Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Intra- versus intermolecular electron transfer in radical nucleophilic aromatic substitution of dihalo(hetero)arenes – a tool for estimating π-conjugation in aromatic systems†
B.
Janhsen
,
C. G.
Daniliuc
and
A.
Studer
 *
*
Organisch Chemisches Institut, Westfälische Wilhelms-Universität Münster, Corrensstraße 40, 48149 Münster, Germany. E-mail: studer@uni-muenster.de
First published on 23rd February 2017
Abstract
In this paper, the application of the double radical nucleophilic aromatic substitution (SRN1) in various dihalogenated, mostly diiodinated, π-conjugated systems as a tool for qualitatively estimating their π-conjugation is described. This approach uses electron delocalisation as a measure of π-conjugation. Electron injection into the π-system is achieved via reaction of an intermediate aryl radical, itself generated from a dihalogenated π-system via SET-reduction of the C–I bond and subsequent reaction with a thiolate anion. The generated arene radical anion can then further react with the second aryl-halogen moiety within the π-system via an intramolecular electron transfer process. The efficiency of this intramolecular electron transfer is related to the π-conjugation of the radical anion. If the π-conjugation within the aromatic unit is weak, the arene radical anion reacts via an intermolecular ET with the starting dihalide. The intramolecular ET process delivers a product of a double SRN1 substitution whereas the intermolecular ET pathway provides a product of a mono- SRN1 substitution. By simple product analysis of mono- versus double substitution, π-conjugation can be qualitatively evaluated. This mechanistic tool is applied to various dihalogenated π-conjugated systems and the results are discussed within the context of π-conjugation. The conjugation mode within the π-system and the length of the aromatic system are varied, and the effect of relative positioning of the two halides within small π-systems is also addressed.
Introduction
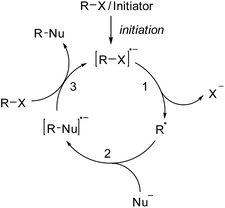
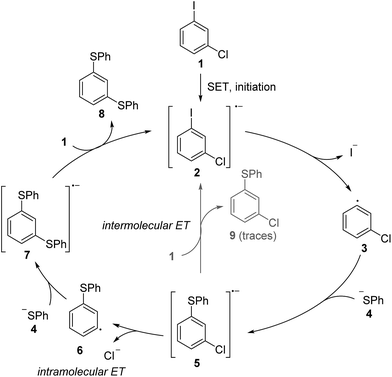
Over the past forty years, radical nucleophilic aromatic substitution (SRN1) has been found to be a highly valuable reaction in organic synthesis.1 This process has been applied to the synthesis of natural products2 and also in neighboring disciplines for the preparation of oligomers3 and polymers.4 Its radical chain mechanism was first proposed by Kornblum5 and Russell6 for activated aliphatic and aromatic substrates. Later Bunnett7 widened the scope by also performing the substitution on unactivated aromatic systems. As for any radical chain reaction, the SRN1 reaction needs to be initiated. In many cases light has been used to initiate the chain via an electron transfer (ET) process from an initiator to the substrate halide.1 The resulting radical anion then fragments to the corresponding C-radical and an anion (step 1, Scheme 1), which is usually a halide.1 In some cases the radical anion is not an intermediate (ET and halide fragmentation are concerted in a so-called dissociative ET).8 The C-radical is then attacked by an anionic nucleophile (step 2) in order to generate a radical anion, which in turn acts as an electron transfer reagent (reductant). Single electron transfer (SET) to the starting substrate halide eventually provides the substitution product along with the substrate halide radical anion, thereby sustaining the chain (step 3). C–C bond formation has been achieved with enolates as nucleophiles, but in most cases the SRN1 reaction has been applied to C-heteroatom bond formation with S-, P-, Sn-, As-, Sb-, Se-, and Te-centered nucleophiles.1Since the SRN1 reaction belongs to an electron-catalyzed process,9 the mechanism depicted in Scheme 1 is drawn accordingly as a catalytic cycle.10 The suggested mechanism of the SRN1 substitution was further supported by Bunnett and Creary using an elegant experiment.11 They showed that the reaction of meta-chloro-iodobenzene (1) with PhSH in ammonia under SRN1 conditions provides the bisthioether 8 as a major product, along with traces of the monosubstituted chlorothioether 9 (Scheme 2). It was also shown that 9 is a sluggish substrate for an SRN1 reaction and therefore 8 cannot be derived from 9 under the applied conditions. The following mechanism was suggested based on these experimental observations. ET initiation generates the radical anion 2, which chemoselectively fragments the iodide anion to give the aryl radical 3. Addition of the thiolate to the aryl radical affords the radical anion adduct 5. This intermediate can now either react via an intramolecular ET (from the π* of 5 to the σ*(C–Cl))12 and subsequent chloride fragmentation to the aryl radical 6 or via an intermolecular ET transfer (from the π* of 5 to the π* of substrate 1) to the monosubstitution product 9. The aryl radical 6 then reacts with the thiolate via7 to the experimentally observed double substitution product 8. Due to the fact that double substitution occurred nearly exclusively, the intramolecular ET in the radical anion 5 is far faster than the intermolecular ET.‡ This result also provided indirect proof for the existence of the radical anion 5 as an intermediate in the chain reaction and strongly supported the general mechanism of the SRN1 reaction that is depicted in Scheme 1.
Dihalo-substituted alkanes including dihalocyclopropanes,13 dihaloadamantanes,14 and dihalobicyclo[2.2.2]octanes15 were also studied with respect to the SRN1 double substitution. For aromatic systems, ortho-,1,16meta-,11,17 and para-dihalobenzenes18 and 1,8-diiodonaphthalene19 were investigated along these lines.
However, more complex aromatic structures bearing two halide substituents in extended π-systems have not been studied to date in the context of a double SRN1 substitution. We assumed this process to be a simple and efficient tool for analyzing the relative ratio of intramolecular versus intermolecular electron transfer in π-conjugated radical anions. Importantly, this ratio should offer a measure for evaluating the π-conjugation in an aromatic system, which is of course an important parameter in various fields of modern materials science.
In this work we introduce the double SRN1 substitution in dihalo(hetero)arenes as a novel qualitative tool for the analysis of the relative intra- versus intermolecular ET efficiency in various π-radical anions. Electron injection into the π-system is achieved via the reaction of an intermediate aryl radical with an anionic nucleophile. Since the aryl radical is generated at a specific site in the molecule, the electron injection occurs site specifically at the σ* orbital of the newly formed C–S bond. The ratio of double versus single SRN1 substitution, readily determined by product analysis at low conversion, will allow the estimation of the relative inter- versus intramolecular ET efficiency (Scheme 3). Importantly, the intramolecular ET efficiency should be related to the π-conjugation of the aromatic system. The conjugation mode within the π-system and the length of the aromatic system will be varied, and the effect of the relative positioning of the two halides within small π-systems will also be addressed.
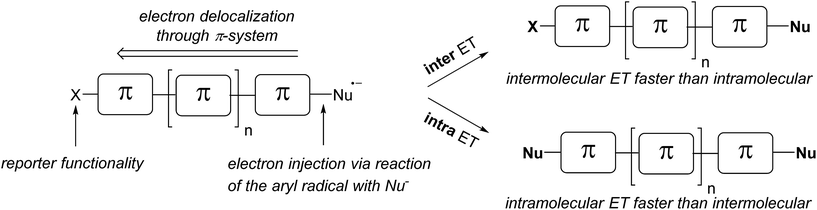 | ||
| Scheme 3 Inter- versus intramolecular ET in π-radical anions – a tool for estimating the π-conjugation of an aromatic system. | ||
Experimental
Materials and methods
All reactions involving air or moisture sensitive reagents were carried out in flame-dried glassware under an atmosphere of argon. All solvents and reagents were purified according to standard procedures or were used as-received from Sigma Aldrich, Acros, Alfa Aesar, or TCI Europe. Mass spectra were recorded on a Finnigan MAT 4200S, a Bruker Daltonics Micro Tof, and a Waters-Micromass Quattro LCZ (ESI) and peaks are given in m/z (% of basis peak). ESI-MS (m/z) and HRMS (m/z) were performed using a Bruker MicroTof (loop injection; resolution: 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), a LTQ Orbitrap XL (nanospray inlet, 1.1 kV, resolution: 30
000), a LTQ Orbitrap XL (nanospray inlet, 1.1 kV, resolution: 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), and an Autoflex Speed TOF-MS (Bruker Daltonics). MALDI spectra were recorded with an Autoflex Speed TOF-MS (Bruker Daltonics) in linear mode. GC-MS (EI, 70 eV) was performed on a combined setup of an Agilent 6890N chromatograph equipped with a HP-5 column, using helium (∼1 bar) as the carrier gas, and a Waters Micromass Quattro Micro spectrometer. Gas chromatography (GC-FID) was performed on an Agilent 7890A chromatograph, which was equipped with a HP-5 column (30 m × 0.32 mm, film thickness 0.25 μm), using H2 (∼1 bar) as the carrier gas.
000), and an Autoflex Speed TOF-MS (Bruker Daltonics). MALDI spectra were recorded with an Autoflex Speed TOF-MS (Bruker Daltonics) in linear mode. GC-MS (EI, 70 eV) was performed on a combined setup of an Agilent 6890N chromatograph equipped with a HP-5 column, using helium (∼1 bar) as the carrier gas, and a Waters Micromass Quattro Micro spectrometer. Gas chromatography (GC-FID) was performed on an Agilent 7890A chromatograph, which was equipped with a HP-5 column (30 m × 0.32 mm, film thickness 0.25 μm), using H2 (∼1 bar) as the carrier gas.
Synthetic procedures
The synthesis of all substrates employed in this work is discussed in detail in the ESI†.Results and discussion
Since the iodide anion is a highly efficient leaving group in SRN1 chemistry we decided to mainly focus on diiodoarenes as substrates. In order to keep the product analysis as simple as possible, we took symmetrical dihaloarenes as coupling partners. To ensure that the disubstitution product does not derive from the monosubstitution compound via a renewed SRN1 process, the reactions were stopped at low conversion (mostly <50%). NaSC6H4CH3, which is readily generated by deprotonation of para-methylthiophenol with NaH, was chosen as a nucleophile and reactions were conducted in acetonitrile under UV irradiation (365 nm) at room temperature, unless otherwise noted. The formation of mono- and/or double SRN1 substitution products was analyzed using gas chromatography or mass spectrometry (see ESI†). The reactions were generally clean and, along with the mono- and/or doubly substituted SRN1 products, unreacted starting material and disulfide derived from oxidation of the thiolate during analysis were identified. In most cases, the mono-reduced (hydrodehalogenated) derivatives of the substrate were also formed as typical side-products in photoinitiated SRN1 reactions.1The simple dihalobenzenes 10a–c were investigated first (Scheme 4). All of them showed the corresponding double substitution products 11a, 11b, and 11c derived from intramolecular ET and only traces of the corresponding monosubstituted products 12b and 12c were identified. These results are in agreement with literature reports.11,18
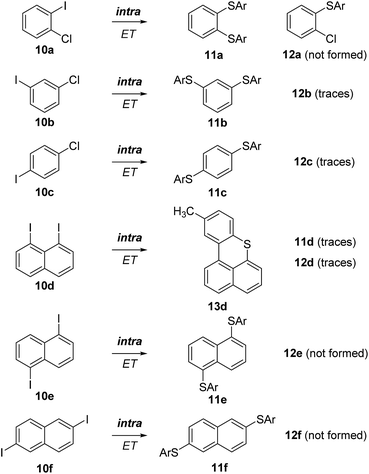 | ||
| Scheme 4 SRN1 substitution on various dihalobenzenes and diiodonaphthalenes. The 11-series, including 13, represents the double substitution products derived from an intramolecular ET. | ||
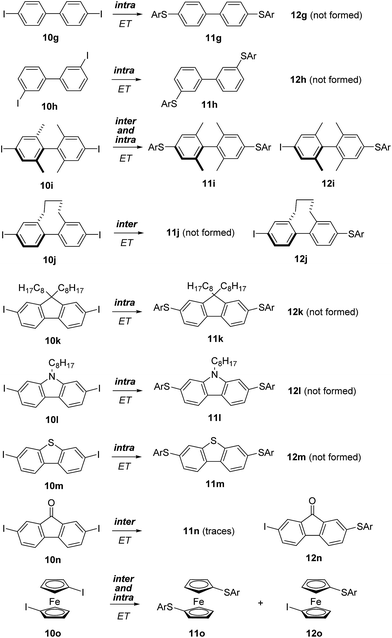
In the naphthalene series, the 1,8-diiodo derivative 10d underwent SRN1 substitution followed by an intramolecular homolytic aromatic substitution to eventually give 13d.19,20 This product results from an intramolecular ET and only traces of the mono SRN1 product 12d, which is formed via an intermolecular ET, were identified in the reaction mixture. A small amount of the double substitution product 11d was formed along with the main product (13d). The 1,5-diiodonaphthalene (10e) afforded only the disubstitution product 11e by an intramolecular ET and monosubstituted 12e was not formed. The 2,6-isomer 10f provided selectively the disubstitution product 11fvia an efficient intramolecular ET in the intermediate radical anion. Hence, as expected, the intramolecular ET in naphthalene radical anions is highly efficient.
We then decided to apply the π-conjugation test to more complex aromatic structures, and started to investigate biphenyl derivatives. Biphenyl is the smallest entity containing two adjacent aromatic rings connected via a π-bond and various derivatives are synthetically readily accessible. The 4,4′-diiodo biphenyl 10g and the 3,3′-diiodo congener 10h exclusively provided the double SRN1 substitution products 11g and 11hvia an intramolecular ET process (Scheme 5). It was shown in previous studies that the conductance of a biphenyl-derived molecular conductor is correlated with the torsion angle along the aryl–aryl bond.21 For 4,4′- and 3,3′-disubstituted biaryls, a conformation with both arene rings oriented in-plane (torsion angle = 0°) should be readily accessible. For intramolecular ET in biaryl radical anions, a planar conformation with a torsion angle of 0° would be an ideal explanation for the exclusive formation of the double substitution products 11g and 11h in the transformations of 10g and 10h.
To further address that important issue, we prepared para-diiodo biaryls 10i and 10j where conformation around the biaryl axis is controlled by the ortho-substitution pattern. The structures of these diiodides were characterized using X-ray crystal structure analysis (Fig. 1). The torsion angle for 10i is 83.0° and for 10j it is 58.1°. Despite the large torsion angle in 10i, both mono- and bis-substitution products 11i and 12i were formed in the SRN1 substitution. However, in the case of 10j only the mono-substitution product was identified, showing that the intramolecular ET process is slower than the intermolecular ET process in such a conformationally rigid biaryl radical anion with a fixed torsion angle that does not allow electronic communication between the two arenes. In the case of the radical anion derived from 10i, despite the larger torsion angle in the crystalline state, intramolecular ET is feasible due to rotational flexibility under the reaction conditions in solution, allowing the two arene moieties in their radical anionic state to interact. The ratio of bis- to monosubstitution (11i![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12i) is dependent on the substrate concentration (see ESI†). The higher the concentration, the more product 12i derived from an intermolecular ET observed, and the concentration linearly correlates with the 11i
12i) is dependent on the substrate concentration (see ESI†). The higher the concentration, the more product 12i derived from an intermolecular ET observed, and the concentration linearly correlates with the 11i![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12i ratio.
12i ratio.
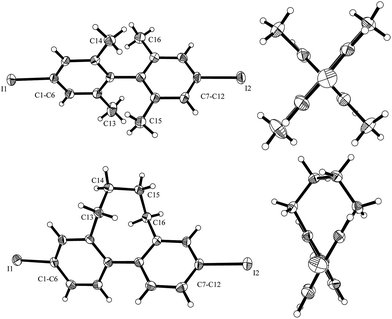 | ||
| Fig. 1 X-ray structure of 10i (top) and 10j (bottom) with the side view (left) and view along the biphenyl system (right; thermal ellipsoids are shown with 30% probability). CCDC numbers for 10i: 1526301 and 10j: 1526302. | ||
Based on these results we assumed that the 2,7-diiodo-9,9′-dioctyl fluorene (10k) with a fixed planar biaryl structural motif would be a good substrate for intramolecular ET. Indeed, selective formation of the double SRN1 product 11k was obtained in the experiment. Along these lines, the carbazole 10l and benzothiophene 10m afforded the corresponding double SRN1 substitution products 11l and 11m with complete selectivity. The additional heteroatom in these conformationally fixed biaryl radical anions do not influence the intramolecular ET to a large extent. In both cases the intramolecular ET is faster than the intermolecular process.
However, incorporating a carbonyl group as a biaryl bridging moiety changes the reactivity towards formation of, nearly exclusively, the monosubstitution product 12n. In this substrate the carbonyl group likely stabilizes the intermediate radical anion so that fragmentation of the iodide is slowed down, allowing for intermolecular ET to occur. As an additional substrate, we tested the diiodoferrocene 10o and found both the mono- (12o) and disubstitution (11o) products. Hence, electronic communication between the two arenes in the radical anion via the Fe-atom is possible but competes with the intermolecular ET.
Our next efforts concentrated on the intra/inter electron transfer efficiency in triphenyl systems. 4,4′′-Diiodo-p-terphenyl (10p) was employed and efficient intramolecular ET in the corresponding intermediate radical anion was observed, leading selectively to 11p as the only substitution product. Meta-connected terphenyl 10q also provided exclusively doubly substituted 11q indicating that the connectivity pattern of the aryl moieties does not produce an observable effect. The triiodide 10r yielded the trisubstitution product 14r as the only substitution product. This result shows that two consecutive intramolecular ETs in this substrate are faster than a single intermolecular ET from this molecule. To our knowledge, this is the first report on a triple substitution in SRN1-type chemistry (Scheme 6).
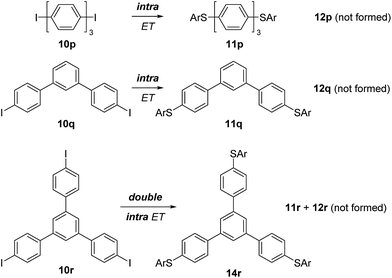 | ||
| Scheme 6 Double SRN1 substitution in triaryl systems and triple SRN1 substitution in 1,3,5-tris(p-iodophenyl)benzene (10r). | ||
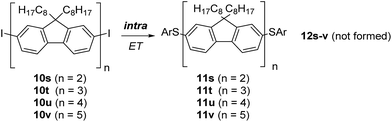
We continued the studies by looking at oligofluorenes, and prepared the dimer 10s, trimer 10t, tetramer 10u, and pentamer 10v (see ESI†).§ The conjugation length in 10s corresponds to a para-quaterphenyl with pairs of phenylene moieties fixed. Accordingly, 10t would be related to a conformationally partly fixed para-sexiphenyl, 10u to a para-octiphenyl, and 10v to a para-deciphenyl. SRN1 substitutions were conducted in DMSO with thermal initiation at 110 °C due to the low solubility of these substrates at room temperature. In place of GC-MS analysis, MALDI-MS was employed for reaction analysis (see ESI†). Reaction with the dimer 10s provided only the disubstitution product 11s, showing that intramolecular ET via the four phenylene moieties is efficient and intermolecular ET cannot compete. Accordingly, 12s was not identified in the reaction mixture. Even for the pentamer 10v the intramolecular ET was highly efficient and only the double SRN1 substitution product 11v was formed at low conversion. These results illustrate the high efficiency of the intramolecular ET in oligofluorenes and underline the conductive properties of such π-radical anions (Scheme 7).22
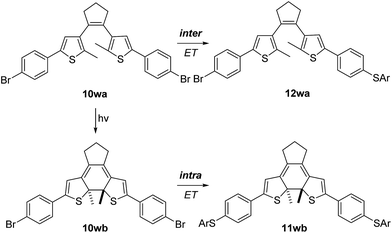
Finally, we examined the electron-transfer efficiency using the photochemically switchable π-system 10w. The SRN1 test was applied to its open (10wa) and closed (10wb) form.23 We chose the bisbromo compound because the corresponding bisiodide24 was not stable under the photochemical conditions used for ring closure. By employing UV light (320 nm) in THF-d8, 10wa could be readily transformed into 10wb. SRN1 substitution of 10wa with NaSC6H4CH3 was initiated thermally in MeCN (see ESI†) to prevent light-mediated ring closure during the SRN1 reaction, whereas the reaction with 10wb was initiated by UV light at room temperature to prevent thermal ring opening. The open form 10wa showed monosubstitution to produce 12wa.¶ This is due to the fact that the π-system is not planar and π-conjugation is disrupted, preventing an efficient intramolecular ET. In contrast, for 10wb, the disubstitution product 11wb was observed as the exclusive product. In the closed form, the polycyclic core renders the whole molecule planar and rigid, so that efficient intramolecular ET is occurring (Scheme 8).
Conclusions
We have introduced double SRN1 substitution as a tool for estimating the π-conjugation in aromatic systems. In dihalogenated π-systems where good π-conjugation is warranted, the SRN1 reaction provides the double substitution product via intramolecular electron transfer through the π-system in the corresponding intermediate radical anion. If π-conjugation is low or disturbed, the radical anion reacts via intermolecular electron transfer to provide the product of mono SRN1 substitution. The product ratio of mono versus double SRN1 substitution is easily determined using standard analytical tools, allowing for ready qualitative estimation of π-conjugation.We have shown that, for benzene derivatives, the intramolecular ET in the corresponding radical anions is nearly the exclusive process and that the relative orientation of the two halides is less important. Also, for the π-extended naphthalene systems, almost exclusively intramolecular ET was observed for all systems investigated. In biphenyl derivatives, the torsion angle between the two planes influences the intramolecular ET efficiency. For systems that can reach a planar π-conjugated conformation, only double SRN1 substitution via intramolecular ET was observed. However, if the two arene moieties in the biaryl radical anions are tilted and conformationally fixed, electron transfer from one arene ring to the other ring is suppressed, and reactions proceed via intermolecular ET. This is very similar to electron conductance through biaryls, where torsion angle effects on conductance were also observed.21 In π-extended systems represented by oligofluorenes we have shown that the intramolecular electron transfer through the π-system can efficiently compete with the intermolecular ET for a conjugation length of up to 10 phenylene moieties (until para-deciphenyl was tested). Moreover, we demonstrated the first example of switchable SRN1 reactivity by employing a photochemical switchable substrate.
Acknowledgements
We thank the Deutsche Forschungsgemeinschaft (DFG) for supporting our work.Notes and references
- R. A. Rossi, A. B. Pierini and A. B. Peñéñory, Chem. Rev., 2003, 103, 71–168 CrossRef CAS PubMed; J. I. Bardagí and V. A. Vaillard, in Encyclopedia of Radicals in Chemistry, Biology and Materials, ed. C. Chatgilialoglu and A. Studer, Wiley, Weinheim, 2009, vol. 1, 13, pp. 333–364 Search PubMed.
- S. M. Barolo, X. Teng, G. D. Cuny and R. A. Rossi, J. Org. Chem., 2006, 71, 8493–8499 CrossRef CAS PubMed.
- W. J. Mijs, O. E. van Lohuizen, J. Bussink and L. Vollbracht, Tetrahedron, 1967, 23, 2253–2264 CrossRef CAS; R. A. Alonso and R. A. Rossi, J. Org. Chem., 1980, 45, 4760–4763 CrossRef; A. B. Chopa, M. T. Lockhart and G. Silbestri, Organometallics, 2001, 20, 3358–3360 CrossRef; E. F. Córsico and R. A. Rossi, J. Org. Chem., 2002, 67, 3311–3316 CrossRef.
- A. C. Archer and P. A. Lovell, Polymer, 1995, 36, 4327–4337 CrossRef CAS; A. C. Archer and P. A. Lovell, Polymer, 1995, 36, 4315–4326 CrossRef; S. Murarka and A. Studer, Angew. Chem. Int. Ed., 2012, 51, 12362–12366 Search PubMed.
- N. Kornblum, R. E. Michel and R. C. Kerber, J. Am. Chem. Soc., 1966, 88, 5660–5662 CrossRef CAS; N. Kornblum, R. E. Michel and R. C. Kerber, J. Am. Chem. Soc., 1966, 88, 5662–5663 CrossRef.
- G. A. Russell and W. C. Danen, J. Am. Chem. Soc., 1966, 88, 5663–5665 CrossRef CAS.
- J. F. Bunnett and J. K. Kim, J. Am. Chem. Soc., 1970, 92, 7463–7464 CrossRef CAS; J. F. Bunnett and J. K. Kim, J. Am. Chem. Soc., 1970, 92, 7464–7466 CrossRef.
- J.-M. Savéant, Acc. Chem. Res., 1993, 26, 455–461 CrossRef.
- A. Studer and D. P. Curran, Nat. Chem., 2014, 6, 765–773 CrossRef CAS PubMed.
- A. Studer and D. P. Curran, Angew. Chem., Int. Ed., 2015, 54, 58–102 Search PubMed.
- J. F. Bunnett and X. Creary, J. Org. Chem., 1974, 39, 3611–3612 CrossRef CAS.
- N. Zhang, S. R. Samanta, B. M. Rosen and V. Percec, Chem. Rev., 2014, 114, 5848–5958 CrossRef CAS PubMed.
- G. F. Meijs, J. Org. Chem., 1986, 51, 606–611 CrossRef CAS.
- A. E. Lukach, A. N. Santiago and R. A. Rossi, J. Phys. Org. Chem., 1994, 7, 610–614 CrossRef CAS.
- A. N. Santiago, V. S. Iyer, W. Adcock and R. A. Rossi, J. Org. Chem., 1988, 53, 3016–3020 CrossRef CAS.
- M. T. Baumgartner, L. B. Jimenez, A. B. Pierini and R. A. Rossi, J. Chem. Soc., Perkin Trans. 2, 2002, 1092–1097 RSC.
- J. F. Bunnett and X. Creary, J. Org. Chem., 1974, 39, 3612–3614 CrossRef CAS.
- J. F. Bunnett and R. P. Traber, J. Org. Chem., 1978, 43, 1867–1872 CrossRef CAS.
- R. K. Norris and J. A. McMahon, ARKIVOC, 2003, 10, 139–155 Search PubMed.
- R. Bolton and G. H. Williams, Chem. Soc. Rev., 1986, 15, 261–289 RSC; A. Studer and D. P. Curran, Angew. Chem., Int. Ed., 2011, 50, 5018–5022 CrossRef CAS PubMed.
- L. Venkataraman, J. E. Klare, C. Nuckolls, M. S. Hybertsen and M. L. Steigerwald, Nature, 2006, 442, 904–907 CrossRef CAS PubMed; D. Vonlanthen, A. Mishchenko, M. Elbing, M. Neuburger, T. Wandlowski and M. Mayor, Angew. Chem., Int. Ed., 2009, 48, 8886–8890 CrossRef PubMed.
- Y. Koizumi, S. Seki, A. Acharya, A. Saeki and S. Tagawa, Chem. Lett., 2004, 33, 1290–1291 CrossRef CAS.
- W. R. Browne, J. J. D. de Jong, T. Kudernac, M. Walko, L. N. Lucas, K. Uchida, J. H. van Esch and B. L. Feringa, Chem.–Eur. J., 2005, 11, 6430–6441 CrossRef CAS PubMed.
- E. C. Harvey, J. Areephong, A. A. Cafolla, C. Long, W. R. Browne, B. L. Feringa and M. T. Pryce, Organometallics, 2014, 33, 447–456 CrossRef CAS.
- C. Costentin, M. Robert and J.-M. Savéant, J. Am. Chem. Soc., 2004, 126, 16051–16057 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details and procedures, 1H and 13C NMR data, GC traces and mass spectra. CCDC 1526301 and 1526302. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7sc00100b |
| ‡ This is not surprising considering the fast kinetics of halide fragmentation in halogen substituted arene radical anions, see ref. 25 and references cited therein. |
| § The starting diiodides contained the monoiodo-derivatives and the doubly hydrodeiodinated oligofluorenes that could not be separated, see ESI.† However, these impurities influence neither the reaction nor the mass spectrometry analysis. |
| ¶ The open form 10wa used contained 10% of the closed form 10wb. In the SRN1 experiment we identified small amounts of the doubly substituted product 11wb and assumed that this doubly substituted product derived from 10wb was present as an impurity in the starting material. This was supported by the fact that upon increasing conversion the relative ratio of 12wa/11wa sharply increased, indicating that the closed form reacts exclusively to the monosubstitution product 12wa (see ESI†). Moreover, we noted 10wb to be a far more reactive substrate in the SRN1 substitution as compared to 10wa. |
| This journal is © The Royal Society of Chemistry 2017 |