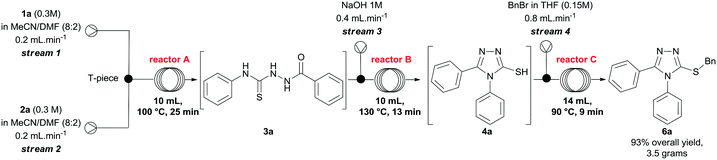
Finally, we investigated the scale up of the telescoped process for the preparation of compound 6a using the parameters previously developed to generate the 3-thio-1,2,4-triazoles. To this end, solutions of 1a and 2a were pumped directly from the corresponding reservoir through streams 1 and 2, respectively. After some experimentation, we reduced the concentrations of the hydrazide and isothiocyanate to 0.3 M, and increased the temperature of reactor A to 100 °C to secure total consumption of the starting materials. Using the conditions presented in Scheme 3, after steady-state operation, we could scale up the process to 3.6 mmol h−1 and, collecting the output flowing stream over 3 hours, we obtained 6a in 93% yield (10.8 mmol, 3.5 grams) after purification. It is important to highlight that this arrangement can also be easily applied to both small- and medium-scale preparations of other 3-thio-1,2,4-triazoles on demand.
Conclusions
In conclusion, we demonstrated the application of a fully integrated continuous flow platform which delivered highly substituted 3-thio-1,2,4-triazoles in a fast, practical and efficient manner. In fact, these compounds were obtained in shorter times and higher yields when compared to batch conditions. These findings confirm the benefits of continuous flow regime over batch, allowing for the use of superheated solvents along with higher mass/heat transfer, leading to the reduction of reaction time and an improvement in conversion/yield. Since the thiosemicarbazide and 1,2,4-triazole-3-thiol intermediates were not isolated, lower solvent consumption and laborious purification steps were avoided. Moreover, we were able to generate the desired products in shorter times and higher yields in comparison to the batch synthesis. The flow process was successfully applied to a range of different substrates (alkyl/aryl hydrazides, isothiocyanates and alkyl/benzyl halides) affording the desired products in up to 91% yield. Additionally, we demonstrated the potential and robustness of our system by running an experiment for more than 3 hours collecting ca. 3.5 grams of the final product after 3 steps. Our work also highlights the application of continuous flow chemistry as a reliable enabling technology for the fast generation of compound libraries for biological screening in drug discovery programs.Experimental section
General
Starting materials, reagents and solvents were obtained from commercial sources and used as received unless otherwise specified. Progress of the reactions was monitored by thin-layer chromatography (TLC) analysis (Merck, silica gel 60 F254 on aluminum plates), unless otherwise stated. Flash chromatography purifications were performed with silica gel 60, 220–440 mesh, Sigma-Aldrich. 1H NMR spectra were recorded on either Bruker Avance-400, Bruker Avance-500, or Bruker Avance-600 instruments and are reported relative to residual solvent: CHCl3 (δ 7.26) or DMSO (δ 2.50). 13C NMR spectra were recorded on the same instruments and are reported relative to CHCl3 (δ 77.16) or DMSO (δ 39.52). Data for 1H NMR are reported as follows: chemical shift (multiplicity, coupling constant in Hz, integration). Multiplicities are reported as follows: s = singlet, d = doublet, t = triplet, q = quartet, quint. = quintet, sext = sextet, dd = doublet of doublets, ddd = doublet of doublets of doublets, appt = apparent triplet, m = multiplet, bs = broad signal. NMR spectra were processed using ACD/NMR Processor Academic Edition version 12.01. FTIR spectra were obtained on an Agilent Cary 630 FTIR spectrometer (neat, ATR sampling) with the intensities of the characteristic signals being reported as weak (w, <30% of tallest signal), medium (m, 31–70% of tallest signal) or strong (s, >71% of tallest signal). High-resolution mass spectrometry data was acquired using an Agilent ifunnel Q-TOF 6550 LC-MS instrument equipped with an electrospray ionization source (Dual Agilent Jet Stream ESI†) operating in the positive mode. Melting points were recorded on a Mettler Toledo MP50 benchtop melting point system with a heating rate of 5 °C min−1 and are uncorrected. IUPAC names of the compounds were generated using ChemBioDraw Ultra 13.0.Batch synthesis of compounds 3a, 4a and 6a
Segmented continuous flow synthesis of compounds 3a, 4a, 4f and 6a
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and filled into loop 1. Isothiocyanate 2a (85 μL, 0.7 mmol) was taken up in 1 mL of MeCN/DMF (8
2) and filled into loop 1. Isothiocyanate 2a (85 μL, 0.7 mmol) was taken up in 1 mL of MeCN/DMF (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and filled into loop 2. The two loops were simultaneously injected into streams of MeCN/DMF (8
2) and filled into loop 2. The two loops were simultaneously injected into streams of MeCN/DMF (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), and the plugs met at a T-piece before passing through a 10 mL coil reactor, to achieve a 5 minutes residence time at 90 °C. The output was collected and the solvent was evaporated under reduced pressure. The residue was dissolved in 10 mL of water and extracted with CHCl3 (3 × 20 mL). The organic layers were combined and extracted with water (3 × 20 mL), and brine (1 × 20 mL). The crude product was further purified by flash chromatography (DCM/MeOH 2–5%) to afford compound 3a in quantitative yield (190 mg), obtained as a white solid.
2), and the plugs met at a T-piece before passing through a 10 mL coil reactor, to achieve a 5 minutes residence time at 90 °C. The output was collected and the solvent was evaporated under reduced pressure. The residue was dissolved in 10 mL of water and extracted with CHCl3 (3 × 20 mL). The organic layers were combined and extracted with water (3 × 20 mL), and brine (1 × 20 mL). The crude product was further purified by flash chromatography (DCM/MeOH 2–5%) to afford compound 3a in quantitative yield (190 mg), obtained as a white solid.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and filled into loop 1. Isothiocyanate 2a (61 μL, 0.5 mmol) was taken up in 1 mL of MeCN/DMF (8
2) and filled into loop 1. Isothiocyanate 2a (61 μL, 0.5 mmol) was taken up in 1 mL of MeCN/DMF (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and filled into loop 2. The two loops were simultaneously injected into streams of MeCN/DMF (8
2) and filled into loop 2. The two loops were simultaneously injected into streams of MeCN/DMF (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), and the plugs met at a T-piece before passing through a coil reactor (10 mL, 25 min) at 90 °C, pressurized by a 100 psi back-pressure regulator. Separately, a solution of 1M NaOH was pumped at 0.4 mL min−1, and mixed via a T-piece with the output stream of reactor A. The combined mixture entered a flow coil (10 mL, 13 min) maintained at 130 ° C. The combined streams gave us a total flow rate of 0.8 mL min−1. The total reactor output was collected, and worked up by neutralization with HCl 1M solution and extraction with CHCl3 (3 × 20 mL). The organic layers were combined and extracted with water (3 × 20 mL), and brine (1 × 20 mL). The crude product was further purified by flash chromatography (DCM/MeOH 1–5%) to afford compound 4a in 85% yield (108 mg), obtained as a white solid.
2), and the plugs met at a T-piece before passing through a coil reactor (10 mL, 25 min) at 90 °C, pressurized by a 100 psi back-pressure regulator. Separately, a solution of 1M NaOH was pumped at 0.4 mL min−1, and mixed via a T-piece with the output stream of reactor A. The combined mixture entered a flow coil (10 mL, 13 min) maintained at 130 ° C. The combined streams gave us a total flow rate of 0.8 mL min−1. The total reactor output was collected, and worked up by neutralization with HCl 1M solution and extraction with CHCl3 (3 × 20 mL). The organic layers were combined and extracted with water (3 × 20 mL), and brine (1 × 20 mL). The crude product was further purified by flash chromatography (DCM/MeOH 1–5%) to afford compound 4a in 85% yield (108 mg), obtained as a white solid.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and filled into loop 1. Isothiocyanate 2a (61 μL, 0.5 mmol) was taken up in 1 mL of MeCN/DMF (8
2) and filled into loop 1. Isothiocyanate 2a (61 μL, 0.5 mmol) was taken up in 1 mL of MeCN/DMF (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and filled into loop 2. The two loops were simultaneously injected into streams of MeCN/DMF (8
2) and filled into loop 2. The two loops were simultaneously injected into streams of MeCN/DMF (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), and the plugs met at a T-piece before passing through a coil reactor at 90 °C (10 mL, 25 min). Separately, a solution of 1M NaOH was pumped at 0.4 mL min−1, and mixed via a T-piece with the output stream of reactor A. The combined mixture entered a flow coil (10 mL, 13 min) maintained at 130 ° C. Subsequently, benzyl bromide (606 μL, 5 mmol) in 20 mL of THF was pumped at 0.8 mL min−1. The reaction streams (stream 4 and the output stream of reactor B) met at a T-piece before passing through a coil reactor at 90 °C (14 mL, 9 min). The system was pressurized by a 100 psi back-pressure regulator, and the combined streams gave us a total flow rate of 1.6 mL min−1. The total reactor output was collected, and extracted with CHCl3 (3 × 20 mL). The organic layers were combined and extracted with water (3 × 20 mL), and brine (1 × 20 mL). The crude product was further purified by flash chromatography (DCM/MeOH 1–5%) to afford compound 6a in 91% yield (156 mg), obtained as a white solid.
2), and the plugs met at a T-piece before passing through a coil reactor at 90 °C (10 mL, 25 min). Separately, a solution of 1M NaOH was pumped at 0.4 mL min−1, and mixed via a T-piece with the output stream of reactor A. The combined mixture entered a flow coil (10 mL, 13 min) maintained at 130 ° C. Subsequently, benzyl bromide (606 μL, 5 mmol) in 20 mL of THF was pumped at 0.8 mL min−1. The reaction streams (stream 4 and the output stream of reactor B) met at a T-piece before passing through a coil reactor at 90 °C (14 mL, 9 min). The system was pressurized by a 100 psi back-pressure regulator, and the combined streams gave us a total flow rate of 1.6 mL min−1. The total reactor output was collected, and extracted with CHCl3 (3 × 20 mL). The organic layers were combined and extracted with water (3 × 20 mL), and brine (1 × 20 mL). The crude product was further purified by flash chromatography (DCM/MeOH 1–5%) to afford compound 6a in 91% yield (156 mg), obtained as a white solid.
Segmented continuous flow synthesis of compounds 6b–i, 7a–d and 8a–d
The flow equipment was set up according to Fig. 3 and the procedure for the synthesis of these compounds followed the conditions described for compound 6a, unless otherwise stated. The corresponding hydrazides, isothiocyanates and alkyl/aryl halides were used in each case.Telescoped continuous flow synthesis of compound 6a
The flow equipment was set up according to Scheme 3. A solution of hydrazide 1a (18 mmol, 2.5 g) in 60 mL of MeCN/DMF (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), and a solution of isothiocyanate 2a (18 mmol, 2.19 mL) in 60 mL of MeCN/DMF (8
2), and a solution of isothiocyanate 2a (18 mmol, 2.19 mL) in 60 mL of MeCN/DMF (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), were pumped at 0.2 mL min−1 through streams 1 and 2, and mixed via a T-piece. The resulting stream passed through a coil reactor at 100 °C (10 mL, 25 min residence time). Separately, a NaOH 1M solution was pumped at 0.4 mL min−1 (stream 3), and mixed via a T-piece with the output stream of reactor A. The combined mixture entered a flow coil (10 mL, 13 min) maintained at 130 ° C. Subsequently, a solution of benzyl bromide (36 mmol, 4.36 mL) in 240 mL of THF was pumped at 0.8 mL min−1. The reaction streams (stream 4 and the output stream of reactor B) met at a T-piece before passing through a coil reactor at 90 °C (14 mL, 9 min residence time). The system was pressurized by a 100 psi back-pressure regulator, and the combined streams gave us a total flow rate of 1.6 mL min−1. After steady-state operation (two residence times, i.e. 110 min), the reactor output was collected for 3 hours, and then extracted with CHCl3 (4 × 100 mL). The organic layers were combined and extracted with water (4 × 100 mL), and brine (1 × 100 mL). The crude product presented a small quantity of impurities, and it was further purified by flash chromatography (DCM/MeOH 1–5%) to afford compound 6a in 93% yield (3.5 g).
2), were pumped at 0.2 mL min−1 through streams 1 and 2, and mixed via a T-piece. The resulting stream passed through a coil reactor at 100 °C (10 mL, 25 min residence time). Separately, a NaOH 1M solution was pumped at 0.4 mL min−1 (stream 3), and mixed via a T-piece with the output stream of reactor A. The combined mixture entered a flow coil (10 mL, 13 min) maintained at 130 ° C. Subsequently, a solution of benzyl bromide (36 mmol, 4.36 mL) in 240 mL of THF was pumped at 0.8 mL min−1. The reaction streams (stream 4 and the output stream of reactor B) met at a T-piece before passing through a coil reactor at 90 °C (14 mL, 9 min residence time). The system was pressurized by a 100 psi back-pressure regulator, and the combined streams gave us a total flow rate of 1.6 mL min−1. After steady-state operation (two residence times, i.e. 110 min), the reactor output was collected for 3 hours, and then extracted with CHCl3 (4 × 100 mL). The organic layers were combined and extracted with water (4 × 100 mL), and brine (1 × 100 mL). The crude product presented a small quantity of impurities, and it was further purified by flash chromatography (DCM/MeOH 1–5%) to afford compound 6a in 93% yield (3.5 g).
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge financial support from the São Paulo Research Foundation – FAPESP (J. C. P., awards No. 2014/26378-2 and 2014/25770-6) and CNPq (J. C. P., award No. 453862/2014-4). We also thank FAPESP (M. C. F. C. B. D., award No. 2015/18572-6) and CAPES (R. G.) for fellowships. We are also thankful to the Obesity and Comorbidities Research Center – OCRC (FAPESP Award No. 2013/07607-8) for providing us with the UNIQSIS flow system used in this work. Prof. Marcos N. Eberlin (University of Campinas, Brazil) is acknowledged for HRMS analyses. We are also grateful to Prof. José Augusto R. Rodrigues (University of Campinas, Brazil) for GC-MS analyses and his friendship.Notes and references
- (a) G. Wess, M. Urmann and B. Sickenberger, Angew. Chem., Int. Ed., 2001, 40, 3341–3350 CrossRef CAS; (b) C. Wiles and P. Watts, Future Med. Chem., 2009, 1, 1593–1612 CrossRef CAS PubMed.
- B. W. Cue and J. Zhang, Green Chem. Lett. Rev., 2009, 2, 193–211 CrossRef CAS.
- (a) C. Jimenez-Gonzalez, A. D. Curzons, D. J. C. Constable and V. L. Cunningham, Int. J. Life Cycle Assess., 2004, 9, 115–121 CrossRef CAS; (b) A. D. Curzons, C. Jimenez-Gonzalez, A. L. Duncan, D. J. C. Constable and V. L. Cunningham, Int. J. Life Cycle Assess., 2007, 12, 272–280 CrossRef CAS.
- (a) B. J. Reizman and Klavs F. Jensen, Org. Process Res. Dev., 2012, 16, 1770–1782 CrossRef CAS; (b) H. G. Jolliffe and D. I. Gerogiorgis, Comput. Chem. Eng., 2016, 91, 269–288 CrossRef CAS; (c) D. Dallinger and C. O. Kappe, Curr. Opin. Green Sustainable Chem., 2017, 7, 6–12 CrossRef; (d) M. B. Plutschack, B. Pieber, K. Gilmore and P. H. Seeberger, Chem. Rev., 2017, 117, 11796–11893 CrossRef CAS PubMed; (e) D. J. C. Constable, C. Jimenez-Gonzalez and R. K. Henderson, Org. Process Res. Dev., 2007, 11, 133–137 CrossRef CAS.
- (a) B. J. Reizman and K. F. Jensen, Org. Process Res. Dev., 2012, 16, 1770–1782 CrossRef CAS; (b) L. Malet-Sanz and F. Susanne, J. Med. Chem., 2012, 55, 4062–4098 CrossRef CAS PubMed; (c) M. Baumann and I. R. Baxendale, Beilstein J. Org. Chem., 2015, 11, 1194–1219 CrossRef CAS PubMed; (d) S. G. Koenig and H. F. Sneddon, Green Chem., 2017, 19, 1418–1419 RSC.
- For recent general reviews on continuous flow chemistry, see (a) R. L. Hartman, J. P. McMullen and K. F. Jensen, Angew. Chem., Int. Ed., 2011, 50, 7502–7519 CrossRef CAS PubMed; (b) J. Wegner, S. Ceylan and A. Kirschning, Adv. Synth. Catal., 2012, 354, 17–57 CrossRef CAS; (c) N. G. Anderson, Org. Process Res. Dev., 2012, 16, 852–869 CrossRef CAS; (d) I. R. Baxendale, J. Chem. Technol. Biotechnol., 2013, 88, 519–552 CrossRef CAS; (e) J. C. Pastre, D. L. Browne and S. V. Ley, Chem. Soc. Rev., 2013, 42, 8849–8869 RSC; (f) R. J. Ingham, C. Battilocchio, D. E. Fitzpatrick, E. Sliwinski, J. M. Hawkins and S. V. Ley, Angew. Chem., Int. Ed., 2015, 54, 144–148 CrossRef CAS PubMed; (g) F. Fanelli, G. Parisi, L. Degennaro and R. Luisi, Beilstein J. Org. Chem., 2017, 13, 520–542 CrossRef PubMed.
- (a) C. F. Carter, I. R. Baxendale, M. O. Brien, B. J. Pavey and S. V. Ley, Org. Biomol. Chem., 2009, 7, 4594–4597 RSC; (b) M. Baumann, I. R. Baxendale, C. H. Hornung, S. V. Ley, M. V. Rojo and K. A. Roper, Molecules, 2014, 19, 9736–9759 CrossRef PubMed; (c) M. Baumann, A. M. Rodriguez Garcia and I. R. Baxendale, Org. Biomol. Chem., 2015, 13, 4231–4239 RSC; (d) H. Kim, H. Lee and D. Kim, Angew. Chem., Int. Ed., 2015, 54, 1877–1880 CrossRef CAS PubMed; (e) A. R. Bogdan and Y. Wang, RSC Adv., 2015, 5, 79264–79269 RSC; (f) M. Therkelsen, M. T. Rasmussen and A. T. Lindhardt, Chem. Commun., 2015, 51, 9651–9654 RSC; (g) S. Roesner and S. L. Buchwald, Angew. Chem., Int. Ed., 2016, 55, 10463–10467 CrossRef CAS PubMed.
- For a discussion on continuous flow chemistry in drug discovery and APIs manufacturing, see (a) M. Colombo and I. Peretto, Drug Discovery Today, 2008, 13, 677–684 CrossRef CAS PubMed; (b) I. R. Baxendale, R. D. Braatz, B. K. Hodnett, K. F. Jensen, M. D. Johnson, P. Sharratt, J. P. Sherlock and A. J. Florence, J. Pharm. Sci., 2015, 104, 781–791 CrossRef CAS PubMed; (c) R. Porta, M. Benaglia and A. Puglisi, Org. Process Res. Dev., 2016, 20, 2–25 CrossRef CAS; (d) P. Bana, R. Örkényi, K. Lövei, Á. Lakó, G. I. Túrós, J. Éles, F. Faigl and I. Greiner, Bioorg. Med. Chem., 2017 DOI:10.1016/j.bmc.2016.12.046.
- (a) X. Zhang, S. Stefanick and F. J. Villani, Org. Process Res. Dev., 2004, 8, 455–460 CrossRef CAS; (b) A. E. Cervera-Padrell, J. P. Nielsen, M. Jønch Pedersen, K. Müller Christensen, A. R. Mortensen, T. Skovby, K. Dam-Johansen, S. Kiil and K. V. Gernaey, Org. Process Res. Dev., 2012, 16, 901–914 CrossRef CAS; (c) B. Gutmann, D. Cantillo and C. O. Kappe, Angew. Chem., Int. Ed., 2015, 54, 6688–6728 CrossRef CAS PubMed; (d) A. Adamo, R. L. Beingessner, M. Behnam, J. Chen, T. F. Jamison, K. F. Jensen, J.-C. M. Monbaliu, A. S. Myerson, E. M. Revalor, D. R. Snead, T. Stelzer, N. Weeranoppanant, S. Yee Wong and P. Zhang, Science, 2016, 352, 61–67 CrossRef CAS PubMed; (e) H. Lin, C. Dai, T. F. Jamison and K. F. Jensen, Angew. Chem., Int. Ed., 2017, 56, 8870–8873 CrossRef CAS PubMed.
- K. P. Cole, J. M. Groh, M. D. Johnson, C. L. Burcham, B. M. Campbell, W. D. Diseroad, M. R. Heller, J. R. Howell, N. J. Kallman, T. M. Koenig, S. A. May, R. D. Miller, D. Mitchell, D. P. Myers, S. S. Myers, J. L. Phillips, C. S. Polster, T. D. White, J. Cashman, D. Hurley, R. Moylan, P. Sheehan, R. D. Spencer, K. Desmond, P. Desmond and O. Gowran, Science, 2017, 356, 1144–1150 CrossRef CAS PubMed.
- For selected recent reviews about heterocyclic compounds in drug discovery, see (a) A. Gomtsyan, Chem. Heterocycl. Compd., 2012, 48, 7–10 CrossRef CAS; (b) R. S. Keri, S. Budagumpi, R. K. Pai and R. G. Balakrishna, Eur. J. Med. Chem., 2014, 78, 340–374 CrossRef CAS PubMed; (c) A. Ibrar Khan and N. Abbas, Arch. Pharm., 2014, 347, 1–20 CrossRef PubMed; (d) C. B. Mishra, S. Kumari and M. Tiwari, Eur. J. Med. Chem., 2015, 92, 1–34 CrossRef CAS PubMed; (e) D. Raffa, B. Maggio, M. V. Raimondi, S. Cascioferro, F. Plescia, G. Cancemi and G. Daidone, Eur. J. Med. Chem., 2015, 97, 732–746 CrossRef CAS PubMed; (f) R. S. Keri, K. Chand, T. Ramakrishnappa and B. M. Nagaraja, Arch. Pharm., 2015, 348, 299–314 CrossRef CAS PubMed; (g) T. Shiro, T. Fukaya and M. Tobe, Eur. J. Med. Chem., 2015, 97, 397–408 CrossRef CAS PubMed; (h) C. S. Demmer and L. Bunch, Eur. J. Med. Chem., 2015, 97, 778–785 CrossRef CAS PubMed; (i) T. V. Sravanthi and S. L. Manju, Eur. J. Pharm. Sci., 2016, 91, 1–10 CrossRef CAS PubMed; (j) A. P. Taylor, R. P. Robinson, Y. M. Fobian, D. C. Blakemore, L. H. Jones and O. Fadeyi, Org. Biomol. Chem., 2016, 14, 6611–6637 RSC.
- For selected examples on the flow synthesis of heterocyclic compounds, see (a) P. P. Lange and K. James, ACS Comb. Sci., 2012, 14, 570–578 CrossRef CAS PubMed; (b) A. Deangelis, D. H. Wang and S. L. Buchwald, Angew. Chem., Int. Ed., 2013, 52, 3434–3437 CrossRef CAS PubMed; (c) J. Jacq and P. Pasau, Chem. – Eur. J., 2014, 20, 12223–12233 CrossRef CAS PubMed; (d) K. Engen, J. Sävmarker, U. Rosenström, J. Wannberg, T. Lundbäck, A. Jenmalm-Jensen and M. Larhed, Org. Process Res. Dev., 2014, 18, 1582–1588 CrossRef CAS; (e) N. J. W. Straathof, Y. Su, V. Hessel and T. Noël, Nat. Protoc., 2015, 11, 10–21 CrossRef PubMed; (f) P. Filipponi and I. R. Baxendale, Eur. J. Org. Chem., 2016, 2016, 2000–2012 CrossRef CAS; (g) M. Baumann and I. R. Baxendale, Synlett, 2016, 27, 159–163 CrossRef CAS; (h) S. Vukelic, B. Koksch, P. H. Seeberger and K. Gilmore, Chem. – Eur. J., 2016, 22, 13451–13454 CrossRef CAS PubMed; (i) L. Mertens, K. J. Hock and R. M. Koenigs, Chem. – Eur. J., 2016, 22, 1–5 CrossRef PubMed; (j) J. Tsoung, A. R. Bogdan, S. Kantor, Y. Wang, M. Charaschanya and S. W. Djuric, J. Org. Chem., 2017, 82, 1073–1084 CrossRef CAS PubMed; (k) M. Baumann and I. R. Baxendale, Bioorg. Med. Chem., 2017 DOI:10.1016/j.bmc.2017.01.022.
- (a) M. Koparir, C. Orek, A. E. Parlak, A. Söylemez, P. Koparir, M. Karatepe and S. D. Dastan, Eur. J. Med. Chem., 2013, 63, 340–346 CrossRef CAS PubMed; (b) G. E. A. A. Abuo-rahma, M. Abdel-aziz, E. A. M. Beshr and T. F. S. Ali, Eur. J. Med. Chem., 2014, 71, 185–198 CrossRef CAS PubMed; (c) S. G. Küçükgüzel and P. Çıkla-Süzgün, Eur. J. Med. Chem., 2015, 97, 830–870 CrossRef PubMed.
- (a) E. Palaska, G. Şahin, P. Kelicen, N. T. Durlu and G. Altinok, Farmaco, 2002, 57, 101–107 CrossRef CAS PubMed; (b) L. Navidpour, H. Shafaroodi, K. Abdi, M. Amini, M. H. Ghahremani, A. R. Dehpour and A. Shafiee, Bioorg. Med. Chem., 2006, 14, 2507–2517 CrossRef CAS PubMed.
- (a) I. R. Ezabadi, C. Camoutsis, P. Zoumpoulakis, A. Geronikaki, M. Sokovic, J. Glamocilija and A. Ciric, Bioorg. Med. Chem., 2008, 16, 1150–1161 CrossRef CAS PubMed; (b) H. Bayrak, A. Demirbas, N. Demirbas and S. A. Karaoglu, Eur. J. Med. Chem., 2009, 44, 4362–4366 CrossRef CAS PubMed.
- H. Foks, M. Janowiec, Z. Zwolska and E. Augustynowicz-Kopeć, Phosphorus, Sulfur Silicon Relat. Elem., 2005, 180, 537–543 CrossRef CAS.
- A. T. Mavrova, D. Wesselinova, Y. A. Tsenov and P. Denkova, Eur. J. Med. Chem., 2009, 44, 63–69 CrossRef CAS PubMed.
- A. C. Flick, H. X. Ding, C. A. Leverett, R. E. Kyne Jr., K. K.-C. Liu, S. J. Fink and C. J. O'Donnell, J. Med. Chem., 2017, 60, 6480–6515 CrossRef CAS PubMed.
- (a) N. Pagano, M. L. Heil and N. D. P. Cosford, Synthesis, 2012, 44, 2537–2546 CrossRef CAS PubMed; (b) J. C. Pastre, D. L. Browne, M. O'Brien and S. V. Ley, Org. Process Res. Dev., 2013, 17, 1183–1191 CrossRef CAS.
- (a) C. J. Smith, N. Nikbin, S. V. Ley, H. Lange and I. R. Baxendale, Org. Biomol. Chem., 2011, 9, 1938–1947 RSC; (b) P. Zhang, M. G. Russell and T. F. Jamison, Org. Process Res. Dev., 2014, 18, 1567–1570 CrossRef CAS.
- L. Guetzoyan, N. Nikbin, I. R. Baxendale and S. V. Ley, Chem. Sci., 2013, 4, 764–769 RSC.
- (a) I. R. Baxendale, S. C. Schou, J. Sedelmeier and S. V. Ley, Chem. – Eur. J., 2010, 16, 89–94 CrossRef CAS PubMed; (b) J.-S. Poh, D. L. Browne and S. V. Ley, React. Chem. Eng., 2016, 1, 101–105 RSC; (c) J. Britton and T. F. Jamison, Angew. Chem., Int. Ed., 2017, 56, 8823–8827 CrossRef CAS PubMed.
- (a) S. A. May, M. D. Johnson, T. M. Braden, J. R. Calvin, B. D. Haeberle, A. R. Jines, R. D. Miller, E. F. Plocharczyk, G. A. Rener, R. N. Richey, C. R. Schmid, R. K. Vaid and H. Yu, Org. Process Res. Dev., 2012, 16, 982–1002 CrossRef CAS; (b) P. F. Carneiro, B. Gutmann, R. O. M. A. De Souza and C. O. Kappe, ACS Sustainable Chem. Eng., 2015, 3, 3445–3453 CrossRef CAS.
- (a) A. Herath and N. D. P. Cosford, Org. Lett., 2010, 12, 5182–5185 CrossRef CAS PubMed; (b) M. Baumann, I. R. Baxendale, J. Wegner, A. Kirschning and S. V. Ley, Heterocycles, 2010, 82, 1297–1316 CrossRef; (c) P. B. Cranwell, M. O'Brien, D. L. Browne, P. Koos, A. Polyzos, M. Peña-López and S. V. Ley, Org. Biomol. Chem., 2012, 10, 5774–5779 RSC.
- S. Glockner, D. N. Tran, R. J. Ingham, S. Fenner, Z. E. Wilson, C. Battilocchio and S. V. Ley, Org. Biomol. Chem., 2015, 13, 207–214 CAS.
- J. I. Yoshida, A. Nagaki and D. Yamada, Drug Discovery Today: Technol., 2013, 10, e53–e59 CrossRef PubMed.
- (a) F. Kurzer, J. Chem. Soc. C, 1971, 2927–2931 RSC; (b) R. Jaiswal, S. Parmar, S. Singh and J. Barthwal, J. Heterocycl. Chem., 1979, 16, 561–565 CrossRef CAS; (c) J. M. Kane, M. A. Staeger, C. R. Dalton, F. P. Miller, M. W. Dudley, A. M. L. Ogden, J. H. Kehne, H. J. Ketteler, T. C. McCloskey, Y. Senyah, P. A. Chmielewski and J. A. Miller, J. Med. Chem., 1994, 37, 125–132 CrossRef CAS PubMed; (d) A. Almasirad, S. A. Tabatabai, M. Faizi, A. Kebriaeezadeh, M. Nazila, A. Dalvandi and A. Shafiee, Bioorg. Med. Chem. Lett., 2005, 15, 1863–1865 CrossRef PubMed; (e) A. Siwek, M. Wujec, M. Dobosz and I. Wawrzycka-Gorczyca, Heteroat. Chem., 2010, 21, 521–532 CrossRef CAS; (f) S. Samanta, T. L. Lim and Y. Lam, ChemMedChem, 2013, 8, 994–1001 CrossRef CAS PubMed; (g) T. Plech, J. J. Luszczki, M. Wujec and J. Flieger, Eur. J. Med. Chem., 2013, 60, 208–215 CrossRef CAS PubMed; (h) E. Kuśmierz, A. Siwek, U. Kosikowska, A. Malm, A. Wróbel, M. Wujec, E. Kuśmierz, A. Siwek, U. Kosikowska, A. Malm, A. Siwek, U. Kosikowska and M. Wujec, Phosphorus, Sulfur Silicon Relat. Elem., 2014, 189, 1539–1545 CrossRef; (i) A. G. Banerjee, N. Das, S. A. Shengule, P. A. Sharma, R. S. Srivastava and S. K. Shrivastava, Bioorg. Chem., 2016, 69, 102–120 CrossRef CAS PubMed; (j) H. Shirinzadeh, E. Ince, A. D. Westwell and H. Gurer-orhan, J. Enzyme Inhib. Med. Chem., 2016, 31, 1312–1321 CrossRef CAS PubMed; (k) L. Yan, J. Liang, C. Yao, P. Wu, X. Zeng, K. Cheng and H. Yin, ChemMedChem, 2016, 11, 822–826 CrossRef CAS PubMed; (l) H. Li, B. Zhang, H.-K. Yang, Q. Li, P.-C. Diao, W.-W. You and P.-L. Zhao, Eur. J. Med. Chem., 2017, 125, 1098–1106 CrossRef PubMed.
- E. Gunic and G. Galvin, Manufacture of 2-(5-Bromo-4 (-cyclo- propylnaphthalen-1-yl)-4H-1,2,4-triazol-3-ylthio) Acetic Acid, WO2014008295A1, 2014 Search PubMed.
- The Uniqis FlowSyn and Vapourtec R2+R4 flow systems used in this work consist of two high-pressure pumps, and electrically heated flow reactors. For more details, please visit www.uniqsis.com and www.vapourtec.com.
- L. Nie, Z. Li, J. Han, X. Zhang, R. Yang, W. X. Liu, F. Y. Wu, J. W. Xie, Y. F. Zhao and Y. B. Jiang, J. Org. Chem., 2004, 69, 6449–6454 CrossRef PubMed.
- R. Chmekh, I. Tapsoba, H. Medini, E. Maisonhaute, M. L. Benkhoud and K. Boujlel, J. Electroanal. Chem., 2007, 599, 85–90 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Procedures for the synthesis of hydrazides 1b–1d and 1H and 13C NMR spectra for all compounds synthesized. See DOI: 10.1039/c7re00125h |
| This journal is © The Royal Society of Chemistry 2017 |