Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Elucidation of the relative and absolute stereochemistry of the kalimantacin/batumin antibiotics†
Iain R. G.
Thistlethwaite
a,
Freya M.
Bull
a,
Chengsen
Cui
 a,
Paul D.
Walker
a,
Paul D.
Walker
 a,
Shu-Shan
Gao
a,
Shu-Shan
Gao
 a,
Luoyi
Wang
a,
Zhongshu
Song
a,
Joleen
Masschelein‡
b,
Rob
Lavigne
a,
Luoyi
Wang
a,
Zhongshu
Song
a,
Joleen
Masschelein‡
b,
Rob
Lavigne
 b,
Matthew P.
Crump
b,
Matthew P.
Crump
 a,
Paul R.
Race
a,
Paul R.
Race
 c,
Thomas J.
Simpson
c,
Thomas J.
Simpson
 a and
Christine L.
Willis
a and
Christine L.
Willis
 *a
*a
aSchool of Chemistry, University of Bristol, Cantock's Close, Bristol BS8 1TS, UK. E-mail: chris.willis@bristol.ac.uk
bLaboratory of Gene Technology, KULeuven, Leuven B-3001, Belgium
cSchool of Biochemistry, University of Bristol, Bristol BS8 1TD, UK
First published on 11th July 2017
Abstract
Kalimantacin A and batumin exhibit potent and selective antibiotic activity against Staphylococcus species including MRSA. Both compounds are formed via a hybrid polyketide synthase/non-ribosomal peptide synthetase (PKS-NRPS) biosynthetic pathway and from comparison of the gene clusters it is apparent that batumin from Pseudomonas batumici and kalimantacin from P. fluorescens are the same compound. The linear structure of this unsaturated acid was assigned by spectroscopic methods, but the relative and absolute stereochemistry of the five stereocentres remained unknown. Herein we describe isolation of kalimantacin A and two further metabolites 17,19-diol 2 and 27-descarbomyl hydroxyketone 3 from cultures of P. fluorescens. Their absolute and relative stereochemistries are rigorously determined using a multidisciplinary approach combining natural product degradation and fragment synthesis with bioinformatics and NMR spectroscopy. Diol 2 has the 5R, 15S, 17S, 19R, 26R, 27R configuration and is the immediate biosynthetic precursor of the bioactive kalimantacin A formed by oxidation of the 17-alcohol to the ketone.
Introduction
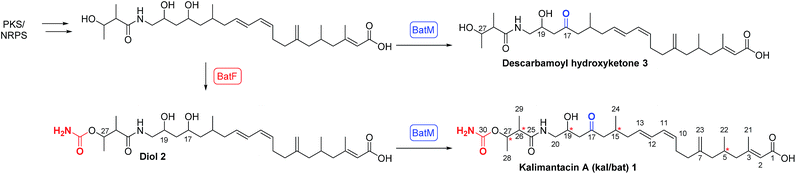
Polyketides isolated from bacteria exhibit a range of important bioactivities making them attractive leads for the development of therapeutics,1 for example mupirocin is used worldwide as a topical antibiotic.2 These compounds are efficiently assembled in the host microorganism via sophisticated multiple enzyme architectures known as modular polyketide synthases. The rational alteration of these biosynthetic pathways offers exciting opportunities to access novel bioactive agents.3In the course of a screening program for novel antibiotics, the kalimantacins were isolated from cultures of Alcaligenes sp. YL-02632S and the major metabolite, kalimantacin A 1 was shown to exhibit activity against Gram-positive bacteria including MRSA (Scheme 1).4,5
A short time later the antibiotic batumin was isolated from Pseudomonas batumici and found to have the same molecular weight and spectroscopic properties as kalimantacin A.6,7 Batumin has been patented in the Ukraine and formulated as Diastaf and used to detect staphylococci by taking advantage of its selectivity for these strains.8 Decreased biofilm formation has also been reported for the majority of S. aureus strains investigated9 and it has been found to reduce nasal S. aureus carriage.10 Peschel and co-workers have recently highlighted the potential scope of narrow spectrum antibiotics in the control of bacterial populations.11
In 2010 Lavigne and co-workers isolated a metabolite from cultures of Pseudomonas fluorescens BCCM_ID9359 which again had spectroscopic properties in accord with the structure of kalimantacin and batumin hence it was named kal/bat (Scheme 1). The kal/bat gene cluster was identified and characterized and shown to consist of 16 open reading frames encoding a hybrid modular polyketide synthase/non-ribosomal peptide synthetase (PKS-NRPS) system.12,13 It has recently been established that the biosynthetic gene clusters of kalimantacin from P. fluorescens and batumin from P. batumici14,15 are identical, confirming that they both produce the same natural product based on a structural framework assembled on a linear unsaturated acid featuring five stereocenters. The PKS-NRPS is a member of the trans-acyltransferase (AT) class of modular polyketide synthases that lack integral AT-domains whereby they gain AT and other tailoring activities in trans.16,17 This can dramatically increase the diversity and complexity of the chemical transformations available to the pathway and offers significant potential for pathway engineering of new compounds. New metabolites diol 2, with a 17-hydroxyl group rather than carbonyl, and descarbamoyl compound 3 were isolated by inactivation of the last two tailoring genes batF (carbamoyl transferase) and batM (oxidoreductase) of the P. fluorescens gene cluster (Scheme 1).
The final conversion by BatM was particularly interesting as it transformed the almost inactive precursor, diol 2, to the bioactive 17-ketone 1. This key step was hypothesized to be essential for bacterial physiology whereby production of the inactive precursor functions as a pro-drug to avoid self-toxicity.12,13 The mode of action of kal/bat was suggested to be inhibition of bacterial fatty acid biosynthesis, due to the presence of a FabI isoform, namely BatG and the antibiotic has high specific activity against staphylococci.13,18 Kalimantacin/batumin has potential value as a lead antibiotic, exhibiting selective and high specific activity against staphylococci (MIC value of 0.064 μg mL−1) and is significantly more active than other antibiotics in commercial use (e.g. mupirocin MIC ca. 0.5 μg mL−1).
The relative and absolute structures of kal/bat 1 and putative biosynthetic intermediates 2 and 3 have remained elusive using spectroscopic methods or via attempts to generate a crystalline derivative for X-ray studies. With 32 possible isomers it is important to determine the full structure of this antibiotic. Herein we report a multidisciplinary approach combining natural product isolation, chemical degradation and fragment synthesis with bioinformatics and extensive NMR studies to determine the relative and absolute configuration of 1 and 2 isolated from P. fluorescens. For simplicity we use the name kalimantacin.
Results and discussion
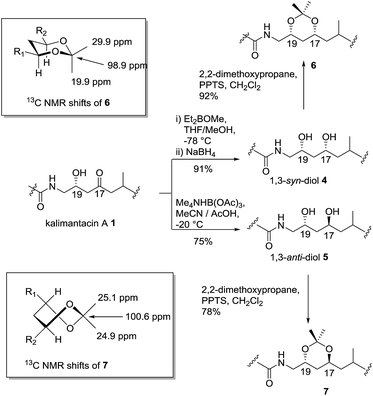
Initially, preparative amounts of pure kalimantacin A 1 were isolated from P. fluorescens for degradation and derivatization studies. We found that higher titres were obtained by growing cultures in a modified L-medium rather than a tryptose broth supplemented with sucrose and glycine as reported previously.12 The natural product was readily purified, using either normal phase followed by reversed phase silica chromatography or HPLC to yield typically 50 mg L−1 of kalimantacin A. A similar protocol was used to grow the ΔbatF and ΔbatM mutants of P. fluorescens giving biosynthetic intermediates 2 and 3. The spectral data of 1, 2 and 3 were in accord with those reported previously12 and these compounds were found to be stable for several weeks at room temperature.The first structural investigation was the relative stereochemistry of the 17,19-diol in 2 isolated from the ΔbatM mutant of P. fluorescens. Directed reductions19,20 of the β-hydroxyketone moiety of kalimantacin A 1 gave 1,3-syn and 1,3-anti diols 4 and 5 for comparison with diol 2 (Scheme 2). The optical rotation of synthetic anti diol 5, [α]D −10.0 and natural product 2, [α]D −10.4 correlated well and their 1H and 13C NMR spectra were identical. In contrast 1,3-syn diol 4 with [α]D −5.9 showed significant differences in the chemical shifts of C-17 (δc 68.4 for 2, δc 70.3 for 4) and C-19 (δc 66.3 for 2, δc 72.0 for 4) (Tables 1 and 2, ESI†).
The assignment of the relative stereochemistry of the 1,3-syn and 1,3-anti diols 4 and 5 was confirmed using the method of Rychnovsky and Evans21 from the characteristic quaternary and methyl group 13C chemical shifts of the corresponding acetonides 6 and 7 (Scheme 2). Similarly the natural diol 2 was converted to its acetonide and was the same by NMR to 7 confirming the anti relationship in the 17,19 diol. Bioinformatic analysis of active site motifs of the appropriate modular ketoreductase (KR) domains predicts that the 17-alcohol should be introduced with the “R” configuration on reduction of the β-keto–thiol ester assembly intermediate.12 Hence taking into account the Cahn–Prelog–Ingold priority change upon further chain elongation, we made a preliminary assignment of the absolute configuration for diol 2 as 17S, 19R.
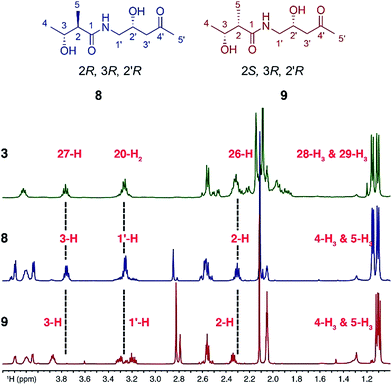
Sequence analysis of the relevant KR domain suggested the R configuration at C27 but this, as well as the relative stereochemistry of C26 and C27, remained to be proven.12 Thus, two fragment mimics, (2R,3R,2′R)-diol 8 and (2S,3R,2′R)-diol 9, of kalimantacin A were prepared and varied in the configuration of the 2-methyl groups (see ESI for synthetic details†). Their NMR spectra were compared with those of the descarbamoyl metabolite 3 isolated from the ΔbatF mutant of P. fluorescens and the best fit was with (2R,3R,2′R)-diol 8 (Fig. 1). To support the proposed relative stereochemistry of the 19-alcohol and 26,27-stereocentres, the analogous (2R,3R,2′S)-diol was also synthesized and, as expected, the NMR data of this compound gave a poor fit with the natural product 3 (ESI, Tables 3 and 4, Fig. S2 and S3†).
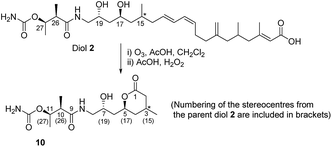
With the above information in hand, it was necessary to determine the configuration of the C-15 stereocenter as well as confirm the tentative assignment of absolute stereochemistry (17S, 19R, 26R, 27R) for diol 2. We turned to natural product degradation and comparison with a synthetic standard (Scheme 3). Ozonolysis of diol 2 followed by treatment with AcOH/H2O2 led to cleavage of the 12,13-double bond to give a dihydroxy acid which cyclized, after purification by HPLC, to give lactone 10 as a single diastereomer with [α]D +25.0.
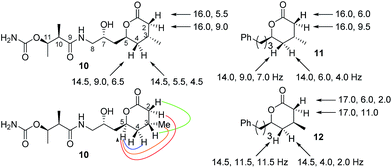
From a combination of TOCSY and NOESY it was apparent that the ring adopted a boat conformation with NOESY correlations from 5-H to 2α-H, 4α-H and 3-CH3 consistent with these being on the same face of the molecule (Fig. 2). A NOESY correlation between 2β-H and 3-H, which was in accord with the methyl group at C-3 having a relative anti relationship with the C-5 side-chain so that in the ‘open chain structure’ it would have a syn relationship with the C-5 alcohol.
Further support came from comparing the coupling constants for the ring protons in lactone 10 with those reported for anti and syn lactones 11 and 1222 (Fig. 2). The signals assigned to 4-H2 in 11 and 12 have significantly different couplings constants and the NMR data for anti lactone 11 correlated well with those for degradation product 10.
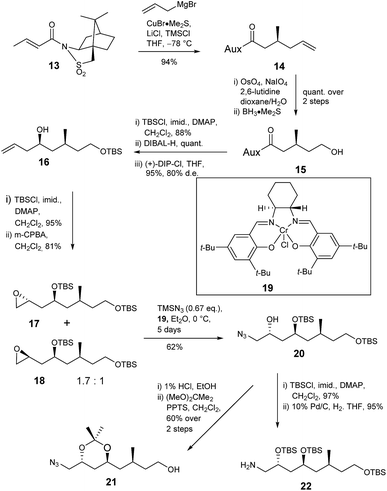
The deduced structure and absolute configuration of degradation product 10 were confirmed by total synthesis (Schemes 4 and 5). Addition of a copper mediated Grignard reagent to chiral crotonate 13 using the conditions reported by Lipshutz23 gave the known allylated sultam 14 generating the 3-methyl stereocenter in the target lactone. Oxidative cleavage of the alkene using OsO4/NaIO4 in the presence of 2,6-lutidine24 followed by reduction of the resultant aldehyde gave alcohol 15 in quantitative yield. Addition of lutidine was important as without it only a 15% yield of aldehyde was obtained. Protection of alcohol 15 as the TBS ether and reductive cleavage of the auxiliary gave an aldehyde which was immediately subjected to Brown allylation conditions with (+)-DIP-Cl25,26 giving alkene 16 in 83% yield over the 3 steps. The stereochemical outcome of the reaction was confirmed by analysis of the (R)- and (S)-Mosher's ester27,28 derivatives of alcohol 16.
Next the amine and the further required protected alcohol were introduced via manipulation of the terminal alkene of 16 (Scheme 4). 1-Azido-2-trimethylsiloxanes have been prepared via kinetic resolution of terminal epoxides using TMSN3 and a chiral bidentate salen ligand.29 Thus alcohol 16 was protected as the TBS ether then treatment with MCPBA gave a 1.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of epoxides 17 and 18 in 81% yield. The mixture was treated with chiral salen complex 19 and TMSN3 giving azide 20 (62%) and unreacted epoxide 18 (31%) which were readily separated by column chromatography. The anti relationship of the protected 1,3-diol was confirmed by deprotection of silyl ether 20 and conversion of the resultant anti 1,3-diol to acetonide 21 which gave characteristic 13C NMR signals at δc 100 for the quaternary carbon and δc 24.7, 24.8 for the methyl groups. TBS protection of secondary alcohol 20 and reduction of the azide gave amine 22 required for coupling to the butanoic acid derivative.
1 mixture of epoxides 17 and 18 in 81% yield. The mixture was treated with chiral salen complex 19 and TMSN3 giving azide 20 (62%) and unreacted epoxide 18 (31%) which were readily separated by column chromatography. The anti relationship of the protected 1,3-diol was confirmed by deprotection of silyl ether 20 and conversion of the resultant anti 1,3-diol to acetonide 21 which gave characteristic 13C NMR signals at δc 100 for the quaternary carbon and δc 24.7, 24.8 for the methyl groups. TBS protection of secondary alcohol 20 and reduction of the azide gave amine 22 required for coupling to the butanoic acid derivative.
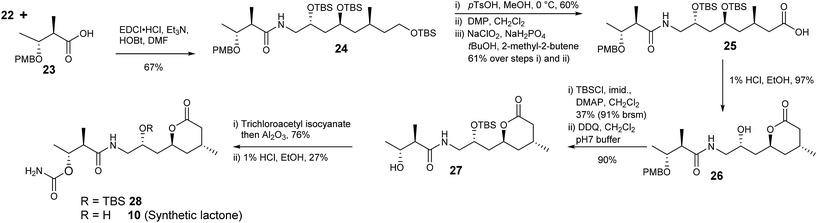
Acid 23 was readily prepared from ethyl (2R,3R)-3-hydroxy-2-methylbutanoate30,31via formation of the p-methoxybenzyl ether followed by hydrolysis of the ester with barium hydroxide (Scheme 5). Amine 22 and acid 23 were coupled giving 24 with the required carbon framework of the target lactone. Further functional group manipulations included selective deprotection of the primary silyl ether 24 then a two-step oxidation protocol to give carboxylic acid 25 using DMP followed by a Pinnick oxidation.32
Treatment of 25 with 1% HCl in EtOH generated lactone 26. To complete the synthesis of 10 the secondary alcohol in 26 was protected as the silyl ether and the benzyl ether removed with 2,3-dichloro-5,6-dicyanobenzoquinone (DDQ) giving 27. The carbamoyl group was introduced using trichloroacetylisocyanate in CH2Cl2 followed by stirring with alumina to give 28 in 76% yield. Finally removal of the silyl ether with 1% HCl in EtOH gave lactone 10.
The 1H- and 13C NMR data of synthetic lactone 10 and the product isolated from ozonolysis of metabolite 2 were the same (Table 5, ESI†). Furthermore the optical rotation of the two samples were entirely consistent. Thus, we can confirm the absolute and relative configuration of the C14–C28 fragment of diol 2 is as shown in Scheme 5.
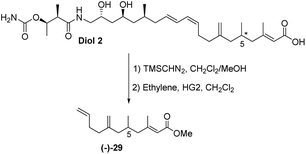
The final challenge was to determine the stereochemistry of the remote C-5 stereocentre. We again applied a combination of degradation studies and chemical synthesis. Diol 2 was methylated with TMS diazomethane then treated with 2nd generation Hoveyda–Grubbs catalyst33 under an ethylene atmosphere to give the degradation product (−)-29 with [α]D −6.8 (Scheme 6).
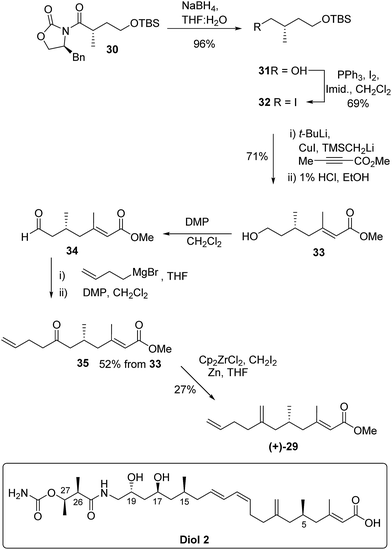
The absolute configuration of 29 was confirmed via the enantioselective synthesis shown in Scheme 7. The stereocentre which would become C-5 in the target was readily generated via an alkylation reaction with NaHMDS/CH3I giving oxazolidinone 30 as previously described.34 Following reductive cleavage of the auxiliary, primary alcohol 31 was converted to iodide 32.
The key synthetic step was formation of the tri-substituted unsaturated ester 33. Preliminary studies using Horner–Wadsworth–Emmons or Julia chain extensions of a methyl ketone proved unsatisfactory giving a mixture of E and Z-alkenes. However, excellent control of alkene geometry was achieved via addition of the cuprate derived from iodide 32 to commercially available methyl but-2-ynoate. Following work up and deprotection, primary alcohol 33 was isolated in 71% yield from iodide 32 and the E-alkene geometry was confirmed by nOe.
Aldehyde 34, prepared by Dess–Martin periodinane (DMP) oxidation35 of alcohol 33, was coupled with but-3-enylmagnesium bromide and further oxidation gave ketone 35 in 52% yield over the 3 steps. In the final methylenation step it was important to avoid bond migration of the exo alkene to an endo position. This was achieved via the zirconium-mediated methylenation36,37 of the ketone. Unsaturated methyl ester (+)-29 had identical NMR spectra to the degradation product (−)-29 but with an optical rotation [α]D +5.0 in accord with the synthetic material being the enantiomer of the degradation product. Thus kalimantacin A 1 has the 5R configuration.
Conclusions
In conclusion, elucidating the absolute configuration of complex polyketides with multiple stereogenic centers is often an ambitious undertaking.38,39 Herein we used a multidisciplinary approach involving natural product isolation, chemical degradation studies, bioinformatics, NMR correlations and fragment synthesis to rigorously determine the absolute and relative stereochemistry of the kalimantacins isolated from P. fluorescens. Diol 2 from the ΔbatM mutant has the 5R, 15S, 17S, 19R, 26R, 27R stereochemistry and is the immediate biosynthetic precursor of the bioactive kalimantacin A 1 formed by oxidation of the secondary alcohol to the 17-ketone. The absolute configurations of the 17, 19 and 27-hydroxyl groups in diol 2 are in good agreement with bioinformatic predictions.12,13The 13C and 1H NMR data for natural product 1 isolated from P. fluorescens are entirely consistent with those reported4,5 for kalimantacin from Alcaligenes sp. YL-02632S in accord with both structures having the same relative stereochemistry. Furthermore it is evident from comparison of PKS/NRPS gene clusters for kalimantacin A from cultures of P. fluorescens12 and batumin from P. batumici14 that the natural products are indeed the same compound. Synergising chemical and biochemical methods have led to the elucidation of the structures of kalimantacin A 1 (and hence batumin) and diol 2.
The inexorable rise of antibiotic resistant bacteria requires a multi-facetted response to meet this global threat to human health. In contrast to broad spectrum antibiotics, narrow spectrum agents can selectively decolonise specific pathogens (so called decolonisation drugs) in the human microbiota and are becoming increasingly important for detecting as well as fighting specific infections. They may also reduce the chance of emergence of antibiotic resistant strains.11 Kalimantacin has been identified as a highly specific agent against staphylococci that will potentially allow its deployment for the detection of resistant strains as well as a potent decolonisation agent. The determination of the absolute stereochemistry of kalimantacin as described in this paper will underpin further functional investigation of this important molecule.
Acknowledgements
We thank Dr Kate de Mattos-Shipley for assistance with our comparison of gene clusters confirming literature. We are grateful to the following for funding: the BBSRC and EPSRC including through BrisSynBio Synthetic Biology Research Centre (BB/L01386X/1) and the Bristol Chemical Synthesis Centre for Doctoral Training which provided PhD studentships for IRGT (EP/G036764/1) and PDW (EP/L015366/1) and BaSe-ics research community from the FWO Vlaanderen (W.0014.12N).Notes and references
- K. J. Weissman and P. F. Leadlay, Nat. Rev. Microbiol., 2005, 3, 925–936 CrossRef CAS PubMed.
- C. M. Thomas, J. Hothersall, C. L. Willis and T. J. Simpson, Nat. Rev. Microbiol., 2010, 8, 281–289 CrossRef CAS PubMed.
- C. Hertweck, Angew. Chem., Int. Ed., 2009, 48, 4688–4716 CrossRef CAS PubMed.
- K. Kamigiri, Y. Suzuki, M. Shibazaki, M. Morioka, K. Suzuki, T. Tokunaga, B. Setiawan and R. M. Rantiatmodjo, J. Antibiot., 1996, 49, 136–139 CrossRef CAS PubMed.
- T. Tokunaga, K. Kamigiri, M. Orita, T. Nishikawa, M. Shimizu and H. Kaniwa, J. Antibiot., 1996, 49, 140–144 CrossRef CAS PubMed.
- V. V. Smirnov, L. N. Churkina, V. I. Perepnikhatka, N. S. Mukvich, A. D. Garagulia, E. A. Kiprianova, A. N. Kravets and S. A. Dovzhenko, Prikl. Biokhim. Mikrobiol., 2000, 36, 55–58 CAS.
- V. V. Smirnov, L. N. Churkina, A. N. Kravets and V. I. Perepnikhatka, Appl. Biochem. Microbiol., 2000, 36, 262–265 CrossRef.
- L. N. Churkina, S. I. Bidnenko, G. Lopes dos Santos Santiago, M. Vaneechoutte, L. V. Avdeeva, O. B. Lutko and N. M. Oserjanskaja, BMC Res. Notes, 2012, 5, 374 CrossRef PubMed.
- L. Churkina, M. Vaneechoutte, E. Kiprianova, N. Perunova, L. Avdeeva and O. Bukharin, Open J. Med. Microbiol., 2015, 5, 193–201 CrossRef.
- P.-Y. Levy, M. Ollivier, M. Drancourt, D. Raoult and J.-N. Argenson, Orthopaedics & Traumatology: Surgery & Research, 2013, 99, 645–651 Search PubMed.
- E. Tacconelli, I. Autenrieth and A. Peschel, Science, 2017, 355, 689–690 CrossRef CAS PubMed.
- W. Mattheus, L. J. Gao, P. Herdewijn, B. Landuyt, J. Verhaegen, J. Masschelein, G. Volckaert and R. Lavigne, Chem. Biol., 2010, 17, 149–159 CrossRef CAS PubMed.
- W. Mattheus, J. Masschelein, L. J. Gao, P. Herdewijn, B. Landuyt, G. Volckaert and R. Lavigne, Chem. Biol., 2010, 17, 1067–1071 CrossRef CAS PubMed.
- V. V. Klochko, Biotechnol. Acta, 2014, 7, 46–50 CrossRef CAS.
- V. V. Klochko, L. B. Zelena, J. Y. Kim, L. V. Avdeeva and O. N. Reva, Int. J. Antimicrob. Agents, 2016, 47, 56–61 CrossRef CAS PubMed.
- J. Piel, Nat. Prod. Rep., 2010, 27, 996–1047 RSC.
- E. J. N. Helfrich and J. Piel, Nat. Prod. Rep., 2016, 33, 231–316 RSC.
- V. E. Lee and A. J. O'Neill, Int. J. Antimicrob. Agents, 2017, 49, 121–122 CrossRef CAS PubMed.
- D. A. Evans, K. T. Chapman and E. M. Carreira, J. Am. Chem. Soc., 1988, 110, 3560–3578 CrossRef CAS.
- K. Narasaka and F. C. Pai, Tetrahedron, 1984, 40, 2233–2238 CrossRef CAS.
- S. D. Rychnovsky, B. N. Rogers and T. I. Richardson, Acc. Chem. Res., 1998, 31, 9–17 CrossRef CAS.
- G. Fronza, C. Fuganti, P. Grasselli and M. Terreni, Tetrahedron, 1992, 48, 7363–7372 CrossRef CAS.
- B. H. Lipshutz and C. Hackmann, J. Org. Chem., 1994, 59, 7437–7444 CrossRef CAS.
- W. Yu, Y. Mei, Y. Kang, Z. Hua and Z. Jin, Org. Lett., 2004, 6, 3217–3219 CrossRef CAS PubMed.
- H. C. Brown and P. K. Jadhav, J. Am. Chem. Soc., 1983, 105, 2092–2093 CrossRef CAS.
- U. S. Racherla and H. C. Brown, J. Org. Chem., 1991, 56, 401–404 CrossRef CAS.
- J. A. Dale, D. L. Dull and H. S. Mosher, J. Org. Chem., 1969, 34, 2543–2549 CrossRef CAS.
- J. A. Dale and H. S. Mosher, J. Am. Chem. Soc., 1973, 95, 512–519 CrossRef CAS.
- J. F. Larrow, S. E. Schaus and E. N. Jacobsen, J. Am. Chem. Soc., 1996, 118, 7420–7421 CrossRef CAS.
- K. Mori and T. Ebata, Tetrahedron, 1986, 42, 4413–4420 CrossRef CAS.
- G. Fráter, U. Müller and W. Günther, Tetrahedron, 1984, 40, 1269–1277 CrossRef.
- B. S. Bal, W. E. Childers and H. W. Pinnick, Tetrahedron, 1981, 37, 2091–2096 CrossRef CAS.
- S. B. Garber, J. S. Kingsbury, B. L. Gray and A. H. Hoveyda, J. Am. Chem. Soc., 2000, 122, 8168–8179 CrossRef CAS.
- R. Bajpai, F. Yang and D. P. Curran, Tetrahedron Lett., 2007, 48, 7965–7968 CrossRef CAS PubMed.
- D. B. Dess and J. C. Martin, J. Org. Chem., 1983, 48, 4155–4156 CrossRef CAS.
- J. M. Tour, P. V. Bedworth and R. Wu, Tetrahedron Lett., 1989, 30, 3927–3930 CrossRef CAS.
- M. Hartmann and E. Zbiral, Tetrahedron Lett., 1990, 31, 2875–2878 CrossRef CAS.
- D. Menche, Nat. Prod. Rep., 2008, 25, 905–918 RSC.
- J. W. Blunt, B. R. Copp, R. A. Keyzers, M. H. G. Munro and M. R. Prinsep, Nat. Prod. Rep., 2016, 33, 382–431 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Complete description of materials and methods and additional tables, figures and schemes including full 1H and 13C NMR data for all new compounds. See DOI: 10.1039/c7sc01670k |
| ‡ Present address: Department of Chemistry, University of Warwick, Coventry CV4 7AL, UK. |
| This journal is © The Royal Society of Chemistry 2017 |