Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Organocatalytic, enantioselective synthesis of benzoxaboroles via Wittig/oxa-Michael reaction Cascade of α-formyl boronic acids†
Gurupada
Hazra
,
Sanjay
Maity
,
Sudipto
Bhowmick
and
Prasanta
Ghorai
 *
*
Department of Chemistry, Indian Institute of Science Education and Research Bhopal, Bhopal By-pass Road, Bhauri, Bhopal-462066, India. E-mail: pghorai@iiserb.ac.in
First published on 30th January 2017
Abstract
An unprecedented enantioselective synthesis of 3-substituted benzoxaboroles has been developed. An in situ generated ortho-boronic acid containing chalcone provides the chiral benzoxaboroles via an asymmetric oxa-Michael addition of hydroxyl group attached to the boronic acid triggered by the cinchona alkaloid based chiral amino-squaramide catalysts. In general, good yields with good to excellent enantioselectivities (up to 99%) were obtained. The resulting benzoxaboroles were converted to the corresponding chiral β-hydroxy ketones without affecting the enantioselectivity.
Introduction
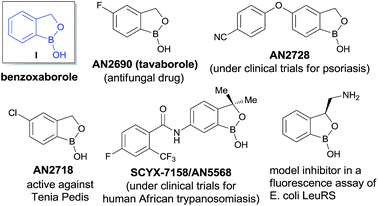
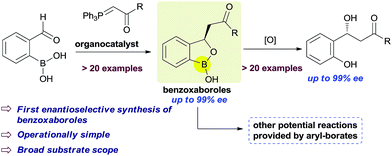
Benzoxaboroles, an important class of boron containing molecules, has recently acquired significant attention towards its applications for the development of new drugs.1,2 The Lewis acidity of the boron and its easy conversion from trigonal to tetrahedral geometry enables benzoxaboroles to bind to the active site of various enzymes and thereby inhibit their activity.1,3 Ever since the discovery of the exceptional sugar-binding properties at physiological conditions4 as well as the finding of antifungal activity of AN2690 (Fig. 1),5 benzoxaboroles have been extensively studied for therapeutic applications. This leads to the development of benzoxaboroles with antibacterial,6 antiviral,7 anti-parasitic,8 anti-inflammatory,9 and antimalarial10 activities as well as β-lactamase inhibitor.1Very recently, KERYDIN was approved for onychomycosis treatment,11 AN2728 (ref. 12) and AN2898 (ref. 13) are currently under clinical trials for psoriasis. SCYX-7158/AN5568 entered into clinical trials for the treatment of human African trypanosomiasis.14 Thus, the intrinsic reactivity and metabolic stability of benzoxaborole motif have made it a new “privileged scaffold” for the design of new drugs.15 Furthermore, the materials conjugated with benzoxaborole exhibit a unique structural assembly which enables to unfold multidentate interactions to improve selective binding.16 This property has extended their applications in supramolecular17 and materials chemistry.18 Apart from these, benzoxaboroles are versatile building blocks for the synthesis of organic molecules of higher complexity.19 Despite the surging applications of benzoxaboroles in various fields and handful attempts directed towards their achiral synthesis, we report herein, for the first time, the catalytic enantioselective synthesis of this class of compounds. In this reaction, the 2-formyl aryl boronic acids react via a Wittig reaction followed by an enantioselective oxa-Michael addition of a hydroxyl group attached to the boronic acid using chiral bifunctional organocatalysts (Scheme 1).
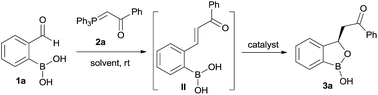
Our strategy is leveraged from the asymmetric oxa-Michael addition using bifunctional organocatalyst consisting of squaramide/thiourea moiety attached to a tertiary nitrogen on a chiral scaffold and the oxo-nucleophilicity of the hydroxy group of organoboronic acids, revealed by Falck et al.20 We hypothesized to utilize an in situ generated ortho-boronic acid containing chalcones (II, Table 1) as the substrate wherein the asymmetric oxa-Michael reaction of hydroxy group of boronic acid is involved in the simultaneous coordination of the carbonyl with squaramide/thiourea (the pull) moiety and the tertiary nitrogen to boron (the push).
| Entry | Catalyst | Solvent | Yieldb (%) | (%) eec |
|---|---|---|---|---|
| a Reactions were performed on a 0.05 mmol scale of aldehyde 1a, 1.5 equiv. of 2a. b Yield was calculated based on 1H NMR spectroscopy of the crude reaction mixture using diphenyl acetonitrile as the internal standard. c Enantiomeric excess was determined by HPLC analysis on a chiral stationary phase after oxidative deborylation. | ||||
| 1 | C1 | CH2Cl2 | 12 | 56 |
| 2 | C2 | CH2Cl2 | <5 | ND |
| 3 | C3 | CH2Cl2 | 26 | 70 |
| 4 | C4 | CH2Cl2 | 16 | −62 |
| 5 | C5 | CH2Cl2 | 26 | 86 |
| 6 | C6 | CH2Cl2 | 53 | 90 |
| 7 | C7 | CH2Cl2 | 55 | 91 |
| 8 | C7 | Toluene | 70 | 90 |
| 9 | C7 | PhCF3 | 85 | 90 |
| 10 | C7 | THF | 15 | 80 |
| 11 | C7 | Chlorobenzene | 86 | 91 |
|
||||
Results and discussion
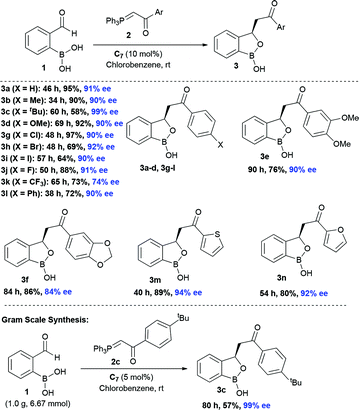
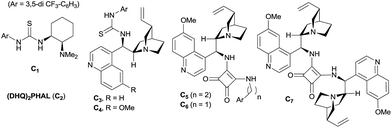
At the outset, we began our investigation with ortho-formyl phenyl boronic acid 1a and benzoyl Wittig olefin 2a aiming towards the synthesis of chiral benzoxaborole 3a (Table 1). A variety of chiral amino-thiourea (C1) and cinchona alkaloid-derived catalysts (C2–C7) were surveyed in dichloromethane at room temperature (entries 1–7) (see ESI†). As shown in entry 7, catalyst C7 was found to catalyze the reaction cleanly to furnish the benzoxaborole 3a (55% NMR yield with 91% ee). A notable increase in yield was observed when the reaction was performed in toluene and trifluoro-toluene (entry 8 and 9, respectively). An immediate solvent study consoled that chlorobenzene was the optimal one, affording 91% ee with 86% yield (entry 11). Under these optimal reaction conditions, we examined the substrate scope of this reaction, and the results are shown in Scheme 2. The effect of substitution on the aryl moiety of Wittig-olefins was first examined. To our delight, electron-donating substituents such as Me– (3b), tBu– (3c) and MeO– (3d–e) underwent smooth cyclization, affording the desired products in good to excellent yields and with excellent enantioselectivities (58–95% yields, 84–99% ee). Similarly, electron-withdrawing substituents such as Cl– (3g), Br– (3h), I– (3i), F– (3j), F3C– (3k) and Ph– (3l), also furnished the desired products with high stereo-induction (64–97% yields, 74–92% ee). Hetero-aromatic groups such as 2-thiophenyl as well as 2-furyl were also well tolerated to provide the corresponding benzoxaboroles 3m (89% yield, 94% ee) and 3n (80% yield, 92% ee), respectively. To illustrate the practical utility of this methodology, a gram scale (1.0 g, 6.67 mmol) reaction was performed to provide the product 3c. Interestingly, it was observed that even 5 mol% of catalyst was enough to complete the reaction without losing any enantioselectivity (57% yield, 99% ee). The absolute stereochemistry (R) of the product 3g was determined by X-ray crystallography (Fig. 2) and the other compounds were assigned by analogy.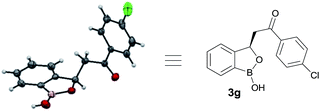 | ||
| Fig. 2 Crystal structure of 3g (CCDC 1487136). | ||
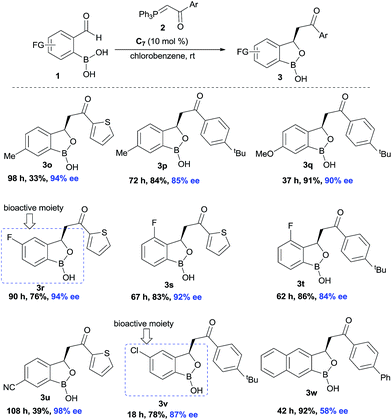
Next, the substitution on the central aryl moiety was examined which was quite general (Scheme 3) concerning enantioselectivities as well as yields. Electron-donating substituents such as Me– (3o–p), MeO– (3q), as well as electron-withdrawing substituents such as F– (3r–t), CN– (3u) and Cl– (3v), worked smoothly, affording the desired benzoxaborole with excellent enantioselectivities. Instead of central phenyl moiety, naphthyl moiety (3w) remained less efficient regarding enantioselectivity. Notably, the F- and Cl-substituted (3r and 3v) benzoxaborole moieties are known to have bioactivity as mentioned in Fig. 1.
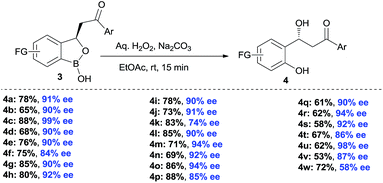
The synthesis of chiral β-hydroxy carbonyls has remained an attractive area of research because of their prevalence in organic synthesis and medicinal chemistry.21 Chiral benzylic alcohol containing phenols were used as chiral precursor for asymmetric synthesis, ligand and chiral auxiliary in asymmetric catalysis.22 However, there are limited methods in literature for the synthesis of such chiral alcohols.23 Moreover, synthesis of chiral β-hydroxy ketones has remained elusive so far. Therefore, we also emphasized on the oxidative deborylation of the products 3 (Scheme 4). Interestingly, the corresponding chiral benzyl alcohol 4 was obtained smoothly after oxidative deborylation of 3 with high yields and excellent enantioselectivities. Thus, via this benzoxaborolane pathway, the synthesis of β-hydroxy ketones shows a considerable advantage with respect to enantioselectivity and substrate generality.
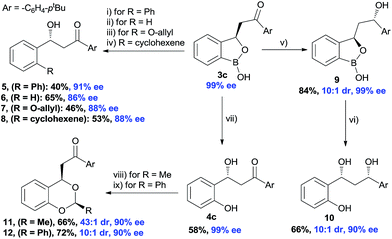
The synthetic potential of the benzoxaborolanes was briefly investigated using oxaborol 3 (Scheme 5). Subjecting 3c to a Pd-catalyzed Suzuki reaction conditions using bromobenzene afforded product 5 in 40% yield and 91% ee. Similarly, Ag-catalyzed deborylation provided the compound 6 and Cu-catalyzed deborylation in the presence of allyl alcohol produced 7 with high ee. A palladium catalyzed olefination with alkenyl triflate furnished the desired 8 with 88% ee. A substantial stereo-induction was observed during the NaBH4 reduction of keto functionality of 3c, an excellent diastereoselectivity (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was achieved in the compound 9. Further, oxidative deborylation of the product 9 produced chiral 1,3-diol2410 with high enantioselectivity and good diastereoselectivity. Finally, chiral benzylic alcohol 4c was treated with aldehydes in the presence of acids which furnished the acetals (11 and 12) with excellent diastereoselectivity (43
1) was achieved in the compound 9. Further, oxidative deborylation of the product 9 produced chiral 1,3-diol2410 with high enantioselectivity and good diastereoselectivity. Finally, chiral benzylic alcohol 4c was treated with aldehydes in the presence of acids which furnished the acetals (11 and 12) with excellent diastereoselectivity (43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 10
1 and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively), albeit a small reduction of enantioselectivity.
1, respectively), albeit a small reduction of enantioselectivity.
To explain the observed absolute stereochemical outcome a bifunctional mechanism similar to those previously proposed for the squaramide/thiourea-catalyzed oxa-Michael reaction of enones may be invoked.25
Conclusions
In summary, a sequential Wittig olefination followed by an enantioselective intramolecular oxa-Michael reaction of ortho-boronic acid containing chalcones have been developed using chiral bifunctional organocatalysts. This process provides the very first and promising approach for the synthesis of benzoxaboroles with excellent enantioselectivities and with a broad substrate scope. The resulting products were converted to the corresponding chiral β-hydroxy ketones without affecting the enantioselectivity.Acknowledgements
This work has been funded by IISER Bhopal. GH and SM thank CSIR and UGC, New Delhi, India, respectively, for the doctoral fellowship. We are thankful to Dr Deepak Chopra, Associate Professor, IISER Bhopal for his useful suggestions. We are grateful to Mr Lalit M. Jha (IISER Bhopal) for X-ray crystallography.Notes and references
- (a) A. Adamczyk-Wozʼniak, K. M. Borys and A. Sporzynśki, Chem. Rev., 2015, 115, 5224 CrossRef PubMed; (b) C. T. Liu, J. W. Tomsho and S. J. Benkovic, Bioorg. Med. Chem., 2014, 22, 4462 CrossRef CAS PubMed; (c) D. C. McKinney, F. Zhou, C. J. Eyermann, A. D. Ferguson, D. B. Prince, J. Breen, R. A. Giacobbe, S. Lahiri and J. C. Verheijen, ACS Infect. Dis., 2015, 1, 310 CrossRef CAS PubMed; (d) V. M. Chellat, L. Raguž and R. Riedl, Angew. Chem., 2016, 128, 6710 ( Angew. Chem., Int. Ed. , 2016 , 55 , 6600 ) CrossRef; (e) S. G. Kurz, S. Hazra, C. R. Bethel, C. Romagnoli, E. Caselli, F. Prati, J. S. Blanchard and R. A. Bonomo, ACS Infect. Dis., 2015, 1, 234 CrossRef CAS PubMed.
- For drug delivery: (a) G. A. Ellis, M. J. Palte and R. T. Raines, J. Am. Chem. Soc., 2012, 134, 3631 CrossRef CAS PubMed; (b) A. Matsumoto, N. Sato, K. Kataoka and Y. Miyahara, J. Am. Chem. Soc., 2009, 131, 12022 CrossRef CAS PubMed; (c) A. Matsumoto, H. Cabral, N. Sato, K. Kataoka and Y. Miyahara, Angew. Chem., 2010, 122, 5626 ( Angew. Chem., Int. Ed. , 2010 , 49 , 5494 ) CrossRef; (d) Q. Peng, F. Chen, Z. Zhong and R. Zhuo, Chem. Commun., 2010, 46, 5888 RSC; (e) S. Biswas, K. Kinbara, T. Niwa, H. Taguchi, N. Ishii, S. Watanabe, K. Miyata, K. Kataoka and T. Aida, Nat. Chem., 2013, 5, 613 CrossRef CAS PubMed.
- S. J. Baker, J. W. Tomsho and S. J. Benkovic, Chem. Soc. Rev., 2011, 40, 4279 RSC.
- (a) M. Dowlut and D. G. Hall, J. Am. Chem. Soc., 2006, 128, 4226 CrossRef CAS PubMed; (b) M. Bérubé, M. Dowlut and D. G. Hall, J. Org. Chem., 2008, 73, 6471 CrossRef PubMed.
- (a) S. J. Baker, Y.-K. Zhang, T. Akama, A. Lau, H. Zhou, V. Hernandez, W. Mao, M. R. K. Alley, V. Sanders and J. J. Plattner, J. Med. Chem., 2006, 49, 4447 CrossRef CAS PubMed; (b) F. L. Rock, W. Mao, A. Yaremchuk, M. Tukalo, T. Crepin, H. Zhou, Y. K. Zhang, V. Hernandez, T. Akama, S. J. Baker, J. J. Plattner, L. Shapiro, S. A. Martinis, S. J. Benkovic, S. Cusack and M. R. K. Alley, Science, 2007, 316, 1759 CrossRef CAS PubMed; (c) E. Seiradake, W. Mao, V. Hernandez, S. J. Baker, J. J. Plattner, M. R. K. Alley and S. Cusack, J. Mol. Biol., 2009, 390, 196 CrossRef CAS PubMed.
- X. Li, S. M. Zhang, Y.-K. Zhang, Y. D. Liu, Z. Charles, Y. Zhou, J. J. Plattner, S. J. Baker, W. Bu, L. Liu, W. M. Kazmierski, M. S. Duan, R. M. Grimes, L. L. Wright, G. K. Smith, R. L. Jarvest, J. J. Ji, J. P. Cooper, M. D. Tallant, R. M. Crosby, K. Creech, J. Z. Ni, W. X. Zou and J. Wright, Bioorg. Med. Chem. Lett., 2011, 21, 2048 CrossRef CAS PubMed.
- Y. Xia, K. Cao, Y. Zhou, M. R. K. Alley, F. Rock, M. Mohan, M. Meewan, S. J. Baker, S. Lux, C. Z. Ding, G. F. Jia, M. Kully and J. Plattner, Bioorg. Med. Chem. Lett., 2011, 21, 2533 CrossRef CAS PubMed.
- R. T. Jacobs, B. Nare, S. A. Wring, M. D. Orr, D. Chen, J. M. Sligar, M. X. Jenks, R. A. Noe, T. S. Bowling, L. T. Mercer, C. Rewerts, E. Gaukel, J. Owens, R. Parham, R. Randolph, B. Beaudet, C. J. Bacchi, N. Yarlett, J. J. Plattner, Y. Freund, C. Ding, T. Akama, Y.-K. Zhang, R. Brun, M. Kaiser, I. Scandale and R. Don, PLoS Neglected Trop. Dis., 2011, 5, e1151 CAS.
- T. Akama, C. Virtucio, C. Dong, R. Kimura, Y.-K. Zhang, J. A. Nieman, R. Sharma, X. Lu, M. Sales, R. Singh, A. Wu, X.-Q. Fan, L. Liu, J. J. Plattner, K. Jarnagin and Y. R. Freund, Bioorg. Med. Chem. Lett., 2013, 23, 1680 CrossRef CAS PubMed.
- For antimalarial activity: Y.-K. Zhang, J. J. Plattner, E. E. Easom, R. T. Jacobs, D. Guo, V. Sanders, Y. R. Freund, B. Campo, P. J. Rosenthal, W. Bu, F.-J. Gamo, L. M. Sanz, M. Ge, L. Li, J. Ding and Y. Yang, J. Med. Chem., 2015, 58, 5344 CrossRef CAS PubMed.
- Press release, July 8, 2014; http://investor.anacor.com/.
- T. Akama, S. J. Baker, Y.-K. Zhang, V. Hernandez, H. Zhou, V. Sanders, Y. R. Freund, R. Kimura, K. R. Maples and J. J. Plattner, Bioorg. Med. Chem. Lett., 2009, 19, 2129 CrossRef CAS PubMed.
- Y. R. Freund, T. Akama, M. R. K. Alley, J. Antunes, C. Dong, K. Jarnagin, R. Kimura, J. A. Nieman, K. R. Maples, J. J. Plattner, F. Rock, R. Sharma, R. Singh, V. Sanders and Y. Zhou, FEBS Lett., 2012, 586, 3410 CrossRef CAS PubMed.
- R. T. Jacobs, J. J. Plattner, B. Nare, S. A. Wring, D. Chen, Y. Freund, E. G. Gaukel, M. D. Orr, J. B. Perales, M. Jenks, R. A. Noe, J. M. Sligar, Y.-K. Zhang, C. J. Bacchi, N. Yarlett and R. Don, Future Med. Chem., 2011, 3, 1259 CrossRef CAS PubMed.
- (a) M. E. Welsch, S. A. Snyder and B. R. Stockwell, Curr. Opin. Chem. Biol., 2010, 14, 347 CrossRef CAS PubMed; (b) S. Sene, J. McLane, N. Schaub, S. Bégu, H. Mutin, L. Ligon, R. J. Gilbert and D. Laurencin, J. Mater. Chem. B, 2016, 4, 257 RSC.
- (a) T. A. Houston, ChemBioChem, 2010, 11, 954 CrossRef CAS PubMed; (b) S. Burroughs and B. Wang, ChemBioChem, 2010, 11, 2245 CrossRef CAS PubMed; (c) H. Kim, Y. J. Kang, S. Kang and K. T. Kim, J. Am. Chem. Soc., 2012, 134, 4030 CrossRef CAS PubMed; (d) Y. Kotsuchibashi, R. V. C. Agustin, J.-Y. Lu, D. G. Hall and R. Narain, ACS Macro Lett., 2013, 2, 260 CrossRef CAS; (e) Y. Zhang, W. Ma, D. Li, M. Yu, J. Guo and C. Wang, Small, 2013, 10, 1379 CrossRef PubMed; (f) D. Dechtrirat, N. Gajovic-Eichelmann, F. Wojcik, L. Hartmann, F. F. Bier and F. W. Scheller, Biosens. Bioelectron., 2014, 58, 1 CrossRef CAS PubMed.
- (a) P. Rodriguez-Cuamatzi, G. Vargas-Diaz and H. Höpfl, Angew. Chem., 2004, 116, 3103 ( Angew. Chem., Int. Ed. , 2004 , 43 , 3041 ) CrossRef; (b) P. Rogowska, M. K. Cyrański, A. Sporzyński and A. Ciesielski, Tetrahedron Lett., 2006, 47, 1389 CrossRef CAS; (c) A. Adamczyk-Woźniak, M. K. Cyrański, M. Jakubczyk, P. Klimentowska, A. Koll, J. Kołodziejczak, G. Pojmaj, A. Żubrowska, G. Z. Żukowska and A. Sporzyński, J. Phys. Chem. A, 2010, 114, 324 CrossRef PubMed.
- S. Sene, S. Bégu, C. Gervais, G. Renaudin, A. Mesbah, M. E. Smith, P. H. Mutin, A. van der Lee, J.-M. Nedelec, C. Bonhomme and D. Laurencin, Chem. Mater., 2015, 27, 1242 CrossRef CAS.
- A. Adamczyk-Woźniak, M. K. Cyrański, A. Żubrowska and A. Sporzyński, J. Organomet. Chem., 2009, 694, 3533 CrossRef.
- For the nucleophilic oxa-addition of organoboronic acids: (a) K. Oshima and Y. Aoyama, J. Am. Chem. Soc., 1999, 121, 2315 CrossRef CAS; (b) J. R. Falck, M. Bondlela, S. K. Venkataraman and D. Srinivas, J. Org. Chem., 2001, 66, 7148 CrossRef CAS PubMed; (c) D. R. Li, A. Murugan and J. R. Falck, J. Am. Chem. Soc., 2008, 130, 46 CrossRef CAS PubMed.
- For the synthesis of chiral β-hydroxy carbonyls: (a) R. Mahrwald, Chem. Rev., 1999, 99, 1095 CrossRef CAS PubMed; (b) B. Schetter and R. Mahrwald, Angew. Chem., Int. Ed., 2006, 45, 7506 CrossRef CAS PubMed; (c) D. A. Evans, S. A. Siska and V. J. Cee, Angew. Chem., 2003, 115, 1803 ( Angew. Chem., Int. Ed. , 2003 , 42 , 1761 ) CrossRef.
- For the use of chiral benzylic alcohol containing phenols: (a) I. Cho, L. Meimetis and R. Britton, Org. Lett., 2009, 11, 1903 CrossRef CAS PubMed; (b) I. Cho, L. Meimetis, L. Belding, M. J. Katz, T. Dudding and R. Britton, Beilstein J. Org. Chem., 2011, 7, 1315 CrossRef CAS PubMed; (c) A. Kišić, M. Stephan and B. Mohara, Adv. Synth. Catal., 2014, 356, 3193 CrossRef.
- For the synthesis of chiral benzylic alcohol: (a) T. Kitanosono and S. Kobayashi, Asian J. Org. Chem., 2013, 2, 961 CrossRef CAS; (b) M. Sai and H. Yamamoto, J. Am. Chem. Soc., 2015, 137, 7091 CrossRef CAS PubMed; (c) S. Kobayashi, P. Xu, T. Endo, M. Ueno and T. Kitanosono, Angew. Chem., 2012, 124, 12935 ( Angew. Chem., Int. Ed. , 2012 , 51 , 12763 ) CrossRef; (d) L. Zhu, T. Kitanosono, P. Xu and S. Kobayashi, Chem. Commun., 2015, 51, 11685 RSC; (e) S. G. Davies, E. W. Hume, P. M. Roberts and J. E. Thomson, Tetrahedron, 2010, 66, 8076 CrossRef CAS; (f) D. B. Ramachary and R. Sakthidevi, Chem.–Eur. J., 2009, 15, 4516 CrossRef CAS PubMed; (g) M. A. Ariger and E. M. Carreira, Org. Lett., 2012, 14, 4522 CrossRef CAS PubMed.
- For the synthesis of chiral 1,3-diol: (a) S. E. Bode, M. Wolberg and M. Müller, Synthesis, 2006, 4, 557 Search PubMed; (b) K. Baer, M. Kraußer, E. Burda, W. Hummel, A. Berkessel and H. Gröger, Ang ew. Chem., 2009, 121, 9519 ( Angew. Chem., Int. Ed. , 2009 , 48 , 9355 ) CrossRef; (c) P. Gogoi, T. Kotipalli, K. Indukuri, S. Bondalapati, P. Saha and A. K. Saikia, Tetrahedron Lett., 2012, 53, 2726 CrossRef CAS; (d) Y. Shimoda, S. Kotani, M. Sugiura and M. Nakajima, Chem.–Eur. J., 2011, 17, 7992 CrossRef CAS PubMed.
- Asymmetric oxa-Michael addition via push/pull-type bifunctional organocatalysis: (a) J. P. Malerich, K. Hagihara and V. H. Rawal, J. Am. Chem. Soc., 2008, 130, 14416 CrossRef CAS PubMed; (b) K. Asano and S. Matsubara, J. Am. Chem. Soc., 2011, 133, 16711 CrossRef CAS PubMed; (c) Y. Kobayashi, Y. Taniguchi, N. Hayama, T. Inokuma and Y. Takemoto, Angew. Chem., 2013, 125, 11320 ( Angew. Chem., Int. Ed. , 2013 , 52 , 11114 ) CrossRef; (d) R. Miyaji, K. Asano and S. Matsubara, Org. Biomol. Chem., 2014, 12, 119 RSC; (e) B. Ravindra, B. G. Das and P. Ghorai, Org. Lett., 2014, 16, 5580 CrossRef CAS PubMed; (f) B. Ravindra, S. Maity, B. G. Das and P. Ghorai, J. Org. Chem., 2015, 80, 7008 CrossRef CAS PubMed; (g) S. Maity, B. Parhi and P. Ghorai, Angew. Chem., 2016, 128, 7854 ( Angew. Chem., Int. Ed. , 2016 , 55 , 7723 ) CrossRef; (h) N. Molleti and V. K. Singh, Org. Biomol. Chem., 2015, 13, 5243 RSC; (i) S. Agrawal, N. Molleti and V. K. Singh, Chem. Commun., 2015, 51, 9793 RSC; (j) S. Kayal and S. Mukherjee, Org. Lett., 2015, 17, 5508 CrossRef CAS PubMed; (k) R. R. Reddy, S. S. Gudup and P. Ghorai, Angew. Chem., 2016, 128, 15339 ( Angew. Chem., Int. Ed. , 2016 , 55 , 15115 ) CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1487136. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6sc04522g |
| This journal is © The Royal Society of Chemistry 2017 |