Open Access Article
Open Access ArticleCu(0), O2 and mechanical forces: a saving combination for efficient production of Cu–NHC complexes†
Audrey
Beillard
 ,
Thomas-Xavier
Métro
,
Thomas-Xavier
Métro
 *,
Xavier
Bantreil
*,
Xavier
Bantreil
 *,
Jean
Martinez
and
Frédéric
Lamaty
*
*,
Jean
Martinez
and
Frédéric
Lamaty
*
Institut des Biomolécules Max Mousseron (IBMM), UMR 5247, CNRS, Université de Montpellier, ENSCM, Campus Triolet, Place Eugène Bataillon, 34095 Montpellier cedex 5, France. E-mail: thomas-xavier.metro@umontpellier.fr; xavier.bantreil@umontpellier.fr; frederic.lamaty@umontpellier.fr
First published on 28th September 2016
Abstract
Mechanical forces induced by ball-milling agitation enabled the highly efficient and widely applicable synthesis of Cu–carbene complexes from N,N-diaryl-imidazolium salts and metallic copper. The required amount of gaseous dioxygen and insoluble copper could be reduced down to stoichiometric quantities, while reaction rates clearly outperformed those obtained in solution. Utilisation of Cu(0) as the copper source enabled the application of this approach to a wide array of N,N-diaryl-imidazolium salts (Cl−, BF4− and PF6−) that transferred their counter anion directly to the organometallic complexes. Cu–NHC complexes could be produced in excellent yields, including utilisation of highly challenging substrates. In addition, five unprecedented organometallic complexes are reported.
Introduction
The application of mechanical forces to solvent-free or solvent-less reaction mixtures through the use of ball-mills offers many advantages over traditional solvent-based strategies,1–5 especially when using poorly soluble, undissolved or insoluble reactants such as metals.6–16 Yet, these examples are limited to reactions between solid chemicals with liquid or soft solid reagents. In addition, the use of ball-mills to promote reactions involving solid reactants with gaseous reagents has been described previously.17,18 Yet, the ball-milling efficiency in reacting chemicals with a high physical state heterogeneity (soft material, gas, liquid and solid) has been explored only scarcely. In 2015, the reduction of organic molecules (soft material) in the presence of H2 (gas), generated by mechano-mediated reduction of water (liquid) with zero-valent chromium (solid), was reported.19 In the same year, a study described the mechanochemical Rh(III)-catalyzed C–H bond functionalization of acetanilides (soft material) in the presence of alkyl acrylates (liquid) and a large excess of molecular oxygen (gas) as the terminal oxidant.20Continuing our efforts to provide the community of chemists with more efficient ways to produce high value molecules,7,21–26 we describe herein the mechanosynthesis of Cu–NHC complexes (NHC = N-heterocyclic carbene). This study includes details on the outstanding efficacy of using ball-mills to react materials with high physical state heterogeneity. Used as catalysts in a wide range of reactions,27–31 Cu–NHC complexes are generally synthesised by deprotonation of imidazolium precursors with a strong base followed by reaction with Cu(I). Although interesting, a less frequent alternative is the use of metallic Cu(0) as the copper source. This route enables the transfer of the imidazolium counter anion directly to the Cu–NHC complexes,32–36 thereby providing easier access to a wider range of copper complexes. Unfortunately, these solvent-based reaction conditions require a large excess of insoluble Cu(0) and long reaction times.
Results and discussion
When IMes·HCl was treated with 5 equivalents of Cu(0) under magnetic stirring in refluxing water (Fig. 1), only 19% conversion was obtained after 8 h of reaction. On the other hand, 80% of IMes·HCl was converted into [CuCl(IMes)] when mixed with 5 equivalents of Cu(0) in a planetary ball-mill (pbm) agitated at 450 rpm for 8 h, without external heating. When adding small amounts of water as a grinding assistant (0.3 μL per mg of reactants), complete conversion could be reached after only 3 h.37 These ball-milling conditions were so efficient that only 1.0 equivalent of insoluble Cu(0) was enough to obtain >95% conversion after 8 h (Fig. 1). This impressive increase in the reaction rate could be explained by the highly concentrated reaction media, along with the highly efficient mixing that prevented mass transfer limitations. Indeed, no conversion could be observed when this reaction was performed with small amounts of water (0.3 μL per mg of reactants) and placed under reflux with magnetic stirring (Fig. 1). Though reactions performed in refluxing water were limited to 100 °C, it is known that under peculiar conditions, the temperature inside a ball-mill can reach much higher levels.38 In our case, the temperature of the reaction mixture after 6 h milling did not exceed 35 °C, in complete accordance with what was previously observed by us and by other research groups using similar milling conditions.38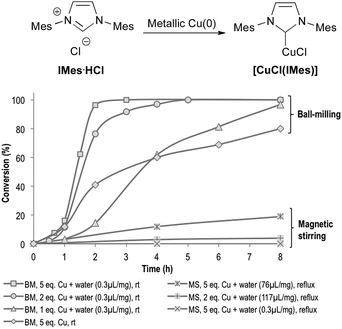 | ||
| Fig. 1 Comparison of reaction conditions for the formation of [CuCl(IMes)]. Mes = mesityl; BM = ball-milling; MS = magnetic stirring. | ||
Conditions involving 2 equivalents of Cu(0) and water as the grinding assistant were selected for further transformations, since the full conversion of IMes·HCl could be reached in only 5 h (Scheme 1). Several [CuCl(NHC)] complexes, featuring unsaturated IMes, IPr and saturated SIMes and SIPr, could be isolated efficiently within a few hours. The 4,5-dimethyl-substituted imidazolium derivatives IMesMe·HCl and IPrMe·HCl, which are known to be less reactive, could also be converted to the corresponding copper complexes in excellent yields with the ball-milling approach. Besides, the previously unreported [CuCl(SIMesMe)] complex was also isolated in excellent yield by applying these reaction conditions.
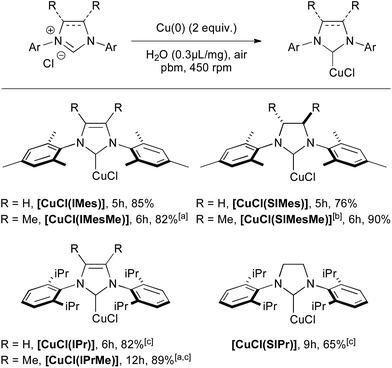 | ||
| Scheme 1 Mechanosynthesis of various [CuCl(NHC)] complexes from Cu(0). [a] Benzonitrile was used in place of H2O. [b] Previously unreported complex. [c] 5 equiv. of Cu(0) were used. | ||
The role of dioxygen in the course of the reaction was next investigated (Fig. 2). When IMes·HCl was treated with 5 equivalents of Cu(0) under inert atmosphere (N2) in a hermetically closed reactor, no conversion into [CuCl(IMes)] could be detected, thus confirming the participation of atmospheric O2 to the overall process. According to eqn (1) (Fig. 2), 1 mmole of O2 could react with 4 mmoles of imidazolium. The reaction was thus repeated under ambient atmosphere with 0.62 mmoles of IMes·HCl and 3.08 mmoles of Cu(0) in a 12 mL reactor for which the free volume contained 79.6 μmoles (0.52 equivalent) of O2 under these conditions.39,40 Conversion of IMes·HCl into [CuCl(IMes)] reached a maximum at 29%, thereby confirming that with these conditions, the reaction was limited by the amount of O2 present in the reactor.39 When the same reaction was performed with the amount of O2 ranging from 0.20 to 1.27 equivalents,39 the conversion and yield were highly proportional to the amount of O2 (Fig. 2). Gratifyingly, quasi-stoichiometric amounts of O2 (1.27 equiv.) were sufficient to convert 100% of IMes·HCl, furnishing [CuCl(IMes)] in 83% yield (Table 1, entry 1). These results showed the impressive capacity of ball-milling to convert high proportions of a gaseous reactant spread among chemicals of high physical state heterogeneity (gas, solid and soft material). To the best of our knowledge, such efficacy in lowering the required quantity of a gaseous reactant in a reaction performed in a ball-mill has never been reported before.
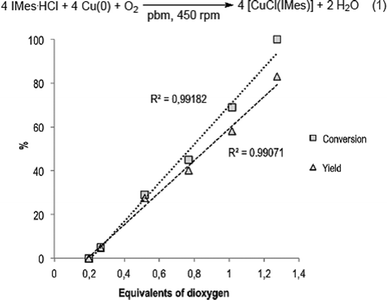 | ||
| Fig. 2 Conversion and yield as a function of O2 equivalents for the reaction of Cu(0), O2 and IMes·HCl performed in a ball-mill. | ||
In 2012, Mack reported the critical importance of the bromoalkane physical state when it was used as an alkylating agent of cyclohexanone in a ball-mill.41 Indeed, it was observed in this situation that gaseous bromoalkanes were less reactive than the liquid ones. In most reports, a different situation is observed, the gaseous nature of a reagent is not an obstacle for its reactivity.17–20 In our case, gaseous O2 is even close to being completely consumed. Although composed of reactants of high physical state heterogeneity, the reaction mixture was homogeneous at the macroscopic scale, as indicated by identical conversions of three samples withdrawn in three different locations inside the reactor. To confirm that such conditions with a low amount of dioxygen were general, [CuCl(SIMes)], [CuCl(IPr)] and [CuCl(SIPr)] were synthesised in good to excellent yields by using only 1.27 equivalents of O2 (Table 1). Notably, the yields obtained under these conditions were very similar to those where the amount of dioxygen was not precisely controlled.
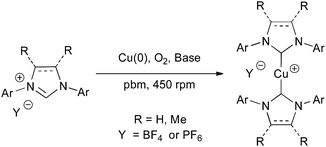
We next turned our attention to the less studied, poorly reactive and thus more challenging tetrafluoroborate and hexafluorophosphate imidazolium salts to produce their corresponding cationic [Cu(NHC)2]+ homoleptic complexes that have previously shown superior catalytic activities than their neutral derivatives in hydrosilylation and 1,3-dipolar cycloaddition reactions.42–44 Unfortunately, these complexes could not be obtained when IMes·HBF4 and IMes·HPF6 were ball-milled with Cu(0), with or without water (Table 2, entries 1 and 2). Yet, ball-milling of these substrates in the presence of 1.1 equivalents of NaOH furnished [Cu(IMes)2]BF4 and [Cu(IMes)2]PF6 in 79% and 87% yield, respectively (Table 2, entry 3). The necessity of using a stronger base could be explained by the weaker hydrogen bonds between the proton at C2 and the BF4− and PF6− counter anions than in Cl− salts, leading to a lower acidity of this proton.30,45 To our delight, the previously unreported [Cu(IMesMe)2]BF4 and [Cu(IMesMe)2]PF6 could be produced in excellent yields, showing the particularly high efficiency and wide applicability of this approach (Table 2, entry 4). Besides, the highly encumbered 1,3-bis-(2,6-diisopropylphenyl)-imidazolium derivatives reacted efficiently under these conditions, yet a stronger base, KHMDS, had to be employed in most cases to obtain acceptable conversion in a reasonable amount of time. By using KHMDS, two additional unprecedented complexes, [Cu(IPrMe)2]BF4 and [Cu(IPrMe)2]PF6, could be produced in satisfying yields (Table 2, entry 6).
| Entry | Substrate | Cu(0) (equiv.) | Additive (1.1 equiv.) | Y = BF4 | Y = PF6 | ||
|---|---|---|---|---|---|---|---|
| t (h) | Yield (%) | t (h) | Yield (%) | ||||
| a 0.3 μL of water per mg of reactants. b Previously unreported complexes. c 5 equiv. were used. d Obtained as a mixture of two structures, one of which is identified as being [Cu(IPrMe)2]PF6. | |||||||
| 1 | IMes·HY | 3 | None | 6 | 0 | 6 | 0 |
| 2 | 2 | H2Oa | 6 | 0 | 6 | 0 | |
| 3 | 3 | NaOH | 3 | 79 | 6 | 87 | |
| 4 | IMesMe·HY | 3 | NaOH | 6 | 91b | 6 | 97b |
| 5 | IPr·HY | 5 | NaOH | 6 | 85 | — | — |
| 5 | KHMDS | — | — | 6 | 89 | ||
| 6 | IPrMe·HY | 5 | KHMDSc | 8 | 72b | 6 | 78b,d |
Conclusions
In conclusion, these results have demonstrated the particularly high efficiency of ball-milling for the production of Cu–carbene complexes from N,N-diaryl imidazolium salts, dioxygen and metallic copper. It was shown for the first time that ball-milling enabled drastic reduction in the amount of gaseous reactant required down to the stoichiometric scale, without hampering conversion and yield. In addition, the rates of reaction clearly outperformed those obtained in traditional solvent-based conditions. Besides, five previously unreported Cu–NHC complexes could be isolated by using this approach. In an era of increasing demand for the discovery of innovative organometallic catalysts, as well as for efficiency and reduction of environmental impact, these results are a clear indication that mechanochemistry is a good choice to achieve these goals.Acknowledgements
Université de Montpellier and CNRS are acknowledged for our funding. François Métro is gratefully acknowledged for the graphical abstract production.Notes and references
- N. R. Rightmire and T. P. Hanusa, Dalton Trans., 2016, 45, 2352–2362 RSC.
- G. N. Hermann, P. Becker and C. Bolm, Angew. Chem., Int. Ed., 2016, 55, 3781–3784 CrossRef CAS PubMed.
- T. Friščić, in Ball Milling Towards Green Synthesis: Applications, Projects, Challenges, The Royal Society of Chemistry, 2015, pp. 151–189 Search PubMed.
- J. G. Hernández and T. Friščić, Tetrahedron Lett., 2015, 56, 4253–4265 CrossRef.
- S. L. James, C. J. Adams, C. Bolm, D. Braga, P. Collier, T. Friščić, F. Grepioni, K. D. M. Harris, G. Hyett, W. Jones, A. Krebs, J. Mack, L. Maini, A. G. Orpen, I. P. Parkin, W. C. Shearouse, J. W. Steed and D. C. Waddell, Chem. Soc. Rev., 2012, 41, 413–447 RSC.
- D. W. Peters and R. G. Blair, Faraday Discuss., 2014, 170, 83–91 RSC.
- J. Bonnamour, T.-X. Métro, J. Martinez and F. Lamaty, Green Chem., 2013, 15, 1116–1120 RSC.
- S.-E. Zhu, F. Li and G.-W. Wang, Chem. Soc. Rev., 2013, 42, 7535–7570 RSC.
- Q. Zhang and F. Jérôme, ChemSusChem, 2013, 6, 2042–2044 CrossRef CAS PubMed.
- C. Hardacre, H. Huang, S. L. James, M. E. Migaud, S. E. Norman and W. R. Pitner, Chem. Commun., 2011, 47, 5846–5848 RSC.
- R. A. Haley, A. R. Zellner, J. A. Krause, H. Guan and J. Mack, ACS Sustainable Chem. Eng., 2016, 4, 2464–2469 CrossRef CAS.
- K. Martina, L. Rinaldi, F. Baricco, L. Boffa and G. Cravotto, Synlett, 2015, 26, 2789–2794 CrossRef CAS.
- M. J. Rak, N. K. Saade, T. Friščić and A. Moores, Green Chem., 2014, 16, 86–89 RSC.
- T. L. Cook, J. A. Walker and J. Mack, Green Chem., 2013, 15, 617–619 RSC.
- D. A. Fulmer, W. C. Shearouse, S. T. Medonza and J. Mack, Green Chem., 2009, 11, 1821–1825 RSC.
- M. Urano, S. Wada and H. Suzuki, Chem. Commun., 2003, 1202–1203 RSC.
- S. Immohr, M. Felderhoff, C. Weidenthaler and F. Schüth, Angew. Chem., Int. Ed., 2013, 52, 12688–12691 CrossRef CAS PubMed.
- S. Mori, W. C. Xu, T. Ishidzuki, N. Ogasawara, J. Imai and K. Kobayashi, Appl. Catal., A, 1996, 137, 255–268 CrossRef CAS.
- Y. Sawama, T. Kawajiri, M. Niikawa, R. Goto, Y. Yabe, T. Takahashi, T. Marumoto, M. Itoh, Y. Kimura, Y. Monguchi, S.-I. Kondo and H. Sajiki, ChemSusChem, 2015, 8, 3773–3776 CrossRef CAS PubMed.
- G. N. Hermann, P. Becker and C. Bolm, Angew. Chem., Int. Ed., 2015, 54, 7414–7417 CrossRef CAS PubMed.
- A. Beillard, E. Golliard, V. Gillet, X. Bantreil, T.-X. Métro, J. Martinez and F. Lamaty, Chem.–Eur. J., 2015, 21, 17614–17617 CrossRef CAS PubMed.
- T.-X. Métro, J. Bonnamour, T. Reidon, A. Duprez, J. Sarpoulet, J. Martinez and F. Lamaty, Chem.–Eur. J., 2015, 21, 12787–12796 CrossRef PubMed.
- T.-X. Métro, E. Colacino, J. Martinez and F. Lamaty, in Ball Milling Towards Green Synthesis: Applications, Projects, Challenges, The Royal Society of Chemistry, 2015, ch. 6, pp. 114–150 Search PubMed.
- T.-X. Métro, X. J. Salom-Roig, M. Reverte, J. Martinez and F. Lamaty, Green Chem., 2015, 17, 204–208 RSC.
- V. Declerck, E. Colacino, X. Bantreil, J. Martinez and F. Lamaty, Chem. Commun., 2012, 48, 11778–11780 RSC.
- V. Declerck, P. Nun, J. Martinez and F. Lamaty, Angew. Chem., Int. Ed., 2009, 48, 9318–9321 CrossRef CAS PubMed.
- F. Lazreg, F. Nahra and C. S. J. Cazin, Coord. Chem. Rev., 2015, 293–294, 48–79 CrossRef CAS.
- J. D. Egbert, C. S. J. Cazin and S. P. Nolan, Catal. Sci. Technol., 2013, 3, 912–926 CAS.
- N. Marion, in N-Heterocyclic Carbenes: From Laboratory Curiosities to Efficient Synthetic Tools, The Royal Society of Chemistry, 2011, pp. 317–344 Search PubMed.
- J. C. Y. Lin, R. T. W. Huang, C. S. Lee, A. Bhattacharyya, W. S. Hwang and I. J. B. Lin, Chem. Rev., 2009, 109, 3561–3598 CrossRef CAS PubMed.
- S. Díez-González and S. P. Nolan, Aldrichimica Acta, 2008, 41, 43–51 Search PubMed.
- B. Liu, X. Ma, F. Wu and W. Chen, Dalton Trans., 2015, 44, 1836–1844 RSC.
- D. N. Barsoum, N. Okashah, X. Zhang and L. Zhu, J. Org. Chem., 2015, 80, 9542–9551 CrossRef CAS PubMed.
- B. R. M. Lake, E. K. Bullough, T. J. Williams, A. C. Whitwood, M. A. Little and C. E. Willans, Chem. Commun., 2012, 48, 4887–4889 RSC.
- B. Liu, B. Liu, Y. Zhou and W. Chen, Organometallics, 2010, 29, 1457–1464 CrossRef CAS.
- B. Liu, Q. Xia and W. Chen, Angew. Chem., Int. Ed., 2009, 48, 5513–5516 CrossRef CAS PubMed.
- G. A. Bowmaker, Chem. Commun., 2013, 49, 334–348 RSC.
- R. Schmidt, H. Martin Scholze and A. Stolle, Int. J. Ind. Chem., 2016, 7, 181–186 CrossRef.
- See ESI† for details.
- Free volume is defined as the volume of the reactor minus the volume of the balls and the reactants.
- D. C. Waddell, T. D. Clark and J. Mack, Tetrahedron Lett., 2012, 53, 4510–4513 CrossRef CAS.
- S. Díez-González, E. D. Stevens, N. M. Scott, J. L. Petersen and S. P. Nolan, Chem.–Eur. J., 2008, 14, 158–168 CrossRef PubMed.
- S. Díez-González and S. P. Nolan, Angew. Chem., Int. Ed., 2008, 47, 8881–8884 CrossRef PubMed.
- S. Díez-González, N. M. Scott and S. P. Nolan, Organometallics, 2006, 25, 2355–2358 CrossRef.
- J. Dupont, J. Braz. Chem. Soc., 2004, 15, 341–350 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Operating procedures and characterization data. See DOI: 10.1039/c6sc03182j |
| This journal is © The Royal Society of Chemistry 2017 |