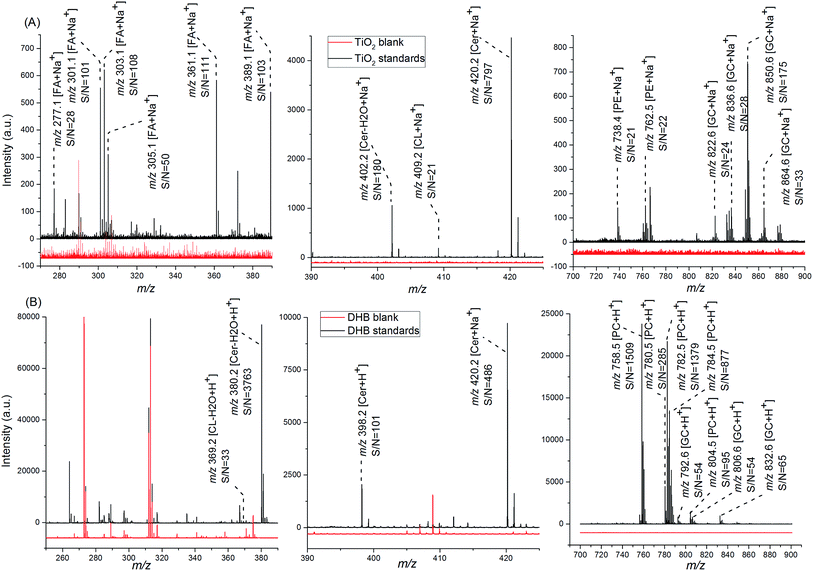
Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Dopamine-modified TiO2 monolith-assisted LDI MS imaging for simultaneous localization of small metabolites and lipids in mouse brain tissue with enhanced detection selectivity and sensitivity†
Qian
Wu
ac,
James L.
Chu
b,
Stanislav S.
Rubakhin
 ac,
Martha U.
Gillette
bc and
Jonathan V.
Sweedler
ac,
Martha U.
Gillette
bc and
Jonathan V.
Sweedler
 *ac
*ac
aDepartment of Chemistry, University of Illinois at Urbana-Champaign, 600 S. Mathews Ave, 63-5, Urbana, Illinois 61801, USA. E-mail: jsweedle@illinois.edu
bDepartment of Cell and Developmental Biology, University of Illinois at Urbana-Champaign, Urbana, Illinois 61801, USA
cBeckman Institute, University of Illinois at Urbana-Champaign, 405 N. Mathews Ave, 63-5, Urbana, Illinois 61801, USA
First published on 21st March 2017
Abstract
Localization of metabolites using multiplexed mass spectrometry imaging (MSI) provides important chemical information for biological research. In contrast to matrix-assisted laser desorption/ionization (MALDI), TiO2-assisted laser desorption/ionization (LDI) for MSI improves detection of low molecular mass metabolites (<500 Da) by reducing matrix background. However, the low UV absorption of TiO2 nanoparticles and their ester hydrolysis catalytic activity hinder the detection of phospholipids and many low-abundance molecules. To address these challenges, we evaluated and optimized the material morphology and composition of TiO2. Dopamine (DA) was found to be an efficient ligand for TiO2, resulting in increased UV light absorption, higher surface pH, and formation of monolithic TiO2-DA structures. The sub-micron scale and higher surface pH of the TiO2 particle sizes led to improved detection of phospholipid signals. Compared to unmodified TiO2 sub-micron particles, the DA-modified TiO2 monolith led to 10- to 30-fold increases in the signal-to-noise ratios of a number of compound peaks. The TiO2-DA monolith-assisted LDI MSI approach has higher selectivity and sensitivity for Lewis basic compounds, such as fatty acids, cholesterols, ceramides, diacylglycerols, and phosphatidylethanolamine, when analyzed in positive mode, than traditional MALDI MS. Using this new method, over 100 molecules, including amino acids, alkaloids, free fatty acids, peptides, and lipids, were localized in mouse brain sections. By comparing the presence and localization of those molecules in young and old mouse brains, the approach demonstrated good performance in the determination of aging-related neurochemical changes in the brain.
Introduction
Mass spectrometry imaging (MSI),1 a sensitive and multiplexed approach for the characterization and localization of a wide range of analytes, is used for both targeted and untargeted analyses. The field encompasses a variety of desorption/ionization techniques, including secondary ion mass spectrometry imaging, laser desorption/ionization (LDI) MSI, matrix-assisted laser desorption/ionization (MALDI) MSI,2 and desorption electrospray ionization MSI. Of these, MALDI MSI enables analyses across the widest molecular mass range3–6 at micrometer-scale spatial resolution, with analyte detection limits comparable to other MS-based approaches.7,8 MALDI MSI is well suited for the detection and localization of small metabolites,4,6 lipids,9 peptides, and proteins10 in tissues. The method can be used to reveal molecular mechanisms of disease,11 facilitate biomarker discovery,12 and unveil the chemical mechanisms of different physiological processes, such as cell-to-cell signaling,13 cell metabolism,14 and brain aging.15Traditional MALDI matrixes, such as 2,5-dihydroxybenzoic acid (DHB), α-cyano-4-hydroxycinnamic acid, and 9-aminoacridine (9-AA), are small molecules with high UV light absorption and good desorption/ionization abilities.16 These widely used matrixes are indispensable in bioanalytical measurements of lipids, peptides, and proteins. However, due to the presence of multiple, intense MALDI matrix-related signals in the resulting mass spectra, their use in the detection of endogenous small molecules is limited.17 As one means to address this challenge, on-tissue derivatization was developed to increase the molecular mass of small analytes and assist targeted analysis.18 Other limitations of MALDI have also been reported, including ion suppression of targeted analytes by compounds that exhibit more efficient ionization and/or are present at high abundance,16,19 leading to lower selectivity.20 To overcome these drawbacks, nanomaterials such as porous silicon,14 carbon nanomaterials,21–23 gold/silver nanoparticles,24 and metal oxide nanoparticles17 have been tested as alternatives to traditional MALDI matrixes and used in approaches that are commonly referred to as surface- or nanoparticle-assisted laser desorption/ionization (LDI).25,26 In contrast to MALDI, the distinct ionization mechanism of nanoparticle-assisted LDI results in fewer background signals. Also, choosing a nanomaterial with an appropriate affinity for a specific analyte may increase the selectivity of the mass spectrometry (MS) analysis. Moreover, the deposition of thin layers of nanoscale-sized particles onto a biological sample results in a uniform coating, which facilitates high spatial resolution in MSI.
Among the available nanomaterials, TiO2 has been widely used to assist LDI.27 Compared with silicon and gold or silver nanoparticles, which are not always stable in an ambient environment (e.g., surface oxidation) or under laser irradiation (resulting in the generation of artifacts such as Aun clusters),24,27 TiO2 creates a stable layer on samples. Carbon nanomaterials for LDI also offer great potential, although they are reported to be hard to disperse in solution28 and can be dislodged, resulting in contamination of an instrument's ion source.21 TiO2-assisted LDI MS has been used in the detection of r-cyclodextrin,29 steroid hormones,28,30 and peptides and small proteins,31 as well as other neutral small molecules such as catechins.32 TiO2-assisted LDI MSI was successfully applied to localize small molecules (molecular mass below 500 Da) in the mouse brain;33 however, few endogenous lipids, which are critical components in biological systems, were detected. The detection of several phosphatidylcholines (PCs) and triacylglycerides with TiO2 particles in soy bean extracts has been reported.34 The authors also proposed that TiO2 acts as a catalyst for phospholipid hydrolysis, and interestingly, the use of TiO2 microparticles instead of TiO2 nanoparticles resulted in relatively higher signals for lipids. In recent MSI studies using TiO2 nanoparticles, the degradation of esters was also found.35
Several studies have demonstrated that modifications to TiO2 can impact its catalytic and other physiochemical properties. Some specific nanostructured TiO2 methods provide faster hydrolysis of organophosphorus esters.36 The Lewis acid site in TiO2 (Ti4+) has a strong affinity to Lewis bases, such as phosphoric salt,37 or organic anchoring groups, including cis-enediol compounds, phosphonate, and hydroxyl carboxylic acid;38–40 the coordination of Ti with these ligands can cause a red shift to its UV absorption. Recently, modified TiO2 particles have been used in TiO2-assisted LDI analysis30,32 for targeted analyte detection, including steroid hormone measurements.
Since it is important to characterize larger lipids41–44 and small metabolites,45–49 many of which are hard to characterize using existing methods, we modified the surface properties of TiO2 to change its catalytic activity and increase its UV absorption, and then evaluated the modifications to the structure of TiO2 using different Lewis base ligands. Dopamine (DA), the optimal ligand, was found to largely increase the UV absorption of TiO2, leading to an increase in the sensitivity of MSI detection. The micro-morphology of TiO2 was also optimized, allowing formation of monolithic structures that led to an increase in active surface area. In addition, the pH environment on the TiO2 monolithic surface was increased, reducing the catalytic hydrolysis of the lipids. Using our approach, over 50 lipids were detected in the mouse brain, together with 35 metabolites with molecular weights under 500 Da. We validated the method using the aging brain model, in which many compounds, including proteins,42 lipids,41–44,48 and small metabolites,45–49 have been reported to change during aging, but as of yet have not been localized together. Our imaging results combined with principal component analysis (PCA) revealed important chemical differences between old and young animals, including the determination of six molecules with signal levels that changed in an age-dependent manner. These results offer a new perspective on the chemical signature of brain aging.
Experimental section
Chemicals and materials
The following chemicals were purchased from Sigma-Aldrich (St. Louis, MO): titanium(IV) n-butoxide, dopamine hydrochloride (>98%), alizarin (>97%), ascorbic acid (>98%), salicylic acid (SA) (>99%), DHB, phospholipid mixture (1 mg mL−1, HPLC grade), cholesterol (>99%), N-hexanoyl-D-sphingosine (>98%), galactocerebrosides mixture (1 mg mL−1, HPLC grade), linolenic acid (purity ≥ 99%), linoleic acid (purity ≥ 99%), erucic acid (purity ≥ 99%), elaidic acid (purity ≥ 99%), and palmitoleic acid (purity ≥ 98.5%). Concentrated nitric acid and phosphoric acid (analytical grade), acetonitrile, ethanol, and water (liquid chromatography (LC)/MS grade) were purchased from Fisher Scientific (Pittsburgh, PA).Synthesis of TiO2 nanoparticles, sub-micron particles, and monolith
TiO2 nanoparticles were prepared using a previously described sol–gel method to hydrolyze titanium(IV) n-butoxide in an ethanol–water solution under acidic conditions.33 Briefly, 3.4 mL of titanium(IV) n-butoxide and 1.6 mL of ethanol were mixed by vortex for 1 min, forming a precursor solution. Then, a solution containing 5 mL of ethanol with 0.1 M nitric acid and 1% HPLC grade water was added dropwise to the vigorously stirred precursor solution, which was cooled in an ice/water bath. TiO2 structures were formed in this solution (solution I) during stirring in an ice/water bath for 3 h.In preparation of the TiO2 nanoparticle deposition mixture, 250 μL of solution I were directly diluted in 5 mL ethanol. Modified TiO2 nanoparticle deposition mixtures were prepared by the addition of 0.005 M ligands (DA, alizarin, ascorbic acid, or SA) into the diluted solution I followed by a 30 min incubation. In preparation of the TiO2 sub-micron particle deposition mixture and DA-modified TiO2 monolith deposition mixture, 250 μL of solution I were diluted in 5 mL of reaction solution for further hydrolysis/condensation (solution II). For TiO2 sub-micron particle deposition mixture, solution II was 1% water–ethanol solution, and mixtures were incubated for different durations, 10 min, 3 h, 12 h, and 24 h (see Fig. S1†). For modified TiO2 monolith deposition mixture, solution II was 5% water–ethanol with 0.005 M of the ligand, and the mixture was incubated for 1 h. The approaches used to characterize the TiO2 structures are described in the ESI.†
Animals and brain sample preparation
Inbred C57BL/6 mice of both genders around the ages of 8 months (young) or 24 months (old) were used. Animals were housed under a 12 h![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 h light–dark cycle at 18–26 °C, 30–70% humidity. Food and water were accessible ab libitum. All animal-related procedures, including euthanasia by cervical dislocation, were performed in compliance with local and federal regulations and according to animal use protocols approved by the University of Illinois Institutional Animal Care and Use Committee.
12 h light–dark cycle at 18–26 °C, 30–70% humidity. Food and water were accessible ab libitum. All animal-related procedures, including euthanasia by cervical dislocation, were performed in compliance with local and federal regulations and according to animal use protocols approved by the University of Illinois Institutional Animal Care and Use Committee.
After quick extraction, brains were chilled at 4 °C in an artificial cerebral spinal fluid tissue slicing solution containing (in mM): 93 N-methyl-D-glucamin, 2.5 KCl, 1.2 NAH2PO4, 30 NaHCO3, 20 HEPES, 25 glucose, 5 sodium ascorbate, 2 thiourea, 3 sodium pyruvate, 10 MgSO4, and 0.5 CaCl2, conditioned with 95% O2/5% CO2 (adapted from a prior report50). Coronal brain slices, 400 μm thick, were cut through the hippocampus and prepared with a Leica VT1000 S vibrating blade microtome (Leica Biosystems, Buffalo Grove, IL). Brain slices were transferred to glass slides covered with Parafilm M (Pechiney Plastic Packaging, Inc., Batavia, IL) and frozen over dry ice for further cryo-sectioning and MS analysis.
Sample preparation for MSI
The 400 μm thick frozen coronal brain slices were further cut into a set of 10 μm thick sections using a cryostat (Leica CM3050 S, Leica Biosystems Inc.). Sections were thaw-mounted on indium-tin-oxide coated glass slides (Delta Technology Ltd, Loveland, CO) and dried in an N2-filled desiccator for 20 min. For TiO2-assisted LDI, all types of TiO2-containing mixtures were applied onto tissue sections by airbrush-assisted deposition. Briefly, 5 mL of the above described, appropriately diluted TiO2-containing solutions were sprayed with a 0.2 mm nozzle airbrush (Paasche Airbrush Company, Chicago, IL), with a nozzle-to-target distance of ∼50 cm and nozzle nitrogen gas pressure set at 35 psi. The final TiO2 material layer was ∼400 μg cm−2, determined by comparing sample weight before and after sublimation.For the MALDI MS experiments, DHB was applied onto tissue slices by sublimation, carried out using a laboratory-constructed system similar to one previously described,51,52 with some modifications.51,52 The sublimation procedure is described in the ESI.† The final matrix layer was ∼500 μg cm−2, determined by comparing sample weights before and after sublimation.
MS analyses
MALDI time-of-flight (TOF)/TOF MSI was performed using an ultrafleXtreme II mass spectrometer (Bruker Daltonics, Billerica, MA) equipped with a solid-state UV Smartbeam II laser. The MSI of tissues was performed using two spatial resolution instrument settings: (1) the “Ultra” laser size setting, with a ∼100 μm diameter footprint and a 100 μm raster step, was used for low spatial resolution MS imaging, and (2) the “Small” laser size setting, with a ∼20 μm diameter laser footprint and a raster step of 30 μm, was used for MSI at high spatial resolution. Due to the large size of the resulting data file and relatively long acquisition time (2 h) for each image, only the hippocampus areas were imaged in the high spatial resolution MSI experiments, whereas a larger brain region was imaged for the lower resolution experiments. Each pixel in the resulting MS images corresponds to a signal in a single mass spectrum acquired as a sum of 1000 laser shots. The laser settings were: 1000 Hz repetition rate, 70% intensity, and a global attenuator offset of 30%. MALDI MS spectra were acquired in the m/z range of 20–3000. A mixture of DHB, bradykinin, and angiotensin II was deposited near to the brain tissue section for mass spectra recalibration, which was needed to address some cases of surface topography-related molecular mass errors. MS image data acquisition, processing, and visualization were performed with flexImaging software (Version 3.0, Bruker Daltonics). The MALDI TOF/TOF mass analyzer was calibrated with the Peptide Calibration Standard II set (Bruker Daltonics) containing bradykinin, angiotensin II, angiotensin I, substance P, bombesin, ACTH clip 1–17, ACTH clip 18–39, renin substrate, and somatostatin 28.Tandem MS (MS/MS) analysis was used to elucidate the structural properties of the detected compounds. MS/MS was carried out in the LIFT mode utilizing argon as a collision gas and a 2 Da precursor isolation window.
Equipped with a MALDI ion source, a solariX™ XR Fourier transform ion cyclotron resonance (FTICR) mass spectrometer (Bruker Daltonics) was used for high mass resolution and accuracy measurements of the samples previously analyzed via MSI. Measurements were performed with the UV Smartbeam II laser set at 50% laser power and medium size laser footprint. Mass spectra were acquired as a sum of 200 laser shots at 500 Hz laser frequency. The m/z range of 20–2000 was investigated with an average accumulation number = 4. Mass spectrometer calibration was performed in tuning mode using the Peptide Mixture II Calibration set.
The methodological details of diffuse reflectance UV-vis spectrometry, UV-vis absorption spectroscopy, and electron microscopy measurements, as well as the statistical analysis, are presented in the ESI.†
Results and discussion
Detection of lipids with TiO2-assisted LDI MS
Particle size has an effect on the observed lipid signals.34 Therefore, to generate TiO2 particles of different sizes, the sol solutions were diluted with the ethanol–water reaction solutions (solution II), and the resulting diluted mixtures were incubated for different times to allow further hydrolysis and condensation. With incubation times equal to or below 3 h, larger particles (500–700 nm in diameter) were formed, along with nanoparticles (Fig. S1A-1–A-3†); lipids were detected with the larger TiO2 particles but no lipid signals were observed when using the TiO2 nanoparticles (Fig. S2†). With a 12 h incubation time, only 500–700 nm TiO2 particles were formed, with no nanoparticles detected (Fig. S1A-4†), and resulted in further increase of the intact lipid signals (Fig. S1B†). No further increases in TiO2 particle sizes or lipid signals were observed with an incubation time of 24 h. However, signals for the head group fragments of hydrolyzed lipids gradually decreased (Fig. S1B†) with increased TiO2 particle sizes, while no similar significant changes in the signal intensities of other small molecules were observed. These results indicate that catalytic hydrolysis of phospholipids on the surface of TiO2 particles is responsible for the absence of intact lipid signals during TiO2 nanoparticle-assisted LDI MS analysis, which is supported by other studies.53–55 Lipid hydrolysis was suppressed with increased TiO2 particle sizes. TiO2 particles with 500–700 nm diameters are referred to as TiO2 sub-micron particles in the following text.
Two inorganic acids, nitric acid (Fig. S3A†) and phosphoric acid (Fig. S3B†), were evaluated at different concentrations as additives to TiO2 sub-micron particle-containing solutions (Fig. S3†). A higher concentration of HNO3 led to lower lipid signal relative peak areas (RPAs) but higher RPAs of the corresponding lipid head group signals (Fig. S3A†). However, the addition of phosphoric acid, which is a known Lewis base with strong affinity to TiO2, led to increased lipid signal RPAs and a decreased ratio of the head group signal RPAs to intact lipids (Fig. S3B†). A Tukey test was performed to determine the statistical significance of the changes (Table S1†). These results can be explained by a decrease in the electrophilicity of the Ti site and a reduction in the catalytic hydrolysis due to coordination of the phosphoric ligands to the Ti site.43 It appears that increasing the pH of the TiO2-containing solution and protecting Ti sites with specific ligands are effective in reducing the lipid hydrolysis.
Effect of ligand additions on TiO2-assisted LDI MS performance
Avoiding the use of nanoscale TiO2 particles can reduce the hydrolysis of lipids; however, the lower surface area of sub-micron particles may decrease the UV absorption of the TiO2 material and its adsorption capacity for analytes, which will decrease the detection sensitivity of TiO2-assisted LDI MS analysis. To increase sensitivity, the modification of TiO2 material with organic ligands was investigated.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, resulting in an increase in UV absorption of TiO2 at a laser wavelength of 355 nm (Fig. S4†). Based on prior studies,39,56 ring structures involving five or seven atoms possibly formed during binding (Fig. 1A and C), and the red shift was due to the formation of significant dipole moments in the TiO2 particles after the binding.39 DA provides the highest dipole moment (16.1 Debye (ref. 39)) and a large shift in UV absorption at 355 nm.
10, resulting in an increase in UV absorption of TiO2 at a laser wavelength of 355 nm (Fig. S4†). Based on prior studies,39,56 ring structures involving five or seven atoms possibly formed during binding (Fig. 1A and C), and the red shift was due to the formation of significant dipole moments in the TiO2 particles after the binding.39 DA provides the highest dipole moment (16.1 Debye (ref. 39)) and a large shift in UV absorption at 355 nm.
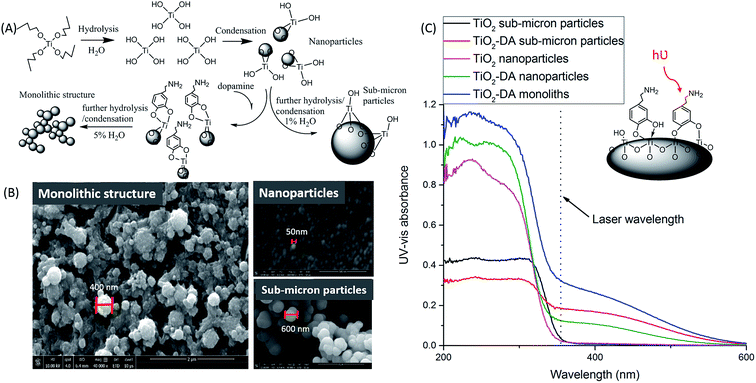 | ||
| Fig. 1 Influence of the addition of DA on the morphology of the TiO2 structure and its UV-vis light absorbance. (A) Schematic illustration of the formation mechanism of the TiO2-DA monolithic structure.59 (B) Scanning electron microscopy images of (left) TiO2-DA monolith, (upper right) TiO2 nanoparticles, and (bottom right) TiO2-DA sub-micron particles. (C) Diffuse reflectance UV-vis spectra of unmodified TiO2 particles, and modified TiO2 particles and monolith, produced with different methods. | ||
For the MSI evaluation, bidentate binding ligands were added into a TiO2 sub-micron particle solution with a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, and the resulting solutions were airbrushed onto mouse brain tissue slices. The acquired MS data demonstrate that the intact lipid to lipid fragment peak area ratios for m/z 844.5: fragment m/z 141.0, and the intact lipid m/z 844.5: fragment m/z 162.9, are much lower with SA-modified TiO2 sub-micron particles than with the other additives tested (Fig. S5B†). As discussed in the prior section, SA, a Brønsted acid, likely reduces the pH of the TiO2 surface, facilitating the catalysis of lipid hydrolysis by TiO2. Additionally, the use of ligands with UV absorption at 355 nm, such as SA and alizarin, for TiO2 sub-micron particle modification led to the detection of intense background signals (Fig. S5A†). Strong desorption and ionization of ligands, their fragments, and different complexes can be responsible for the formation of those background signals. In contrast, the addition of DA, which is a Brønsted base, was most efficient in reducing lipid hydrolysis without significant complication of the mass spectra with background peaks. Thus, we selected DA as the bidentate binding ligand for TiO2 because it provided the highest UV absorption, a basic environment, and reduced lipid hydrolysis.
10, and the resulting solutions were airbrushed onto mouse brain tissue slices. The acquired MS data demonstrate that the intact lipid to lipid fragment peak area ratios for m/z 844.5: fragment m/z 141.0, and the intact lipid m/z 844.5: fragment m/z 162.9, are much lower with SA-modified TiO2 sub-micron particles than with the other additives tested (Fig. S5B†). As discussed in the prior section, SA, a Brønsted acid, likely reduces the pH of the TiO2 surface, facilitating the catalysis of lipid hydrolysis by TiO2. Additionally, the use of ligands with UV absorption at 355 nm, such as SA and alizarin, for TiO2 sub-micron particle modification led to the detection of intense background signals (Fig. S5A†). Strong desorption and ionization of ligands, their fragments, and different complexes can be responsible for the formation of those background signals. In contrast, the addition of DA, which is a Brønsted base, was most efficient in reducing lipid hydrolysis without significant complication of the mass spectra with background peaks. Thus, we selected DA as the bidentate binding ligand for TiO2 because it provided the highest UV absorption, a basic environment, and reduced lipid hydrolysis.
To investigate the kinetics of the coordination reaction involved in the formation of TiO2-DA bidentate complexes, UV spectra from solutions of TiO2-DA nanoparticles with different reaction times were acquired (Fig. S6A†). A time curve of UV absorbance at 355 nm (Fig. S6B†) demonstrates that reaction equilibrium can be reached in 30 min; therefore, a 1 h incubation time for TiO2 modification was selected.
A comparison of the mass spectra acquired from mouse brain sections using TiO2 and TiO2-DA sub-micron particle-assisted LDI MS revealed that similar sets of signals were detected (Table S2†) with both materials, with some exceptions. Additional signals were observed with TiO2-DA sub-micron particle-assisted LDI MS, including the Na and K adducts of DA as well as oxidized DA (DA-o-quinone),57 and also several putatively identified amino acids and dipeptides obtained by matching measured and theoretical accurate molecular masses (representative mass spectra are shown in Fig. S7†). Dipeptides may form in the mass spectrometer source by photocatalytic redox reactions, resulting in fragmentation of longer peptides.58 Importantly, except for the additional signals mentioned above, the LDI MS using TiO2-DA sub-micron particles resulted in up to a 10-fold increase in the signal-to-noise ratios (S/Ns) for peaks at m/z 200–1000 in mouse brain tissue compared to untreated TiO2 sub-micron particles, without changing their spatial distribution (Fig. 2C).
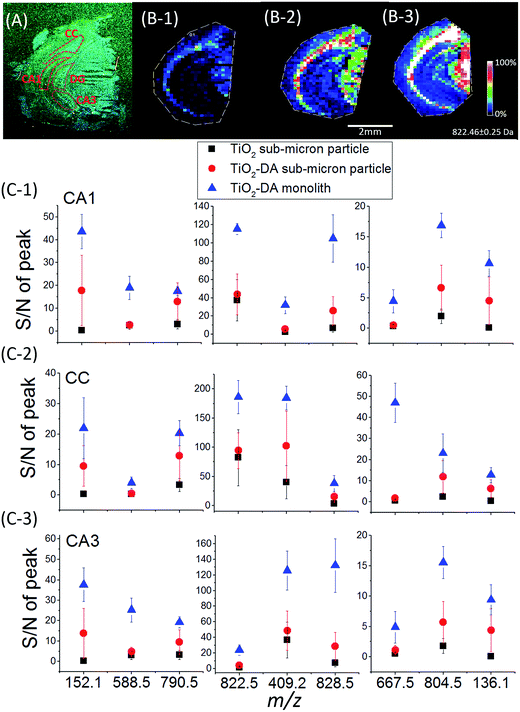 | ||
| Fig. 2 Sensitivity of MSI analyses for endogenous metabolites using different TiO2 materials. (A) Optical image of a mouse coronal brain hemisphere section. The anatomical regions are outlined in accordance with existing brain maps.64 (B) Ion images of a representative lipid at m/z 822.5 (PE(p-40:2)) acquired from the mouse brain hemisphere using LDI MS assisted by (1) unmodified TiO2 sub-micron particles, (2) TiO2-DA sub-micron particles, and (3) TiO2-DA monolith. (C) Averaged S/Ns of molecular signals detected in the different brain regions using the three methods mentioned above (n = 3; p values of the Tukey test are listed in Table S3†). CA1, region I of hippocampus proper; CA3, region III of hippocampus proper; CC, corpus callosum. | ||
We assessed the sensitivity of our LDI MS analyses using the various TiO2 materials (Fig. 2). With the TiO2-DA monolith, the S/Ns of most of the peaks in the range of 100–1000 m/z increased between 10- to 30-fold as compared to the results with unmodified TiO2 sub-micron particles. In addition, a lot of compounds with average S/Ns below the detection limit when using unmodified TiO2 were detected with S/Ns up to 40 with the TiO2-DA monolith. These improvements in the S/Ns are attributed to the higher surface area and higher concentration of bound Ti sites within the TiO2-DA monolith. The diffuse reflectance UV-vis spectroscopy analysis of TiO2 and TiO2-DA materials shown in Fig. 1C demonstrates that such changes lead to an increase in the UV absorption efficiency of the materials. The diffuse reflectance UV-vis spectrum of a TiO2-DA sub-micron particle has a red shift compared with that of an unmodified TiO2 sub-micron particle. The TiO2-DA monolith has a much higher UV absorbance compared to the DA-modified TiO2 sub-micron particles.
In addition to improving the analyte detection sensitivity, the hydrolysis of lipids on TiO2-DA materials was also investigated. The ratio of peak areas for a representative lipid signal and one characteristic fragment signal was calculated (Fig. S9†). The ratios are higher with the TiO2-DA-modified materials than the unmodified materials. However, the ratios are similar when comparing datasets obtained with TiO2-DA sub-micron particle- and TiO2-DA monolith-assisted LDI MS. The data indicate that the presence of DA in the TiO2 sub-micron particles and monolith can lead to lower lipid hydrolysis. This can be explained by the fact that DA binding can decrease the electrophilicity of the Ti site.
Moreover, it can be seen from the scanning electron microscopy images (Fig. 1B and S10†) that the TiO2-DA monolith layer is more uniform and provides high sample surface coverage (93%); the coverage area was calculated using Adobe Photoshop CC (2014). This uniformity can be also be beneficial in high spatial resolution MSI analysis. After deposition on the TiO2 gel sample surface, small visible cracks developed in the coating (Fig. S10†), likely caused by shrinkage during drying. Since the crack widths (<1 μm) are much smaller than the imaging raster gap (30–100 μm) and the laser's footprint size, these cracks should not affect the spatial resolution of the MSI, as discussed further in next section.
Comparison of TiO2-DA monolith-assisted LDI MSI with traditional MALDI MSI
TiO2-DA monolith-assisted LDI MSI
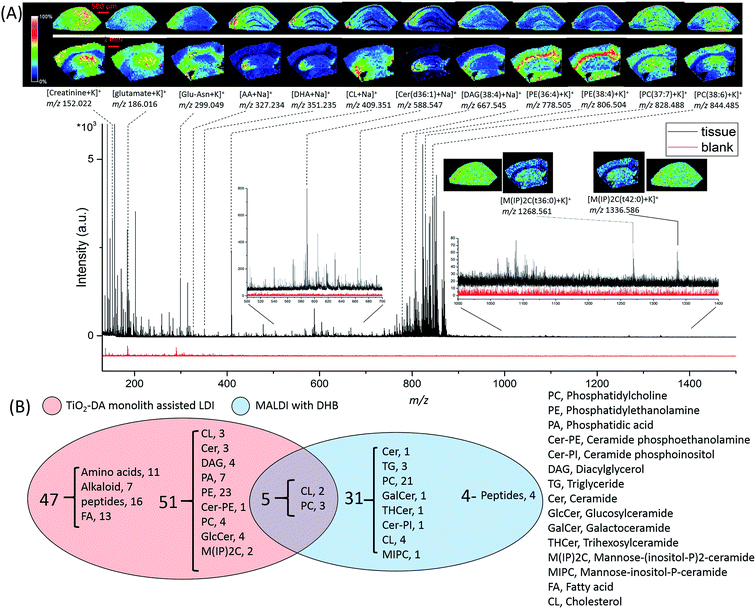
A large number of signals were detected in the mass range of 100 to 1500 Da in hippocampal samples using TiO2-DA monolith-assisted LDI MSI (Fig. 4). Detected compounds were identified with different levels of confidence by comparing metabolite information available in the online databases (METLIN, HMDB, and LIPID MAPS) with one or a combination of several measured analyte characteristics, including accurate molecular mass determined with high mass resolution FTICR MS and molecule fragmentation patterns acquired using MALDI-TOF/TOF MS/MS (Fig. S12 and Table S4†). For example, the identity of the detected PE lipids was determined by their presence in the MS/MS spectra of these lipid fragment signals, with m/z 180 and m/z 141 corresponding to the potassiated and monoprotonated HPO3C2H4–NH2 head group, and characteristic of the C2H4–NH2 neutral loss of 43 Da (Fig. S12A†). PCs were identified by observation of a neutral loss of 183 Da, corresponding to the detachment of the HPO3C2H4–N(CH3)3+ head group, and the neutral loss of 59 Da, matching the m/z N(CH3)3 fragment ion (Fig. S12B†). The sphingoid base ion at m/z 265 is characteristic for ceramides (Fig. S12E†). The 384 Da loss corresponds to the detachment of the mannose–phosphate–inositol group, characteristic for fragmentation of mannose–(inositol-P)2-ceramide (M(IP)2C) molecules (Fig. S12C†). Assignments of the fragment identities for other molecules are shown in (Fig. S12D and F–J†). When the fragmentation of targeted analytes was not conclusive, assignments of molecular identities were done by matching detected and predicted m/z values with the lowest mass error (Δppm). For candidates with the same Δppm, compounds that have been previously reported in animal tissues and organs, including the brain, were chosen and listed in Table S4.† The presented identifications of the detected molecules have different levels of confidence.
Molecules detected in the m/z range of 100 to 500 Da are categorized as amino acids, peptides, alkaloids, fatty acids, and cholesterols (Table S4†). In the molecular mass range of 500 to 700 Da, most of the detected molecules are ceramides and diacylglycerols (DAGs). The mass range of 700 to 1200 Da is populated by phospholipids, including PEs and PCs. The use of TiO2-DA monolith-assisted LDI MSI allowed detection of both PEs and PCs, with PEs dominating the mass spectra. Several low intensity M(IP)2C signals were observed in the mass range of 1000 to 1500 Da. In contrast, positive mode MALDI MS detected predominantly PC species. Analysis of the data acquired with TiO2-DA monolith-assisted LDI MS revealed that 103 molecules were detected and identified with different levels of confidence using this approach; in contrast, only 40 endogenous molecules could be characterized with DHB-based MALDI MSI (Fig. 4B). Even with negative mode MALDI MS using the MALDI matrix 9-AA, only 6 PEs, 3 phosphatidylserines and 9 sulfatides were identified (Table S4†). A literature search suggests that 19 of the 103 compounds observed have not been detected previously with MALDI MS or LDI MS, but have been measured with other MS methods in mouse and rat brains (corresponding literature citations are labeled “c” in Table S4†). Moreover, to the best of our knowledge, 30 of the compounds detected here have not been reported in the mouse brain with other MS methods (citations are labeled “d” in Table S4†).
Acquired ion images with low (100 μm) and high (30 μm) spatial resolutions depict localizations of different compounds, including lipids, in specific sub-regions and cell layers of the hippocampus (Fig. 4A).
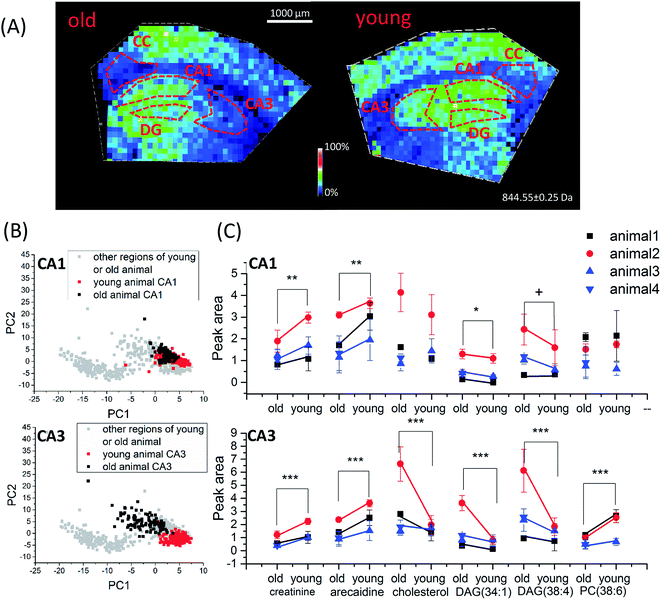 | ||
| Fig. 5 Age-dependent spatio-chemical differences in the hippocampus detected with TiO2-DA monolith-assisted LDI MSI. (A) Representative ion images of cholesterol (m/z 409.3). (B) PCA score plots of TiO2-DA monolith-assisted LDI MSI datasets acquired from different hippocampal and surrounding regions of young and old mice. (C) Statistical comparison of average peak areas of identified molecular signals (data points acquired from the same batch of old and young animal samples are marked by the same color). p values for datasets collected from animals of different ages; samples were calculated using two-way ANOVA, and related detailed information is provided in Table S5.† ***, p value < 0.001, **, p value < 0.01, *, p value < 0.05, +, p value < 0.1. CA1, region I of hippocampus proper; CA3, region III of hippocampus proper; DG, dentate gyrus; CC, corpus callosum. | ||
Conclusions
TiO2-assisted LDI MSI approaches using TiO2 materials with various morphologies and surface properties were systematically evaluated. It was found that lipid hydrolysis on a TiO2 surface can be largely reduced by increasing TiO2 particle size from the nanoscale (<50 nm) to sub-micron scale (>500 nm), and also by increasing the pH of the evaluated TiO2-containing suspensions. The sensitivity of TiO2-assisted LDI MSI detection for a variety of endogenous molecules was largely increased by modifying TiO2 materials with DA, and optimizing the TiO2 morphology to be monolithic with DA binding. In comparison with unmodified TiO2 sub-micron particle-assisted LDI MS, the peak S/Ns for both small molecule and large lipid signals in the molecular mass range of 100 to 1500 Da were increased up to 30-fold with TiO2-DA monolith-assisted LDI MS. By comparing the new method described here with traditional MALDI MS, TiO2-DA monolith-assisted LDI MS performed in positive mode demonstrated higher selectivity and sensitivity for Lewis basic lipids, such as fatty acids, cholesterols, ceramides, DAGs, and PEs. Importantly, the approach is less sensitive for the detection of high-abundance PCs. Investigation of age-related chemical changes in the hippocampus of mice confirmed the utility of TiO2-DA monolith-assisted LDI MSI measurements for fundamental neuroscience. Thirty-five small molecules (including amino acids, alkaloids, free fatty acids, and dipeptides) and over 50 lipids (including cholesterols, ceramides, DAGs, glucosylceramide, PA, ceramide phosphoethanolamine, PCs, and PEs) were detected with TiO2-DA monolith-assisted LDI MSI and identified with MALDI FTICR MS and/or MALDI MS/MS with different levels of confidence. PCA of datasets acquired from brain samples using TiO2-DA monolith-assisted LDI MSI identified several different molecules in young and aging mice.In summary, traditional MALDI matrixes work efficiently in the MSI of mostly large and abundant molecules such as lipids and peptides. Previously reported nanomaterial-assisted LDI, which provides spectra with low background noise, is useful for the detection of small molecules, but may have limitations when characterizing larger compounds and larger sets of chemical classes. TiO2-DA monolith-assisted LDI MSI enhances the efficacy of nanomaterials for lipid detection and their high selectivity for Lewis basic compounds. In all, this approach presents an opportunity for the detection of both small (<500 Da) and large molecules (500 to 1000 Da), and complements traditional MALDI MS approaches by providing additional information on the chemical composition of complex biological tissues.
Acknowledgements
Funding was provided by Abbott Nutrition through the Center for Nutrition, Learning, and Memory at the Beckman Institute, University of Illinois at Urbana-Champaign, and the National Institute on Drug Abuse by Award No. P30 DA018310. We thank Mia Yu for care of the aged mice. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.References
- R. M. Caprioli, T. B. Farmer and J. Gile, Anal. Chem., 1997, 69, 4751–4760 CrossRef CAS PubMed.
- S. A. Schwartz and R. M. Caprioli, in Mass Spectrometry Imaging: Principles and Protocols, ed. S. S. Rubakhin and J. V. Sweedler, 2010, vol. 656, pp. 3–19 Search PubMed.
- P. Chaurand, J. L. Norris, D. S. Cornett, J. A. Mobley and R. M. Caprioli, J. Proteome Res., 2006, 5, 2889–2900 CrossRef CAS PubMed.
- T. J. Garrett, M. C. Prieto-Conaway, V. Kovtoun, H. Bui, N. Izgarian, G. Stafford and R. A. Yost, Int. J. Mass Spectrom., 2007, 260, 166–176 CrossRef CAS.
- A. A. Aksenov and M. E. Bier, J. Am. Soc. Mass Spectrom., 2008, 19, 219–230 CrossRef CAS PubMed.
- H. L. Liu, J. Y. Dai, J. H. Zhou, H. Y. Huang, F. Chen and Z. L. Liu, Int. J. Mass Spectrom., 2015, 376, 85–89 CrossRef CAS.
- D. S. Cornett, M. L. Reyzer, P. Chaurand and R. M. Caprioli, Nat. Methods, 2007, 4, 828–833 CrossRef CAS PubMed.
- L. A. McDonnell and R. M. A. Heeren, Mass Spectrom. Rev., 2007, 26, 606–643 CrossRef CAS PubMed.
- K. A. Z. Berry, J. A. Hankin, R. M. Barkley, J. M. Spraggins, R. M. Caprioli and R. C. Murphy, Chem. Rev., 2011, 111, 6491–6512 CrossRef PubMed.
- R. J. A. Goodwin, S. R. Pennington and A. R. Pitt, Proteomics, 2008, 8, 3785–3800 CrossRef CAS PubMed.
- T. J. A. Dekker, B. D. Balluff, E. A. Jones, C. D. Schoene, M. Schmitt, M. Aubele, J. R. Kroep, V. T. H. B. M. Smit, R. A. E. M. Tollenaar, W. E. Mesker, A. Walch and L. A. McDonnell, J. Proteome Res., 2014, 13, 4730–4738 CrossRef CAS PubMed.
- S. S. Rubakhin, J. C. Jurchen, E. B. Monroe and J. V. Sweedler, Drug Discovery Today, 2005, 10, 823–837 CrossRef CAS PubMed.
- J. r. Hanrieder, N. T. N. Phan, M. E. Kurczy and A. G. Ewing, ACS Chem. Neurosci., 2013, 4, 666–679 CrossRef CAS PubMed.
- M. P. Greving, G. J. Patti and G. Siuzdak, Anal. Chem., 2011, 83, 2–7 CrossRef CAS PubMed.
- Y. Sugiura, S. Shimma, Y. Konishi, M. K. Yamada and M. Setou, PLoS One, 2008, 3, e3232 Search PubMed.
- R. Knochenmuss and R. Zenobi, Chem. Rev., 2003, 103, 441–452 CrossRef CAS PubMed.
- T. Kinumi, T. Saisu, M. Takayama and H. Niwa, J. Mass Spectrom., 2000, 35, 417–422 CrossRef CAS.
- Q. Wu, T. J. Comi, B. Li, S. S. Rubakhin and J. V. Sweedler, Anal. Chem., 2016, 88, 5988–5995 CrossRef CAS PubMed.
- R. J. A. Goodwin, J. Proteomics, 2012, 75, 4893–4911 CrossRef CAS PubMed.
- M. Glückmann and M. Karas, J. Mass Spectrom., 1999, 34, 467–477 CrossRef.
- S.-f. Ren, L. Zhang, Z.-h. Cheng and Y.-l. Guo, J. Am. Soc. Mass Spectrom., 2005, 16, 333–339 CrossRef CAS PubMed.
- M. Lu, Y. Lai, G. Chen and Z. Cai, Anal. Chem., 2011, 83, 3161–3169 CrossRef CAS PubMed.
- S. Chen, H. Zheng, J. Wang, J. Hou, Q. He, H. Liu, C. Xiong, X. Kong and Z. Nie, Anal. Chem., 2013, 85, 6646–6652 CrossRef CAS PubMed.
- M. Dufresne, A. Thomas, J. Breault-Turcot, J.-F. Masson and P. Chaurand, Anal. Chem., 2013, 85, 3318–3324 CrossRef CAS PubMed.
- E.-M. Ratai, L. Annamalai, T. Burdo, C.-G. Joo, J. P. Bombardier, R. Fell, R. Hakimelahi, J. He, M. R. Lentz, J. Campbell, E. Curran, E. F. Halpern, E. Masliah, S. V. Westmoreland, K. C. Williams and R. G. González, Magn. Reson. Med., 2011, 66, 625–634 CrossRef CAS PubMed.
- M. Waki, E. Sugiyama, T. Kondo, K. Sano and M. Setou, in Mass Spectrometry Imaging of Small Molecules, ed. L. He, Springer New York, New York, NY, 2015, DOI:10.1007/978-1-4939-1357-2, vol. 16, pp. 159–173.
- C.-K. Chiang, N.-C. Chiang, Z.-H. Lin, G.-Y. Lan, Y.-W. Lin and H.-T. Chang, J. Am. Soc. Mass Spectrom., 2010, 21, 1204–1207 CrossRef CAS PubMed.
- C. Shi, C. Deng, X. Zhang and P. Yang, ACS Appl. Mater. Interfaces, 2013, 5, 7770–7776 CAS.
- C.-T. Chen and Y.-C. Chen, Anal. Chem., 2004, 76, 1453–1457 CrossRef CAS PubMed.
- T.-C. Chiu, Talanta, 2011, 86, 415–420 CrossRef CAS PubMed.
- C.-T. Chen and Y.-C. Chen, Rapid Commun. Mass Spectrom., 2004, 18, 1956–1964 CrossRef CAS PubMed.
- K.-H. Lee, C.-K. Chiang, Z.-H. Lin and H.-T. Chang, Rapid Commun. Mass Spectrom., 2007, 21, 2023–2030 CrossRef CAS PubMed.
- K. Shrivas, T. Hayasaka, Y. Sugiura and M. Setou, Anal. Chem., 2011, 83, 7283–7289 CrossRef CAS PubMed.
- P. Lorkiewicz and M. C. Yappert, Anal. Chem., 2009, 81, 6596–6603 CrossRef CAS PubMed.
- L. Morosi, P. Spinelli, M. Zucchetti, F. Pretto, A. Carrá, M. D'Incalci, R. Giavazzi and E. Davoli, PLoS One, 2013, 8, e72532 CAS.
- S.-J. Lee, C.-H. Huang, S. Shian and K. H. Sandhage, J. Am. Ceram. Soc., 2007, 90, 1632–1636 CrossRef CAS.
- R. Asapu, V. M. Palla, B. Wang, Z. Guo, R. Sadu and D. H. Chen, J. Photochem. Photobiol., A, 2011, 225, 81–87 CrossRef CAS.
- G. Guerrero, P. H. Mutin and A. Vioux, Chem. Mater., 2001, 13, 4367–4373 CrossRef CAS.
- T. Rajh, L. X. Chen, K. Lukas, T. Liu, M. C. Thurnauer and D. M. Tiede, J. Phys. Chem. B, 2002, 106, 10543–10552 CrossRef CAS.
- B. Jiang, H. Yin, T. Jiang, Y. Jiang, H. Feng, K. Chen, W. Zhou and Y. Wada, Mater. Chem. Phys., 2006, 98, 231–235 CrossRef CAS.
- S. Yang, T. Liu, S. Li, X. Zhang, Q. Ding, H. Que, X. Yan, K. Wei and S. Liu, Neuroscience, 2008, 154, 1107–1120 CrossRef CAS PubMed.
- I. Begcevic, H. Kosanam, E. Martínez-Morillo, A. Dimitromanolakis, P. Diamandis, U. Kuzmanov, L.-N. Hazrati and E. P. Diamandis, Clin. Proteomics, 2013, 10, 1–7 CrossRef PubMed.
- K. Smiljanic, T. Vanmierlo, A. M. Djordjevic, M. Perovic, N. Loncarevic-Vasiljkovic, V. Tesic, L. Rakic, S. Ruzdijic, D. Lutjohann and S. Kanazir, Lipids, 2013, 48, 1069–1077 CrossRef CAS PubMed.
- J. H. Hong, J. W. Kang, D. K. Kim, S. H. Baik, K. H. Kim, S. R. Shanta, J. H. Jung, I. Mook-Jung and K. P. Kim, J. Lipid Res., 2016, 57, 36–45 CrossRef CAS PubMed.
- S. Gruber, K. Pinker, F. Riederer, M. Chmelík, A. Stadlbauer, M. Bittšanský, V. Mlynárik, R. Frey, W. Serles, O. Bodamer and E. Moser, Eur. J. Radiol., 2008, 68, 320–327 CrossRef CAS PubMed.
- O. U. Scremin, M. Roch, K. M. Norman, S. Djazayeri and Y. Y. Liu, Neuroscience, 2015, 301, 520–528 CrossRef CAS PubMed.
- S. A. Fuchs, M. G. M. de Sain-van der Velden, M. M. J. de Barse, M. W. Roeleveld, M. Hendriks, L. Dorland, L. W. J. Klomp, R. Berger and T. J. de Koning, Clin. Chem., 2008, 54, 1443–1450 CAS.
- P. Liu, N. Gupta, Y. Jing and H. Zhang, Neuroscience, 2008, 155, 789–796 CrossRef CAS PubMed.
- K. R. Amaya, J. V. Sweedler and D. F. Clayton, J. Neurochem., 2011, 118, 499–511 CrossRef CAS PubMed.
- J. T. Ting, T. L. Daigle, Q. Chen and G. Feng, Methods Mol. Biol., 2014, 1183, 221–242 Search PubMed.
- J. A. Hankin, R. M. Barkley and R. C. Murphy, J. Am. Soc. Mass Spectrom., 2007, 18, 1646–1652 CrossRef CAS PubMed.
- E. J. Lanni, S. J. B. Dunham, P. Nemes, S. S. Rubakhin and J. V. Sweedler, J. Am. Soc. Mass Spectrom., 2014, 25, 1897–1907 CrossRef CAS PubMed.
- P. A. Sutton and D. A. Buckingham, Acc. Chem. Res., 1987, 20, 357–364 CrossRef CAS.
- J. Chin, Acc. Chem. Res., 1991, 24, 145–152 CrossRef CAS.
- J. Suh, Acc. Chem. Res., 1992, 25, 273–279 CrossRef CAS.
- Q. Ye, F. Zhou and W. Liu, Chem. Soc. Rev., 2011, 40, 4244–4258 RSC.
- D. Sulzer and L. Zecca, Neurotoxic. Res., 2000, 1, 181–195 CrossRef CAS.
- L. Qiao, H. Bi, J.-M. Busnel, J. Waser, P. Yang, H. H. Girault and B. Liu, Chem.–Eur. J, 2009, 15, 6711–6717 CrossRef CAS PubMed.
- Y. Chen, Y. Yi, J. D. Brennan and M. A. Brook, Chem. Mater., 2006, 18, 5326–5335 CrossRef CAS.
- G. L. Drisko, A. s. Zelcer, X. Wang, R. A. Caruso and G. J. d. A. A. Soler-Illia, ACS Appl. Mater. Interfaces, 2012, 4, 4123–4130 CAS.
- W. Zhu, H. Yang, Y. Xie, S. Sun and X. Guo, Mater. Res. Bull., 2016, 73, 48–55 CrossRef CAS.
- J. Konishi, K. Fujita, K. Nakanishi and K. Hirao, Chem. Mater., 2006, 18, 6069–6074 CrossRef CAS.
- J. r. m. Randon, J.-F. o. Guerrin and J.-L. Rocca, J. Chromatogr., 2008, 1214, 183–186 CrossRef CAS PubMed.
- E. S. Lein, M. J. Hawrylycz, N. Ao, M. Ayres, A. Bensinger, A. Bernard, A. F. Boe, M. S. Boguski, K. S. Brockway, E. J. Byrnes, L. Chen, L. Chen, T.-M. Chen, M. Chi Chin, J. Chong, B. E. Crook, A. Czaplinska, C. N. Dang, S. Datta, N. R. Dee, A. L. Desaki, T. Desta, E. Diep, T. A. Dolbeare, M. J. Donelan, H.-W. Dong, J. G. Dougherty, B. J. Duncan, A. J. Ebbert, G. Eichele, L. K. Estin, C. Faber, B. A. Facer, R. Fields, S. R. Fischer, T. P. Fliss, C. Frensley, S. N. Gates, K. J. Glattfelder, K. R. Halverson, M. R. Hart, J. G. Hohmann, M. P. Howell, D. P. Jeung, R. A. Johnson, P. T. Karr, R. Kawal, J. M. Kidney, R. H. Knapik, C. L. Kuan, J. H. Lake, A. R. Laramee, K. D. Larsen, C. Lau, T. A. Lemon, A. J. Liang, Y. Liu, L. T. Luong, J. Michaels, J. J. Morgan, R. J. Morgan, M. T. Mortrud, N. F. Mosqueda, L. L. Ng, R. Ng, G. J. Orta, C. C. Overly, T. H. Pak, S. E. Parry, S. D. Pathak, O. C. Pearson, R. B. Puchalski, Z. L. Riley, H. R. Rockett, S. A. Rowland, J. J. Royall, M. J. Ruiz, N. R. Sarno, K. Schaffnit, N. V. Shapovalova, T. Sivisay, C. R. Slaughterbeck, S. C. Smith, K. A. Smith, B. I. Smith, A. J. Sodt, N. N. Stewart, K.-R. Stumpf, S. M. Sunkin, M. Sutram, A. Tam, C. D. Teemer, C. Thaller, C. L. Thompson, L. R. Varnam, A. Visel, R. M. Whitlock, P. E. Wohnoutka, C. K. Wolkey, V. Y. Wong, M. Wood, M. B. Yaylaoglu, R. C. Young, B. L. Youngstrom, X. Feng Yuan, B. Zhang, T. A. Zwingman and A. R. Jones, Nature, 2007, 445, 168–176 CrossRef CAS PubMed.
- I. Popovic, M. Nesic, M. Nisavic, M. Vranjes, T. Radetic, Z. Saponjic, R. Masnikosa and M. Petkovic, Mater. Lett., 2015, 150, 84–88 CrossRef CAS.
- C. Valongo, M. L. s. Cardoso, P. Domingues, L. g. Almeida, N. Verhoeven, G. Salomons, C. Jakobs and L. Vilarinho, Clin. Chim. Acta, 2004, 348, 155–161 CrossRef CAS PubMed.
- P. Singer, U. Gerike, W. Gödicke, V. Moritz and R. Baumann, Biomed. Biochim. Acta, 1984, 43, 233–235 CAS.
- A. Gaiti, M. Brunetti, D. Sitkiewicz, G. Porcellati and H. Woelk, in Aging of the Brain and Dementia, ed. L. Amaducci, A. N. Davison and P. Antuono, Raven Press, New York, NY, 1980, pp. 65–74 Search PubMed.
- M. Shinitzky, Gerontology, 1987, 33, 149–154 CrossRef CAS.
- S. J. Pasquare, M. G. I. de Boschero and N. M. Giusto, Exp. Gerontol., 2001, 36, 1387–1401 CrossRef CAS PubMed.
- C. J. Galton, K. Patterson, J. H. Xuereb and J. R. Hodges, Brain, 2000, 123, 484–498 CrossRef PubMed.
- J. Hardy and D. J. Selkoe, Science, 2002, 297, 353–356 CrossRef CAS PubMed.
- A. Konkel and N. J. Cohen, Front. Neurosci., 2009, 3, 166–174 CrossRef PubMed.
- I. Rappley, D. S. Myers, S. B. Milne, P. T. Ivanova, M. J. LaVoie, H. A. Brown and D. J. Selkoe, J. Neurochem., 2009, 111, 15–25 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7sc00937b |
| This journal is © The Royal Society of Chemistry 2017 |