Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Phosphine-catalyzed enantioselective [3 + 2] cycloadditions of γ-substituted allenoates with β-perfluoroalkyl enones†
Wei
Zhou
,
Huamin
Wang
,
Mengna
Tao
,
Chao-Ze
Zhu
,
Tao-Yan
Lin
and
Junliang
Zhang
 *
*
Shanghai Key Laboratory of Green Chemistry and Chemical Processes, School of Chemistry and Molecular Engineering, East China Normal University, Shanghai, 200062, P. R. China. E-mail: jlzhang@chem.ecnu.edu.cn; Web: http://faculty.ecnu.edu.cn/s/1811/main.jspy
First published on 19th April 2017
Abstract
The enantioselective construction of densely functionalized cyclopentene bearing contiguous three stereocenters has been a challenging task in organic synthesis. Herein, we present a phoshine-catalyzed highly regio-, diastereo- and enantioselective [3 + 2] cycloaddition of γ-substituted allenoates with β-perfluoroalkyl enones, delivering a wide range of densely functionalized perfluoroalkylated cyclopentenes with three contiguous chiral stereocenters.
Introduction
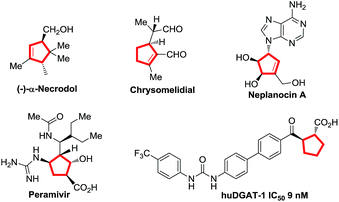
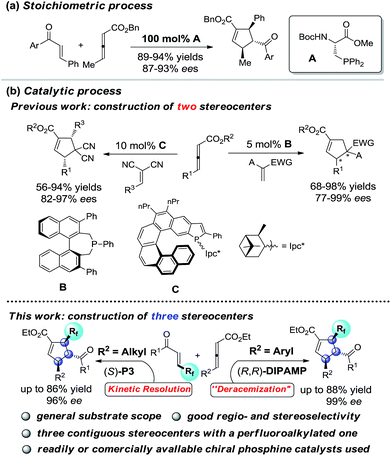
Cyclopentenes (or cyclopentanes) are valuable skeletons found in several natural products and pharmaceuticals (Fig. 1).1 Among existing methodologies for their preparation, phosphine-catalyzed [3 + 2] cycloaddition of allenoates with electron-deficient olefins was first reported by Lu in 1995 as a powerful and straightforward strategy for the construction of functionalized cyclopentene rings.2,3 As a result of tremendous effort from numerous research groups, Lu's enantioselective [3 + 2] cycloaddition reaction of terminal allenoates with electron-deficient olefins has been well established over the past years.4 However, asymmetric [3 + 2] cycloaddition reaction of γ-substituted allenoates with electron-deficient olefins has been less explored despite the increase in stereochemical diversity of the cycloaddition products. In 2007, Miller's group first realized a unique “deracemization” reaction upon cycloaddition of chalcone with racemic γ-methyl allenoates but requisite the use of a stoichiometric amount of chiral phosphine catalyst A (Scheme 1a).4c Subsequently, Fu and co-workers have accomplished the cycloaddition reaction of racemic γ-substituted allenoates with heteroatom-bearing olefins with the use of a catalytic amount of chiral phosphine B, furnishing a facile access to functionalized cyclopentenes with two adjacent stereo centers (Scheme 1b).5 Recently, Marinetti and coworkers have reported a highly enantioselective [3 + 2] cycloaddition of γ-substituted allenoates with benzylidenemalononitrile by utilizing chiral phosphahelicenes catalyst C (Scheme 1b).6Despite this progress, the scope of γ-substituted allenoates and electron-deficient olefin partner for enantioselective Lu's annulation is very limited, and the construction of cyclopentene derivatives with three contiguous chiral stereocenters has been a major challenge but a highly desirable task. Moreover, introduction of perfluoroalkylated, particularly trifluoromethylated, stereocenters into chiral compounds have garnered special attention in pharmaceutical and pesticide industry since the polarity, bioavailability, metabolic stability and other properties of the parent molecules could be influenced greatly by these perfluoroalkyl groups.7 During the course of our continuous interest in design, synthesis and application of novel chiral β-aminephosphines8,9 in asymmetric catalysis and the synthesis of enantio-enriched trifluoromethylated building blocks,8d,g we envisaged that the challenging enantioselective [3 + 2] cycloadditions of γ-substituted allenoates with β-perfluoralkyl α,β-enones might be addressed by systematic screening of known phosphines or rational design of new catalysts (Scheme 1b). In the present study, we report our efforts in addressing this challenging reaction by identifying two phosphine catalysts, commercially available bisphosphine (R,R)-DIPAMP and novel multifunctional (S)-P3 which have been developed in our group. Further control experiments have shown that the reaction under the catalysis of (R,R)-DIPAMP was a deracemization process, while the kinetic resolution reaction was observed under the multifunctional phosphine catalyst (Scheme 1).
Results and discussion
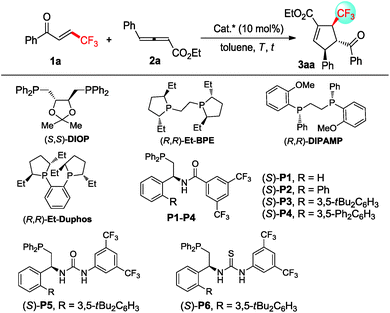
In order to validate the feasibility of the asymmetric [3 + 2] cycloaddition of γ-substituted allenoates with β-perfluoralkyl α,β-enones, allenoate 2a and enone 1a were exposed to a range of commercially available chiral bisphosphine catalysts (Table 1). A small amount of the desired 3aa was observed when (S,S)-DIOP or (R,R)-Et-DUPHOS was utilized as the catalyst (Table 1, entries 1 and 2). Interestingly, (R,R)-Et-BPE exhibited a promising level of reactivity with 64% yield and stereoinduction with 39% ee (Table 1, entry 3). Fortunately, 86% yield of 3aa with 89% ee was obtained using (R,R)-DIPAMP as a catalyst (Table 1, entry 4). It can be noted that multifunctional chiral phosphines (S)-P1–P6 bearing hydrogen bond donors, such as amide and (thio) urea groups, could deliver higher chemical yield but with unacceptable enantioselectivity (Table 1, entries 5–10). Gratifyingly, the enantioselectivity was improved to 92%, albeit with a slightly lower yield when decreasing the reaction temperature from 25 °C to −20 °C (Table 1, entries 11–13). However, much lower reaction temperature was not beneficial for enantioselectivity and reactivity (Table 1, entry 14). In addition, much lower yield and enantioselectivity was observed when (Z)-1a was utilized in the reaction, indicating that the configuration of enone also affected the reaction significantly (Table 1, entry 15). Further screening of solvents demonstrated that toluene was the best reaction medium for this transformation (see ESI† for details). Then, the optimized reaction conditions were identified: 10 mol% (R,R)-DIPAMP as the catalyst and toluene as the reaction medium at −20 °C.| Entry | Cat. | T (°C) | t (h) | Yieldb (%) | eec (%) |
|---|---|---|---|---|---|
a Unless otherwise specified, all reactions were carried out with (E)-1a (0.1 mmol), racemic 2a (0.15 mmol) in toluene (1 mL).
b Yield of isolated products; d.r. and r.r. > 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
c Determined by HPLC analysis.
d (Z)-1a was used. 1.
c Determined by HPLC analysis.
d (Z)-1a was used.
|
|||||
| 1 | (S,S)-DIOP | 25 | 12 | <10 | — |
| 2 | (R,R)-Et-Duphos | 25 | 12 | <10 | — |
| 3 | (R,R)-Et-BPE | 25 | 8 | 64 | 39 |
| 4 | (R,R)-DIPAMP | 25 | 6 | 81 | 89 |
| 5 | (S)-P1 | 25 | 3 | 79 | 3 |
| 6 | (S)-P2 | 25 | 3 | 85 | 14 |
| 7 | (S)-P3 | 25 | 3 | 84 | 40 |
| 8 | (S)-P4 | 25 | 3 | 86 | 21 |
| 9 | (S)-P5 | 25 | 0.5 | 91 | 31 |
| 10 | (S)-P6 | 25 | 0.5 | 88 | 38 |
| 11 | (R,R)-DIPAMP | 0 | 6 | 84 | 90 |
| 12 | (R,R)-DIPAMP | −10 | 8 | 81 | 91 |
| 13 | (R,R)-DIPAMP | −20 | 12 | 78 | 92 |
| 14 | (R,R)-DIPAMP | −25 | 20 | 63 | 92 |
| 15d | (R,R)-DIPAMP | −20 | 24 | 23 | 50 |
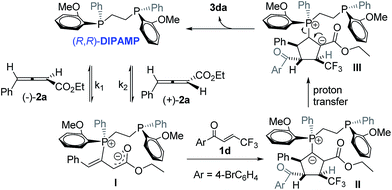
Under optimal reaction conditions, we investigated the scope of the enantioselective [3 + 2] cycloaddition reaction (Scheme 2). Remarkably, a wide range of β-trifluoromethyl substituted enones containing different electron nature functional groups worked well with allenoate 2a, thereby resulting in a highly regioselective α-addition products 3ba–3ha in good yields with 88–94% ee. However, the introduction of an ortho substituent, such as Cl and Br, to the phenyl ring of enone led to dramatic decrease in the enantioselectivity (3ia and 3ja). To our delight, naphthyl- and heteroaryl-containing substrates 1k–1o were also compatible, efficiently furnishing a set of trifluoromethylated cyclopentenes containing naphthyl- and heteroaryl frameworks 3ka–3oa. In addition, the present protocol could be readily extended to the challenging synthesis of cyclohexenyl and cyclohexyl based trifluoromethyl enone 1p and 1q. It was noteworthy that both β-pentafluoroethyl and β-heptafluoropropyl enone were particularly effective in the present transformation, forming valuable perfluoroalkyl substituted cyclopentene 3ra and 3sa in good yields with 94% ee. Furthermore, γ-aryl allenoates 2b–2d with substituted aryl and hetereoaryl groups were well applicable and formed corresponding products 3ab–3ad with high regioselectivity and enantioselectivity. The absolute configuration of product 3aa was confirmed by single-crystal X-ray diffraction analysis.10
 | ||
| Scheme 2 Enantioselective [3 + 2] cycloadditions of γ-aryl substituted allenoates with β-perfluoro substituted enonea. | ||
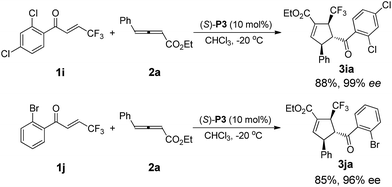
After intensive screening of various chiral phosphine catalysts, it was found that multifunctional phosphine (S)-P3 displayed good performance in the substrates with ortho-substituent, and the desired products 3ia and 3ja could be isolated in 85–88% yields with 96% and 99% ee, respectively (Scheme 3).
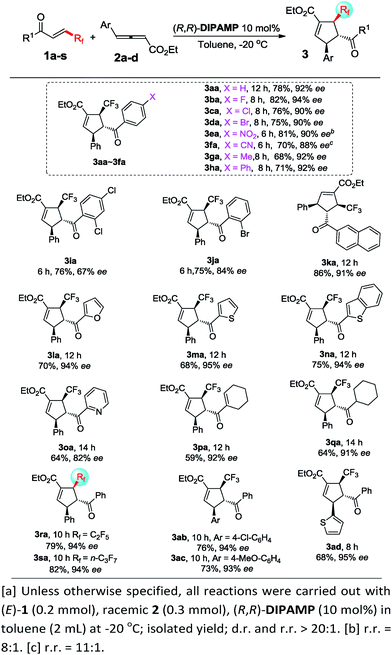
Unfortunately, the performance of (R,R)-DIPAMP in the cycloaddition of γ-alkyl substituted allenoates was not as good as that in the cases of γ-aryl substituted allenoates. For example, the reaction of 2e with 1c resulted in the formation of desired 3ce in 67% yield but with only 86% ee. After further screening of a series of chiral phosphine catalysts, solvents and reaction temperature, it was found that (S)-P3 was a privileged catalyst for cycloaddition of γ-alkyl allenoates. In general, allenoates 2e–2g with different alkyl substituents at γ position participated in the annulation process with good regio- and enantioselectivity. In addition, diverse alkyl substituents such as benzyl, halogen and ester group were well tolerant, furnishing the corresponding cycloadducts 3ch–3cj in moderate to good yields with high enantioselectivity. Furthermore, allenoates with bulky substituents such as isopropyl, cyclopentyl and cyclohexyl at γ position worked well, thereby forming the desired 3ck–3cm in good yields with 92–94% ee. Good to excellent regioselectivity and enantioselectivity were also obtained in the cycloaddition reactions of allenoate 2g with a wide range of β-trifluoromethyl substituted enones (Scheme 4).
 | ||
| Scheme 4 Enantioselective [3 + 2] cycloadditions of γ-alkyl substituted allenoates with β-perfluoro substituted enonea. | ||
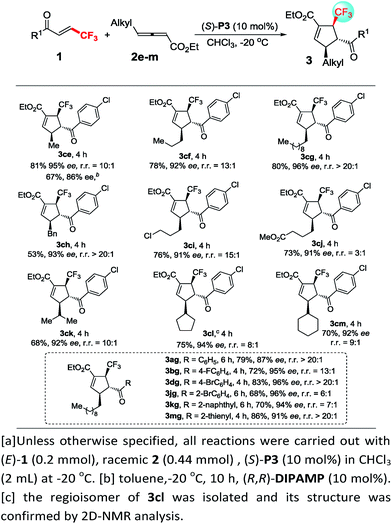
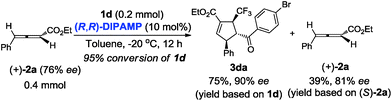
Next, we turned our attention to gain insight into catalytic process for the proposed [3 + 2] cycloaddition reaction. In case of (R,R)-DIPAMP catalysed cycloaddition of 1d and racemic 2a, the starting material 2a was recovered in 38% yield (based on 2a) with 0% ee (eqn (1)). Furthermore, when optically active allenoate (+)-2a (76% ee) served as the substrate, ee of 3da did not improve but the recovered (+)-2a had a higher ee (eqn (2)). These results have supported that a deracemization process was followed in the (R,R)-DIPAMP catalysed cycloaddition of 1d and 2a.
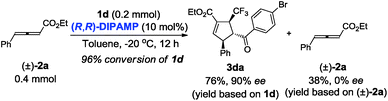 | (1) |
 | (2) |
To examine both the phosphines in (R,R)-DIPAMP induce enantioselectivity independently or cooperatively, (R,R)-SDIPAMP that contained only one nucleophilic phosphine was synthesized and subjected to the reaction of 1d and racemic 2a (Scheme 5). Although the reaction became slower, enantio-selectivity of 3da remained unchanged, demonstrating that both the phosphines in (R,R)-DIPAMP might induce enantioselectivity independently (Scheme 5b).
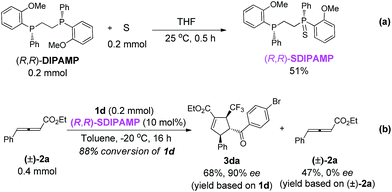 | ||
| Scheme 5 Synthesis of (R,R)-SDIPAMP and its application in the asymmetric [3 + 2] cycloaddition of 2a and 1d. | ||
Based on the abovementioned results and earlier reports,11 a plausible catalytic cycle for (R,R)-DIPAMP catalysed asymmetric [3 + 2] cycloaddition reaction of γ-aryl allenoates with trifluoromethyl enones has been illustrated in Scheme 6. The zwitterionic intermediate I was formed through nucleophilic addition of (R,R)-DIPAMP to racemic 2a. The deracemization process resulted from the same nucleophilic attack rate (K1 = K2) of (R,R)-DIPAMP to both the enantiomers of allenoates 2a. The subsequent [3 + 2] cycloaddition favoured α-addition to provide intermediate II, which then underwent proton transfer to provide intermediate III. Finally, (R,R)-DIPAMP and cyclopetene 3da were released from intermediate III.
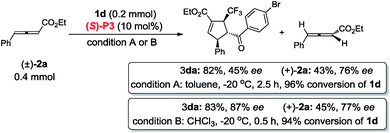
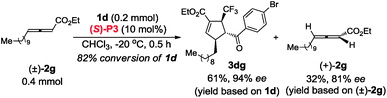
In contrast to (R,R)-DIPAMP, a kinetic resolution reaction takes place with multifunctional chiral phosphine (S)-P3 as the catalyst and (+)-2a12 and (+)-2g13 is recovered in 76% ee (in toluene, 77% ee in CHCl3) and 81% ee respectively (eqn (3) and (4)). In order to confirm the possible hydrogen-bonding interaction during the catalytic process, (S)-P7 without hydrogen-bond donor was synthesized and subjected to the cycloaddition reaction (Scheme 7). The conversion decreased dramatically under higher catalyst loading and higher reaction temperature. The ee value of the recovered 2g also vanished (Scheme 7b). These results demonstrated that the hydrogen-bond donor in (S)-P3 was crucial for enantioselective formation of cycloaddition product via kinetic resolution process.
 | (3) |
 | (4) |
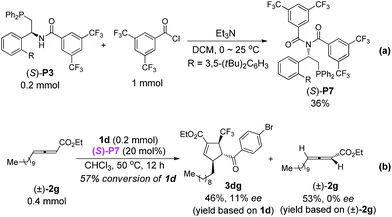 | ||
| Scheme 7 Synthesis of (S)-P7 and its application in the asymmetric [3 + 2] cycloaddition of 2g and 1d. | ||
On the basis of above control experiments and recent excellent mechanistic studies11 on the [3 + 2] cycloaddition of allenoates with electron-deficient olefins, a tentatively proposed catalytic cycle for (S)-P3 catalysed asymmetric [3 + 2] cycloaddition reaction of racemic allenoate with trifluoromethyl enone is shown in Scheme 8. (−)-2 might prefer a configuration that would facilitate hydrogen-bonding interactions of N–H and carbonyl group (Scheme 8, TS-1). On the other hand, the nucleophilic attack of (S)-P3 with (+)-2 might be suppressed by the steric interaction of the bulky R2 group with the phenyl moiety (Scheme 8, TS-2). Accordingly, different nucleophilic attack rates (K1 > K2) of (S)-P3 to both the enantiomers of allenoates 2 contribute to the kinetic resolution process. It should be note that further experimental and theoretical studies are required to gain insights into kinetic resolution process.
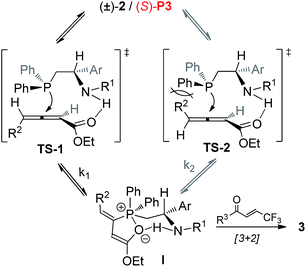 | ||
| Scheme 8 Possible catalytic cycle for (S)-P3 catalysed asymmetric [3 + 2] cycloaddition reaction of racemic allenoate with trifluoro-methyl enone. | ||
Conclusions
In conclusion, we have developed a highly regio-, diastereo- and enantioselective [3 + 2] cycloaddition of γ-substituted allenoates with β-perfluoroalkyl enones with (R,R)-DIPAMP or (S)-P3 as a catalyst; it provides a facile access to a wide range of trifluoromethylated cyclopentenes with three contiguous chiral centers (up to 88% yield with 99% ee). In case of γ-aryl allenoate, commercially available chiral phosphine (R,R)-DIPAMP was identified as an efficient catalyst. In contrast, presently developed multifunctional phosphine (S)-P3 has displayed high performance in the asymmetric cycloaddition of γ-alkyl allenoates with trifluoromethyl enones. In addition, control experiments have demonstrated that under the catalysis of (R,R)-DIPAMP, racemic allenoate reacted with trifluoromethyl enone through a “deracemization” process, whereas a clearly kinetic resolution reaction takes place with multifunctional chiral phosphine (S)-P3 as a catalyst due to the hydrogen-bonding interaction between catalyst and the allenoate. Efforts toward other transformations of allenoate under the catalysis of our developed catalysts P1–P6 are currently underway and will be reported in due course.Acknowledgements
We are grateful to 973 Programs (2015CB856600), the National Natural Science Foundation of China (21372084, 21425205, 21672067) and the Changjiang Scholars and Innovative Research Team in University (PCSIRT) for financial supports.Notes and references
- (a) J. Meinwald and T. H. Jones, J. Am. Chem. Soc., 1978, 100, 1883 CrossRef CAS; (b) B. Roach, T. Eisner and J. Meinwald, J. Org. Chem., 1990, 55, 4047 CrossRef CAS; (c) M. Ono, K. Nishimura, H. Tsubouchi, Y. Nagaoka and K. Tomioka, J. Org. Chem., 2001, 66, 8199 CrossRef CAS PubMed; (d) G. Zhao, A. J. Souers, M. Voorbach, H. D. Falls, B. Droz and S. Brodjian, J. Med. Chem., 2008, 51, 380 CrossRef CAS PubMed; (e) M. Lawrenz, R. Baron and J. A. McCammon, J. Chem. Theory Comput., 2009, 5, 1106 CrossRef CAS PubMed.
- (a) C. Zhang and X. Lu, J. Org. Chem., 1995, 60, 2906 CrossRef CAS; (b) Y. Du, X. Lu and Y. Yu, J. Org. Chem., 2002, 67, 8901 CrossRef CAS PubMed; (c) Y. Du and X. Lu, J. Org. Chem., 2003, 68, 6463 CrossRef CAS PubMed.
- For selected reviews related to the cycloaddition of allenes with olefins, see: (a) X. Lu, C. Zhang and Z. Xu, Acc. Chem. Res., 2001, 34, 535 CrossRef CAS PubMed; (b) L.-W. Ye, J. Zhou and Y. Tang, Chem. Soc. Rev., 2008, 37, 1140 RSC; (c) B. J. Cowen and S. J. Miller, Chem. Soc. Rev., 2009, 38, 3102 RSC; (d) Q.-Y. Zhao, Z. Lian, Y. Wei and M. Shi, Chem. Commun., 2012, 48, 1724 RSC; (e) Y. C. Fan and O. Kwon, Chem. Commun., 2013, 49, 11588 RSC; (f) Z. Wang, X. Xu and O. Kwon, Chem. Soc. Rev., 2014, 43, 2927 RSC; (g) L. Yang and J. Ma, Acta Chim. Sin., 2016, 74, 130 CrossRef CAS.
- For enantioselective Lu's [3 + 2] cycloaddition reaction of terminal allenoates with electron-deficient olefins see: (a) G. Zhu, Z. Chen, Q. Jiang, D. Xiao, P. Cao and X. Zhang, J. Am. Chem. Soc., 1997, 119, 3836 CrossRef CAS; (b) J. E. Wilson and G. C. Fu, Angew. Chem., Int. Ed., 2006, 45, 1426 CrossRef CAS PubMed; (c) B. J. Cowen and S. J. Miller, J. Am. Chem. Soc., 2007, 129, 10988 CrossRef CAS PubMed; (d) A. Voituriez, A. Panossian, N. Fleury-Brégeot, P. Retailleau and A. Marinetti, J. Am. Chem. Soc., 2008, 130, 14030 CrossRef CAS PubMed; (e) A. Voituriez, A. Panossian, N. Fleury-Brégeot, P. Retailleau and A. Marinetti, Adv. Synth. Catal., 2009, 351, 1968 CrossRef CAS; (f) H. Xiao, Z. Chai, C.-W. Zheng, Y.-Q. Yang, W. Liu, J.-K. Zhang and G. Zhao, Angew. Chem., Int. Ed., 2010, 49, 4467 CrossRef CAS PubMed; (g) M. Neel, J. Gouin, A. Voituriez and A. Marinetti, Synthesis, 2011, 2003 CAS; (h) X. Han, Y. Wang, F. Zhong and Y. Lu, J. Am. Chem. Soc., 2011, 133, 1726 CrossRef CAS PubMed; (i) X. Han, S.-X. Wang, F. Zhong and Y. Lu, Synthesis, 2011, 1859 CAS; (j) Q. Zhao, X. Han, Y. Wei, M. Shi and Y. Lu, Chem. Commun., 2012, 48, 970 RSC; (k) D. Duvvuru, N. Pinto, C. Gomez, J.-F. Betzer, P. Retailleau, A. Voituriez and A. Marinetti, Adv. Synth. Catal., 2012, 354, 408 CrossRef CAS; (l) M. Steurer, K. L. Jensen, D. Worgull and K. A. Jørgensen, Chem.–Eur. J., 2012, 18, 76 CrossRef CAS PubMed; (m) X.-N. Zhang and M. Shi, ACS Catal., 2013, 3, 507 CrossRef CAS.
- Y. Fujiwara and G. C. Fu, J. Am. Chem. Soc., 2011, 133, 12293 CrossRef CAS PubMed.
- M. Gicquel, Y. Zhang, P. Aillard, P. Retailleau, A. Voituriez and A. Marinetti, Angew. Chem., Int. Ed., 2015, 54, 5470 CrossRef CAS PubMed.
- (a) K. Müller, C. Faeh and F. Diederich, Science, 2007, 317, 1881 CrossRef PubMed; (b) W. K. Hagmann, J. Med. Chem., 2008, 51, 4359 CrossRef CAS PubMed; (c) N. A. Meanwell, J. Med. Chem., 2011, 54, 2529 CrossRef CAS PubMed; (d) J. Nie, H.-C. Guo, D. Cahard and J.-A. Ma, Chem. Rev., 2011, 111, 455 CrossRef CAS PubMed; (e) T. Liang, C. N. Neumann and T. Ritter, Angew. Chem., Int. Ed., 2013, 52, 8214 CrossRef CAS PubMed; (f) X.-Y. Yang, T. Wu, R. J. Phipps and F. D. Toste, Chem. Rev., 2015, 115, 826 CrossRef CAS PubMed; (g) C. Alonso, E. Martínez de Marigorta, G. Rubiales and F. Palacios, Chem. Rev., 2015, 115, 1847 CrossRef CAS PubMed.
- For the new chiral β-aminephosphine developed by our group see: (a) Z.-M. Zhang, P. Chen, W. Li, Y. Niu, X.-L. Zhao and J. Zhang, Angew. Chem., Int. Ed., 2014, 53, 4350 CrossRef CAS PubMed; (b) X. Su, W. Zhou, Y. Li and J. Zhang, Angew. Chem., Int. Ed., 2015, 54, 6874 CrossRef CAS PubMed; (c) W. Zhou, X. Su, M. Tao, C. Zhu, Q. Zhao and J. Zhang, Angew. Chem., Int. Ed., 2015, 54, 14853 CrossRef CAS PubMed; (d) Z.-M. Zhang, B. Xu, S. Xu, H.-H. Wu and J. Zhang, Angew. Chem., Int. Ed., 2016, 55, 6324 CrossRef CAS PubMed; (e) P. Chen, Z. Yue, J. Zhang, X. Lv, L. Wang and J. Zhang, Angew. Chem., Int. Ed., 2016, 55, 13316 CrossRef CAS PubMed; (f) W. Zhou, P. Chen, M. Tao, X. Su, Q. Zhao and J. Zhang, Chem. Commun., 2016, 52, 7612 RSC; (g) B. Xu, Z.-M. Zhang, S. Xu, Y. Xiao and J. Zhang, ACS Catal., 2017, 7, 210 CrossRef CAS.
- For reviews related to chiral phosphines catalysis, see: (a) Y. Wei and M. Shi, Acc. Chem. Res., 2010, 43, 1005 CrossRef CAS PubMed; (b) A. Marinetti and A. Voituriez, Synlett, 2010, 174 CrossRef CAS; (c) S.-X. Wang, X. Han, F. Zhong, Y. Wang and Y. Lu, Synlett, 2011, 2766 CAS; (d) Q.-Y. Zhao, Z. Lian, Y. Wei and M. Shi, Chem. Commun., 2012, 48, 1724 RSC; (e) L.-W. Xu, ChemCatChem, 2013, 5, 2775 CrossRef CAS; (f) Y. Wei and M. Shi, Chem. Rev., 2013, 113, 6659 CrossRef CAS PubMed; (g) Y. Wei and M. Shi, Chem.–Asian J., 2014, 9, 2720 CrossRef CAS PubMed; (h) W. Li and J. Zhang, Chem. Soc. Rev., 2016, 45, 1657 RSC; (i) T. Wang, X. Han, F. Zhong, W. Yao and Y. Lu, Acc. Chem. Res., 2016, 49, 1369 CrossRef CAS PubMed.
- ESI.†.
- (a) T. Dudding, O. Kwon and E. Mercier, Org. Lett., 2006, 8, 3643 CrossRef CAS PubMed; (b) Y. Xia, Y. Liang, Y. Chen, M. Wang, L. Jiao, F. Huang, S. Liu, Y. Li and Z.-X. Yu, J. Am. Chem. Soc., 2007, 129, 3470 CrossRef CAS PubMed; (c) E. Mercier, B. Fonovic, C. Henry, O. Kwon and T. Dudding, Tetrahedron Lett., 2007, 48, 3617 CrossRef CAS; (d) Y. Liang, S. Liu, Y. Xia, Y. Li and Z.-X. Yu, Chem.–Eur. J, 2008, 14, 4361 CrossRef CAS PubMed; (e) G.-T. Huang, T. Lankau and C.-H. Yu, J. Org. Chem., 2014, 79, 1700 CrossRef CAS PubMed.
- The absolute configuration of (+)-2a were assigned by comparison with optical rotation in previous report: T. Inokuma, M. Furukawa, T. Uno, Y. Suzuki, K. Yoshida, Y. Yano, K. Matsuzaki and Y. Takemoto, Chem.–Eur. J., 2011, 17, 10470 CrossRef CAS PubMed.
- The absolute configuration of (+)-2g were assigned by comparison with optical rotation in previous report: C.-Y. Li, X.-L. Sun, Q. Jing and Y. Tang, Chem. Commun., 2006, 2980 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, analytical data, NMR spectra of products. CCDC 1503840 (3aa). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7sc01432e |
| This journal is © The Royal Society of Chemistry 2017 |