Open Access Article
Open Access ArticlePsychosine variants as antigens for natural killer T cells†
S.
Deng
a,
L.
Kain
b,
C. S.
Pereira
cd,
S.
Mata
a,
M. F.
Macedo
 cde,
A.
Bendelac
f,
L.
Teyton
b and
P. B.
Savage
cde,
A.
Bendelac
f,
L.
Teyton
b and
P. B.
Savage
 *a
*a
aDepartment of Chemistry and Biochemistry, Brigham Young University, Provo, UT84602, USA. E-mail: paul_savage@byu.edu
bDepartment of Immunology, The Scripps Research Institute, La Jolla, CA 92037, USA
cInstituto de Investigação e Inovação em Saúde, Universidade do Porto, Portugal
dIBMC – Instituto de Biologia Molecular e Celular, Universidade do Porto, Porto, Portugal
eDepartment of Medical Sciences, University of Aveiro, Aveiro, Portugal
fCommittee on Immunology and Department of Pathology, Howard Hughes Medical Institute, University of Chicago, Chicago, IL 60637, USA
First published on 9th December 2016
Abstract
Natural killer T (NKT) cells play a central role in the interface between innate and adaptive immunity, and alpha-galactosylceramide was recently shown to be an endogenous antigen for these cells. The source of alpha-galactosylceramide has not yet been determined; however, in vivo degradation of alpha-galactosylceramide involves generation of alpha-psychosine (alpha-galactosylsphingosine). Alpha-psychosine stimulates cytokine release from NKT cells and constitutes an endogenous antigen for these cells. Alpha-psychosine contains a single lipid chain, while most antigens for NKT cells have two lipid chains, and we have investigated if other glycolipids with one lipid chain, derived from know antigens for NKT cells, stimulate cytokine release from NKT cells. Only psychosine variants derived from the most potent NKT cell antigens cause stimulation, and this stimulation occurs in vitro as well as in vivo. Truncated forms of weak antigens for NKT cells are not stimulatory.
Introduction
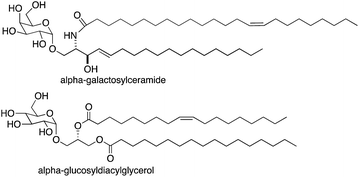
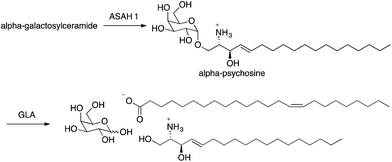
Natural killer T (NKT) cells play a central role in the interface between innate and adaptive immunity.1,2 These cells respond to specific glycolipid structures presented by the protein CD1d on antigen presenting cells by releasing cytokines, which in turn can lead to inflammatory or immune-modulatory responses. NKT cells can mediate immune responses to bacterial, viral and parasitic infections and tumor cells. They also influence the development of autoimmune diseases. As the role of NKT cells has been elucidated, substantial efforts have been made to understand sources of glycolipid antigens for NKT cells. Multiple exogenous glycolipid antigens have been identified from bacteria and fungi,3 and a majority of these include either glucose or galactose with an alpha anomeric linkage to either ceramide or diacylglycerol (for examples, see alpha-galactosylceramide and alpha-glucosyldiacylglycerol in Fig. 1). Efforts have been made to identify endogenous glycolipid antigens for NKT cells as well, and it has generally been assumed that glycosyl-ceramide linkages of endogenous antigens would have to be beta because there is no mammalian enzyme known to transfer glucose or galactose onto ceramide or diacylglycerol giving alpha glycosidic bonds.Recently, we discovered that alpha-galactosylceramide and alpha-glucosylceramide are both endogenous antigens for NKT cells.4,5 While it is not yet fully understood how these compounds are generated in mammals, the degradation pathway of alpha-galactosylceramide has been established, and the impact of this pathway on immune responses has been demonstrated. Degradation of alpha-galactosylceramide requires cleavage of both the glycosidic bond and the amide between the acyl chain and sphingosine. Alpha-galactosidase (GLA) cleaves alpha-galactosidic bonds, but alpha-galactosylceramide is not a substrate for this enzyme. However, cleavage of the amide bond by acid ceramidases ASAH1 gives the corresponding deacylated compound (alpha-psychosine). Psychosine is comprised of galactose linked to sphingosine through a beta-glycosidic bond. The alpha form of psychosine is the product of ASAH1 action on alpha-galactosylceramide, and this compound is a substrate for GLA, which catalyzes the final step in the full degradation of the parent compound to its component subunits (Fig. 2).
Because alpha-psychosine is an intermediate in the degradation of alpha-galactosylceramide and it maintains much of the structure of the parent compound, we tested the ability of this compound to bind to CD1d, the protein that presents alpha-galactosylceramide to NKT cells. We found that alpha-psychosine was presented well by CD1d and that this presentation resulted in stimulation of NKT cells, measured as cytokine release.4 This was the first observation that glycolipids comprised of a sugar alpha linked to sphingosine caused stimulation of NKT cells. And, it showed that degradation products of glycosylceramides and glycosyl diacylglycerols may represent potential antigens for NKT cells.
Results and discussion
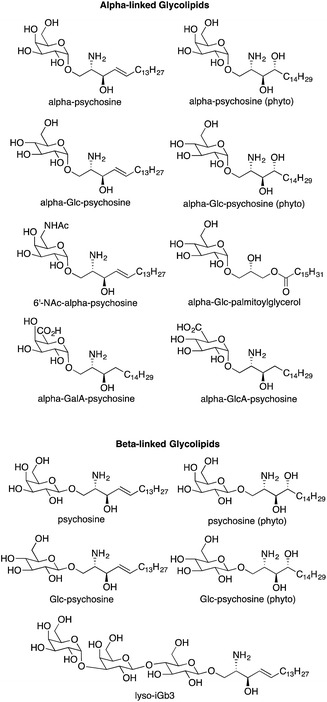
Our initial investigation included only alpha-psychosine (Fig. 2) derived from alpha-galactosylceramide by loss of an acyl chain.4 A logical extension of this investigation was to determine if truncated forms of other NKT cell antigens (i.e., loss of an acyl chain) would also act as antigens for NKT cells. Because a wide array of glycolipids have been demonstrated to be antigens for NKT cells, this effort required synthesis of a series of compounds lacking an acyl chain, relative to their parent glycolipids (Fig. 3).In mammals, sphingosine is prevalent, while phytosphingosine is found in plants. Nevertheless, both types of bases have been used in antigens for NKT cells, and we used both in the psychosine derivatives to determine the impact of the differences in these bases on NKT cell stimulation. Alpha-galactosylceramide derived from phytosphingosine is commonly used to stimulate NKT cells, and ASAH1 mediated cleavage of its acyl chain would yield alpha-psychosine (phyto); the sphingosine variant is alpha-psychosine (Fig. 3). In the context of glycosylceramides, glucose-containing glycolipids have been proven to be weaker antigens as compared to their galactose-containing isomers.3 Nevertheless, we prepared the glucose-derived forms of alpha-psychosines, termed as alpha-Glc-psychosine and alpha-Glc-psychosine (phyto). In earlier research,6 we found that replacement of the hydroxyl group at C6 in galactose with an –NHAc group gave a glycolipid that was a more potent antigen for NKT cells than the parent compound. To discover if this replacement would impact NKT cell stimulation by alpha-psychosine, we prepared 6′-NAc-alpha-psychosine.
As described above, multiple glycolipids of bacterial origin have been shown to stimulate NKT cells. Alpha-glucosyldiacylglycerol (Fig. 1) was identified as an NKT cell antigen from Streptococcus pneumoniae.7 Loss of an acyl chain from the sn2 position gives alpha-Glc-palmitoylglycerol (Fig. 3), and this compound was prepared for comparison purposes. Glycosphingolipids from Sphingomonas spp. are also antigens for NKT cells,8–10 and these glycolipids contain either galacturonose or glucuronose sugars. Loss of acyl chains from these glycolipids gives alpha-GalA-psychosine and alpha-GlcA-psychosine, which were prepared and tested for NKT cell stimulatory properties.
Few beta-linked glycosylceramides have been described as antigens for NKT cells,11–13 and compounds lacking an N-acyl chain were prepared for comparison purposes. These include psychosine and a truncated form of iGb3, termed lyso-iGb3 (Fig. 3).
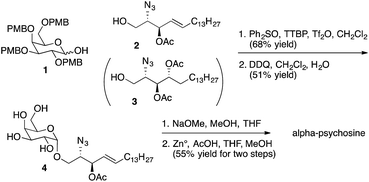
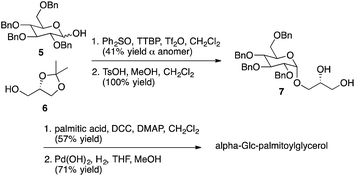
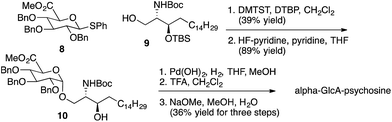
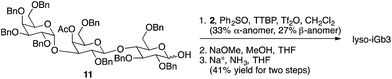
Representative syntheses of selected psychosine variants shown in Fig. 3 are described in Schemes 1–4 (full synthetic schemes are included in the ESI†). Preparation of alpha-psychosine (Scheme 1) began with coupling of protected galactose 1 (ref. 14) with sphingosine variant 2.15 Deprotection using DDQ gave 4. Further deprotection with methoxide and reduction of the azide provided the desired glycolipid in moderate yield. Phytosphingosine glycolipids (e.g., alpha-psychosine (phyto)) were prepared by substitution of 2 with protected phytosphingosine 3. Glucose-containing glycolipids were prepared following a similar route with appropriately protected glucose substituting for 1. Alpha-Glc-palmitoylglycerol was prepared by coupling 5 with 6 followed by deprotection, acylation with palmitic acid, and further deprotection (Scheme 2). Synthesis of the truncated form of GSL-1 from Sphingomonas spp. began with coupling of 8 (ref. 16) with sphinganine derivative 9.15 Sequential deprotection of the TBS group, benzyl groups, the BOC group, and the methyl ester gave alpha-GlcA-psychosine (Scheme 3). The lyso form of iGb3 was generated by coupling trisaccharide 11 (ref. 17) with 2. As expected, the alpha anomer was the major product, but the beta anomer was produced in a modest amount, and the two anomers could be separated chromatographically. Carrying the beta anomer forward, sequential deprotections gave lyso-iGb3 in moderate yield (Scheme 4).
With the psychosine derivatives described in Fig. 3 in hand, we were prepared to test their abilities to stimulate cytokine release from NKT cells. For the initial experiments, a murine NKT cell line (DN32.D3)18 was used. Antigen presentation occurs via CD1d, and rat basophilic leukemia (RBL) cells, which produce CD1d, were used for presentation of the glycolipids. Release of the cytokine IL-2 was used to quantify NKT cell stimulation (Fig. 4). As anticipated, alpha-galactosylceramide stimulated IL-2 release at relatively low concentrations. As observed with glycosylceramides, galactose-containing alpha-psychosines (alpha-psychosine and alpha-psychosine (phyto)) proved to be more strongly stimulating than glucose-containing alpha-psychosines (alpha-Glc-psychosine and alpha-Glc-psychosine (phyto)). Replacement of the hydroxyl group at C6′ with an –NHAc group led to a substantial loss of stimulatory activity.
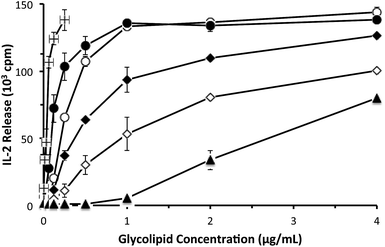 | ||
| Fig. 4 Stimulation of NKT cells (DN32.D3 cells) by the indicated glycolipids presented by CD1d on RBL cells. Stimulation measured by tritium incorporation into a natural killer cell line.4 Alpha-galactosylceramide (■); alpha-psychosine (●); alpha-psychosine (phyto) (❍); alpha-Glc-psychosine (◊); alpha-Glc-psychosine (phyto) (◆); 6′-NAc-alpha-psychosine (▲). | ||
The relative strength of stimulation of the murine NKT cell line by the glycolipids included in Fig. 4 roughly follows the pattern seen with alpha-glycosylceramides. That is, galactose-19 and phytosphingosine-20 containing glycolipids are more potent stimulators than their glucose- and sphingosine-containing counterparts. The only result that was unexpected was the impact of the –NHAc group, which in the context of alpha-galactosylceramide increased stimulatory activity,6 yet decreased activity in the context of alpha-psychosine.
Each of the glycolipids shown in Fig. 3 was evaluated for NKT cell stimulatory activity, but only the five compounds described for Fig. 4 demonstrated stimulation. All of the beta-linked glycolipids, including lyso-iGb3 showed no detectable stimulation of the NKT cell line. Relative to alpha-galactosylceramide, iGb3 is a weak antigen for NKT cells;3 consequently, it is understandable that further weakening of the stimulatory nature of iGb3 by loss of an acyl chain yields a non-stimulatory glycolipid.
Truncated forms of bacterially-derived glycolipids (alpha-Glc-palmatoylglycerol, alpha-GalA-psychosine and alpha-GlcA-psychosine) were also non-stimulatory. As with iGb3, each of the parent glycolipids (i.e., glycolipids with two lipid chains) is weakly stimulatory relative to alpha-galactosylceramide.3 And loss of an acyl chain from weak stimulators is sufficient to eliminate detectable NKT cell stimulatory properties.
We also evaluated the NKT cell stimulatory properties of alpha-psychosine and alpha-Glc-psychosine using two different human NKT cell lines.21 C1R cells transfected with CD1d were used for antigen presentation, and these cells were cultured with varied concentrations of the two glycolipids followed by addition of human NKT cells. Release of the cytokine GM-CSF was determined by ELISA and used to quantify NKT cell stimulation (Fig. 5). Both psychosine variants stimulated cytokine release, although at concentrations higher than those required for stimulation of these cell lines with alpha-galactosylceramide (see ESI†). Notably, and in contrast to the results with murine NKT cells, alpha-Glc-psychosine stimulated cytokine release at lower concentrations than alpha-psychosine. At higher concentrations of the glycolipids, these differences became insignificant. Nevertheless, these differences in responses between murine and human NKT cells to alpha-psychosines suggest that glucose and galactose-containing psychosines may play distinct roles in these two organisms. With other glycolipids, human and murine NKT cells have been shown to respond differently.20
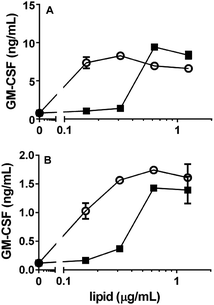 | ||
| Fig. 5 Response of two human NKT cell lines (A and B) to alpha-psychosine (■) and alpha-Glc-psychosine (❍). CD1d-transfected C1R cells were used for antigen presentation. | ||
A property of glycolipids that stimulate NKT cells in vivo is that following stimulation, NKT cells proliferate.2 To verify that alpha-psychosine derivatives retain the ability to stimulate NKT cells in vivo, small amounts of two of the more potent compounds (alpha-psychosine (phyto) and alpha-Glc-psychosine (phyto)) were injected (IP) into mice. After three days, the percentage of NKT cells in the spleen was determined via flow cytometry. These percentages are included in Fig. 6. Compared to vehicle, alpha-psychosine (phyto) caused a substantial increase in the percentage of NKT cells. Because alpha-Glc-psychosine (phyto) is a weaker antigen for NKT cells, injection of this compound led to a lower expansion of NKT cells. No adverse effects were observed upon injection of these glycolipids, and cytokine levels increased in the blood plasma (Fig. S2 in ESI†).
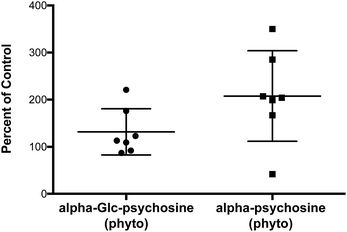 | ||
| Fig. 6 Expansion of NKT cells in wild-type C57BL/6J mice three days after injection (IV) with the indicated glycolipid (2.5 μg per mouse). | ||
Conclusions
Because NKT cells play an important role in influencing the immune states of humans, in particular the interplay between innate and adaptive immunity, substantial efforts have been made in identifying glycolipid antigens for NKT cells.1–3 Most naturally occurring antigens come from bacteria;3 however, it has been clear that endogenous antigens are present in humans.2 The nature and origins of these endogenous antigens have been an area of intense investigation for many years. The recent discovery that alpha-galactosyl- and alpha-glucosylceramides are endogenous antigens4,5 has opened the possibility that not only these glycolipids but also their degradation products may play a role in controlling immune responses. We have demonstrated that alpha-psychosine is produced as alpha-galactosylceramide is degraded, and the observation that this truncated glycolipid stimulates NKT cells identifies it as an endogenous antigen for NKT cells.This observation inspired our efforts to determine the structural features of other psychosines, derived from other known NKT cell antigens, that are necessary for NKT cell stimulation. An alpha glycosidic bond between galactose or glucose and sphingosine or phytosphingosine appears to be the primary requirements; beta glycosidic bonds yield non-stimulatory compounds. Oxidation of glucose or galactose to the corresponding glycuronic acids also gives non-stimulatory glycolipids, and replacement of sphingosine or phytosphingosine with monoacylglycerol causes loss of stimulatory properties.
Identification of alpha-galactosylceramide as an endogenous antigen, elucidation of its degradation pathway, and the studies presented herein lead to three key conclusions: (1) alpha-psychosine and alpha-Glc-psychosine are antigens for NKT cells; (2) alpha-psychosine is an endogenous antigen for NKT cells; and (3) few structural variations among these glycolipids are tolerated for NKT cell stimulation. These conclusions provide a clearer picture of how NKT cells are stimulated by endogenous antigens and influence immune responses.
Acknowledgements
This work was supported by National Institutes of Health grant AI102892. Financial support was also given by project Norte-01-0145-FEDER-000012, supported by Norte Portugal Regional Operational Programme (NORTE 2020), under the PORTUGAL 2020 Partnership Agreement, through the European Regional Development Fund (ERDF).Notes and references
- L. Mori, M. Lepore and G. De Libero, Annu. Rev. Immunol., 2016, 34, 479–510 CrossRef PubMed.
- A. Bendelac, P. B. Savage and L. Teyton, Annu. Rev. Immunol., 2007, 25, 297–336 CrossRef CAS PubMed.
- B. L. Anderson, L. Teyton, A. Bendelac and P. B. Savage, Molecules, 2013, 18, 15662–15688 CrossRef CAS PubMed.
- L. Kain, B. Webb, B. L. Anderson, S. Deng, M. Holt, A. Castanzo, M. Zhao, K. Self, A. Teyton, C. Everett, M. Kronenberg, D. M. Zajonc, A. Bendelac, P. B. Savage and L. Teyton, Immunity, 2014, 41, 543–554 CrossRef CAS PubMed.
- L. Kain, A. Constanzo, B. Webb, M. Holt, A. Bendelac, P. B. Savage and L. Teyton, Mol. Immunol., 2015, 68, 94–97 CrossRef CAS PubMed.
- Y. Liu, R. D. Goff, D. Zhou, J. Mattner, B. A. Sullivan, A. Khurana, C. Cantu, J. D. Altman, L. Teyton, A. Bendelac and P. B. Savage, J. Immunol. Methods, 2006, 312, 34–39 CrossRef CAS PubMed.
- Y. Kinjo, P. Illarionov, J. L. Vela, B. Pei, E. Girardi, X. M. Li, Y. L. Li, M. Imamura, Y. Kaneko, A. Okawara, Y. Miyazaki, A. Gómez-Velasco, P. Rogers, S. Dahesh, S. Uchiyama, A. Khurana, K. Kawahara, H. Yesilkaya, P. W. Andrew, C.-H. Wong, K. Kawakami, V. Nizet, G. S. Besra, M. Tsuju, D. M. Zajonic and M. Kronenberg, Nat. Immunol., 2011, 12, 966–U972 CrossRef CAS PubMed.
- Y. Kinjo, D. Wu, G. S. Kim, G. W. Xing, M. A. Poles, D. D. Ho, M. Tsuji, K. Kawahara, C.-H. Wong and M. Kronenberg, Nature, 2005, 434, 520–525 CrossRef CAS PubMed.
- J. Mattner, K. L. DeBord, R. D. Goff, C. Cantu, D. Zhou, P. Saint-Mezard, V. Wang, Y. Gao, N. Yin, K. Hoebe, O. Schneewind, N. Ismail, D. Walker, B. Buetler, L. Teyton, P. B. Savage and A. Bendelac, Nature, 2005, 434, 525–528 CrossRef CAS PubMed.
- X. Long, S. Deng, Z. Zang, J. Mattner, D. Zhou, N. McNary, R. D. Goff, L. Teyton, A. Bendelac and P. B. Savage, Nat. Chem. Biol., 2007, 3, 559–564 CrossRef CAS PubMed.
- D. Zhou, J. Mattner, C. Cantu, N. Schrantz, N. Yin, Y. Gao, Y. Sagiv, K. Hudspeth, Y. Wu, T. Yamashita, S. Teneberg, D. Wang, R. Proia, S. B. Levery, P. B. Savage, L. Teyton and A. Bendelac, Science, 2004, 306, 1786–1789 CrossRef CAS PubMed.
- P. J. Brennan, R. V. V. Tatituri, M. Brigl, E. Y. Kim, A. Tuli, J. P. Sanderson, S. D. Gadola, F. F. Hsu, G. S. Besra and M. B. Brenner, Nat. Immunol., 2011, 12, 1202–U1207 CrossRef CAS PubMed.
- L. A. Albacker, V. Chaudary, Y.-J. Chang, H. Y. Kim, Y.-T. Chuang, M. Pichuvant, R. H. DeKruyff, P. B. Savage and D. T. Umetsu, Nat. Med., 2013, 19, 1297–1304 CrossRef CAS PubMed.
- L. J. Whalen and R. L. Halcomb, Org. Lett., 2004, 6, 3221–3224 CrossRef CAS PubMed.
- Preparation described in ESI.†.
- J. Dinkelaar, A. R. de Jong, R. van Meer, M. Somers, G. Lodder, H. S. Overkleeft, J. D. C. Codée and G. A. van der Marel, J. Org. Chem., 2009, 74, 4982–4991 CrossRef CAS PubMed.
- N. Yin, X. Long, R. D. Goff, D. Zhou, C. Cantu, J. Mattner, P. Saint Mezard, L. Teyton, A. Bendelac and P. B. Savage, ACS Chem. Biol., 2009, 4, 191–197 CrossRef CAS PubMed.
- S. H. Park, J. H. Roark and A. Bendelac, J. Immunol., 1998, 160, 3128–3134 CAS.
- T. Kawano, J. Cui, Y. Koezuka, I. Toura, Y. Kaneko, K. Motoki, H. Ueno, R. Nakagawa, H. Sato, E. Kondo, H. Koseki and M. Taniguchi, Science, 1997, 278, 1626–1629 CrossRef CAS PubMed.
- E. M. Dangerfield, J. M. H. Cheng, D. A. Knight, R. Weinkove, P. R. Dunbar, I. F. Hermans, M. S. M. Timmer and B. L. Stocker, ChemBioChem, 2012, 13, 1349–1356 CrossRef CAS PubMed . Species-specific activity of glycolipids ligands for invariant NKT cells.
- C. S. Pereira, C. Sa-Miranda, G. De Libero, L. Mori and M. F. Macedo, Eur. J. Immunol., 2016, 46, 147–153 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details for synthesis of glycolipids shown in Fig. 3, cytokine quantification, and NKT cell percentage measurements. GM-SCF release from human NKT cell lines stimulated by alpha-galactosylceramide. Cytokine release (plasma) from mice injected with alpha-Glc-psychosine (phyto) and alpha-psychosine (phyto). See DOI: 10.1039/c6sc04218j |
| This journal is © The Royal Society of Chemistry 2017 |