Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Integration of aerobic oxidation and intramolecular asymmetric aza-Friedel–Crafts reactions with a chiral bifunctional heterogeneous catalyst†
Hong-Gang
Cheng‡
,
Javier
Miguélez‡
,
Hiroyuki
Miyamura
,
Woo-Jin
Yoo
and
Shū
Kobayashi
*
Department of Chemistry, School of Science, The University of Tokyo, Hongo, Bunkyo-ku, Tokyo 113-0033, Japan. E-mail: shu_kobayashi@chem.s.u-tokyo.ac.jp
First published on 5th October 2016
Abstract
A new class of chiral bifunctional heterogeneous materials composed of Au/Pd nanoparticles and chiral phosphoric acids as active orthogonal catalysts was prepared by utilizing a facile pseudo-suspension co-polymerization method. It was found that this heterogeneous catalyst was capable of facilitating the sequential aerobic oxidation-asymmetric intramolecular aza-Friedel–Crafts reaction between benzyl alcohols and N-aminoethylpyrroles. Moreover, the designed chiral heterogeneous catalyst could be recovered and reused several times without significant loss of activity or enantioselectivity.
Introduction
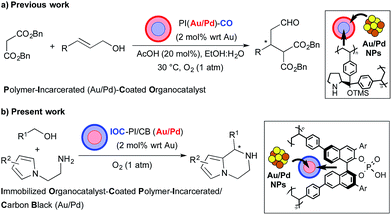
Multicatalyst-promoted asymmetric tandem reactions1 are an emerging subset in the family of one-pot processes2 that can provide access to complex organic substrates of high enantiopurity in an efficient and practical manner. Despite the promise of these reactions, one of the major challenges that has limited their development is the problem associated with catalyst incompatibility. A potential strategy to overcome this obstacle is by applying the principle of site separation to prevent mutual deactivation of catalysts, and successful tandem asymmetric processes have been achieved with chiral catalysts immobilized or encapsulated on polymers3 and sol–gel materials.4 Another challenge facing multicatalyst-promoted tandem reactions is the unselective interaction of starting materials and reaction intermediates with the catalysts to generate unwanted by-products. While it is difficult to achieve catalyst selectivity in one-pot reaction process, the use of heterogeneous catalysts in continuous-flow systems is a potential solution to overcome this problem.5Our group has a long-standing interest in the immobilization of metal nanoparticles (NPs) onto polymer supports6 and its application to tandem oxidation processes (TOPs) with oxygen gas as the terminal oxidant.7 Previously, we reported the fabrication of a layered heterogeneous bifunctional chiral catalyst consisting of Au/Pd NPs and a Jørgensen–Hayashi-type organocatalyst supported on separate polymeric materials (PI(Au/Pd)–CO, polymer-incarcerated Au/Pd NP-coated organocatalyst), and its application as a catalyst for the sequential aerobic oxidation-asymmetric Michael reaction of primary allylic alcohols and dibenzyl malonate (Scheme 1a).3c While we were able to demonstrate that our fabrication method prevented catalyst deactivation between the Au/Pd NPs and the chiral organocatalyst to enable the asymmetric TOP, it was discovered that the chiral secondary amine catalyst was deactivated under aerobic conditions and that the heterogeneous catalyst could not be reused. Based on our preliminary experimental studies and related literature,8 we concluded that the use of chiral heterogeneous secondary amines as organocatalysts for asymmetric TOPs was not a viable strategy due to the propensity of the covalent intermediates to undergo aerobic oxidation and become degraded. Therefore, we hypothesized that the use of chiral organocatalysts which activate the organic substrates through non-covalent interactions would lead to a more robust heterogeneous system that could be recovered and reused even under aerobic conditions.
Over the past decade, chiral phosphoric acids (CPAs) have been shown to be highly efficient catalysts for a wide range of asymmetric transformations.9,10 Of particular note is their ability to activate imine derivatives, via hydrogen bonding or ion pair interactions (non-covalent interactions), and to promote high levels of stereoinduction of the prochiral electrophiles. Based on these considerations, we rationalized that CPAs might represent the ideal chiral component for a recyclable heterogeneous catalyst that could facilitate asymmetric TOPs. Herein, we report the preparation of a heterogeneous chiral bifunctional catalyst, consisting of Au/Pd NPs and a CPA, and its application to the sequential aerobic oxidation-asymmetric aza-Friedel–Crafts (FC) reaction (Scheme 1b).
Results and discussion
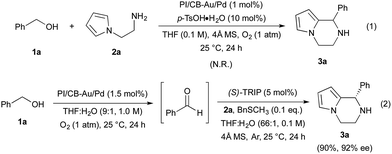
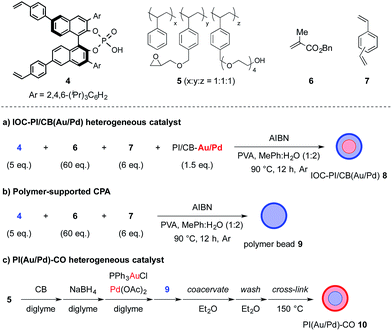
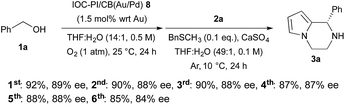
We began our investigations by examining the feasibility of a TOP that integrated aerobic oxidation with the aza-FC reaction, using benzyl alcohol (1a) and N-aminoethylpyrrole (2a) as model substrates and PI/CB–Au/Pd and p-toluenesulfonic acid as co-catalysts (Scheme 2, eqn (1)). It was found that the expected piperazine 3a was not obtained under our initial conditions. This was unexpected given that control studies showed that both the aerobic oxidation of 1a and the aza-FC reaction between 2a and benzaldehyde proceeded with good yields under these initial conditions.11 It was later revealed that the aerobic oxidation of 1a did not occur in the presence of 2a, most likely due to the strong coordination of the primary amine moiety of 2a to the Au/Pd NPs, causing catalyst deactivation. To overcome this limitation, we found that performing the TOP through a one-pot, sequential addition process, in which the aerobic oxidation of 1a was allowed to proceed prior to the addition of 2a, was key to successfully obtaining 3a. Optimization of the aerobic oxidation step and the asymmetric TOP using (S)-3,3′-bis(2,4,6-triisopropylphenyl)-1,1′-binaphthyl-2,2′-diyl hydrogenphosphate ((S)-TRIP)12 as the CPA was performed in order to improve the yield and enantioselectivity of the desired chiral piperazine 3a. It was found that water was essential to promote the aerobic oxidation of 1a, while the introduction of BnSCH3 as an additive to deactivate the Au/Pd NPs was important to prevent oxidation of 3a to its imine form. Based on these two major modifications, the asymmetric TOP proceeded well to deliver chiral piperazine 3a in high yield and enantioselectivity (Scheme 2, eqn (2)).With these results in hand, we began the process of fabricating chiral bifunctional heterogeneous catalysts that would be capable of facilitating the sequential aerobic oxidation-asymmetric aza-FC reaction. We began by developing a reliable synthetic route for (S)-TRIP-type monomer 4,13 and then utilized this as a chiral feedstock to construct the chiral composite material 8 through a pseudo-suspension co-polymerization method (Fig. 1a). With this heterogeneous bifunctional chiral catalyst in hand, we examined the asymmetric TOP between benzyl alcohol (1a) and N-aminoethylpyrrole 2a (Scheme 3). It was found that after slight modification of the optimized reaction conditions previously determined for the combined catalyst system of PI/CB–Au/Pd and (S)-TRIP, the desired piperazine 3a could be obtained in excellent yield and enantioselectivity. We also examined our model reaction using the layered heterogeneous catalyst 10 with inversed placement of the Au/Pd NPs and CPA (Fig. 1c), and it was found to catalyze the asymmetric TOP with similar results (85%, 88% ee of 3a).
 | ||
| Scheme 3 Sequential aerobic oxidation of 1a and asymmetric aza-FC reaction with 2a catalyzed by IOC–PI/CB(Au/Pd) 8. | ||
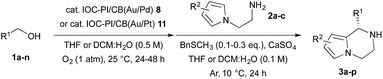
After establishing the optimal reaction conditions for the sequential aerobic oxidation-asymmetric aza-FC reaction, we examined the substrate scope for this one-pot process (Table 1). It was found that substituted benzyl alcohols 1a–h, bearing electron-donating substituents, could be utilized for the asymmetric TOP to furnish the desired chiral piperazines 3a–h in good yields and enantioselectivities (entries 1–8). On the other hand, when we examined 4-fluorobenzyl alcohol (1i) as a substrate, only a trace amount of the expected product 3i was detected under the current reaction conditions. Control studies revealed that the origin of this problem was the aerobic oxidation step, and we found that Au/Pt NPs in DCM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1
H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were more effective for the aerobic oxidation of benzyl alcohols possessing electron-withdrawing substituents. Thus, we prepared a new chiral bifunctional heterogeneous catalyst, IOC–PI/CB(Au/Pt) 11, and found that this new chiral composite material could effectively catalyze the sequential oxidation-asymmetric aza-FC reaction for 4-substituted benzyl alcohols with a wide range of functional groups, such as F, Cl, CF3, CO2Me and CN, to furnish the corresponding chiral piperazines 3i–n in good yields and enantioselectivities (entries 9–14). We also examined the asymmetric TOP with methyl substituted N-aminoethylpyrroles 2b–c, and it was found that these substrates were also suitable, providing chiral bicyclic heterocycles 3o–p with high yields and enantioselectivities (entries 15 and 16).
1) were more effective for the aerobic oxidation of benzyl alcohols possessing electron-withdrawing substituents. Thus, we prepared a new chiral bifunctional heterogeneous catalyst, IOC–PI/CB(Au/Pt) 11, and found that this new chiral composite material could effectively catalyze the sequential oxidation-asymmetric aza-FC reaction for 4-substituted benzyl alcohols with a wide range of functional groups, such as F, Cl, CF3, CO2Me and CN, to furnish the corresponding chiral piperazines 3i–n in good yields and enantioselectivities (entries 9–14). We also examined the asymmetric TOP with methyl substituted N-aminoethylpyrroles 2b–c, and it was found that these substrates were also suitable, providing chiral bicyclic heterocycles 3o–p with high yields and enantioselectivities (entries 15 and 16).
| Entry | 1: R1; 2: R2 | 3 | Yieldd (%) | eee (%) |
|---|---|---|---|---|
a Reaction conditions: benzyl alcohol 1 (0.3 mmol), IOC–PI/CB(Au/Pd) 8 (1.5 mol% wrt Au) in THF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (v/v = 0.56 H2O (v/v = 0.56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.04 mL) under a balloon of oxygen gas at room temperature for 24 h (aerobic oxidation step). Then N-aminoethylpyrrole 2 (0.2 mmol), CaSO4 (200 mg), BnSCH3 (2.8 mg) and THF (1.4 mL) were added under a balloon of Ar at 10 °C for 24 h (aza-FC step).
b Standard reaction conditions except for the use of IOC–PI/CB(Au/Pd) 8 (3.0 mol% wrt Au) for 48 h (aerobic oxidation step) and BnSCH3 (5.6 mg) (aza-FC step).
c Standard reaction conditions except for the use of IOC–PI/CB(Au/Pt) 11 (3.0 mol% wrt Au) in DCM 0.04 mL) under a balloon of oxygen gas at room temperature for 24 h (aerobic oxidation step). Then N-aminoethylpyrrole 2 (0.2 mmol), CaSO4 (200 mg), BnSCH3 (2.8 mg) and THF (1.4 mL) were added under a balloon of Ar at 10 °C for 24 h (aza-FC step).
b Standard reaction conditions except for the use of IOC–PI/CB(Au/Pd) 8 (3.0 mol% wrt Au) for 48 h (aerobic oxidation step) and BnSCH3 (5.6 mg) (aza-FC step).
c Standard reaction conditions except for the use of IOC–PI/CB(Au/Pt) 11 (3.0 mol% wrt Au) in DCM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (v/v = 0.54 H2O (v/v = 0.54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.06 mL) (aerobic oxidation step) and BnSCH3 (5.6 mg) (aza-FC step).
d Isolated yield based on 2a–c and determined by weight of the isolated product 3a–p.
e The ee value was determined by chiral HPLC analysis. 0.06 mL) (aerobic oxidation step) and BnSCH3 (5.6 mg) (aza-FC step).
d Isolated yield based on 2a–c and determined by weight of the isolated product 3a–p.
e The ee value was determined by chiral HPLC analysis.
|
||||
| 1 | 1a: Ph; 2a: H | 3a | 89 | 94 |
| 2 | 1b: 4-Me–C6H4; 2a: H | 3b | 89 | 84 |
| 3 | 1c: 2-Me–C6H4; 2a: H | 3c | 91 | 92 |
| 4 | 1d: 4-MeO–C6H4; 2a: H | 3d | 80 | 80 |
| 5b | 1e: 3-MeO–C6H4; 2a: H | 3e | 83 | 93 |
| 6b | 1f: 2-MeO–C6H4; 2a: H | 3f | 85 | 91 |
| 7 | 1g: 3,4-(OCH2O)–C6H3; 2a: H | 3g | 84 | 73 |
| 8b | 1h: 1-naphthyl; 2a: H | 3h | 85 | 93 |
| 9c | 1i: 4-F–C6H4; 2a: H | 3i | 91 | 90 |
| 10c | 1j: 2-F–C6H4; 2a: H | 3j | 88 | 91 |
| 11c | 1k: 4-Cl–C6H4; 2a: H | 3k | 83 | 90 |
| 12c | 1l: 4-CF3–C6H4; 2a: H | 3l | 82 | 86 |
| 13c | 1m: 4-CN–C6H4; 2a: H | 3m | 89 | 95 |
| 14c | 1n: 4-CO2Me–C6H4; 2a: H | 3n | 84 | 90 |
| 15 | 1a: Ph; 2b: 2-Me | 3o | 91 | 85 |
| 16 | 1a: Ph; 2c: 2,4-(Me)2 | 3p | 83 | 70 |
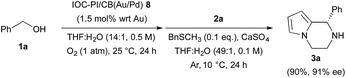
Finally, we examined the possibility of recycling our chiral bifunctional heterogeneous catalyst, and found that it could be recovered and reused several times without significant loss of yield or enantioselectivity for the asymmetric TOP with benzyl alcohol 1a and N-aminoethylpyrrole 2a as substrates (Scheme 4). The key to recycling the heterogeneous catalyst was the treatment of the spent IOC–PI/CB(Au/Pd) 8 with an aqueous solution of H2O2 to remove BnSCH3 and reactivate the Au/Pd NP catalyst.
Conclusions
In conclusion, we have developed a new chiral bifunctional heterogeneous catalyst, composed of metal NPs and CPAs, that was capable of facilitating the sequential one-pot aerobic oxidation-intramolecular asymmetric aza-FC reaction to provide chiral 1,2,3,4-tetrahydropyrrolo[1,2-a]pyrazines 3a–p in high yields and enantioselectivities. Interestingly, we found a chemical system (H2O2/BnSCH3) to switch on/off the catalytic activity14 of the Au/Pd and Au/Pt NPs. By controlling the catalytic activity of the heterogeneous catalyst through the use of a chemical modifier, the undesired oxidation of chiral piperazines 3a–p was avoided, and the facile reactivation of the deactivated catalyst allowed the chiral composite material to be recovered and reused several times without significant losses in yield or enantioselectivity of the desired product 3a. Moreover, we were able to demonstrate that heterogeneous CPAs could retain their catalytic ability in the presence of metal NPs under oxidative conditions (O2 and H2O2). Further investigations into the use of these robust chiral heterogeneous catalysts in other types of asymmetric TOPs are currently underway in our laboratory.Acknowledgements
This work was partially supported by Grant-in-Aids for Scientific Research from JSPS, the Global COE Program, the University of Tokyo, MEXT (Japan), and the Japan Science and Technology Agency (JST). We also thank Noriaki Kuramitsu (The University of Tokyo) for STEM and EDS analysis.Notes and references
- (a) H. Pellissier, Tetrahedron, 2013, 69, 7171 CrossRef CAS; (b) Z. Du and Z. Shao, Chem. Soc. Rev., 2013, 42, 1337 RSC; (c) C. A. Denard, J. F. Hartwig and H. Zhao, ACS Catal., 2013, 3, 2856 CrossRef CAS; (d) H. Clavier and H. Pellissier, Adv. Synth. Catal., 2012, 354, 3347 CrossRef CAS; (e) S. Piovesana, D. M. Scarpino Schietroma and M. Bella, Angew. Chem., Int. Ed., 2011, 50, 6216 CrossRef CAS PubMed; (f) J. Zhou, Chem.–Asian J., 2010, 5, 422 CrossRef CAS PubMed.
- (a) Y. Hayashi, Chem. Sci., 2016, 7, 866 RSC; (b) T. L. Lohr and T. J. Marks, Nat. Chem., 2015, 7, 477 CrossRef CAS PubMed; (c) N. T. Patil, V. S. Shinde and B. Gajula, Org. Biomol. Chem., 2012, 10, 211 RSC.
- (a) J. Lu, J. Dimroth and M. Weck, J. Am. Chem. Soc., 2015, 137, 12984 CrossRef CAS PubMed; (b) X. Fan, C. Rodríguez-Escrich, S. Wang, S. Sayalero and M. A. Pericàs, Chem.–Eur. J., 2014, 20, 13089 CrossRef CAS PubMed; (c) H. Miyamura, G. C. Y. Choo, T. Yasukawa, W.-J. Yoo and S. Kobayashi, Chem. Commun., 2013, 49, 9917 RSC; (d) X. Fan, C. Rodríguez-Escrich, S. Sayalero and M. A. Pericàs, Chem.–Eur. J., 2013, 19, 10814 CrossRef CAS PubMed; (e) Y. Chi, S. T. Scroggins and J. M. J. Fréchet, J. Am. Chem. Soc., 2008, 130, 6322 CrossRef CAS PubMed; (f) S. L. Poe, M. Kobašlija and D. T. McQuade, J. Am. Chem. Soc., 2007, 129, 9216 CrossRef CAS PubMed; (g) S. J. Broadwater, S. L. Roth, K. E. Price, M. Kobašlija and D. T. McQuade, Org. Biomol. Chem., 2005, 3, 2899 RSC.
- (a) A. Leyva-Pérez, P. García-García and A. Corma, Angew. Chem., Int. Ed., 2014, 53, 8687 CrossRef PubMed; (b) P. García-García, A. Zagdoun, C. Copéret, A. Lesage, U. Díaz and A. Corma, Chem. Sci., 2013, 4, 2006 RSC; (c) F. Gelman, J. Blum and D. Avnir, J. Am. Chem. Soc., 2002, 124, 14460 CrossRef CAS PubMed.
- For examples of immobilized multicatalyst-promoted asymmetric reactions in flow, see: (a) H. Ishitani, Y. Saito, T. Tsubogo and S. Kobayashi, Org. Lett., 2016, 18, 1346 CrossRef CAS PubMed; (b) T. Tsubogo, H. Oyamada and S. Kobayashi, Nature, 2015, 520, 329 CrossRef CAS PubMed.
- (a) H. Miyamura and S. Kobayashi, Acc. Chem. Res., 2014, 47, 1054 CrossRef CAS PubMed; (b) S. Kobayashi and H. Miyamura, Aldrichimica Acta, 2013, 46, 3 CAS; (c) S. Kobayashi and H. Miyamura, Chem. Rec., 2010, 10, 271 CrossRef CAS PubMed; (d) R. Akiyama and S. Kobayashi, Chem. Rev., 2009, 109, 594 CrossRef CAS PubMed.
- (a) H. Miyamura, A. Suzuki, T. Yasukawa and S. Kobayashi, Adv. Synth. Catal., 2015, 357, 3815 CrossRef CAS; (b) H. Miyamura, H. Min, J.-F. Soulé and S. Kobayashi, Angew. Chem., Int. Ed., 2015, 54, 7564 CrossRef CAS PubMed; (c) J.-F. Soulé, H. Miyamura and S. Kobayashi, Chem.–Asian J., 2013, 8, 2614 CrossRef PubMed; (d) J.-F. Soulé, H. Miyamura and S. Kobayashi, Chem. Commun., 2013, 49, 355 RSC; (e) J.-F. Soulé, H. Miyamura and S. Kobayashi, Asian J. Org. Chem., 2012, 1, 319 CrossRef; (f) H. Yuan, W.-J. Yoo, H. Miyamura and S. Kobayashi, Adv. Synth. Catal., 2012, 354, 2899 CrossRef CAS; (g) J.-F. Soulé, H. Miyamura and S. Kobayashi, J. Am. Chem. Soc., 2011, 133, 18550 CrossRef PubMed; (h) W.-J. Yoo, H. Miyamura and S. Kobayashi, J. Am. Chem. Soc., 2011, 133, 3095 CrossRef CAS PubMed; (i) T. Yasukawa, H. Miyamura and S. Kobayashi, Chem.–Asian J., 2011, 6, 621 CrossRef CAS PubMed; (j) K. Kaizuka, H. Miyamura and S. Kobayashi, J. Am. Chem. Soc., 2010, 132, 15096 CrossRef CAS PubMed; (k) H. Miyamura, T. Yasukawa and S. Kobayashi, Green Chem., 2010, 12, 776 RSC.
- O. V. Maltsev, A. O. Chizhov and S. G. Zlotin, Chem.–Eur. J., 2011, 17, 6109 CrossRef CAS PubMed.
- For representative reviews of CPA-catalyzed asymmetric transformations, see: (a) A. K. Mutyala and N. T. Patil, Org. Chem. Front., 2014, 1, 582 RSC; (b) D. Parmar, E. Sugiono, S. Raja and M. Rueping, Chem. Rev., 2014, 114, 9047 CrossRef CAS PubMed; (c) M. Terada, Synthesis, 2010, 1929 CrossRef CAS; (d) M. Terada, Chem. Commun., 2008, 4097 RSC; (e) T. Akiyama, Chem. Rev., 2007, 107, 5744 CrossRef CAS PubMed; (f) T. Akiyama, J. Itoh and K. Fuchibe, Adv. Synth. Catal., 2006, 348, 999 CrossRef CAS.
- Multicatalyst-promoted asymmetric tandem reactions involving homogeneous CPAs and metal complexes have been reported. For representative examples, see: (a) P.-S. Wang, H.-C. Lin, Y.-J. Zhai, Z.-Y. Han and L.-Z. Gong, Angew. Chem., Int. Ed., 2014, 53, 12218 CrossRef CAS PubMed; (b) S.-Y. Yu, H. Zhang, Y. Gao, L. Mo, S. Wang and Z.-J. Yao, J. Am. Chem. Soc., 2013, 135, 11402 CrossRef CAS PubMed; (c) Z.-L. Tao, W.-Q. Zhang, D.-F. Chen, A. Adele and L.-Z. Gong, J. Am. Chem. Soc., 2013, 135, 9255 CrossRef CAS PubMed; (d) Z. Chai and T. J. Rainey, J. Am. Chem. Soc., 2012, 134, 3615 CrossRef CAS PubMed; (e) C. Wang, Z.-Y. Han, H.-W. Luo and L.-Z. Gong, Org. Lett., 2010, 12, 2266 CrossRef CAS PubMed; (f) M. Terada and Y. Toda, J. Am. Chem. Soc., 2009, 131, 6354 CrossRef CAS PubMed; (g) M. E. Muratore, C. A. Holloway, A. W. Pilling, R. I. Storer, G. Trevitt and D. J. Dixon, J. Am. Chem. Soc., 2009, 131, 10796 CrossRef CAS PubMed; (h) Z.-Y. Han, H. Xiao, X.-H. Chen and L.-Z. Gong, J. Am. Chem. Soc., 2009, 131, 9182 CrossRef CAS PubMed; (i) X.-Y. Liu and C.-M. Che, Org. Lett., 2009, 11, 4204 CrossRef CAS PubMed; (j) S. Mukherjee and B. List, J. Am. Chem. Soc., 2007, 129, 11336 CrossRef CAS PubMed; (k) H. Alper and N. Hamel, J. Am. Chem. Soc., 1990, 112, 2803 CrossRef CAS.
- Details regarding reaction optimization and control studies can be found in the ESI.†.
- For asymmetric intramolecular aza-FC reactions of N-aminoethylpyrroles with aldehydes catalyzed by (R)-TRIP, see: Y. He, M. Lin, Z. Li, X. Liang, G. Li and J. C. Antilla, Org. Lett., 2011, 13, 4490 CrossRef CAS PubMed.
- Relatively few examples of immobilized CPAs have been reported: (a) B. Zhang, L. Shi and R. Guo, Catal. Lett., 2015, 145, 1718 CrossRef CAS; (b) L. Osorio-Planes, C. Rodríguez-Escrich and M. A. Pericàs, Chem.–Eur. J., 2014, 20, 2367 CrossRef CAS PubMed; (c) D. S. Kundu, J. Schmidt, C. Bleschke, A. Thomas and S. Blechert, Angew. Chem., Int. Ed., 2012, 51, 5456 CrossRef CAS PubMed; (d) C. Bleschke, J. Schmidt, D. S. Kundu, S. Blechert and A. Thomas, Adv. Synth. Catal., 2011, 353, 3101 CrossRef CAS; (e) M. Rueping, E. Sugiono, A. Steck and T. Theissmann, Adv. Synth. Catal., 2010, 352, 281 CrossRef CAS.
- The modulation of the catalytic activity of metal NPs using an external stimulus is not well-established. For a review on artificial switchable catalysts, see: V. Blanco, D. A. Leigh and V. Marcos, Chem. Soc. Rev., 2015, 44, 5341 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: General procedures, materials, and instrumentation; synthesis, characterization and relevant spectra/charts; procedures and results for optimization and additional experiments. See DOI: 10.1039/c6sc03849b |
| ‡ These authors made equal contributions. |
| This journal is © The Royal Society of Chemistry 2017 |