Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Superconductivity and magnetism in iron sulfides intercalated by metal hydroxides†
Xiuquan
Zhou
 ab,
Christopher
Eckberg
bc,
Brandon
Wilfong
ab,
Sz-Chian
Liou
d,
Hector K.
Vivanco
a,
Johnpierre
Paglione
bc and
Efrain E.
Rodriguez
*ac
ab,
Christopher
Eckberg
bc,
Brandon
Wilfong
ab,
Sz-Chian
Liou
d,
Hector K.
Vivanco
a,
Johnpierre
Paglione
bc and
Efrain E.
Rodriguez
*ac
aDepartment of Chemistry and Biochemistry, University of Maryland, College Park, MD 20742, USA. E-mail: efrain@umd.edu
bDepartment of Physics, University of Maryland, College Park, MD 20742, USA
cCenter for Nanophysics and Advanced Materials, University of Maryland, College Park, MD 20742, USA
dAIM Lab, Maryland NanoCenter, University of Maryland, College Park, MD 20742, USA
First published on 13th March 2017
Abstract
Inspired by naturally occurring sulfide minerals, we present a new family of iron-based superconductors. A metastable form of FeS known as the mineral mackinawite forms two-dimensional sheets that can be readily intercalated by various cationic guest species. Under hydrothermal conditions using alkali metal hydroxides, we prepare three different cation and metal hydroxide-intercalated FeS phases including (Li1−xFexOH)FeS, [(Na1−xFex)(OH)2]FeS, and KxFe2−yS2. Upon successful intercalation of the FeS layer, the superconducting critical temperature Tc of mackinawite is enhanced from 5 K to 8 K for the (Li1−xFexOH)δ+ intercalate. Layered heterostructures of [(Na1−xFex)(OH)2]FeS resemble the natural mineral tochilinite, which contains an iron square lattice interleaved with a hexagonal hydroxide lattice. Whilst heterostructured [(Na1−xFex)(OH)2]FeS displays long-range magnetic ordering near 15 K, KxFe2−yS2 displays short range antiferromagnetism.
Introduction
The chemistry of iron-based superconductors has been dominated by the arsenide,1–5 selenide,6–9 and telluride10–12 compounds since their discovery nearly a decade ago. Many high-temperature superconductors exhibit layered structures, and rich chemistry can be applied to modify their structures that may result in the increase of their critical temperatures (Tc).13,14 We demonstrate that iron sulfides prepared by hydrothermal routes provide a new series of superconductors that could further elucidate the structure–property relationships across closely related phases. Mainly, we isolate FeS layers to enhance their two-dimensional (2D) electronic character by inserting metal hydroxide spacers that also act as electron donating layers.The tetragonal form of FeS known as mackinawite is a metastable mineral recently shown to be superconducting with a Tc near 4 K.15,16 Mackinawite FeS adopts the anti-PbO structure where FeS4 tetrahedra edge-share to form 2D layers held by weak van der Waals interactions. Consequently, these layered chalcogenides are excellent hosts for intercalation chemistry.17 In the selenide case, the Tc can be increased from 8 K (ref. 6) to 42–44 K by intercalation of alkali metal in liquid ammonia18,19 or (Li1−xFexOH)δ+ under hydrothermal conditions.20,21 Therefore, our goal was to extend this type of chemistry to the sulfides.
We have found the intercalation chemistry of layered FeS to be quite versatile, and we illustrate in Fig. 1 the various guest–host phases that can be prepared via hydrothermal routes. Inspired by recent studies on the hydrothermally prepared 42 K superconductor, (Li1−xFexOH)FeSe,20–25 we applied similar intercalation chemistry for FeS using different alkali metal hydroxides. Herein, we report newfound superconductivity in the Li-intercalated FeS phases, and magnetic ordering in the Na-intercalated FeS phases. We find that the superconducting properties depend on preserving an iron square lattice and in electron doping the metallic FeS layer.
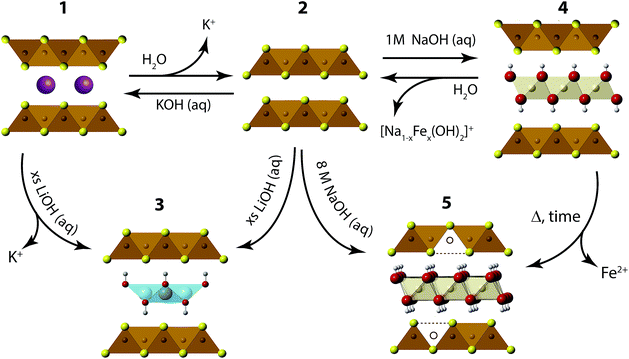 | ||
| Fig. 1 Synthetic scheme for the intercalation chemistry of FeS with metal hydroxides and K+ cations via hydrothermal preparations. | ||
Synthesis and characterization
For a typical preparation of (Li1−xFexOH)FeS via the route from 2 to 3 in Fig. 1, 5 mmol Fe powder, 8 mmol of Li2S (or thiourea/Na2S·9H2O), 1 mmol Sn metal plate and 72 mmol LiOH·H2O were mixed with 10 mL de-ionized (DI) water in a Teflon-lined stainless steel autoclave at 120–200 °C for 3 days. Mainly Li2S was used as the sulfur source to avoid possible contamination from other alkali cations such as sodium. Afterwards, the content in the autoclave was washed and centrifuged several times until the supernatant was clear. The remaining product was collected, vacuumed dried, and stored in a nitrogen-filled glove box.For (Li1−xFexOH)FeS prepared via the cation exchange route from 1 to 3 in Fig. 1, KxFe2−yS2 single crystals grown from high temperature reactions were mixed with 3 mmol Fe powder, 3 mmol of sulfur source (Li2S, thiourea or Na2S·9H2O), 1 mmol Sn metal plate and 72 mmol LiOH·H2O. The KxFe2−yS2 precursors and reagents were reacted under hydrothermal conditions at 120 °C for 1–3 days. For the growth of the KxFe2−yS2 single crystals, 1.2 g of FeS powder was mixed with 0.266 g of potassium metal to match the nominal composition of KFe2S2. The FeS/K mixtures were loaded in a quartz ampoule inside a nitrogen-filled glovebox, and the ampoules flame sealed under vacuum. In order to avoid oxidation of the samples from breaking of the ampoule due to potassium-induced corrosion of the quartz walls, the sample container was sealed in a larger ampoule. For crystal growth of KxFe2−ySe2, the mixture was heated to 1030 °C over 10 h and held at 1030 °C for 3 hours to form a homogeneous melt. Subsequently, the melt was slowly cooled at a rate of 6 °C per hour to 650 °C to allow crystal growth.
For the preparation of Na-intercalated phases, we combined 10 mmol of Fe powder, 10–12 mmol of Na2S·9H2O, and 5–10 mmol of NaOH in an autoclave with 10 mL of DI water and heated the mixture for 7 days at 120 °C. As described later in the Results section, these samples labeled inc-Na-tochilinite are compound 4 in Fig. 1. A different series of Na-intercalated samples (5 in Fig. 1) were prepared by utilizing a larger amount of base. The series labeled Na-tochilinite was prepared by combining 10 mmol of Fe powder, 15–20 mmol of Na2S·9H2O, 50–80 mmol of NaOH, and 2 mmol of Sn metal plate in an autoclave with 10 mL DI water and heated to 120 °C for 3–7 days.
We also utilized hydrothermal synthesis for the preparation of K-intercalated phases labeled 1 in Fig. 1. Phase pure polycrystalline material was prepared by combining 10 mmol of Fe powder, 15 mmol of thiourea, 50–100 mmol of KOH, and 2 mmol of Sn metal plate with 10 mL DI water in an autoclave and heated to 160 °C for 5–7 days.
Experimental details on the diffraction, magnetization, transport measurements, and other characterization techniques can be found in ESI† file.
Results and discussions
Li-intercalated phases
We first describe our results utilizing LiOH to intercalate the FeS host. Our starting point is to utilize KxFe2−yS2 (1) crystals grown from congruently melting the constituent elements. Under hydrothermal and basic conditions, these crystals can either de-intercalate the potassium cations to form mackinawite FeS (2), or cation exchange the potassium for cationic layers of (Li1−xFexOH)δ+ as traced in the reaction from 1 to 3. Alternatively, we can isolate (Li1−xFexOH)FeS (3) via the method used by previous workers,21,26,27 whereby polycrystalline material is prepared by the oxidation of iron metal in the presence of a sulfide source and excess amounts of LiOH base. In this reaction (2 to 3 in Fig. 1), mackinawite FeS forms in situ with the hydroxide layers to yield (Li1−xFexOH)FeS. We note that Lu et al.26 and Pachmayr et al.21 had previously observed superconductivity in some of their mixed solid-solutions, (Li1−xFexOH)FeS1−zSez, but both studies had concluded that their pure sulfide samples (z = 0) were nonsuperconducting.We found that superconductivity can be established in the intercalated sulfides for both our cation exchange and polycrystalline routes if two conditions are met: (1) the reaction temperature must be less than 160 °C, i.e. mild hydrothermal conditions, and (2) the environment must remain reducing. The latter condition was maintained by the inclusion of tin metal plate as a way to dynamically change the hydrothermal conditions from oxidizing to more reducing.25 No tin was found in the products as determined from energy dispersive X-ray spectroscopy (EDS).
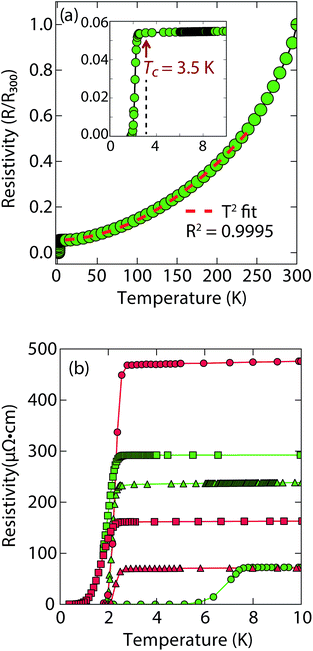
Magnetization and electrical resistivity measurements revealed that the Tc of the (Li1−xFexOH)FeS phases can vary from 3 K to 8 K (Fig. 2), with some samples showing superconducting volume fractions up to 40%, indicative of bulk superconductivity (Fig. S1a†). We must note, however, that due to the proximity of Tc to the base temperature of our magnetometer (1.8 K) we could not reach full saturation of the diamagnetic signal. Therefore, it is possible that the volume fraction is even higher than 40%.
Heat capacity measurements were also carried out in a sample with a Tc near 3 K, but a large signal peaked near 4.5 K whose intensity is independent of applied magnetic field seems to mask any superconducting signal (Fig. S7a†). In the similar selenides (Li1−xFexOH)FeSe, which have a Tc near 42 K, magnetic ordering in the superconducting state takes place near 10 K due to the iron substituted for lithium in the hydroxide layer.23,24,28 Seemingly, a magnetic signal proximate to the Tc of the (Li1−xFexOH)FeS makes it difficult to evaluate the superconducting properties from heat capacity measurements.
Remarkably, for (Li1−xFexOH)FeS samples prepared via the cation exchange route, we observed Tc's both above and below that of bulk FeS (Fig. 2). This result indicates that charge doping into the FeS layer is controlling the critical temperatures in (Li1−xFexOH)FeS. From our various samples, intercalation by (Li1−xFexOH)δ+ could increase the Tc of FeS up to 8 K. Fig. 2b shows the low temperature data near Tc for various samples and the sample with the lowest residual resistivity ratio also led to the highest Tc in the series. From M vs. H hysteresis loops, the upper critical field (Hc2) of the sample at 2 K is 180 Oe whilst Hc1 was found to be approximately 40 Oe (Fig. S1b†). Magnetotransport measurements find a slightly higher Hc2 near 220 Oe for H∥c at 1.8 K (Fig. S2†). Therefore, the intercalated compound was found to have an even smaller Hc2 than pure FeS where it is approximately 1600 Oe along the c-direction and 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 along the ab-plane.16
000 along the ab-plane.16
It is also interesting to note the normal state properties of the intercalated samples. Unlike pristine FeS,16 (Li1−xFexOH)FeS samples with the lower Tc (≈3.5 K) displayed nonlinear temperature dependence in the electrical resistivity above Tc up to approximately 250 K, as shown by the T2-fit in Fig. 2a. Typically, T2 dependence is associated with Fermi liquid behavior, and linear temperature dependence takes over at higher temperatures (approximately above the Debye temperature) due to electron–phonon scattering.29 The samples with the lower Tc exhibit this quadratic behaviour more pronouncedly (Fig. 2a and S3†). Similar Fermi liquid behaviour has been observed for the normal state in select cuprate superconductors that were overdoped in either electron and hole carriers.30–32 Another superconductor that exhibits such quadratic dependence of its resistivity near room temperature is Ag5Pb2O6, which is a three-dimensional electron-gas system.33 Yonezawa and Maeno ascribe the T2 behaviour to enhanced electron–electron scattering that arises in superconductors with low electron carrier densities with respect to elements such as alkali and noble metals.33 Therefore, it is possible that both the lower Tc and T2-behaviour for the sample presented in Fig. 2a and S3† are related to having non-optimal charge doping in the FeS layers, and indeed lower carrier concentrations.
To determine the structure of our superconducting (Li1−xFexOH)FeS samples, we performed high-resolution synchrotron X-ray powder diffraction (sXRD) as shown in Fig. 3. From quantitative analysis of the data, we have provided detailed crystallographic information for two samples in Table 1. Upon intercalation, the Fe–Fe bond distances increased from 2.604 Å in bulk FeS25 to 2.619 Å in (Li1−xFexOH)FeS, but the FeS4 tetrahedron remains virtually unchanged both in bond distances and bond angles. There is also an increase in the distance between the iron square sublattices. In mackinawite, that interlayer distance is ≈5.03 Å,16 whereas in the (Li1−xFexOH)-intercalated phase it is 8.89–8.93 Å, further enhancing the two-dimensionality of its electronic structure. Due to the subtle changes in the (Li1−xFexOH)δ+ layer, Rietveld refinements for the superconducting and non-superconducting samples did not show significant differences in their stoichiometries (both close to (Li0.85Fe0.15OH)FeS).
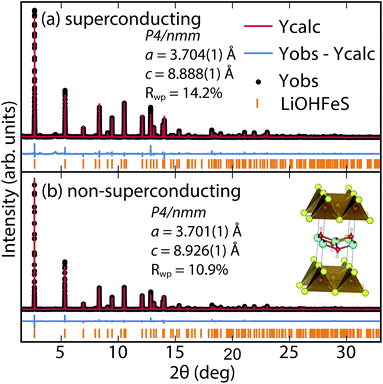 | ||
| Fig. 3 Synchrotron XRD patterns of (a) superconducting and (b) non-superconducting (Li1−xFexOH)FeS prepared under hydrothermal conditions at 160 °C and 200 °C, respectively. | ||
| a = 3.7041(1) Å, c = 8.8877(1) Å, Rwp = 14.27%, Tc = 3 K | ||||||
|---|---|---|---|---|---|---|
| Atom | Wyckoff site | x | y | z | Occ. | U iso (Å2) |
| Li | 2b | 0 | 0 | 0.5 | 0.848(1) | 0.0398(11) |
| Fe1 | 2b | 0 | 0 | 0.5 | 0.152(1) | 0.0398(11) |
| Fe2 | 2a | 0.5 | 0.5 | 0 | 1 | 0.0091(2) |
| O | 2c | 0.5 | 0 | 0.4184(3) | 1 | 0.0174(7) |
| S | 2c | 0 | 0.5 | 0.1444(2) | 1 | 0.0104(3) |
| α 1 (°) | α 2 (°) | Fe–Fe (Å) | Fe–S (Å) | F.U. |
|---|---|---|---|---|
| 110.55(5) | 108.93(3) | 2.6192(1) | 2.2534(7) | (Li0.85Fe0.15OH)FeS |
| a = 3.7011(1) Å, c = 8.9257(1) Å, Rwp = 10.91%, non-superconducting | ||||||
|---|---|---|---|---|---|---|
| Atom | Wyckoff site | x | y | z | Occ. | U iso (Å2) |
| Li | 2b | 0 | 0 | 0.5 | 0.846(1) | 0.0380(7) |
| Fe1 | 2b | 0 | 0 | 0.5 | 0.154(1) | 0.0380(7) |
| Fe2 | 2a | 0.5 | 0.5 | 0 | 1 | 0.0092(1) |
| O | 2c | 0.5 | 0 | 0.4182(2) | 1 | 0.0141(5) |
| S | 2c | 0 | 0.5 | 0.1439(1) | 1 | 0.0102(2) |
| α 1 (°) | α 2 (°) | Fe–Fe (Å) | Fe–S (Å) | F.U. |
|---|---|---|---|---|
| 110.47(3) | 108.98(2) | 2.6171(1) | 2.2527(4) | (Li0.85Fe0.15OH)FeS |
For a more accurate analysis of chemical composition of the (Li1−xFexOH)FeS phases, we performed inductively coupled plasma atomic emission spectroscopy (ICP-AES). For superconducting and non-superconducting samples, ICP-AES afforded Fe/Li ratios of 1.132 and 1.093, respectively. Since Rietveld refinements for their high-resolution synchrotron data suggested no Fe vacancy in the FeS layers (Table 1), the excess amounts of Fe likely resided in the LiOH layers. Therefore, the superconducting samples contains more Fe in the hydroxide layer and consequently more electron doping (0.13 e−vs. 0.09 e−) into the FeS layer. Similarly, Zhou et al.25 have reported that for the selenide analogues, higher Tc's were achieved with lower reaction temperatures so that more iron cations could incorporate into the lithium hydroxide layer. Studies on the same system by Clarke et al. demonstrated that the iron in the hydroxide layer is Fe2+ and that iron vacancies in the FeSe layer degraded the superconducting properties.22 Through the cation exchange method demonstrated here, vacancy formation in the FeS layer is less of a factor and achieving sufficient electron doping from the hydroxide layer is the bigger challenge. We detail in the ESI (Table S1†) the synthesis conditions for various superconducting and non-superconducting samples.
Na-intercalated phases
Our next objective was to explore larger alkali metal hydroxides as intercalates. Unlike LiOH, which favors a square lattice commensurate with that of mackinawite FeS, a similar structure for NaOH was not reproduced. Instead, we found a new phase with very few reflections in the XRD powder pattern and its first peak corresponded to a d-spacing of 5.38 Å. This phase is reminiscent of a natural mineral known as tochilinite, which consists of brucite-type Mg(OH)2 layers between mackinawite-like FeS sheets. Natural tochilinite is quasi-commensurate and its (001) reflection has a d-spacing of 10.72 Å, which is close to twice our first reflection. Therefore, if the first peak of our new phase is the (002) reflection, it would indicate that the FeS layers are stacked in a body-centered fashion. Since we only observe (00l) reflections in our new phase, the square and hexagonal sheets are completely incommensurate to each other in the ab-plane. Henceforth, we refer to this phase as inc-Na-tochilinite (4 in Fig. 1).We found the new inc-Na-tochilinite to always coexist with some residual mackinawite FeS (Fig. S4†). The ratio between inc-Na-tochilinite and mackinawite FeS was increased by using less Na2S·9H2O and decreased with prolonged ultrasonication, indicating conversion of inc-Na-tochilinite to FeS by de-intercalation and dissolution of the metal hydroxide layer. The equilibrium between the two phases is indicated in the steps between 2 and 4 in Fig. 1.
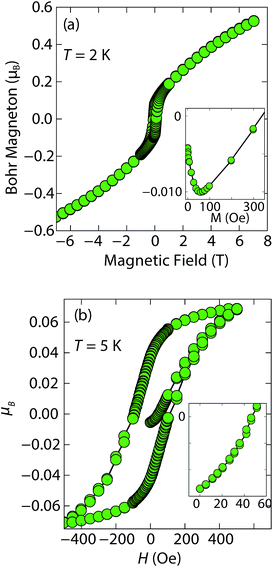
At low fields, we observed two transitions at 5 K and 15 K (Fig. 4a). The 5 K transition was more pronounced for a sample that contained less inc-Na-tochilinite and more mackinawite FeS impurity (Fig. S4 and S5 in ESI†). Therefore, the 5 K anomaly likely corresponds to the superconducting transition of FeS (Tc ≈ 4.5 K). Although the transition at ≈15 K in the zero-field cooled (ZFC) curve (Fig. 4a) appears to indicate Meissner screening due to superconductivity, the negative signal may actually correspond to long-range ordering such as ferro- or ferrimagnetism. If the internal moment of a ferromagnetic material is of sufficient strength and aligned opposite to a weak external field, then the ZFC curve will display negative susceptibility below the Curie temperature. To resolve this ambiguity, we increased the external field of the magnetization measurements from 5 Oe to 10 Oe (Fig. 4). The field cooled (FC) curves better indicate a clear ferromagnetic transition in inc-Na-tochilinite near 15 K. Therefore, inc-Na-tochilinite does not appear to be a superconductor based on the current magnetization data.
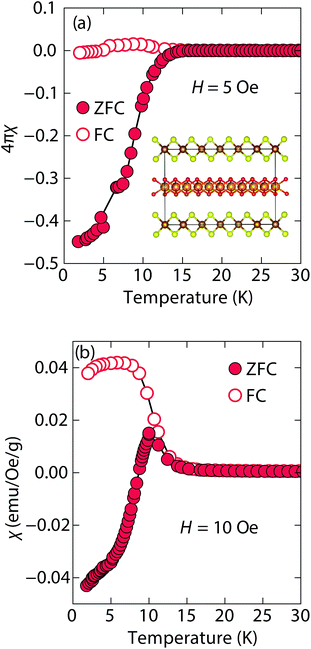 | ||
| Fig. 4 Magnetic susceptibility of inc-Na-tochilinite, [(Na1−xFex)(OH)2]FeS, as a function of temperature with an applied external fields of (a) 5 Oe and (b) 10 Oe. | ||
We also performed temperature-dependent resistivity measurement down to 2 K on a pressed pellet of inc-Na-tochilinite. We did not observe a superconducting transition, but instead semiconducting behaviour (Fig. S6†). We note, however, that similar temperature-dependent behaviour was observed for pressed pellets of FeS powders,34 even though our recent studies of single crystal FeS samples demonstrated that it is indeed metallic in the normal state.16 We attribute this disparity between polycrystalline and single crystal transport measurements of FeS to effects from grain boundaries and surface oxidations, typical for pressed pellets of micaceous materials. Therefore, although the current resistivity data of polycrystalline inc-Na-tochilinite displays semiconducting behaviour, its true state could be metallic, similar to the Li-intercalated FeS phases in the current study.
Magnetization (M) versus applied field (H) measurements further clarify the true ground state of inc-Na-tochilinite (Fig. 5). The M vs. H curves suggest ferromagnetic behavior as the isotherm of the field sweep at 5 K (Fig. 5b) displayed the typical hysteresis loop of ferro- and ferrimagnets. The diamagnetic signal observed for the isotherm at 2 K (Fig. 5a inset) was therefore likely due to the superconducting FeS phase present as an impurity, which has a Tc near 4.5 K.16 At 5 K, this diamagnetic signal is lost (Fig. 5b inset). Although the new inc-Na-tochilinite phase is likely to be either ferro- or ferrimagnetic below 15 K, it does exhibit other interesting anomalies. The low temperature transition likely due to long-range magnetic ordering did not appear as a well defined transition in the heat capacity measurements (Fig. S7b†). Instead, a broad anomaly occurred below 15 K, which was suppressed with a field of 3 T.
By changing the synthesis conditions of the hydrothermal reactions, the NaOH-intercalated FeS system can be stabilized into a quasi-commensurate tochilinite phase (Fig. 6a), which we refer to as Na-tochilinite. This quasi-commensurate phase was prepared by utilizing more concentrated solutions of NaOH (5 to 8 M) in the hydrothermal reactions. Significantly less tetragonal FeS was recovered (Fig. 6a) with Na-tochilinite, and this phase did not easily revert to FeS by ultrasonication, indicating its stability with respect to inc-Na-tochilinite. Using the crystal structure of the naturally occurring mineral known as ferrotochilinite (2(Fe1−xS)·1.8[(Mg, Fe)(OH)2]),35 with lattice parameters, a = 5.37 Å, b = 15.65 Å, c = 10.72 Å, we extracted by Pawley fits the lattice parameters of our Na-tochilinite (Fig. 6a) The lattice parameters after convergence were found to be a = 5.18(1) Å b = 15.62(4) Å, c = 11.14(4) Å and β = 95.07(10)° at room temperature.
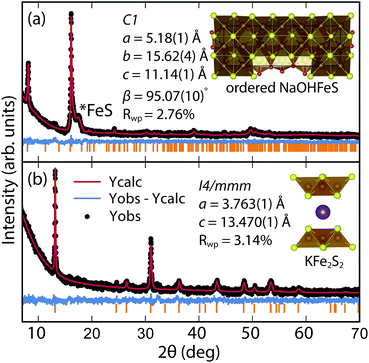 | ||
| Fig. 6 (a) Pawley fit to the XRD pattern of hydrothermally prepared Na-tochilinite and (b) Rietveld fit to the XRD data of KxFe2−yS2. | ||
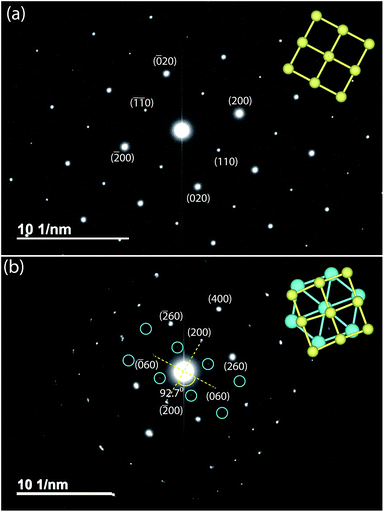
Given the difficulty in elucidating the structure of these heterolayered materials by powder XRD, we have also performed electron diffraction (ED). We present two ED patterns with the (hk0) reflections that were difficult to resolve from powder XRD – one for mackinawite FeS and the other for Na-tochilinite. Along the [001] zone axis, the ED pattern of FeS (Fig. 7a) clearly demonstrates a square lattice corresponding to its simple primitive tetragonal structure. For Na-tochilinite, additional satellite reflections emerge in addition to the square lattice of FeS (Fig. 7b). Upon closer inspection the seemingly 4-fold symmetry of the brighter reflections in Na-tochilinite is actually a 2-fold axis. The angle between the cross-sections connecting the (200) to (![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 00) and (060) to (0
00) and (060) to (0![[6 with combining macron]](https://www.rsc.org/images/entities/char_0036_0304.gif) 0) reflections is about 93°, which is close to the monoclinic angle found from XRD (β = 95.07(10)°). Therefore, the monoclinic distortion of the FeS square lattice in Na-tochilinite is clearly reproduced in the ED along with the satellite reflections indicating the intercalation of the FeS layers. The lattice constants a and b extracted from ED are 5.2(2) Å and 15.9(2) Å, respectively – in good agreement with the XRD analysis.
0) reflections is about 93°, which is close to the monoclinic angle found from XRD (β = 95.07(10)°). Therefore, the monoclinic distortion of the FeS square lattice in Na-tochilinite is clearly reproduced in the ED along with the satellite reflections indicating the intercalation of the FeS layers. The lattice constants a and b extracted from ED are 5.2(2) Å and 15.9(2) Å, respectively – in good agreement with the XRD analysis.
Next, we discuss the nature of the chemical composition of Na-tochilinite. As in some natural minerals,36 we can formulate the stoichiometry as [(Na1−xFex)–(OH)2]FeS, and ICP-AES analysis provided an Fe/Na ratio of 2.99. Therefore, [(Na0.5Fe)(OH)2]FeS is the proposed chemical formula since the ratio of Fe to Na in the tochilinite is (1 + x)/(1 − x) = 2.99. We can modify the formula by considering the number of iron vacancies in the FeS layers, y, and the phase fraction of mackinawite FeS impurity, f. The formula is therefore re-written as (1 + x − y)/(1 − x) = 2.99 × (1 − f). If we estimate the limits based on diffraction data as y < 0.2 and 0.1 < f < 0.2, then x can vary between 0.41 < x < 0.52. This result suggests that approximately half of the cations in the hydroxide layers are filled by iron cations, and in order to charge balance the two OH− groups of the hexagonal brucite layer, the nature of that iron site must be in the form of Fe3+. Whilst ICP-AES could not determine the number of hydroxide groups, crystal chemistry arguments support M(OH)2 for the spacer layer since this is how the hexagonal brucite is formulated. Furthermore, the highly reactive and pyrophoric mineral known as “white rust” consists of Fe(OH)2 layers that crystallize in the CdI2-type structure.37 By oxidizing to Fe3+, such a layer would be stabilized by the presence of either Na+ or vacancies, and indeed natural tochilinites exhibit significant amounts of Fe vacancies (up to 20%).38,39
Rather than displaying superconductivity as in the LiOH-intercalated systems or long-range ferro- or ferrimagnetism as in inc-Na-tochilinite, Na-tochilinite displays broad features in the magnetization reminiscent of short-range antiferromagnetic behavior (Fig. 8a). The splitting of the ZFC and FC curves likely indicate some degree of spin glassiness. The presence of iron vacancies and distortion of the iron square sublattice are some the likely reasons that Na-tochilinite does not produce a well-defined transition in the magnetization data. Interestingly, Parise et al. found through neutron diffraction that Fe(OH)2 exhibits long-range magnetic ordering with each sheet consisting of ferromagnetically coupled iron centers, and each sheet anti-aligned to each other.37 Future neutron diffraction experiments on both incommensurate and quasi-commensurate Na-tochilinites would reveal the nature of the interesting evolution of long-range magnetic ordering arising from the hydroxide layer.
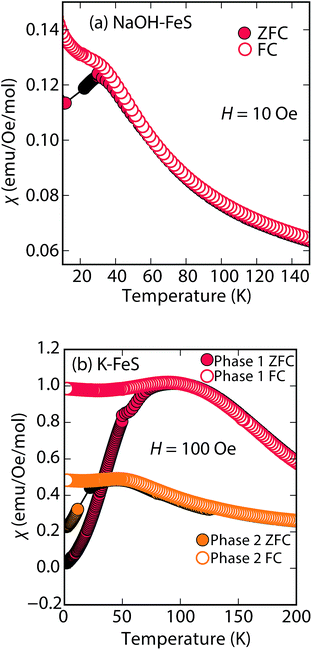 | ||
| Fig. 8 Magnetic susceptibility measurements of (a) Na-tochilinite and (b) KxFe2−yS2, respectively. The lattice constant c for phase 1 and phase 2 in (b) is 13.627 and 13.470 Å, respectively. | ||
K-intercalated phases
Efforts to incorporate KOH layers into FeS hosts resulted in cationic K+ intercalation instead. When hydrothermal reactions of Fe powder with KOH and a sulfide source were undertaken, the XRD pattern revealed a phase pure sample similar to the KxFe2−yS2 prepared using solid-state routes (Fig. 6b). In addition, its pattern could be well fit by Rietveld refinement using the crystal structure of KxFe2−yS2 with the space group I4/mmm. Its layer spacing (lattice constant c) is 13.47 Å, which is comparable to the reported 245-type (I4/m, 13.599 Å)40 and 122-type (I4/mmm, 13.546 Å) compounds.41 EDS analysis gave a composition of K1.1Fe1.6S2 and its magnetic susceptibility displayed broad antiferromagnetic features around 45 K (c = 13.470 Å) and 96 K (c = 13.627 Å) for samples with different layer spacings (Fig. 8b). The ZFC and FC curves do not trace each other as well, which raises the possibility that these materials may display some spin glassiness as well. Since the transitions are fairly broad, it is likely that long-range antiferromagnetic ordering is never observed but rather some form of low-dimensional or short-range antiferromagnetic order. Although not superconducting, it is remarkable that we could prepare via hydrothermal routes such ternary phases since these have previously been prepared only by high temperature solid state techniques.Conclusions
In conclusion, we have demonstrated that metal hydroxides can be intercalated into tetragonal mackinawite-type FeS via hydrothermal routes, and that new superconductors can be prepared in this manner. Given that FeS is a metastable phase, it is of paramount importance that we continue to explore novel low temperature routes towards mineral-inspired superconductors. Whilst we have enhanced Tc to 8 K through these charge-doping hydroxide layers, we have also demonstrated that FeS can serve as a suitable host for various guests species acting as bases. The differences in going from Li+ to Na+ to K+ are remarkable in the vastly different structure types that were stabilized and the physical properties that are manifested. These results point to the exciting possibility of utilizing both size and charge parameters of other guests species, such as amines, to ultimately enhance the superconductivity of sulfide-based materials. Furthermore, the fact that heterostructures could be stabilized points to mackinawite-type FeS as a possible new 2D chalcogenide to be incorporated into other functional 2D materials. The field of vertical 2D heterostructures has exciting possibilities for constructing entirely new functional materials,42 and mackinawite-type FeS could be a new building block in such structures.Acknowledgements
We thank Dr Igor Pekov at Lomonosov Moscow State University for discussions with mineral ferrotochilinite. Research at the University of Maryland was supported by the NSF Career DMR-1455118 and the AFOSR Grant No. FA9550-14-1-0332. Use of the Advanced Photon Source at Argonne National Laboratory was supported by the U. S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DE-AC02-06CH11357. We also acknowledge support from the Maryland Nanocenter and Center for Nanophysics and Advanced Materials.References
- Y. Kamihara, T. Watanabe, M. Hirano and H. Hosono, J. Am. Chem. Soc., 2008, 130, 3296–3297 CrossRef CAS PubMed.
- J. Bos, G. Penny, J. Rodgers, D. Sokolov, A. Huxley and J. P. Attfield, Chem. Commun., 2008, 3634–3635 RSC.
- X. H. Chen, T. Wu, G. Wu, R. H. Liu, H. Chen and D. F. Fang, Nature, 2008, 435, 761–762 CrossRef PubMed.
- C. de La Cruz, Q. Huang, J. W. Lynn, J. Li, W. Ratcliff, J. L. Zarestky, H. A. Mook, G. F. Chen, J. L. Luo, N. L. Want and P. Dai, Nature, 2008, 453, 899 CrossRef CAS PubMed.
- H. Luetkens, H.-H. Klauss, M. Kraken, F. J. Litterst, T. Dellmann, R. Klingeler, C. Hess, R. Khasanov, A. Amato, C. Baines, M. Kosmala, O. J. Schumann, M. Braden, J. Hamann-Borrero, N. Leps, A. Kondrat, G. Behr, J. Werner and B. Buchner, Nat. Mater., 2009, 8, 305 CrossRef CAS PubMed.
- F.-C. Hsu, J.-Y. Luo, K.-W. Yeh, T.-K. Chen, T.-W. Huang, P. M. Wu, Y.-C. Lee, Y.-L. Huang, Y.-Y. Chu, D.-C. Yan and M.-K. Wu, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 14262–14264 CrossRef CAS PubMed.
- H. Kotegawa, S. Masaki, Y. Awai, H. Tou, Y. Mizuguchi and Y. Takano, J. Phys. Soc. Jpn., 2008, 77, 113703 CrossRef.
- S. Margadonna, Y. Takabayashi, M. T. McDonald, K. Kasperkiewicz, Y. Mizuguchi, Y. Takano, A. N. Fitch, E. Suard and K. Prassides, Chem. Commun., 2008, 5607–5609 RSC.
- T. Imai, K. Ahilan, F. L. Ning, T. M. McQueen and R. J. Cava, Phys. Rev. Lett., 2009, 102, 177005 CrossRef CAS PubMed.
- T. Hanaguri, S. Niitaka, K. Kuroki and H. Takagi, Science, 2010, 328, 474–476 CrossRef CAS PubMed.
- P. Zajdel, P.-Y. Hsieh, E. E. Rodriguez, N. P. Butch, J. D. Magill, J. Paglione, P. Zavalij, M. R. Suchomel and M. A. Green, J. Am. Chem. Soc., 2010, 132, 13000–13007 CrossRef CAS PubMed.
- E. E. Rodriguez, C. Stock, P.-Y. Hsieh, N. P. Butch, J. Paglione and M. A. Green, Chem. Sci., 2011, 2, 1782–1787 RSC.
- J. P. Attfield, J. Mater. Chem., 2011, 21, 4756–4764 RSC.
- T. C. Ozawa and S. M. Kauzlarich, Sci. Technol. Adv. Mater., 2008, 9, 033003 CrossRef PubMed.
- X. Lai, H. Zhang, Y. Wang, X. Wang, X. Zhang, J. Lin and F. Huang, J. Am. Chem. Soc., 2015, 137, 10148–10151 CrossRef CAS PubMed.
- C. K. H. Borg, X. Zhou, C. Eckberg, D. J. Campbell, S. R. Saha, J. Paglione and E. E. Rodriguez, Phys. Rev. B, 2016, 93, 094522 CrossRef.
- H. K. Vivanco and E. E. Rodriguez, J. Solid State Chem., 2016, 242, 3–21 CrossRef CAS.
- M. Burrard-Lucas, D. G. Free, S. J. Sedlmaier, J. D. Wright, S. J. Cassidy, Y. Hara, A. J. Corkett, T. Lancaster, P. J. Baker, S. J. Blundell and S. J. Clarke, Nat. Mater., 2013, 12, 15–19 CrossRef CAS PubMed.
- T. Ying, X. Chen, G. Wang, S. Jin, X. Lai, T. Zhou, H. Zhang, S. Shen and W. Wang, J. Am. Chem. Soc., 2013, 135, 2951–2954 CrossRef CAS PubMed.
- X. F. Lu, N. Z. Wang, G. H. Zhang, X. G. Luo, Z. M. Ma, B. Lei, F. Q. Huang and X. H. Chen, Phys. Rev. B: Condens. Matter Mater. Phys., 2014, 89, 020507 CrossRef.
- U. Pachmayr and D. Johrendt, Chem. Commun., 2015, 51, 4689–4692 RSC.
- H. Sun, D. N. Woodruff, S. J. Cassidy, G. M. Allcroft, S. J. Sedlmaier, A. L. Thompson, P. A. Bingham, S. D. Forder, S. Cartenet, N. Mary, S. Ramos, F. R. Foronda, B. H. Williams, X. Li, S. J. Blundell and S. J. Clarke, Inorg. Chem., 2015, 54, 1958–1964 CrossRef CAS PubMed.
- J. W. Lynn, X. Zhou, C. K. H. Borg, S. R. Saha, J. Paglione and E. E. Rodriguez, Phys. Rev. B: Condens. Matter Mater. Phys., 2015, 92, 060510 CrossRef.
- X. F. Lu, N. Z. Wang, H. Wu, Y. P. Wu, D. Zhao, X. Z. Zeng, X. G. Luo, T. Wu, W. Bao, G. H. Zhang, F. Q. Huang, Q. Z. Huang and X. H. Chen, Nat. Mater., 2015, 14, 325–329 CrossRef CAS PubMed.
- X. Zhou, C. K. H. Borg, J. W. Lynn, S. R. Saha, J. Paglione and E. E. Rodriguez, J. Mater. Chem. C, 2016, 4, 3934–3941 RSC.
- X. F. Lu, N. Z. Wang, X. G. Luo, G. H. Zhang, X. L. Gong, F. Q. Huang and X. H. Chen, Phys. Rev. B: Condens. Matter Mater. Phys., 2014, 90, 214520 CrossRef.
- X. Zhang, X. Lai, N. Yi, J. He, H. Chen, H. Zhang, J. Lin and F. Huang, RSC Adv., 2015, 5, 38248–38253 RSC.
- U. Pachmayr, F. Nitsche, H. Luetkens, S. Kamusella, F. Brückner, R. Sarkar, H.-H. Klauss and D. Johrendt, Angew. Chem., Int. Ed., 2015, 54, 293–297 CrossRef CAS PubMed.
- C. Kittel, Introduction to Solid State Physics, Wiley, 8th edn, 2005 Search PubMed.
- K. Levin, J. H. Kim, J. Lu and Q. Si, Phys. C, 1991, 175, 449–522 CrossRef CAS.
- T. Manako, Y. Kubo and Y. Shimakawa, Phys. Rev. B: Condens. Matter Mater. Phys., 1992, 46, 11019–11024 CrossRef CAS.
- R. A. Cooper, Y. Wang, B. Vignolle, O. J. Lipscombe, S. M. Hayden, Y. Tanabe, T. Adachi, Y. Koike, M. Nohara, H. Takagi, C. Proust and N. E. Hussey, Science, 2009, 323, 603–607 CrossRef CAS PubMed.
- S. Yonezawa and Y. Maeno, Phys. Rev. B: Condens. Matter Mater. Phys., 2013, 88, 205143 CrossRef.
- S. Denholme, S. Demura, H. Okazaki, H. Hara, K. Deguchi, M. Fujioka, T. Ozaki, T. Yamaguchi, H. Takeya and Y. Takano, Mater. Chem. Phys., 2014, 147, 50–56 CrossRef CAS.
- N. Organova, V. Drits and A. Dmitrik, Crystallogr. Rep., 1972, 17, 667 Search PubMed.
- I. V. Pekov, E. V. Sereda, Y. S. Polekhovsky, S. N. Britvin, N. V. Chukanov, V. O. Yapaskurt and I. A. Bryzgalov, Geol. Ore Deposits, 2013, 55, 567–574 CrossRef.
- J. B. Parise, W. G. Marshall, R. I. Smith, H. D. Lutz and H. Möller, Am. Mineral., 2000, 85, 189–193 CrossRef CAS.
- Y. Peng, G. Xi, C. Zhong, L. Wang, J. Lu, X. Sun, L. Zhu, Q. Han, L. Chen, L. Shi, M. Sun, Q. Li, M. Yu and M. Yin, Geochim. Cosmochim. Acta, 2009, 73, 4862–4878 CrossRef CAS.
- G. A. Kakos, T. W. Turney and T. B. Williams, J. Solid State Chem., 1994, 108, 102–111 CrossRef CAS.
- H. Lei, M. Abeykoon, E. S. Bozin and C. Petrovic, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 83, 180503 CrossRef.
- J. J. Ying, Z. J. Xiang, Z. Y. Li, Y. J. Yan, M. Zhang, A. F. Wang, X. G. Luo and X. H. Chen, Phys. Rev. B: Condens. Matter Mater. Phys., 2012, 85, 054506 CrossRef.
- B. V. Lotsch, Annu. Rev. Mater. Res., 2015, 45, 85–109 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6sc05268a |
| This journal is © The Royal Society of Chemistry 2017 |