Open Access Article
Open Access ArticleA multi-signal fluorescent probe for simultaneously distinguishing and sequentially sensing cysteine/homocysteine, glutathione, and hydrogen sulfide in living cells†
Longwei
He
 ,
Xueling
Yang
,
Kaixin
Xu
,
Xiuqi
Kong
,
Xueling
Yang
,
Kaixin
Xu
,
Xiuqi
Kong
 and
Weiying
Lin
and
Weiying
Lin
 *
*
Institute of Fluorescent Probes for Biological Imaging, School of Chemistry and Chemical Engineering, School of Materials Science and Engineering, University of Jinan, Shandong 250022, P. R. China. E-mail: weiyinglin2013@163.com
First published on 30th June 2017
Abstract
Biothiols, which have a close network of generation and metabolic pathways among them, are essential reactive sulfur species (RSS) in the cells and play vital roles in human physiology. However, biothiols possess highly similar chemical structures and properties, resulting in it being an enormous challenge to simultaneously discriminate them from each other. Herein, we develop a unique fluorescent probe (HMN) for not only simultaneously distinguishing Cys/Hcy, GSH, and H2S from each other, but also sequentially sensing Cys/Hcy/GSH and H2S using a multi-channel fluorescence mode for the first time. When responding to the respective biothiols, the robust probe exhibits multiple sets of fluorescence signals at three distinct emission bands (blue-green-red). The new probe can also sense H2S at different concentration levels with changes of fluorescence at the blue and red emission bands. In addition, the novel probe HMN is able to discriminate and sequentially sense biothiols in biological environments via three-color fluorescence imaging. We expect that the development of the robust probe HMN will provide a powerful strategy to design fluorescent probes for the discrimination and sequential detection of biothiols, and offer a promising tool for exploring the interrelated roles of biothiols in various physiological and pathological conditions.
Introduction
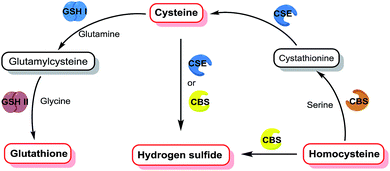
Biothiols are essential reactive sulfur species (RSS) in cells and they play vital roles in human physiology, such as the regulation of oxidative stress, signal transduction, and chelation of metal ions.1–5 Hydrogen sulfide (H2S), the simplest biothiol, has been considered as the gaseous signal molecule following nitric oxide (NO) and carbon monoxide (CO).6 The physiological level of H2S maintains the balance of intracellular redox status and regulates fundamental signaling processes including cardiovascular functional regulation, insulin secretion, neurotransmission, and apoptosis.7–10 Cysteine (Cys) and homocysteine (Hcy) are two very similar biothiols, both of which are precursors of H2S, which can be endogenously produced by enzymes such as cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE), displaying vital functions in the regulation of matrix degradation and cell motility.11–13 Glutathione (GSH), the most abundant intracellular non-protein biothiol, is synthesized from its constituent amino acids by the consecutive actions of γ-glutamylcysteine synthetase (GSH I) and glutamine synthetase (GSH II).14 GSH also plays important roles in combating oxidative stress and defending against free radicals to protect thiol proteins and enzymes from oxidation.15 As shown in Fig. 1, the H2S level is tightly associated with the Cys, Hcy, and GSH levels in living systems, and the level fluctuations of one biothiol may have a significant impact on others. Hence, it is of great significance to simultaneously distinguish and sequentially detect Cys/Hcy, GSH, and H2S in bio-systems, and this is beneficial for gaining a better understanding of their generation and metabolic mechanisms and physiological functions in biological systems.Recently, fluorescent probes have attracted a great deal of attention due to the advantages of high selectivity, high sensitivity, and real-time and non-destructive visual detection.16–22 Therefore, it is interesting to engineer fluorescent probes for simultaneously distinguishing and sequentially detecting Cys/Hcy, GSH, and H2S. Owing to the similar chemical structures and properties of these biothiols, it is very challenging to distinguish Cys/Hcy, GSH, and H2S from each other. For instance, all of them have a nucleophilic sulfhydryl group (–SH) and the pKa of H2S (6.9) is lower than that of Cys (8.3), Hcy (8.9), or GSH (9.2), indicating that H2S has a stronger nucleophilicity than other biothiols under physiological conditions. Hence, it is very difficult to distinguish Cys/Hcy/GSH from H2S simply via nucleophilic reaction-based strategies. Pluth et al. found that the dark 4-chloro-7-nitro-1,2,3-benzoxadiazole (NBD-Cl) could perform a turn-on fluorescence in response to Cys via consecutive nucleophilic substitution and intramolecular rearrangement reactions, while the emission would be quenched again via another stronger nucleophilic substitution reaction by H2S.23 Three independent groups led by Guo, Goswami, and Zhao reported three merocyanine-based fluorescent probes for the ratiometric detection of H2S without interference from other biothiols through the addition reaction of S2−/HS− with an indolium moiety.24–26 To the best of our knowledge, although a number of fluorescent probes for selectively detecting Cys/Hcy, GSH, or H2S have been designed (Table S1†),27–34 there is no reported probe that can simultaneously distinguish Cys/Hcy, GSH, and H2S from each other.
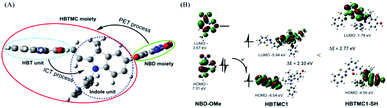
Herein, we subtly connected a hydroxyphenyl benzothiazole merocyanine (HBTMC) fluorophore and a nitro benzoxadiazole (NBD) chromophore using an ether linker as a new hybrid fluorescent dyad (HMN) (Scheme 1). The HBTMC moiety served as a recognition unit for the selective response to H2S, and the functional NBD moiety was designed for the selective detection of Cys/Hcy and the sequential sensing of Cys/Hcy/GSH and H2S. The HMN probe might be inherently non-fluorescent due to the protected hydroxyl NBD chromophore and quenching of the fluorescence of the HBTMC moiety by the NBD moiety. As shown in Scheme S1,† reaction of HMN with Cys/Hcy may cut off the ether bond, releasing the HBTMC unit and forming an amino substituted NBD chromophore (NBD-Cys/Hcy) after sequential nucleophilic substitution and intramolecular rearrangement, which will result in lighting up in green and red emission. Meanwhile, reaction of HMN with GSH may produce free HBTMC1 and a dark mono-substituted sulfhydryl NBD (NBD-GSH) via a one step nucleophilic substitution reaction, giving off only red emission (Schemes 1 and S1†). If HMN reacts with the stronger nucleophilic reagent H2S, the ether bond would also be cut off to yield a dark non-substituted sulfhydryl NBD chromophore (NBD-SH) and a free HBTMC chromophore (HBTMC1). In addition, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N double bond of HBTMC1 would be further added by HS− to afford an interrupted HBTMC derivative (HBTMC-SH), generating a new blue emission. Thus, HMN can discriminate Cys/Hcy, GSH, and H2S with three different sets of fluorescence signals in three channels. In addition, if H2S continues to be added to the mixture of HMN and Cys/Hcy, the intermediate product NBD-Cys/Hcy would be further substituted and HBTMC1 would be attacked by the HS− anion to yield dark NBD-SH and blue fluorescent HBTMC-SH, accompanied by quenching of both green and red emission (Schemes 1 and S1†). Similarly, the intermediate product HBTMC1 from the reaction of HMN with GSH could also react with H2S, and synchronously light up fluorescence in the blue emission channel (Schemes 1 and S1†). Taken together, the dyad HMN is capable of not only simultaneously distinguishing Cys/Hcy, GSH, and H2S from each other but also sequentially detecting Cys/Hcy (or GSH) and H2S through the changes of three well-defined emission bands (blue-green-red).
N double bond of HBTMC1 would be further added by HS− to afford an interrupted HBTMC derivative (HBTMC-SH), generating a new blue emission. Thus, HMN can discriminate Cys/Hcy, GSH, and H2S with three different sets of fluorescence signals in three channels. In addition, if H2S continues to be added to the mixture of HMN and Cys/Hcy, the intermediate product NBD-Cys/Hcy would be further substituted and HBTMC1 would be attacked by the HS− anion to yield dark NBD-SH and blue fluorescent HBTMC-SH, accompanied by quenching of both green and red emission (Schemes 1 and S1†). Similarly, the intermediate product HBTMC1 from the reaction of HMN with GSH could also react with H2S, and synchronously light up fluorescence in the blue emission channel (Schemes 1 and S1†). Taken together, the dyad HMN is capable of not only simultaneously distinguishing Cys/Hcy, GSH, and H2S from each other but also sequentially detecting Cys/Hcy (or GSH) and H2S through the changes of three well-defined emission bands (blue-green-red).
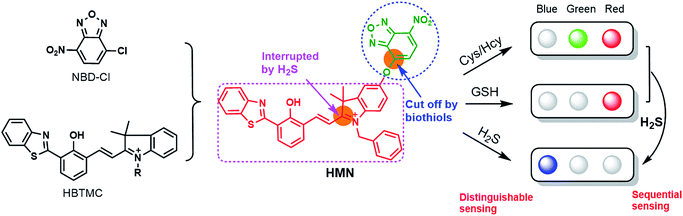 | ||
| Scheme 1 The design strategy of the probe HMN and the proposed fluorescence signal changes in response to respective biothiols or sequential biothiols with three well-defined emission bands. | ||
Results and discussion
Design and synthesis of probes
Due to the strong electron withdrawing ability of the 7-position nitro group, the functional group at the 4-position is easily substituted by nucleophilic reagents, such as sulfhydryl-containing biothiols, in the NBD chromophore. Many NBD derivatives have been employed for the selective detection of Cys/Hcy or the sequential detection of biothiols.32,33,36–38 In addition, the group of carbon–nitrogen double bonds in electropositive merocyanine and cyanine dyes can be interrupted by strongly nucleophilic reagents (such as S2− and HS− anions), while it is barely attacked by the relatively moderate nucleophile like mono-substituted sulfhydryl reagents (RSH).24–26,39 Accordingly, merocyanine dyes are the preferred platforms for designing fluorescent probes for distinguishing H2S from other biothiols. Thus, we rationally designed a hybrid fluorophore (HMN) of hydroxyphenyl benzothiazole merocyanine (HBTMC) and nitro benzoxadiazole (NBD) through an ether group for simultaneously distinguishing and sequentially sensing Cys/Hcy/GSH and H2S. The synthetic route of probe HMN is outlined in Scheme S2,† and intermediate products 1, 3, and HBTQ were prepared according to previous methods.40–42 The target product was synthesized through a nucleophilic substitution reaction of dihydroxyl HBTMC1 and NBD-Cl with desirable yield, and the results of the control experiment indicate that monohydroxyl HBTMC2 can barely react with NBD-Cl, due to the considerable steric hindrance. All new compounds were well characterized using mass spectrometry and NMR spectroscopy (for synthesis and characterization details, see the ESI†).Spectral response of the probe to biothiols
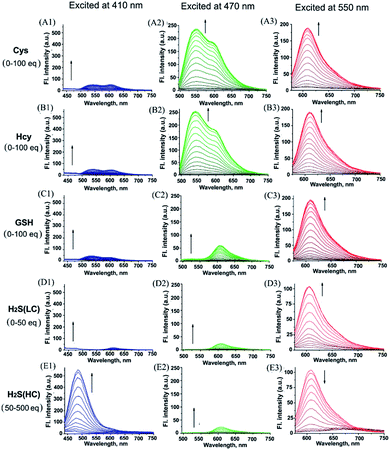
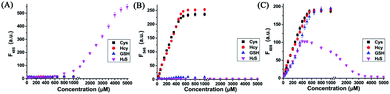
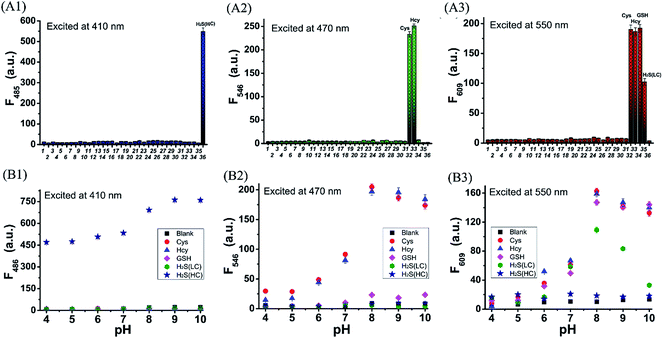
With probe HMN in hand, we firstly investigated the spectra of HMN (10 μM) in order to separate biothiols in phosphate buffer solution (25 mM, pH 7.4, containing 20% acetonitrile). In spectrofluorometric titrations, free HMN has no fluorescence in the visible region. Upon addition of increasing dosages of Cys or Hcy (from 0 to 1.0 mM), the mixture exhibits two new green and red emission bands centered at 546 nm (excited at 470 nm) and 609 nm (excited at 550 nm), respectively (Fig. 2A and B). This may be attributed to the products HBTMC1 and amino NBD (NBD-Cys/NBD-Hcy) from the sequential nucleophilic substitution and intramolecular rearrangement reactions of HMN with Cys/Hcy. When GSH (from 0 to 1.0 mM) was added to the probe solution, only a gradual increase of the red emission was observed (Fig. 2C), indicating that the reaction of the probe with GSH generates red fluorescent HBTMC1 and dark mono-substituted sulfhydryl NBD (NBD-GSH). When probe HMN encounters increasing amounts of H2S (from 0 to 5.0 mM), the mixture responds with the emergence of red emission, like GSH as the H2S concentration ranged from 0–0.5 mM (Fig. 2D). However, as the H2S concentration continues to increase, the emerging red emission begins to weaken and synchronously generates a new blue emission band centered at 485 nm (excited at 410 nm). These results can be interpreted to mean that probe HMN is cut off by H2S at a low concentration (abbreviated as H2S(LC)) and produces the red fluorescent chromophore HBTMC1 and non-fluorescent non-substituted sulfhydryl NBD (NBD-SH), while the intermediate product HBTMC1 is further attracted by H2S at a high concentration (abbreviated as H2S(HC)) and yields the blue fluorescent compound HBTMC1-SH. As the intensity of the newly generated emission reaches the maximum values, Cys/Hcy induces a 53.4/57.6 and 32.2/32.9-fold enhancement of the fluorescence intensity at 546/609 nm, GSH induces 33.6-fold enhancement of intensity at 609 nm, and H2S(LC)/H2S(HC) generates a 17.7/52.7-fold increase of intensity at 609/485 nm (Fig. 3). Accordingly, the fluorescent colour of HMN was changed from dim to orange, pink, and blue by the addition of Cys/Hcy, GSH/H2S(LC), and H2S(HC), respectively (Fig. S1†). Furthermore, all biothiols have good linear relationships with the maximum intensity values of newly generated red emission bands in a certain concentration range (Fig. S2†), and accordingly the limit of detection (defined by 3σ/k, where σ is the standard deviation of the blank sample and k is the slope of the linear regression equation) is calculated to be 4.25, 5.11, 4.30, and 6.74 μM for Cys, Hcy, GSH, and H2S, respectively. In the reaction-time study, when the changes of fluorescence intensity are completed, the intensity values at 546 and 609 nm reach plateaus 12/20 and 15/20 min after adding 55 equivalents of Cys/Hcy, respectively (Fig. S2b and c†). The new red emission induced by GSH (65 equiv.) or H2S(LC) (50 equiv.) reaches the steady state within 20 and 4 min, respectively, and the value of intensity at 485 nm induced by H2S(HC) (500 equiv.) reaches a maximum within about 2 min (Fig. S3a and c†). Therefore, probe HMN is capable of simultaneously distinguishing Cys/Hcy, GSH, and H2S from each other and sensing H2S at different concentration levels through the changes of three well-defined emission bands (blue-green-red), which possess 61 and 63 nm interval distances between two adjacent bands respectively.To shed light on the response process, the absorption spectra of HMN were also investigated in aqueous solution. As shown as in Fig. S4a,† free HMN exhibits two characteristic absorption peaks of NBD and HBTMC at 373 and 577 nm, respectively. Upon addition of Cys/Hcy, the absorption peak of HMTMC slightly blue shifts to 563 nm and a shoulder peak simultaneously emerges at about 485 nm, which can be attributed to the products HBTMC1 and NBD-Cys/Hcy from reaction of HMN with Cys/Hcy. Similarly, the absorption peaks of HBTMC1 and NBD-GSH are observed at 563 and 435 nm in the presence of GSH. When adding increasing dosages of H2S, both of the two peaks of HMN gradually fade, and this is accompanied by the emergence of a broad peak at about 554 nm, which should contain two absorption peaks of HBTMC1 and NBD-SH. To confirm the original attribution of those newly generated absorption peaks, the absorption spectra of control compounds NBD-Cl and HBTMC1 in the presence of the respective biothiols were studied, and the results are coincident with the spectral performance of HMN (Fig. S4b and c†). Furthermore, the mass spectra of HMN in the respective biothiols were also measured in order to confirm the proposed reaction processes. As shown in Fig. S5,† the peaks of the key products HMBMC1 (m/z = 503.2) and NBD-Cys (m/z = 284.3) are observed in the mixture of HMN and Cys. In a similar way, the peaks of NBD-Hcy (m/z = 299.2), NBD-GSH (m/z = 471.1), and NBD-SH (m/z = 197.1) appear in the spectra of HMN in the presence of Hcy, GSH, and H2S respectively, while treatment with a high concentration of H2S generates compound HBTMC1-SH (m/z = 536.2) (Fig. S6–9†). Therefore, the results of the measured absorption spectra and mass spectra of HMN in the presence of various biothiols agree with the proposed reaction mechanism.
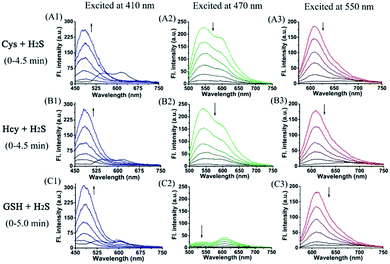
Furthermore, we also investigated the spectral response of HMN upon successive addition of Cys/Hcy/GSH, and H2S in aqueous solution. Upon continuing addition of greatly excessive amounts of H2S to the mixture of HMN and Cys, both green and red emission gradually fade away and, synchronously, significant emission in the blue channel is generated (Fig. 4A). A similar phenomenon occurs in the mixture of HMN and Hcy (Fig. 4B). This could be ascribed to the idea that products HBTMC1 and NBD-Cys/Hcy from the reaction of the probe with Cys/Hcy further react with the subsequent H2S, yielding an interrupted merocyanine dye HBTMC1-SH and non-fluorescent NBD-SH. When adding excessive H2S to the HMN and GSH mixture, the increasing blue emission accompanied by gradually weakening red emission is also observed (Fig. 4C). Accordingly, the orange (or pink) fluorescence of HMN induced by Cys/Hcy (or GSH) was finally changed to bright blue emission by the subsequent addition of H2S (Fig. S10†). In the absorption spectra, the weakness of the HBTMC1 absorption and disappearance of the NBD-Cys/Hcy absorption proves that HMN can react with Cys/Hcy/GSH and H2S sequentially (Fig. S11†). The results indicated that probe HMN not only can simultaneously distinguish Cys/Hcy, GSH, and H2S from each other, but also can sequentially sense Cys/Hcy/GSH and H2S with three-channel fluorescence signal changes.
To evaluate the selectivity of probe HMN for biothiols, the specificity of HMN was tested in the presence of various relevant biological amino acids, anions, cations, ROS, and RNS, including alanine (Ala), arginine (Arg), aspartic acid (Asp), glutamic acid (Glu), isoleucine (Ile), phenylalanine (Phe), serine (Ser), threonine (Thr), tryptophan (Trp), valine (Val), histidine (His), VC, AcO−, Br−, Cl−, NO3−, NO2−, N3−, SO32−, S2O32−, Cu+, Ca2+, Fe2+, Fe3+, Zn2+, HClO, O2−, H2O2, TBHP, and NO. As shown in Fig. 5A, HMN exhibits negligible fluctuation of fluorescence intensity in all three emission bands upon treatment with 1 mM of these interfering species. However, remarkable enhancement of the fluorescence intensity at both 546 and 609 nm is observed after treatment with 550 μM Cys or Hcy; addition of GSH (650 μM) only produces a significant increase of intensity at 609 nm, while treatment of H2S(LC) (500 μM) or H2S(HC) (5000 μM) generates an obvious enhancement of intensity at 609 or 485 nm, respectively. This suggests that probe HMN can selectively detect Cys/Hcy, GSH, and H2S without interference from relevant amino acids, ROS, and RNS through changes of three well-defined emission bands. In addition, the effect of environmental pH on the response of HMN was also studied. As shown in Fig. 5B, the fluorescence intensity of free HMN at all three emission bands is nearly not fluctuant in aqueous solutions with different pH values. However, at pH ranging from 7.0 to 8.0, probe HMN generates significant enhancement of the fluorescence intensity in both blue and red emission bands in the presence of Cys/Hcy; addition of GSH induces fluorescence enhancement in only the red emission band, and an increase of red or blue fluorescence is observed in the presence of H2S(LC) or H2S(HC), respectively. Thus, HMN has the potential to discriminatively detect biothiols under physiological conditions.
Theoretical study on the fluorescence mechanism
To better understand the spectral changes of probe HMN responding to biothiols, theoretical calculations were performed using density functional theory (DFT) with the B3LYP(d) exchange functional employing 6-31G* basis sets using a suite of Gaussian 09 programs. In the optimized structures of HMN, the dihedral angle of the HBT unit and indole unit is 92.6° in the conjugated HBTMC moiety, and the NBD moiety is also nearly perpendicular to indole group (86.3° angle), which suggests that the NBD moiety has a minimal impact on the electron delocalization degree of the HBTMC moiety (Fig. 6A). Consequently, the emission of the HBTMC moiety should be regulated by the NBD unit via the photoinduced electron transfer (PET) process. To validate the proposed fluorescence mechanism, the frontier orbital energy of both separate NBD and HBTMC fluorophores was also calculated. As shown in Fig. 6B, the energy magnitude of the highest occupied molecular orbital (HOMO) of the oxyalkyl NBD chromophore (methoxyl NBD derivative, NBD-OMe) is between that of the lowest unoccupied molecular orbital (LUMO) and HOMO of the HBTMC chromophore (HBTMC1), indicating that the electron tends to transfer from the HOMO of NBD to the transition HOMO of HBTMC at the excited state. What’s more, the π electrons on both the HOMO and LUMO efficiently distribute in the respective HBT and indole units in HBTMC1, suggesting that HBTMC is a typical intramolecular charge transfer (ICT)-based fluorophore. However, the product HBTMC1-SH from the addition reaction between HBTMC1 and H2S possesses a bigger energy difference between the LUMO and HOMO (2.77 eV) than that of HBTMC1 (2.10 eV) (Fig. 6B), which can be ascribed to the interruption of the ICT process from the electron-donating HBT group to the electron-withdrawing indole group. Thus, the response emission of HMN to biothiols is regulated by the PET and ICT mechanisms together.Discriminative detection of biothiols in living cells
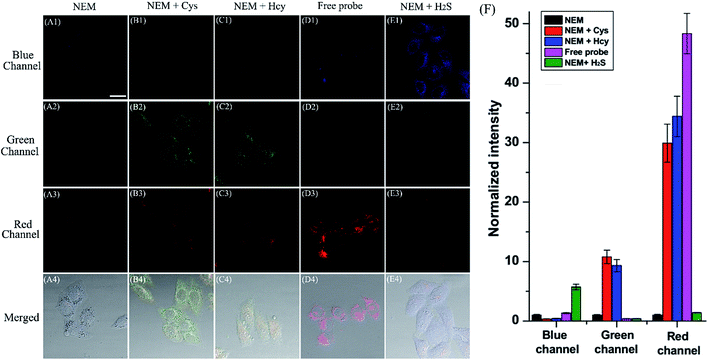
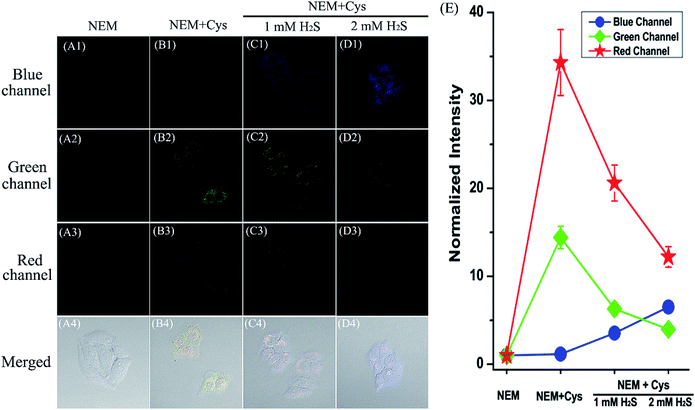
Encouraged by the desirable spectral response of the probe to biothiols in aqueous solution, we then investigated the capability of HMN to detect biothiols in biological systems in a three-color manner (blue-green-red) using confocal fluorescence microscopy. Before that, the cell cytotoxicity of HMN was evaluated using standard MTT assays, and these demonstrate that HMN has low cytotoxicity to living HeLa cells (Fig. S12†). For probe HMN, thiol- and amino-containing species are the most likely potential interferents for the imaging of biothiols in living systems. The selectivity assay suggested that treatment with amino-containing species (such as various amino acids) induced a very small amount of interference in the detection of biothiols (Fig. 5). In addition, we selected bovine serum albumin (BSA) as a model thiol-containing protein, and the spectral response of HMN to BSA was investigated. The results showed that negligible fluorescence fluctuation of the probe in the blue/green emission band and a small fluorescence fluctuation in the red emission band was generated in the presence of BSA (0–1000 μg mL−1) in PBS (Fig. S13†). This might be because most of the sulfhydryls in BSA are present in the oxidized form (S–S), but both the reduced form (–SH) and the disulfide bonds exist stably in the physiological environment.43,44 Thus, thiol-containing proteins have little effect on the detection of biothiols in living cells. In the control experiment, after being pre-treated with N-ethylmaleimide (NEM, a thiol scavenger, 0.5 mM), HeLa cells loaded with HMN (5 μM) exhibit very dim fluorescence in all three channels (Fig. 7A), which is in agreement with the emission spectral performance of the free probe in an aqueous system. For the experimental groups, the cells continued to be incubated with respective exogenous biothiols (Cys/Hcy/H2S) after being pre-treated with NEM for 30 min. Upon continued treatment with Cys/Hcy (250 μM), significant enhancement of fluorescence in both the green (about 10.8/9.3-fold) and red (about 29.9/34.4-fold) channels is observed in the cells loaded with HMN (Fig. 7B and C). However, the cells treated with free HMN (5 μM) show a bright emission in the red channel (Fig. 7D), which indicates that HMN reacts with the most abundant cellular biothiol, GSH, and yields the strongly fluorescent HBTMC1 chromophore. The cells pre-incubated with successive NEM and Na2S (a donor for H2S,45 2 mM) generate a remarkable increase in blue fluorescence (about 5.7-fold), while almost no fluorescence emission is observed in the green and red channels (Fig. 7E). Thus, HMN can discriminate cellular Cys/Hcy, GSH, and H2S (at relatively high concentration levels) with three different sets of fluorescence signals via three-color fluorescence imaging.In addition, we also carried out fluorescence imaging of exogenous H2S and endogenous H2S induced by Cys at a relatively low level in cells. As shown in Fig. S14,† after pre-treatment with NEM, the cells incubated with Na2S (200 μM) show a slight and remarkable enhancement of fluorescence in the blue and red channels, respectively. However, upon pre-incubation with Cys (a precursor of H2S,46 200 μM) for 1 hour, a similar fluorescence performance is observed to that resulting from the addition of a small dosage of Na2S in living cells (Fig. S14c†), and this can be attributed to the endogenous production of H2S stimulated by Cys. In brief, HMN can generate three or two distinct sets of fluorescence signals different from the control experiment in response to cellular biothiols (Cys/Hcy, GSH, and H2S (at relatively high or low concentration levels)) in three-channel fluorescence imaging, which is in accordance with the spectral performances in aqueous solution. Thus, HMN not only can simultaneously distinguish three groups of biothiols (Cys/Hcy, GSH, and H2S (at relatively high concentration levels)) in aqueous and biological systems via three-channel fluorophotometric assay, but is also appropriate for imaging applications able to simultaneously distinguish Cys/Hcy and endogenous H2S (at relatively low concentration levels) using the green and blue channels.
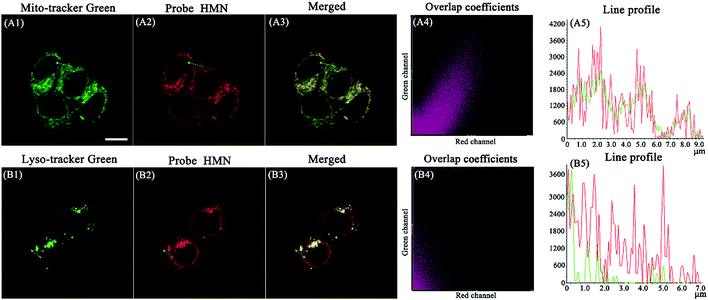
To confirm the distribution of HMN at subcellular levels, co-localization experiments were carried out in HeLa cells. The cells were pre-treated with HMN (5 μM) for 25 min and Mito-Tracker Green or Lyso-Tracker Green (1 μM) was subsequently added and incubated for another 5 min. As shown in Fig. 8A, as expected, the fluorescence image of HMN in the red channel almost completely overlaps with that of the mitochondrial probe (Mito-Tracker Green) in the green channel, with a high overlap coefficient (0.869). In contrast, the fluorescence image of HMN has an extremely inefficient overlap with that of the lysosomal probe (Lyso-Tracker Green) and the overlap coefficient is as low as 0.476 (Fig. 8B). It might be that the electropositive HMN tends to localize to the cellular mitochondria with a negative potential.22,47 Thus, probe HMN is a potentially excellent tool for studying mitochondrial biothiols.
Sequential sensing of biothiols in living cells
After reaching the goal of simultaneous discrimination of biothiols, we further employed HMN to sequentially sense biothiols in living cells. The cells were firstly treated with NEM to scavenge the inherent thiols, followed by the addition of Cys (250 μM) and incubation for 20 min. Cells were subsequently incubated for another 30 min with HMN (5 μM). The bright emission induced by Cys is observed in both the green and red channels (Fig. 9A and B). However, with increasing dosages of Na2S (1–2 mM), the emission is gradually weakened in the green and red channels, and at the same time a significant enhancement of fluorescence appears in the blue channel (Fig. 9C and D). This could be interpreted to mean that the encounter of HMN with cellular Cys produces the red fluorescent compound HBTMC1 and a green fluorescent NBD derivative NBD-Cys, which further reacts with H2S to yield a fluorescent compound HBTMC1-SH with blue emission and a non-fluorescent compound NBD-SH, respectively. Similarly, the red emission generated by the reaction of HMN with cellular GSH is quenched by the subsequently added H2S, and a gradual increase of blue fluorescence arises simultaneously (Fig. S15†). These results indicated that probe HMN is capable of sequentially sensing biothiols in biological contexts using three-color fluorescence imaging, in addition to simultaneous discrimination of biothiols from each other.Conclusions
In summary, by hybridizing a hydroxyphenyl benzothiazole merocyanine (HBTMC) dye and a nitro benzoxadiazole (NBD) chromophore, we have developed a single fluorescent probe, HMN, for simultaneously distinguishing Cys/Hcy, GSH, and H2S from each other and sequentially sensing Cys/Hcy/GSH and H2S via a multi-channel fluorometric method for the first time. When responding to respective biothiols, HMN exhibits multiple sets of fluorescence signals in three distinct emission bands (blue-green-red), which possess 61 and 63 nm interval distances between two adjacent bands, respectively. The probe can also sense H2S at different concentration levels with changes of fluorescence in the blue and red emission bands. The excellent performance of HMN in the three-channel fluorescence imaging indicates that the probe can be applied to simultaneously discriminate biothiols from each other and sequentially sense Cys/Hc/GSH and H2S in biological contexts. The development of this unique probe may open an avenue to design fluorescent probes for the discrimination and sequential detection of biothiols, and provides a promising tool for unveiling the interrelated roles of biothiols in various physiological and pathological conditions.Acknowledgements
This work was financially supported by NSFC (21472067, 21672083, and 21605059), SDNSF (ZR2016BB26), Taishan Scholar Foundation (TS 201511041), and the start-up fund of the University of Jinan (309-10004 and 160100136).Notes and references
- G. I. Giles, K. M. Tasker and C. Jacob, Free Radical Biol. Med., 2001, 31, 1279 CrossRef CAS PubMed.
- Z. A. Wood, E. Schröder, J. R. Harris and L. B. Poole, Trends Biochem. Sci., 2003, 28, 32 CrossRef CAS PubMed.
- G. I. Giles and C. Jaco, Biol. Chem., 2005, 383, 375 Search PubMed.
- M. Kemp, Y.-M. Go and D. P. Jones, Free Radical Biol. Med., 2008, 44, 921 CrossRef CAS PubMed.
- M. T. V. ishanina, M. Libiad and R. Banerjee, Nat. Chem. Biol., 2015, 11, 457 CrossRef PubMed.
- L. Li, P. Rose and P. K. Moore, Annu. Rev. Pharmacol., 2011, 51, 169 CrossRef CAS PubMed.
- G. Yang, L. Wu, B. Jiang, B. Yang, J. Qi, K. Cao, Q. Meng, A. K. Mustafa, W. Mu, S. Zhang, S. H. Snyder and R. Wang, Science, 2008, 322, 587 CrossRef CAS PubMed.
- O. Kabil and R. Banerjee, J. Biol. Chem., 2010, 285, 21903 CrossRef CAS PubMed.
- N. C. Sturgess, R. Z. Kozlowski, C. A. Carrington, C. N. Hales and J. M. L. Ashford, Br. J. Pharmacol., 1988, 95, 83 CrossRef CAS PubMed.
- G. Yang, L. Wu and R. Wang, FASEB J., 2006, 20, 553 CAS.
- S. Singh, D. Padovani, R. A. Leslie, T. Chiku and R. Banerjee, J. Biol. Chem., 2009, 284, 22457 CrossRef CAS PubMed.
- J. E. Dominy and M. H. Stipanuk, Nutr. Rev., 2004, 62, 348 Search PubMed.
- S. Y. Zhang, C.-N. Ong and H.-M. Shen, Cancer Lett., 2004, 208, 143 CrossRef CAS PubMed.
- M. E. Anderson, Chem.-Biol. Interact., 1998, 111, 1 CrossRef PubMed.
- T. D. Dalton, H. G. Shertzer and A. Puga, Annu. Rev. Pharmacol. Toxicol., 1999, 39, 67 CrossRef CAS PubMed.
- P. N. Prasad, Bioimaging: Principles and Techniques, in Introduction to Biophotonics, John Wiley & Sons, Inc., 2003, ch. 7 Search PubMed.
- J. Chan, S. C. Dodani and C. J. Chang, Nat. Chem., 2012, 4, 973 CrossRef CAS PubMed.
- X. Chen, T. Pradhan, F. Wang, J. S. Kim and J. Yoon, Chem. Rev., 2012, 112, 1910 CrossRef CAS PubMed.
- A. Chattopadhyay, S. Shrivastava and A. Chaudhuri, Membrane Fluorescent Probes: Insights and Perspectives, in Fluorescent Analogues of Biomolecular Building Blocks: Design and Applications, John Wiley & Sons, Inc., 2016, ch. 15 Search PubMed.
- K. P. Carter, A. M. Young and A. E. Palmer, Chem. Rev., 2014, 114, 4564 CrossRef CAS PubMed.
- A. P. Demchenko, Introduction to fluorescence sensing, Springer, 2015 Search PubMed.
- H. Zhu, J. Fan, J. Du and X. Peng, Acc. Chem. Res., 2016, 49, 2115 CrossRef CAS PubMed.
- L. A. Montoya and M. D. Pluth, Anal. Chem., 2014, 86, 6032 CrossRef CAS PubMed.
- Y. Chen, C. Zhu, Z. Yang, J. Chen, Y. He, Y. Jiao, W. He, L. Qiu, J. Cen and Z. Guo, Angew. Chem., Int. Ed., 2013, 52, 1688 CrossRef CAS PubMed.
- S. Paul, S. Goswami and C. D. Mukhopadhyay, New J. Chem., 2015, 39, 8940 RSC.
- X. Feng, T. Zhang, J.-T. Liu, J.-Y. Miao and B.-X. Zhao, Chem. Commun., 2016, 52, 3131 RSC.
- H. S. Jung, X. Chen, J. S. Kim and J. Yoon, Chem. Soc. Rev., 2013, 42, 6019 RSC.
- L.-Y. Niu, Y.-Z. Chen, H.-R. Zheng, L.-Z. Wu, C.-H. Tung and Q.-Z. Yang, Chem. Soc. Rev., 2015, 44, 6143 RSC.
- V. S. Lin, W. Chen, M. Xian and C. J. Chang, Chem. Soc. Rev., 2015, 44, 4596 RSC.
- J. Liu, Y.-Q. Sun, Y. Huo, H. Zhang, L. Wang, P. Zhang, D. Song, Y. Shi and W. Guo, J. Am. Chem. Soc., 2014, 136, 574 CrossRef CAS PubMed.
- H. Zhang, R. Liu, J. Liu, L. Li, P. Wang, S. Q. Yao, Z. Xu and H. Sun, Chem. Sci., 2016, 7, 256 RSC.
- W. Chen, H. Luo, X. Liu, J. W. Foley and X. Song, Anal. Chem., 2016, 88, 3638 CrossRef CAS PubMed.
- Q. Hu, C. Yu, X. Xia, F. Zeng and S. Wu, Biosens. Bioelectron., 2016, 81, 341 CrossRef CAS PubMed.
- H. Li, W. Peng, W. Feng, Y. Wang, G. Chen, S. Wang, S. Li, H. Li, K. Wang and J. Zhang, Chem. Commun., 2016, 52, 4628 RSC.
- Z.-H. Fu, X. Han, Y. Shao, J. Fang, Z.-H. Zhang, Y.-W. Wang and Y. Peng, Anal. Chem., 2017, 89, 1937 CrossRef CAS PubMed.
- L.-Y. Niu, H.-R. Zheng, Y.-Z. Chen, L.-Z. Wu, C.-H. Tunga and Q.-Z. Yang, Analyst, 2014, 139, 1389 RSC.
- M. D. Hammers and M. D. Pluth, Anal. Chem., 2014, 86, 7135 CrossRef CAS PubMed.
- X. Gao, X. Li, L. Li, J. Zhou and H. Ma, Chem. Commun., 2015, 51, 9388 RSC.
- M. Ren, B. Deng, X. Kong, K. Zhou, K. Liu, G. Xu and W. Lin, Chem. Commun., 2016, 52, 6415 RSC.
- K. Schöller, S. Küpfer, L. Baumann, P. M. Hoyer, D. de Courten, R. M. Rossi, A. Vetushka, M. Wolf, N. Bruns and L. J. Scherer, Adv. Funct. Mater., 2014, 24, 5194 CrossRef.
- D. Oushiki, H. Kojima, T. Terai, M. Arita, K. Hanaoka, Y. Urano and T. Nagano, J. Am. Chem. Soc., 2010, 132, 2795 CrossRef CAS PubMed.
- P. Xu, T. Gao, M. Liu, H. Zhang and W. Zeng, Analyst, 2015, 140, 1814 RSC.
- S. F. Betz, Protein Sci., 1993, 2, 1551 CrossRef CAS PubMed.
- P. J. Hogg, Trends Biochem. Sci., 2003, 28, 210 CrossRef CAS PubMed.
- G. Yang, L. Wu, B. Jiang, W. Yang, J. Qi, K. Cao, Q. Meng, A. K. Mustafa, W. Mu, S. Zhang, S. H. Snyder and R. Wang, Science, 2008, 322, 587 CrossRef CAS PubMed.
- Y. Qian, J. Karpus, O. Kabil, S.-Y. Zhang, H.-L. Zhu, R. Banerjee, J. Zhao and C. He, Nat. Commun., 2011, 2, 1506 Search PubMed.
- B. Kadenbach, Biochim. Biophys. Acta, Bioenerg., 2003, 1604, 77 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details for chemical synthesis of all compounds, chemical structure characterization, supplementary spectra of probe, and fluorescence imaging methods and data. See DOI: 10.1039/c7sc00423k |
| This journal is © The Royal Society of Chemistry 2017 |