Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
CoOx nanoparticle anchored on sulfonated-graphite as efficient water oxidation catalyst†
Jingqi
Guan
,
Chunmei
Ding
,
Ruotian
Chen
,
Baokun
Huang
,
Xianwen
Zhang
,
Fengtao
Fan
,
Fuxiang
Zhang
 * and
Can
Li
* and
Can
Li
 *
*
State Key Laboratory of Catalysis, iChEM, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian National Laboratory for Clean Energy, Dalian, 116023, China. E-mail: fxzhang@dicp.ac.cn; canli@dicp.ac.cn
First published on 26th June 2017
Abstract
Development of efficient, robust and earth-abundant water oxidation catalysts (WOCs) is extremely desirable for water splitting by electrolysis or photocatalysis. Herein, we report cobalt oxide nanoparticles anchored on the surface of sulfonated graphite (denoted as “CoOx@G-Ph-SN”) to exhibit unexpectedly efficient water oxidation activity with a turnover frequency (TOF) of 1.2 s−1; two or three orders of magnitude higher than most cobalt-based oxide WOCs reported so far. The CoOx@G-Ph-SN nanocomposite can be easily prepared by a soft hydrothermal route to have an average CoOx size below 2 nm. Additionally, the loading of CoOx@G-Ph-SN catalyst on the surface of a BiVO4 or Fe2O3 photoanode can boost remarkably the photoanode currents for robust photocatalytic water oxidation under visible light irradiation. Its excellent activity and photochemical stability for water oxidation suggest that this ultrasmall cobalt-based composite is a promising candidate for solar fuel production.
Introduction
Current energy resources are derived mainly from fossil fuels (oil, coal and gas), and the utilization of these has caused numerous environmental problems, such as the increasing emission of greenhouse gas, most notably carbon dioxide (CO2).1 To address this issue, an increased amount of clean, renewable and sustainable energy resources should be presented for energy resource distribution.2 Solar energy is the largest clean and renewable energy source on the Earth.3 Solar-driven water splitting can store energy in the form of hydrogen fuel,2 and water oxidation is considered to be the key reaction for overall water splitting.4,5 Therefore, the development of highly efficient and robust water oxidation catalysts (WOCs) is crucial to the implementation of solar fuel production.In the past decades, continuous efforts have been devoted to developing efficient and robust WOCs. Mn-,6–8 Fe-,9 Co-,10–13 Ni-,14 Ru-,15–17 and Ir-based18 complexes or metal oxides have been examined for water oxidation by chemical, electrochemical or photocatalytic approaches. Some homogeneous molecular complexes, such as [Ru(bda)(isoq)2] (H2bda = 2,2′-bipyridine-6,6′-dicarboxylic acid; isoq = isoquinoline)15 and halogen substituted [Ru(bda)(isoq)2]17 exhibited comparable catalytic activity for water oxidation to the CaMn4O5 complex in PSII of natural photosynthesis. In addition, cobalt-based polyoxometalates [Co4(H2O)2(PW9O34)2]10− and Na10[Co4(H2O)2(VW9O34)2]·35H2O showed very high catalytic activity (TOF = 5 s−1) for water oxidation in the [Ru(bpy)3]2+-sulfate system under light irradiation.11,13 Although the homogeneous complexes have exhibited satisfactory water oxidation activity, they are seldom applied in real solar water oxidation systems, mostly due to their poor photochemical stability. Comparatively, heterogeneous WOCs are robust and thus frequently supported on the surface of semiconductors for solar water oxidation.19,20
Nocera's group reported an in situ electrochemical synthesis of cobalt phosphate films on an indium tin oxide anode, which can oxidize water well under a neutral pH environment.21 Since then, the development of cobalt-based WOCs as well as their application in the fabrication of artificial photosynthesis devices has gained considerable attention. For example, Frei et al. synthesized ∼25 nm Co3O4 nanoclusters supported inside mesoporous silica (SBA-15 and KIT-6), and found that smaller Co3O4 clusters showed higher water oxidation activity.22 The surface of g-C3N4 modified with layered Co(OH)2 can not only accelerate the interface transfer rate of charge carriers, but also reduce the excessive energy barrier for O–O formation, thus leading to enhanced reaction kinetics for photocatalytic water oxidation.23 CoOx as O2-evolving cocatalysts supported on the surface of a LaTiO2N photocatalyst also showed remarkable promotion of water oxidation performance under visible light irradiation.19,24 However, the size of CoOx nanoparticles reported in most of the previous literature is usually larger than 5 nm, with a catalytic activity that is at least 2 orders of magnitude lower than those of homogeneous Co-based catalysts.10
Recently, many heterogeneous catalysts with single atoms or nanocluster structures have exhibited significantly enhanced catalytic activities with respect to conventional bulk catalysts.25,26 Inspired by this, one possible strategy to obtain highly active heterogeneous Co-based WOC is to reduce further the size of the cobalt oxide. However, the reduction of particle size is generally accompanied by an enhancement of surface energy, causing aggregation and instability of the WOC. Thus, further efforts on how to stabilize the ultrasmall nanoparticles should be considered. To address this, loading of ultrasmall nanoparticles onto the surface of a solid support has been known as effective way to stabilize nanoparticles.10 In addition, as for the application of WOC in photo(electro)catalytic water splitting, another design consideration is that the support should possess good conductivity for efficient carrier transfer. Based on these findings, the synthesis of ultrasmall Co-based WOCs anchored on the surface of a conductor is highly desirable. Graphite not only possesses good mechanical stability, but also exhibits excellent conductivity, and has been used widely in the field of solar cell and electrochemical water splitting.27–29 Accordingly, graphite is expected to be a good support to stabilize ultrasmall Co-based nanoparticles for further solar water splitting.
Herein, we report functionalized graphite-anchored CoOx nanoparticles with an average size of sub-2 nm to exhibit unexpectedly efficient heterogeneous water oxidation activity. The sub-2 nm CoOx nanoparticles were hydrothermally synthesized by anchoring them on the surface of phenylsulfonic acid-functionalized graphite. The TOF value of water oxidation on the optimized CoOx@G-Ph-SN sample can reach as high as 1.2 s−1, over two orders of magnitude higher than most heterogeneous transition metal oxides. In addition, the direct coupling of CoOx@G-Ph-SN with an Fe2O3 photoanode demonstrates its good photochemical stability under an artificial photosynthesis environment.
Experimental
Materials and reagents
All chemicals were analytical grade and used as purchased without further purification. Solutions were prepared using high purity water (Millipore Milli-Q purification system, resistivity > 18 MΩ cm). The fluorine-doped tin oxide (FTO) conductive glass was purchased from Nippon Sheet Glass Company (Japan) and was ultrasonically cleaned with acetone, ethanol and deionized water for 20 min each in sequence prior to use.Synthesis of samples
As described in Scheme 1, the synthesis of the CoOx@G-Ph-SN sample mainly involves three steps: (i) immobilization of phenyl on the surface of graphite (G-Ph); (ii) sulfonation of phenyl (G-Ph-SO3H); and (iii) hydrothermal synthesis of CoOx anchored on the surface of phenylsulfonic acid functionalized graphite (CoOx@G-Ph-SN). The first two steps were achieved by referring to previous work.30 As for the first step on immobilization of phenyl on the surface of graphite, typically, the dark gray graphite powder (0.5 g, 41.6 mmol of carbon) was dispersed in benzene (400 mL) in a 500 mL three necked round-bottom flask equipped with a magnetic stir bar. The contents were then stirred vigorously after benzoyl peroxide (10.1 g, 41.6 mmol) was added. The mixture was then heated at 80 °C for 12 h with continuous vigorous stirring. After cooling down, the contents of the flask were centrifuged and washed with ethanol for four times. The black solid was dried at 60 °C overnight, which was nominated as G-Ph.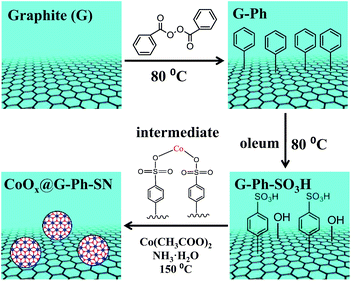 | ||
| Scheme 1 Schematic illustration of the synthesis of CoOx nanoparticles anchored on sulfonated graphite (CoOx@G-Ph-SN). | ||
As for the second step on phenylsulfonic acid functionalized graphite, a typical experimental process was as follows: phenylated graphite (G-Ph) (200 mg) was dispersed in oleum (70 mL, H2SO4, 25% as free SO3), and heated at 80 °C for 5 h to yield phenyl sulfonated graphite. After cooling down, 300 g of ice block was then carefully added into the suspension. The mixture was then centrifuged and washed with water several times until the pH value of the filtrate reached ∼7. The obtained solid was dried at 60 °C overnight, which was nominated as G-Ph-SO3H. For comparison, the pristine graphite was also treated with oleum in a similar process with the synthesis of G-Ph-SO3H, which was nominated as G–O.
As for the third step, the hydrothermal anchoring of CoOx on the functionalized graphite (G-Ph-SO3H), typically 13.2 mg of Co(CH3COO)2·4H2O and 150 mg of G-Ph-SO3H were mixed in 20.0 mL of ethanol solution containing 50 µL of deionized water. After about 10 min stirring, 75 µL of 28% ammonia were added and mixed for another 10 min. Afterwards, the mixture was transferred into a 30 mL Teflon autoclave and heated at 150 °C for 2 h. After heat treatment, the autoclave was cooled to room temperature, and the product was washed with deionized water for more than three times. The final product was dried at 60 °C overnight, which was nominated as CoOx@G-Ph-SN.
In this work, the immobilization of phenyl and sulfonation of phenyl were confirmed by FTIR (ESI Fig. S1†), UV-Vis (ESI Fig. S2†) and XPS (ESI Table S1†), and the possible formation of cobalt benzenesulfonate intermediate can be verified by UV/Vis diffuse reflectance spectra (ESI Fig. S2 and S3†).31 As for discussion and comparison, Co3O4 nanoparticles (Co3O4-nano), CoOx@G–O and CoOx@graphite nanocomposites were similarly synthesized by following the above hydrothermal process (the third step) except that the former was free of G-Ph-SO3H, the middle employed G–O to substitute G-Ph-SO3H, and the latter employed graphite to substitute G-Ph-SO3H. Commercial Co3O4 was also employed for comparison.
Preparation of photoanodes
The preparation of the nanoporous BiVO4 photoanode was as described in previous work.32 Typically, F-doped SnO2 (FTO) substrates were first electrochemically deposited with BiOI, and then a dimethylsulfoxide (DMSO) solution of vanadyl acetylacetonate [VO(acac)2] was dropped onto their surface and heated in air at 450 °C for 2 hours to obtain BiVO4 photoanodes. Afterwards, a calculated amount of pre-dispersed CoOx@G-Ph-SN ethanol solution was dropped onto the BiVO4 photoanode, which was then heated in air at 80 °C for 1 h to obtain the CoOx@G-Ph-SN/BiVO4 photoanode.As for preparation of Fe2O3 photoanodes, Fe2O3 was deposited on an FTO substrate electrode using a modified chemical bath deposition method reported elsewhere.33 Afterwards, a calculated amount of pre-dispersed CoOx@G-Ph-SN ethanol solution was dropped onto the surface of the Fe2O3 photoanode, which was further heated in air at 65 °C for 10 min to produce the CoOx@G-Ph-SN/Fe2O3 photoanode.
Characterizations of samples
The as-prepared samples were characterized by X-ray powder diffraction (XRD) on a Rigaku D/Max-2500/PC powder diffractometer. The sample powder was scanned using Cu Kα radiation with an operating voltage of 40 kV and current of 200 mA. A scan rate of 5° min−1 was applied to record the patterns in the range of 10–80°. Transmission electron microscope (TEM) images were observed by a Hitachi HT7700. High resolution TEM (HRTEM) images were recorded on a JEM-2100 transmission electron microscope (Tokyo, Japan) at 200 kV. The loading amount of cobalt oxide in the catalyst was determined using an inductively coupled plasma atomic emission spectrometer (ICP-AES) on a Shimadzu ICPS-8100. Prior to ICP-AES measurement, the supported cobalt oxide was dissolved in aqua regia. FT-IR spectra were obtained using a Varian 3100 FTIR spectrophotometer in DRIFT mode (diffuse reflectance infrared Fourier transform). The spectra were collected in the wavenumber range from 3900–400 cm−1 with 2 cm−1 resolution (average of 32 scans). The valence state of the cobalt oxide cluster was determined using XPS recorded on a Thermo ESCALAB 250Xi. The X-ray source selected was a monochromatized Al Kα source (15 kV, 10.8 mA). Region scans were collected using a 20 eV pass energy. Peak positions were calibrated relative to the C 1s peak position at 284.6 eV.Tests of chemical water oxidation
Typically, a calculated amount of 0.03–0.3 g L−1 CoOx@G-Ph-SN was added to a borate buffer solution (pH 9.0, 3.0 mL) containing Na2S2O8 (5.0 mM) and [Ru(bpy)3](ClO4)2 (1.0 mM). After 5 min stirring, the mixture was then irradiated with a halogen lamp through a coloured filter glass transmitting λ > 420 nm at room temperature. The photon flux of the incident light was determined to be 1.28 × 10−7 mol cm−2 s−1 using an EKO LS-100 spectroradiometer. Evolved oxygen gas was monitored by using a Clark-type electrode (Strathkelvin SI130 UK). The turnover frequency (TOF) was calculated by using the initial constant O2 evolution rate and assuming all the cobalt atoms were active sites. The contents of cobalt on typical samples were analyzed by ICP-AES measurement, which are 1.2 wt% and 1.29 wt% for CoOx@graphite and CoOx@G-Ph-SN, respectively.Tests of electrochemical water oxidation
The electrochemical water oxidation performances of all the cobalt-based electrodes were tested in a conventional three-electrode electrochemical cell with a platinum plate as the auxiliary electrode and a saturated calomel electrode (SCE, saturated KCl) as the reference electrode. A 1 M NaOH aqueous solution was used as the electrolyte with a pH measured at ca. 13.6. The scanning rate was 10 mV s−1. All potentials measured were calibrated to RHE using the following equation: E(RHE) = E(SCE) + 0.241V + 0.0591pH.Photoelectrochemical (PEC) measurements
The PEC tests were conducted in a three electrode system under simulated AM 1.5G solar light irradiation (100 mW cm−2, Newport Sol3A, Class AAA Solar simulator) or a 300 W xenon lamp (PLS-SXE300C, Perfectlight Company). The fabricated electrode, a platinum electrode, and a SCE were used as the working, counter and reference electrodes, respectively. 0.5 M lithium borate buffered solution (pH 9) or 1 M NaOH aqueous solution (pH = 13.6) was used as an electrolyte after saturation with Ar gas for 30 min. The photocurrent was measured by linear sweep voltammetry with a scan rate of 10 mV s−1. The light irradiation came from the front side of the electrodes in all cases.Kelvin probe force microscopy measurements
Kelvin Probe Force Microscopy (KPFM), which measures the contact potential difference between the probe and sample, was employed for potential imaging.34 An amplitude-modulated mode of the KPFM in lift mode was used. For each scan, a SCM-PIT Pt/Ir-coated conductive probe passes over the surface twice. On the first pass, a feedback loop controls the sample height to acquire the topography and phase signal of the sample. On the second pass, the cantilever is held at 100 nm of tip–sample distance to record the surface potential. The scan rate is 0.5 Hz.Results and discussion
As seen in the TEM images of Fig. 1a and b, the CoOx nanoparticles on the surface of CoOx@G-Ph-SN (Fig. 1a) are more homogeneously dispersed compared to the CoOx@graphite (Fig. 1b), CoOx@G–O (Fig. S4†) and Co3O4-nano (Fig. S4†); based on which their average sizes are calculated to be 1.6, 3.2, 2.5 and 5.3 nm, respectively. This demonstrates that compared to the synthesis of Co3O4-nano, the addition of graphite can inhibit the growth of CoOx nanoparticles (CoOx@graphite) to a certain extent, but its aggregation (Fig. 1b) is clearly observed because of the hydrophobic surface feature of graphite itself. However, the surface of graphite after the functionalization of phenylsulfonic acid is changed from hydrophobic to hydrophilic according to the measurement of its contact angle (Fig. S5†). A hydrophilic surface is generally favorable for the homogeneous dispersion of CoOx nanoparticles.19 In addition, the phenylsulfonic group is expected to complex with Co2+ according to comparison of the UV-Vis absorption results on typical samples (Fig. S2†), which should be another factor inhibiting its growth kinetics, causing the reduction of particle size. As an extended discussion, we also synthesized the oleum modified graphite for the loading of CoOx nanoparticles. Similarly, the surface of graphite after treatment by oleum changes from hydrophobic to hydrophilic according to the measurement of its contact angle (Fig. S5†), and after loading the same amount of CoOx on the oleum treated graphite, the dispersion of CoOx can be improved and the particle size can be reduced compared with those on the pristine graphite (Fig. S4†). However, the average particle size of CoOx on the oleum treated graphite is 2.5 nm, which is a little larger than that (ca. 1.6 nm) loaded on the surface of GE-Ph-SO3H. This means that surface wettability modification caused by oleum treatment can improve the dispersion and decrease the size of CoOx to a certain extent, but its influence is not as obvious as that caused by the surface phenyl-sulfonation modification. This difference may lie in the complex effect of Co2+ ions with phenylsulfonic groups, as this may change the number of crystal nuclei as well as the concentration of Co2+ ions remaining in the solution, leading to different growth kinetics.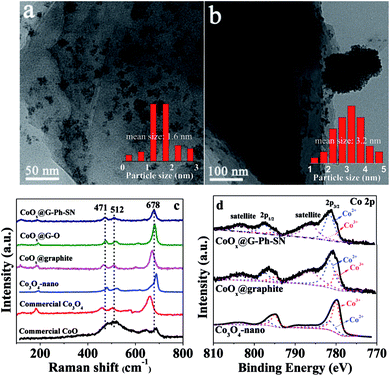 | ||
| Fig. 1 Representative TEM images of typical samples: (a) CoOx@G-Ph-SN, (b) CoOx@graphite; and their Raman (c) and XPS (d) spectra. | ||
The states of the cobalt species existing on the samples were analyzed by Raman and XPS spectra. As seen in Fig. 1c, three prominent Raman peaks, located at 678, 512, and 471 cm−1 can be observed for the CoOx@G-Ph-SN, CoOx@G–O and CoOx@graphite samples, which are not consistent with the pure CoO phase, Co3O4 phase, or nano-Co3O4 phase.35 This demonstrates that the grafted cobalt species differ from the single phase of CoO or Co3O4. The valence state of CoOx in CoOx@G-Ph-SN is further investigated by Co 2p XPS (Fig. 1d), in which two prominent shake-up satellite peaks, indicative of Co2+ ions,36 are clearly observed, and the Co 2p1/2–Co 2p3/2 energy separation is approximately 16.0 eV. Based on our fitted curves of Co 2p3/2 peak, the surface atomic ratios of Co3+/Co2+ are calculated to be ca. 0.35, 0.38 and 2.0 for CoOx@G-Ph-SN, CoOx@graphite and Co3O4-nano, respectively. The high concentration of Co2+ in CoOx@graphite and CoOx@G-Ph-SN can be further verified by EPR spectra (Fig. S6†).
To characterize further the CoOx particles on graphite, we carried out XRD and HRTEM measurements. No obvious XRD peaks assigned to the CoOx can be observed even for the sample with a CoOx loading content up to ca. 10.0 wt% (Fig. S7†). Based on the HRTEM image (Fig. S8†), the space distance of the CoOx can be calculated to be 0.251 nm, which is not in accordance with those of CoO, Co(OH)2, CoOOH and Co3O4. Thus, the CoOx phase in this work should be different from each of them. Based on the above analysis, we thus label the cobalt species in this work as CoOx for simplicity. The surface element contents of CoOx@graphite, CoOx@G–O and CoOx@G-Ph-SN samples were analyzed and are given Table S1.†
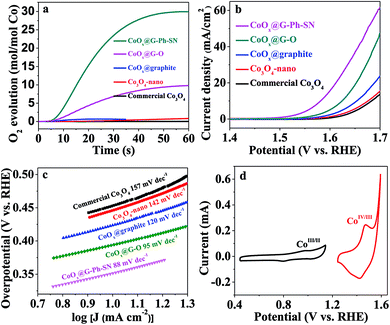
To evaluate the potential of the as-obtained CoOx@G-Ph-SN sample as a WOC, a visible-light-driven water oxidation system containing [Ru(bpy)3]Cl2 and Na2S2O8 in the presence of a borate-buffered solution was examined with oxygen detected by a Clark electrode. Fig. 2a gives their typical activity curves as a function of reaction time, based on which their TOF values of water oxidation are calculated. As a comparison, Co3O4-nano (TOF of 0.012 s−1) shows an obvious promotion of water oxidation activity compared to the commercial Co3O4 (TOF of 0.0013 s−1). The photocatalytic water oxidation activity can be enhanced by the CoOx@graphite sample, showing a TOF of 0.058 s−1. The photocatalytic water oxidation activity can be further enhanced by improving the dispersion of CoOx and decreasing the particle size of CoOx. A TOF of 0.31 s−1 can be achieved over CoOx@G–O. Comparatively, the CoOx@G-Ph-SN sample shows the best TOF value of 1.2 s−1, an unexpectedly efficient water oxidation activity with respect to previously reported cobalt-based oxides (Table S2†). It should be pointed out that the TOF value is related to the light intensity (Fig. S9†). In addition, the water oxidation performances are normally affected by the surface reaction and charge separation processes, so factors beyond the size of CoOx should have an effect on the activity; these probably include the mass transfer at the interface of the catalyst and aqueous solution, the structures of the active species and the the good conductivity of graphite etc.
The water oxidation performance was also evaluated by electrochemical water oxidation. Their linear sweep voltammetry (LSV) curves are depicted in Fig. 2b. The glassy carbon electrode (GCE) or graphite itself shows a negligible current and very high onset potential. Similarly, the water oxidation activity trend on typical electrodes can be described as follows: CoOx@G-Ph-SN > CoOx@G–O > CoOx@graphite > Co3O4-nano > commercial Co3O4, whose overpotential values at a current density of 10 mA cm−2 are 350 mV, 395 mV, 450 mV, 470 mV, and 475 mV, respectively. The excellent O2-evolving activity of the CoOx@G-Ph-SN composite was further confirmed by its much smaller Tafel slope (88 mV per decade) at lower overpotentials than that measured for CoOx@G–O (95 mV per decade), CoOx@graphite (120 mV per decade), Co3O4-nano (142 mV per decade), and commercial Co3O4 (157 mV per decade) (Fig. 2c). The cyclic voltammogram (CV) of CoOx@G-Ph-SN in 1.0 M NaOH solution at a scan rate of 10 mV s−1 (Fig. 2d) revealed two reversible reduction waves at E1/2 = 0.89 V and 1.44 V versus RHE, assigned to two sequentially occurring one-electron redox reactions involving CoII/CoIII and CoIII/CoIV couples, respectively.37–39
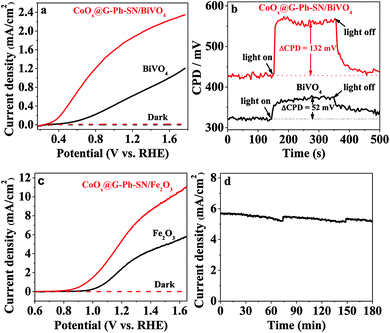
Encouraged by the extraordinary water oxidation activity on the CoOx@G-Ph-SN sample, we thus further evaluated its potential use in a practical artificial photosynthesis system. As an initial attempt, we loaded the CoOx@G-Ph-SN onto the surface of a BiVO4 electrode for photoelectrochemical water oxidation. Here the CoOx@G-Ph-SN WOC can be considered as a cocatalyst of the BiVO4 photoanode for water oxidation.40Fig. 3a gives the typical linear sweep voltammetry (LSV) curves of BiVO4 photoanodes with and without loading of CoOx@G-Ph-SN, in which loading of CoOx@G-Ph-SN not only obviously promotes the current of the BiVO4 photoanode, but also causes a negative shift of the onset potential. This clearly demonstrates that the CoOx@G-Ph-SN is not only more active for water oxidation than BiVO4 itself, but also efficient for the extraction of holes reaching the surface of BiVO4 for efficient transfer as well as promoted charge separation. The efficient transfer of photo-generated carriers between BiVO4 and CoOx@G-Ph-SN is confirmed by analysis of KPFM. As given in Fig. 3b, the steady state contact potential difference (ΔCPD) of the BiVO4 photo-electrode with and without light irradiation is more significantly increased after the loading of CoOx@G-Ph-SN.
The effectiveness of CoOx@G-Ph-SN as a cocatalyst of water oxidation to promote the performance of artificial photocatalysts can be further revealed by loading it on the surface of another robust Fe2O3 photoanode. As revealed by the LSV curves of Fig. 3c, the loading of CoOx@G-Ph-SN on the surface of Fe2O3 photoanode not only negatively shifts the onset potential of the photocurrent by ca. 150 mV, but also promotes the photocurrent at 1.23 eV (vs. RHE) by about 2.1 times. It is worth noting that the photocurrent of the CoOx@G-Ph-SN/Fe2O3 electrode at 1.23 V vs. RHE can be maintained by over 90% after 3 h irradiation, indicating the good photochemical stability of the CoOx@G-Ph-SN catalyst as a water oxidation cocatalyst in artificial photosynthesis. Together with the promotion of charge separation, we can reasonably deduce that CoOx@G-Ph-SN, with high activity and stability, is a promising WOC for artificial photosynthesis.
Conclusions
In summary, ultrasmall CoOx nanoparticles (around 1.6 nm) anchored on the surface of sulfonated graphite have been synthesized by a simple hydrothermal process. The success of the synthesis in reducing particle size mainly originates from the improvement of the surface hydrophilicity of graphite as well as its possible complex effect with Co2+ ions, leading to homogeneous dispersion and retarded growth. The as-obtained nanocomposite exhibits unexpected photochemical water oxidation activity with an optimal TOF of 1.2 s−1, at least two orders of magnitude higher than that of conventional cobalt-based oxides. Furthermore, the CoOx@G-Ph-SN can be grafted on the surface of artificial photocatalysts like BiVO4 and Fe2O3 for promoted photoelectrochemical water oxidation, demonstrating its bright future in constructing solar-to-chemical energy conversion systems.Acknowledgements
This work was supported by the Basic Research Program of China (973 Program: 2014CB239403), and the Natural Science Foundation of China (No. 21633009, 21522306, 21373210). F. Zhang thanks the priority support from the “Hundred Talents Program” of the Chinese Academy of Sciences.Notes and references
- N. S. Lewis and D. G. Nocera, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 15729 CrossRef CAS PubMed.
- A. J. Esswein and D. G. Nocera, Chem. Rev., 2007, 107, 4022 CrossRef CAS PubMed.
- L. Hammarstrom, J. R. Winkler, H. B. Gray and S. Styring, Science, 2011, 333, 288 CrossRef CAS PubMed.
- P. Du and R. Eisenberg, Energy Environ. Sci., 2012, 5, 6012 CAS.
- V. Artero, M. Chavarot-Kerlidou and M. Fontecave, Angew. Chem., Int. Ed., 2011, 50, 7238 CrossRef CAS PubMed.
- R. Lomoth, A. Magnuson, M. Sjodin, P. Huang, S. Styring and L. Hammarstrom, Photosynth. Res., 2006, 87, 25 CrossRef CAS PubMed.
- Y. Umena, K. Kawakami, J.-R. Shen and N. Kamiya, Nature, 2011, 473, 55 CrossRef CAS PubMed.
- M. Wiechen, I. Zaharieva, H. Dau and P. Kurz, Chem. Sci., 2012, 3, 2330 RSC.
- W. C. Ellis, N. D. McDaniel, S. Bernhard and T. J. Collins, J. Am. Chem. Soc., 2010, 132, 10990 CrossRef CAS PubMed.
- X. Deng and H. Tueysuez, ACS Catal., 2014, 4, 3701 CrossRef CAS.
- Q. Yin, J. M. Tan, C. Besson, Y. V. Geletii, D. G. Musaev, A. E. Kuznetsov, Z. Luo, K. I. Hardcastle and C. L. Hill, Science, 2010, 328, 342 CrossRef CAS PubMed.
- M. Zhang, M. de Respinis and H. Frei, Nat. Chem., 2014, 6, 362 CrossRef CAS PubMed.
- H. Lv, J. Song, Y. V. Geletii, J. W. Vickers, J. M. Sumliner, D. G. Musaev, P. Koegerler, P. F. Zhuk, J. Bacsa, G. Zhu and C. L. Hill, J. Am. Chem. Soc., 2014, 136, 9268 CrossRef CAS PubMed.
- M. J. Kenney, M. Gong, Y. Li, J. Z. Wu, J. Feng, M. Lanza and H. Dai, Science, 2013, 342, 836 CrossRef CAS PubMed.
- L. Duan, F. Bozoglian, S. Mandal, B. Stewart, T. Privalov, A. Llobet and L. Sun, Nat. Chem., 2012, 4, 418 CrossRef CAS PubMed.
- L. Duan, L. Wang, F. Li, F. Li and L. Sun, Acc. Chem. Res., 2015, 48, 2084 CrossRef CAS PubMed.
- L. Wang, L. Duan, Y. Wang, M. S. G. Ahlquist and L. Sun, Chem. Commun., 2014, 50, 12947 RSC.
- J. A. Woods, R. Lalrempuia, A. Petronilho, N. D. McDaniel, H. Mueller-Bunz, M. Albrecht and S. Bernhard, Energy Environ. Sci., 2014, 7, 2316 CAS.
- S. Chen, S. Shen, G. Liu, Y. Qi, F. Zhang and C. Li, Angew. Chem., Int. Ed., 2015, 54, 3047 CrossRef CAS PubMed.
- J. Yang, D. Wang, H. Han and C. Li, Acc. Chem. Res., 2013, 46, 1900 CrossRef CAS PubMed.
- M. W. Kanan and D. G. Nocera, Science, 2008, 321, 1072 CrossRef CAS PubMed.
- F. Jiao and H. Frei, Angew. Chem., Int. Ed., 2009, 48, 1841 CrossRef CAS PubMed.
- G. Zhang, S. Zang and X. Wang, ACSCatal., 2015, 5, 941 CAS.
- F. Zhang, A. Yamakata, K. Maeda, Y. Moriya, T. Takata, J. Kubota, K. Teshima, S. Oishi and K. Domen, J. Am. Chem. Soc., 2012, 134, 8348 CrossRef CAS PubMed.
- P. X. Liu, Y. Zhao, R. X. Qin, S. G. Mo, G. X. Chen, L. Gu, D. M. Chevrier, P. Zhang, Q. Guo, D. D. Zang, B. H. Wu, G. Fu and N. F. Zheng, Science, 2016, 352, 797 CrossRef CAS PubMed.
- H. S. Wei, X. Y. Liu, A. Q. Wang, L. L. Zhang, B. T. Qiao, X. F. Yang, Y. Q. Huang, S. Miao, J. Y. Liu and T. Zhang, Nat. Commun., 2014, 5, 5634 CrossRef CAS PubMed.
- T. F. Yeh, J. M. Syu, C. Cheng, T. H. Chang and H. S. Teng, Adv. Funct. Mater., 2010, 20, 2255 CrossRef CAS.
- T. F. Yeh, S. J. Chen, C. S. Yeh and H. S. Teng, J. Phys. Chem. C, 2013, 117, 6516 CAS.
- X. Yu, X. Han, Z. H. Zhao, J. Zhang, W. B. Guo, C. F. Pan, A. X. Li, H. Liu and Z. L. Wang, Nano Energy, 2015, 11, 19 CrossRef CAS.
- A. Mukherjee, J. Kang, O. Kuznetsov, Y. Sun, R. Thaner, A. S. Bratt, J. R. Lomeda, K. F. Kelly and W. E. Billups, Chem. Mater., 2011, 23, 9 CrossRef CAS.
- A. Datta, K. Das, C. Massera, J. K. Clegg, M. C. Pfrunder, E. Garribba, J.-H. Huang, C. Sinha, T. K. Maji, T. Akitsu and S. Orita, Inorg. Chem. Front., 2015, 2, 157 RSC.
- T. W. Kim and K.-S. Choi, Science, 2014, 343, 990 CrossRef CAS PubMed.
- H. K. Mulmudi, N. Mathews, X. C. Dou, L. F. Xi, S. S. Pramana, Y. M. Lam and S. G. Mhaisalkar, Electrochem. Commun., 2011, 13, 951 CrossRef CAS.
- J. Zhu, F. Fan, R. Chen, H. An, Z. Feng and C. Li, Angew. Chem., Int. Ed., 2015, 54, 9111 CrossRef CAS PubMed.
- C.-W. Tang, C.-B. Wang and S.-H. Chien, Thermochim. Acta, 2008, 473, 68 CrossRef CAS.
- K. Cao, L. Jiao, Y. Liu, H. Liu, Y. Wang and H. Yuan, Adv. Funct. Mater., 2015, 25, 1082 CrossRef CAS.
- J. B. Gerken, J. G. McAlpin, J. Y. C. Chen, M. L. Rigsby, W. H. Casey, R. D. Britt and S. S. Stahl, J. Am. Chem. Soc., 2011, 133, 14431 CrossRef CAS PubMed.
- M. S. Burke, S. Zou, L. J. Enman, J. E. Kellon, C. A. Gabor, E. Pledger and S. W. Boettcher, J. Phys. Chem. Lett., 2015, 6, 3737 CrossRef CAS PubMed.
- E. L. Tae, J. Song, A. R. Lee, C. H. Kim, S. Yoon, I. C. Hwang, M. G. Kim and K. B. Yoon, ACS Catal., 2015, 5, 5525 CrossRef CAS.
- D. Guevarra, A. Shinde, S. K. Suram, I. D. Sharp, F. M. Toma, J. A. Haber and J. M. Gregoire, Energy Environ. Sci., 2016, 9, 565 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7sc01756a |
| This journal is © The Royal Society of Chemistry 2017 |