Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Molecular catalysis at polarized interfaces created by ferroelectric BaTiO3†
Eugene S.
Beh‡
 a,
Sergey A.
Basun
bc,
Xiaofeng
Feng§
a,
Ighodalo U.
Idehenre
bd,
Dean R.
Evans
b and
Matthew W.
Kanan
a,
Sergey A.
Basun
bc,
Xiaofeng
Feng§
a,
Ighodalo U.
Idehenre
bd,
Dean R.
Evans
b and
Matthew W.
Kanan
 *a
*a
aDepartment of Chemistry, Stanford University, 337 Campus Drive, Stanford, California 94305, USA. E-mail: mkanan@stanford.edu
bAir Force Research Laboratory, Materials and Manufacturing Directorate, Wright-Patterson Air Force Base, Ohio 45433, USA
cAzimuth Corporation, 4134 Linden Avenue, Suite 300, Dayton, Ohio 45432, USA
dUniversity of Dayton, Department of ECE and Electro-Optics Program, Dayton, Ohio 45469, USA
First published on 6th February 2017
Abstract
The local environment at polarized solid–liquid interfaces provides a unique medium for chemical reactions that could be exploited to control the selectivity of non-faradaic reactions. Polarized interfaces are commonly prepared by applying a voltage to an electrode in an electrolyte solution, but it is challenging to achieve high surface charge densities while suppressing faradaic reactions. Ferroelectric materials have permanent surface charge densities that arise from the dipole moments of ferroelectric domains and can be used to create polarized solid–liquid interfaces without applying a voltage. We studied the effects of ferroelectric oxides on the selectivity of a Rh porphyrin-catalyzed carbene rearrangement. The addition of ferroelectric BaTiO3 nanoparticles to the reaction solution changed the product ratio in the same direction and by a similar magnitude as performing the reaction at an electrode–electrolyte interface polarized by a voltage. The results demonstrate that colloidal suspensions of BaTiO3 nanoparticles act as a dispersible polarized interface that can influence the selectivity of non-faradaic reactions.
Introduction
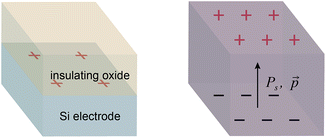
The double layer region at a polarized solid–liquid interface has electrostatic and chemical properties that differ significantly from a bulk solution.1,2 These properties affect the rates of electrochemical and photocatalytic reactions, which entail charge transfer across the interface.3–6 We previously investigated the effects of a polarized electrode–electrolyte interface on the selectivity of catalytic non-faradaic reactions.7,8 These experiments employed a custom reaction vessel—the “parallel plate cell”—whose walls consist of Si electrodes coated with thin layers of an insulating oxide. Polarization of the oxide–electrolyte interface by applying a voltage across the Si electrodes was found to change the selectivity of reactions confined to the interface, including an epoxide rearrangement catalyzed by Al2O3 and an intramolecular carbene rearrangement catalysed by Rh porphyrins. The selectivity changes were dependent on the charge density at the oxide–electrolyte interface, which was determined from the applied voltage and the interfacial capacitance.The study of non-faradaic reactions at voltage-polarized interfaces has limitations. The need to block faradaic processes necessitates fabricating electrodes with thin insulating layers and dielectric breakdown of these layers limits the maximum interfacial charge density that can be achieved. To address these issues, we sought to replace an electrode polarized by a voltage with a material that permanently has a large surface charge density. Inorganic ferroelectrics are crystalline materials composed of domains that have permanent dipole moments below a characteristic Curie temperature.9 The surfaces of ferroelectric particles have regions of high charge density where the dipole moment of the domain at the surface has a normal component, leading to the formation of double layers.10 Previous studies of chemical reactivity at ferroelectric surfaces have utilized the polarization to localize and enhance photoredox reactions.11–14 We hypothesized that ferroelectric materials would provide polarized solid–liquid interfaces that affect the selectivity of non-faradaic reactions in the same manner as an electrode–electrolyte interface polarized by a voltage (Fig. 1).
Barium titanate (BaTiO3) is a readily accessible ferroelectric metal oxide with a Curie temperature of 120 °C.15 The polarization of domains in BaTiO3 arises from a small displacement of the Ti4+ cation from the centre of the tetragonal unit cell. The polarization of BaTiO3 is stable at room temperature.16–18
The charge density on a BaTiO3 surface that is normal to the dipole moment of a polarized domain can be estimated from previous measurements of the spontaneous polarization (Ps) of BaTiO3 nanoparticles. The Ps of a BaTiO3 nanoparticle composed of a single ferroelectric domain corresponds to the charge densities on the opposing surfaces of the domain that are normal to the dipole moment (Fig. 1). Ball milling of bulk BaTiO3 powder produces nanoparticles that are composed of a small number of ferroelectric domains. We have measured Ps values of 20–120 μC cm−2 for ball milled BaTiO3 nanoparticles by performing triangular wave ac voltammetry on nanoparticle suspensions.19,20 (These values for Ps assume that each nanoparticle is a cube composed of a single ferroelectric domain; particle aggregation will lower the observed Ps).21,22 Notably, surface charge densities of 20–120 μC cm−2 are considerably larger than what is accessible in the parallel plate cell at voltages below the dielectric breakdown limit (at most ∼14 μC cm−2).8 Larger BaTiO3 particles that have not been subjected to ball milling have little or no measurable Ps because they are composed of many ferroelectric domains that are randomly oriented and therefore cancel each other out. Nevertheless, the average magnitude of surface charge density on large BaTiO3 particles is expected to be similar to BaTiO3 nanoparticles because the charge density at any point on the surface is dominated by the orientation of the closest domain.
Results and discussion
A Rh porphyrin-catalyzed intramolecular carbene reaction was used as a model reaction. Diazoketone 1 reacts at ambient temperature with Rh porphyrin catalysts to form a mixture of products 2 and 3 (Scheme 1). In the absence of a polarized interface, the reaction favours 2 over 3 by an approximately 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. We previously studied this same reaction in the parallel plate cell using an applied voltage to create polarized electrode–electrolyte interfaces.8 Rh porphyrin catalysts were localized to these interfaces using either covalent attachment or spontaneous physisorption. With Al2O3-coated electrodes or alkylphosphonate-coated electrodes, the 2
1 ratio. We previously studied this same reaction in the parallel plate cell using an applied voltage to create polarized electrode–electrolyte interfaces.8 Rh porphyrin catalysts were localized to these interfaces using either covalent attachment or spontaneous physisorption. With Al2O3-coated electrodes or alkylphosphonate-coated electrodes, the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio decreased as the magnitude of the voltage was increased, reaching 2
3 ratio decreased as the magnitude of the voltage was increased, reaching 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at the maximum voltage that could be applied before dielectric breakdown. We attributed this change to an effect of the local solvation and electrostatic environment at the polarized interface on the competing activation barriers leading to 2 and 3.
1 at the maximum voltage that could be applied before dielectric breakdown. We attributed this change to an effect of the local solvation and electrostatic environment at the polarized interface on the competing activation barriers leading to 2 and 3.
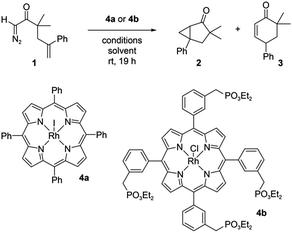 | ||
| Scheme 1 The reaction of diazo compound 1 catalyzed by Rh porphyrins 4a or 4b produces products 2 and 3. | ||
To see whether the effect of an interface polarized by a voltage could be recapitulated by a dispersed, permanently polarized interface, we compared the selectivity of the Rh porphyrin-catalyzed reaction of 1 to products 2 and 3 in the presence or absence of various ferroelectric (BaTiO3, PbTiO3, and LiNbO3) and non-ferroelectric (CaTiO3, SrTiO3, and TiO2) nanoparticles. The nanoparticle samples were produced as colloidal suspensions by ball milling the corresponding bulk powders in the presence of heptane and oleic acid.19 Transmission electron microscopy (TEM) analysis indicated average particle sizes in the range of 7–10 nm for all the milled materials except for LiNbO3 (50 nm; Fig. S1–S12†). The average Ps for the nanoparticles was calculated from the ac displacement current of nanoparticle suspensions subjected to a periodic ac electric field within a narrow cell.20 A Ps of 20 μC cm−2 was measured for the BaTiO3 nanoparticles, indicating substantial alignment of the ferroelectric domains. As expected for non-ferroelectric materials, the Ps values calculated from the ac displacement measurements for CaTiO3, SrTiO3, and TiO2 were all at least two orders of magnitude smaller than BaTiO3 (Fig. S13†).
Reactions were performed by diluting freshly ball milled 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w/w heptane/oleic acid/nanoparticle suspensions to the appropriate nanoparticle concentration with a solution of Rh porphyrin catalyst 4a in CH2Cl2, followed by a solution of 1 in CH2Cl2. The reactions were allowed to proceed for 19 h and then analysed using NMR and HPLC. 2 and 3 were the only major products observed (see ESI main text and Fig. S14†).
1 w/w/w heptane/oleic acid/nanoparticle suspensions to the appropriate nanoparticle concentration with a solution of Rh porphyrin catalyst 4a in CH2Cl2, followed by a solution of 1 in CH2Cl2. The reactions were allowed to proceed for 19 h and then analysed using NMR and HPLC. 2 and 3 were the only major products observed (see ESI main text and Fig. S14†).
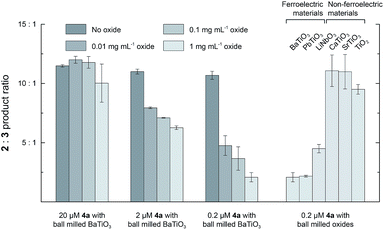
Fig. 2 shows the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 product ratio for reactions performed with different concentrations of BaTiO3 nanoparticles, 1, and 4a in CH2Cl2. In the absence of nanoparticles, the 2
3 product ratio for reactions performed with different concentrations of BaTiO3 nanoparticles, 1, and 4a in CH2Cl2. In the absence of nanoparticles, the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio was ∼11
3 ratio was ∼11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in all cases. The largest changes were observed with 0.2 mM 1 and 0.2 μM 4a, to which the addition of increasing concentrations of BaTiO3 nanoparticles up to 1 mg mL−1 resulted in a 5.1-fold decrease of the product ratio from 10.7
1 in all cases. The largest changes were observed with 0.2 mM 1 and 0.2 μM 4a, to which the addition of increasing concentrations of BaTiO3 nanoparticles up to 1 mg mL−1 resulted in a 5.1-fold decrease of the product ratio from 10.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 to a minimum of 2.1
1.0 to a minimum of 2.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0. These changes are in the same direction as what was observed with externally polarized electrode–electrolyte interfaces in the parallel plate cell.8 Smaller changes were observed with higher ratios of catalyst to nanoparticles, most likely because of incomplete localization of the catalyst to the ferroelectric–solution interface (see below).
1.0. These changes are in the same direction as what was observed with externally polarized electrode–electrolyte interfaces in the parallel plate cell.8 Smaller changes were observed with higher ratios of catalyst to nanoparticles, most likely because of incomplete localization of the catalyst to the ferroelectric–solution interface (see below).
Under the same conditions with 1 mg mL−1 of nanoparticles, similar changes in the product ratio were also seen with other ferroelectric materials like PbTiO3 (2.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0) and LiNbO3 (4.5
1.0) and LiNbO3 (4.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0). In contrast, very little effect on the product ratio was seen with chemically similar non-ferroelectric nanoparticles such as CaTiO3 (11.1
1.0). In contrast, very little effect on the product ratio was seen with chemically similar non-ferroelectric nanoparticles such as CaTiO3 (11.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0), SrTiO3 (11.0
1.0), SrTiO3 (11.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0), or TiO2 (9.5
1.0), or TiO2 (9.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0).
1.0).
Control experiments in which 1 was combined with BaTiO3 nanoparticles in the absence of catalyst 4a showed no conversion, which shows that the BaTiO3 is not itself a catalyst for the diazo decomposition. In addition, reactions performed in the presence of heptane and oleic acid without any nanoparticles gave a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio of 11.3
3 ratio of 11.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0, indicating that these additives themselves do not impact the product ratio.
1.0, indicating that these additives themselves do not impact the product ratio.
The ability of the polarized BaTiO3–solution interface to affect the selectivity of 4a requires that a substantial portion of the catalyst partitions at or near the BaTiO3 surface during the reaction. The concentration dependence of the BaTiO3 effect in the results above is consistent with an interface-dependent effect: as the ratio of BaTiO3 surface area to 4a is increased, a larger change in product ratio is observed. Notably, the surface coverage of long aliphatic capping ligands at colloidal nanoparticles is much lower than one monolayer,23 which may be critical to allow the reactant and catalyst molecules to localize near the surface.
To further test this model, a reaction was performed in the presence of an excess of un-metalated 5,10,15,20-tetraphenylporphyrin (TPP), which could compete with 4a for localizing to the surface. When 100 μM TPP was added to a reaction with 2 mM 1, 2 μM 4a and 1 mg mL−1 of BaTiO3 nanoparticles, the product ratio was 10.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 compared to 6.3
1.0 compared to 6.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 when TPP was omitted and 11.0
1.0 when TPP was omitted and 11.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 when both TPP and BaTiO3 were absent. This result shows that the BaTiO3 effect is lost in the presence of a species that can block the surface localization of the catalyst. Furthermore, when 2 μM 4a was first stirred with 1 mg mL−1 of BaTiO3 nanoparticles for 24 h before the introduction of 100 μM TPP and 2 mM 1, the ratio was 10.2
1.0 when both TPP and BaTiO3 were absent. This result shows that the BaTiO3 effect is lost in the presence of a species that can block the surface localization of the catalyst. Furthermore, when 2 μM 4a was first stirred with 1 mg mL−1 of BaTiO3 nanoparticles for 24 h before the introduction of 100 μM TPP and 2 mM 1, the ratio was 10.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 after a further 19 h of reaction time. This experiment demonstrates that simply exposing the catalyst to BaTiO3 nanoparticles does not result in an irreversible change to a species with different selectivity. Notably, if the anion of 4a is replaced by chloride, a smaller change in product ratio from 8.1
1.0 after a further 19 h of reaction time. This experiment demonstrates that simply exposing the catalyst to BaTiO3 nanoparticles does not result in an irreversible change to a species with different selectivity. Notably, if the anion of 4a is replaced by chloride, a smaller change in product ratio from 8.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 (no BaTiO3) to 5.1
1.0 (no BaTiO3) to 5.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 (1 mg mL−1 BaTiO3) is obtained, which may reflect reduced localization of the Cl-bound Rh porphyrin at the BaTiO3 surface.
1.0 (1 mg mL−1 BaTiO3) is obtained, which may reflect reduced localization of the Cl-bound Rh porphyrin at the BaTiO3 surface.
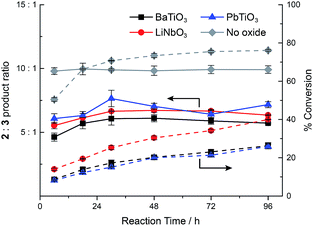
In order to understand the timescale on which ferroelectric nanoparticles affect the catalyst, the reaction kinetics were studied in the presence or absence of the various ball milled ferroelectric oxides (Fig. 3). Solutions of 2 mM 1, 2 μM 4a, and 1 mg mL−1 of ball milled oxide (BaTiO3, PbTiO3, or LiNbO3) in approximately 10 mL of CH2Cl2 were prepared, as well as an identical reaction with no oxide. Ball milled oxides were used within 60 minutes after ball milling had concluded. Over the course of 96 h, aliquots from each of the reaction mixtures were quenched with acetonitrile and immediately analysed by HPLC to determine the conversion and ratio of products 2 and 3. In each case, the product ratio stayed roughly constant throughout the course of 96 h, which suggests that catalyst molecules localize to the polarized ferroelectric surface within, at most, several hours. The slight increase in product ratio after the 6 h mark is attributed to some aggregation of the ball milled nanoparticles over the course of several hours, which resulted in a lowered surface area that was accessible to the catalyst.
The effect of BaTiO3 nanoparticles was also examined in solvents other than CH2Cl2. Reactions were performed with 2 mM 1 and 2 μM 4a in PhCF3, THF, EtOAc, and PhCH3. In the absence of BaTiO3 nanoparticles, the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio ranged from 9.4
3 ratio ranged from 9.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 to 17.9
1.0 to 17.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0, demonstrating that the strong preference for cyclopropanation is maintained across diverse solvents. The addition of 1 mg mL−1 BaTiO3 nanoparticles caused a reduction in the 2
1.0, demonstrating that the strong preference for cyclopropanation is maintained across diverse solvents. The addition of 1 mg mL−1 BaTiO3 nanoparticles caused a reduction in the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio in all cases, with a substantial variation in the magnitude of the effect (Fig. 4). The largest effect was a 6.6-fold reduction from 17.9
3 ratio in all cases, with a substantial variation in the magnitude of the effect (Fig. 4). The largest effect was a 6.6-fold reduction from 17.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 to 2.7
1.0 to 2.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 observed in THF. Solvents more polar than PhCF3 could not be used because they caused immediate flocculation of the nanoparticles.
1.0 observed in THF. Solvents more polar than PhCF3 could not be used because they caused immediate flocculation of the nanoparticles.
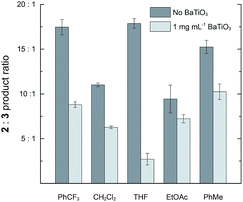 | ||
Fig. 4 The effect of adding 1 mg mL−1 ball milled BaTiO3 nanoparticles on the product ratio 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 in a variety of solvents. Reactions were run with 2 mM 1 and 2 μM 4a at room temperature for 19 h. 3 in a variety of solvents. Reactions were run with 2 mM 1 and 2 μM 4a at room temperature for 19 h. | ||
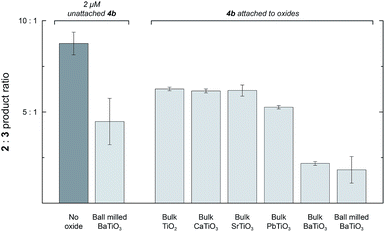
Unmilled BaTiO3 particles have no measurable Ps because they are composed of randomly oriented domains (see above), but they still have regions of high surface charge densities. Adding 1 mg mL−1 of unmilled BaTiO3 particles to a reaction with 2 mM 1 and 2 μM 4a caused only a 1.2-fold reduction in the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio, but the surface area of these relatively large particles (∼900 nm) is too low for effective physisorption of 4a in solution. To test for an interfacial polarization effect on selectivity with unmilled BaTiO3, we prepared Rh porphyrin 4b with four phosphonate groups to enable covalent surface attachment. The results of reactions performed with 4b are summarized in Fig. 5. When used as a homogeneous catalyst, 2 μM 4b reacted with 2 mM 1 in CH2Cl2 to give a 2
3 ratio, but the surface area of these relatively large particles (∼900 nm) is too low for effective physisorption of 4a in solution. To test for an interfacial polarization effect on selectivity with unmilled BaTiO3, we prepared Rh porphyrin 4b with four phosphonate groups to enable covalent surface attachment. The results of reactions performed with 4b are summarized in Fig. 5. When used as a homogeneous catalyst, 2 μM 4b reacted with 2 mM 1 in CH2Cl2 to give a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 product ratio of 8.8
3 product ratio of 8.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0. As with catalyst 4a, the introduction of 1 mg mL−1 ball milled BaTiO3 resulted in a 2.0-fold decrease of the product ratio to 4.5
1.0. As with catalyst 4a, the introduction of 1 mg mL−1 ball milled BaTiO3 resulted in a 2.0-fold decrease of the product ratio to 4.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0. Next, 4b was attached to various ferroelectric and non-ferroelectric oxides studied. Reactions performed with 2 mM 1 and 1 mg mL−1 unmilled BaTiO3 functionalized with 4b gave a 2
1.0. Next, 4b was attached to various ferroelectric and non-ferroelectric oxides studied. Reactions performed with 2 mM 1 and 1 mg mL−1 unmilled BaTiO3 functionalized with 4b gave a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 product ratio of 2.2
3 product ratio of 2.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0, corresponding to a 4.0-fold reduction. The 2
1.0, corresponding to a 4.0-fold reduction. The 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio was further reduced to 1.8
3 ratio was further reduced to 1.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 if the BaTiO3 functionalized with 4b was first subjected to ball milling and then used at the same oxide concentration.
1.0 if the BaTiO3 functionalized with 4b was first subjected to ball milling and then used at the same oxide concentration.
In contrast, when 4b was attached to the non-ferroelectric oxides TiO2, CaTiO3, and SrTiO3, a small (1.4-fold) effect on the reaction to approximately 6.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was observed. Measurements of zeta potentials for TiO2 particles in hexane in the presence of surfactant indicate surface charge densities on the order of −0.1 μC cm−2, which is much lower than the surface charge density for ferroelectric nanoparticles.24 The small change in selectivity observed upon attachment of 4b to nonferroelectric nanoparticles may arise from the interaction of the catalyst with the oxide surface or a subtle conformational biasing for attached catalysts.25,26 When 4b was attached to ferroelectric PbTiO3, the ratio was 5.3
1 was observed. Measurements of zeta potentials for TiO2 particles in hexane in the presence of surfactant indicate surface charge densities on the order of −0.1 μC cm−2, which is much lower than the surface charge density for ferroelectric nanoparticles.24 The small change in selectivity observed upon attachment of 4b to nonferroelectric nanoparticles may arise from the interaction of the catalyst with the oxide surface or a subtle conformational biasing for attached catalysts.25,26 When 4b was attached to ferroelectric PbTiO3, the ratio was 5.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0, which was only slightly lower than the other non-ferroelectric oxides. This result may arise from decreased robustness of the phosphonate linkage to the PbTiO3 surface and increased leaching of catalyst molecules into the bulk solution. Nevertheless, attachment to ferroelectric oxides, especially BaTiO3, effected a substantially larger change in selectivity than attachment to other non-ferroelectric oxides.
1.0, which was only slightly lower than the other non-ferroelectric oxides. This result may arise from decreased robustness of the phosphonate linkage to the PbTiO3 surface and increased leaching of catalyst molecules into the bulk solution. Nevertheless, attachment to ferroelectric oxides, especially BaTiO3, effected a substantially larger change in selectivity than attachment to other non-ferroelectric oxides.
The results above demonstrate that the polarized interface between a ferroelectric oxide and an organic solvent can change the selectivity of catalytic reactions in the same way as an electrically polarized electrode–electrolyte interface. By attaching the catalyst to BaTiO3 surfaces or adding BaTiO3 nanoparticles to the reaction solution, the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio is reduced by up to a factor of 4.9. Similar effects are seen with ball milled ferroelectric PbTiO3 and LiNbO3; chemically similar but non-ferroelectric CaTiO3, SrTiO3, or TiO2 nanoparticles had essentially no effect, which indicates that the selectivity change requires the permanently polarized domains in BaTiO3.
3 ratio is reduced by up to a factor of 4.9. Similar effects are seen with ball milled ferroelectric PbTiO3 and LiNbO3; chemically similar but non-ferroelectric CaTiO3, SrTiO3, or TiO2 nanoparticles had essentially no effect, which indicates that the selectivity change requires the permanently polarized domains in BaTiO3.
Conclusions
By using ball milled ferroelectric nanoparticles such as BaTiO3, a polarized interface can now be introduced to a reaction medium without the need for physical electrodes. The use of ferroelectric nanoparticles provides an accessible way to investigate and exploit the effects of interfacial polarization on catalysis.Acknowledgements
We thank the Air Force Office of Scientific Research (FA9550-14-1-0132) for support of this research.Notes and references
- J. O. M. Bockris, A. K. N. Reddy and M. Gamboa-Aldeco, in Modern Electrochemistry Volume 2, Springer, US, 1970, pp. 623–843 Search PubMed.
- R. Parsons, Chem. Rev., 1990, 90, 813–826 CrossRef CAS.
- A. J. Bard and L. R. Faulkner, Electrochemical Methods: Fundamentals and Applications, John Wiley & Sons, New York, 2001 Search PubMed.
- E. Gileadi, Electrode Kinetics for Chemists, Engineers, and Materials Scientists, Wiley-VCH, New York, 1993 Search PubMed.
- E. J. F. Dickinson and R. G. Compton, J. Electroanal. Chem., 2011, 661, 198–212 CrossRef CAS.
- S. Chen, Y. Liu and J. Chen, Chem. Soc. Rev., 2014, 43, 5372–5386 RSC.
- C. F. Gorin, E. S. Beh and M. W. Kanan, J. Am. Chem. Soc., 2012, 134, 186–189 CrossRef CAS PubMed.
- C. F. Gorin, E. S. Beh, Q. M. Bui, G. R. Dick and M. W. Kanan, J. Am. Chem. Soc., 2013, 135, 11257–11265 CrossRef CAS PubMed.
- M. J. Polking, M. G. Han, A. Yourdkhani, V. Petkov, C. F. Kisielowski, V. V. Volkov, Y. Zhu, G. Caruntu, A. P. Alivisatos and R. Ramesh, Nat. Mater., 2012, 11, 700–709 CrossRef CAS PubMed.
- R. J. Ferris, S. Lin, M. Therezien, B. B. Yellen and S. Zauscher, ACS Appl. Mater. Interfaces, 2013, 5, 2610–2617 CAS.
- S. V. Kalinin, D. A. Bonnell, T. Alvarez, X. Lei, Z. Hu, J. H. Ferris, Q. Zhang and S. Dunn, Nano Lett., 2002, 2, 589–593 CrossRef CAS.
- J. L. Giocondi and G. S. Rohrer, J. Phys. Chem. B, 2001, 105, 8275–8277 CrossRef CAS.
- M. Stock and S. Dunn, J. Phys. Chem. C, 2012, 116, 20854–20859 CAS.
- Y. Cui, J. Briscoe and S. Dunn, Chem. Mater., 2013, 25, 4215–4223 CrossRef CAS.
- S. Roberts, Phys. Rev., 1947, 71, 890–905 CrossRef CAS.
- G. H. Kwei, A. C. Lawson, S. J. L. Billinge and S.-W. Cheong, J. Phys. Chem., 1993, 97, 2368–2377 CrossRef CAS.
- M. B. Smith, K. Page, T. Siegrist, P. L. Redmond, E. C. Walter, R. Seshadri, L. E. Brus and M. L. Steigerwald, J. Am. Chem. Soc., 2008, 130, 6955–6963 CrossRef CAS PubMed.
- K. Page, T. Proffen, M. Niederberger and R. Seshadri, Chem. Mater., 2010, 22, 4386–4391 CrossRef CAS.
- H. Atkuri, G. Cook, D. R. Evans, C. I. Cheon, A. Glushchenko, V. Reshetnyak, Y. Reznikov, J. West and K. Zhang, J. Opt. A: Pure Appl. Opt., 2009, 11, 024006 CrossRef.
- S. A. Basun, G. Cook, V. Y. Reshetnyak, A. V. Glushchenko and D. R. Evans, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 84, 024105 CrossRef.
- D. R. Evans, S. A. Basun, G. Cook, I. P. Pinkevych and V. Y. Reshetnyak, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 84, 174111 CrossRef.
- P. Lagos, R. Hermans, N. Velasco, G. Tarrach, F. Schlaphof, C. Loppacher and L. M. Eng, Surf. Sci., 2003, 532, 493–500 CrossRef.
- C. N. Valdez, A. M. Schimpf, D. R. Gamelin and J. M. Mayer, ACS Nano, 2014, 8, 9463–9470 CrossRef CAS PubMed.
- M. Kosmulski, P. Próchniak and J. B. Rosenholm, Colloids Surf., A, 2009, 348, 298–300 CrossRef CAS.
- C. M. Queffélec, M. Petit, P. Janvier, D. A. Knight and B. Bujoli, Chem. Rev., 2012, 112, 3777–3807 CrossRef PubMed.
- J. M. Notestein and A. Katz, Chem.–Eur. J., 2006, 12, 3954–3965 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6sc05032h |
| ‡ Current address: Department of Chemistry and Chemical Biology, Harvard University, 12 Oxford Street, Naito 118, Cambridge, Massachusetts 02138, USA. |
| § Current address: Department of Physics, University of Central Florida, Orlando, Florida 32816, USA. |
| This journal is © The Royal Society of Chemistry 2017 |