Open Access Article
Open Access ArticleHeterocyclic boronic acids display sialic acid selective binding in a hypoxic tumor relevant acidic environment†
A.
Matsumoto
 *a,
A. J.
Stephenson-Brown
b,
T.
Khan
c,
T.
Miyazawa
a,
H.
Cabral
c,
K.
Kataoka
bd and
Y.
Miyahara
a
*a,
A. J.
Stephenson-Brown
b,
T.
Khan
c,
T.
Miyazawa
a,
H.
Cabral
c,
K.
Kataoka
bd and
Y.
Miyahara
a
aInstitute of Biomaterials and Bioengineering, Tokyo Medical and Dental University, 2-3-10 Kanda-Surugadai, Chiyoda-ku, Tokyo 101-0062, Japan. E-mail: matsumoto.bsr@tmd.ac.jp
bSchool of Chemical Engineering, University of Birmingham, Edgbaston, Birmingham, B15 2TT, UK
cDepartment of Bioengineering, Graduate School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656, Japan
dDepartment of Materials Engineering, Graduate School of Engineering, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-8656, Japan
First published on 5th July 2017
Abstract
Boronic acids are well known for their ability to reversibly interact with the diol groups found in sugars and glycoproteins. However, they are generally indiscriminate in their binding. Herein we describe the discovery of a group of heterocyclic boronic acids demonstrating unusually high affinity and selectivity for sialic acids (SAs or N-acetylneuraminic acid), which are sugar residues that are intimately linked with tumor growth and cancer progression. Remarkably, these interactions strengthen under the weakly acidic pH conditions associated with a hypoxic tumoral microenvironment. In vitro competitive binding assays uncovered a significantly higher ability of 5-boronopicolinic acid, one of the derivatives identified in this work as a strong SA-binder, to interact with cell surface SA in comparison to a gold-standard structure, 3-propionamidophenylboronic acid, which has proven to be an efficient SA-binder in numerous reports. This structure also proved to be suitable for further chemical conjugation with a well-preserved SA-binding capability. These findings suggest an attractive alternative to other ongoing boronic acid based chemistry techniques aiming to achieve tumor-specific chemotherapies and diagnoses.
Introduction
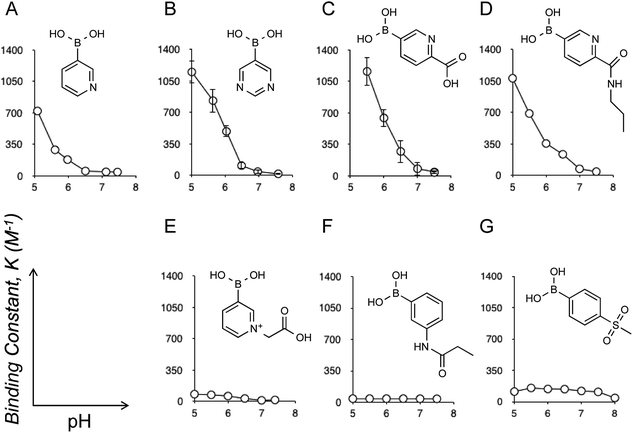
Eukaryotic cells are surrounded by a dense layer of sugar polymers, known as glycans, that are attached to cell surfaces via glycoproteins and glycolipids. Despite their prevalence in all mammalian tissues, the precise role of glycans remains to be completely elucidated, largely due to their tremendous diversity in structure; at present there is no overarching “code” to predict the roles of each specific glycan structure.1 One family of sugars that is known to make up a significant proportion of glycan structures is sialic acids (SAs or N-acetylneuraminic acid). Given their outermost (chain-end) arrangement and uniquely anionic polarity, SAs can mediate a wide variety of physiological and pathological cell processes, and thus can provide an accessible “code” to tell the state of glycans in the context of cellular events. For instance, sialylation is typically altered in cancers; increased expression of sialylated glycans, with some exceptions, is a common manifestation of cancer progression, poor prognosis, and higher metastatic potential.2 Therefore, determination of the glycan SA is relevant to the diagnosis of these cancerous conditions.3 Additionally, SA-specific molecular recognition leads to the ability to target therapeutic agents to highly sialylated epitopes or tumor cells.4 To this end, it is worth noting that the localized pH of a hypoxic tumoral microenvironment is commonly lower than the surrounding tissues, typically around 6.5 while that for healthy tissues is 7.4, due to disturbances in the metabolic balance of neoplastic cells.5 Thus, the presence of SAs under such weakly acidic conditions provides a highly selective marker for tumors and other cancerous changes. Likewise, SA-specific molecular recognition, if achieved in a way that is dependent on such acidification, would further validate the synthetic strategies of highly tumor-specific chemotherapies and diagnoses.3,4Boronic acids are compounds that display the ability to form reversible covalent interactions with sugars6 including SAs.3,4,7 Herein we report the discovery of a novel boronic acid motif, which is able to demonstrate highly specific interactions with SA under weakly acidic pH conditions, and is thus relevant to tumoral sites. Recently, there have been several reports of the novel properties of a heterocyclic class of boronic acids based on the pyridine structures.8 While screening a number of these compounds for their sugar binding activity by B11 NMR (see also ESI†), a curious, and to our knowledge hitherto undescribed, pH dependent interaction with SA was noted. As pH was decreased, the interaction between SA and these derivatives significantly increased, producing binding constants orders of magnitude higher than those previously reported (Fig. 1). Note that the binding constant between SA and meta-amide substituted phenylboronic acid (Fig. 1F), a gold-standard structure which has proven to be an efficient SA-binder for the above-mentioned therapeutic and diagnostic applications,3,4d–g was at most 40 M−1,7c whereas some derivatives displayed in Fig. 1 reveal values well exceeding 1000 M−1, to our knowledge the highest levels ever reported. Also worthy of mention is that 4-(methylsulfonyl)benzene boronic acid (Fig. 1G) is the one previously known to have the strongest SA interaction of all structures reported in the literature (see also ESI Fig. 9†).6g However, the level of interaction by this derivative is far below that observed for the heterocyclic derivatives (Fig. 1A–D). In addition, there is no appreciable acidification-dependent increase in the SA interaction as is the case for the heterocyclic derivatives (Fig. 1A–D).
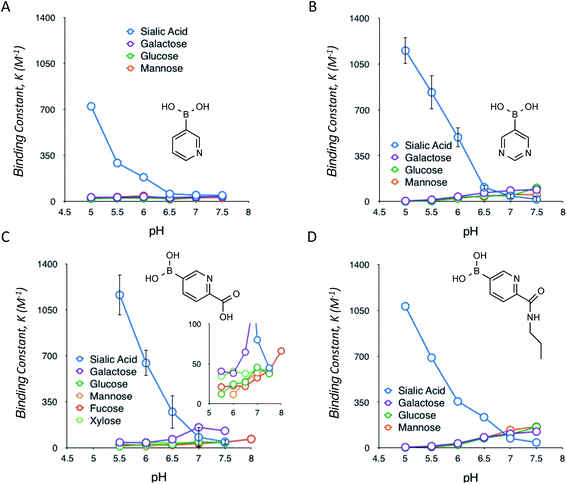
Following the identification of this special class of derivatives showing unprecedented levels of interaction with SA, we further investigated how these compounds interact with other glycan-present sugars, which are commonly found in biological samples, under a range of pH conditions (Fig. 2). The interactions with these sugars turned out to be typical of those observed for other well-documented types of (phenyl)boronic acids; formation of boronate complexes with these sugars was favoured by increases in the pH.7f As a result, these heterocyclic boronic acids appear to be highly selective for SA under acidic conditions, with almost no interference from the other common sugars present in biological samples. However, under normal physiological conditions, the binding of SA was much reduced. Some of these interactions were also assessed via a dye displacement assay using Alizarin Red S (ARS),9 thereby confirming a good agreement in both the quantities and the trend of the pH-dependency between the two independent methods (ESI Fig. 14†).
Given this unique behavior, an investigation was commenced on methods to conjugate these structures such that they could be further assessed for their suitability for medical applications (e.g., via polymer conjugation), and also to elucidate the mechanisms of the interactions observed. Of all of the compounds investigated, the most successful structure was found to be the 5-boronopicolinic acid (Fig. 1C) along with its propylamine-conjugate ((6-propylcarbamoyl)pyridine-3-boronic acid, Fig. 1D). The conjugate was found to maintain the high binding constant of the parent compounds, remaining almost unchanged at pH 6.5 (Fig. 1C and D), which is relevant to the tumoral microenvironment as stated above. Thus, this structure establishes a rational candidate for drug and polymer conjugations, and provides room for further chemical tailoring. Furthermore, an investigation on a series of compounds was able to shed some light on the mechanism by which this interaction occurs. As ascertained by B11 NMR, the observed interaction always involves boronate (or boronic acid)-ester formation and, therefore, there is a loose correlation between each boronic acid pKa and the SA-binding strength (ESI Table 1†). However, the results obtained here and elsewhere suggest that the nitrogen species in the compound must also play an important role in stabilizing the complex.7c,f,10 Indeed, N-substituted 3-pyridylboronic acid revealed a dramatic decrease in the SA-binding strength (Fig. 1E) as compared to its parent structure (Fig. 1A), despite the fact that this substitution causes negligible electronic effects on the boronate itself, i.e. no change of pKa.8a We found that these boronate–SA interactions are not interfered with even by excessively increased salt concentrations reaching up to 600 mM (see ESI Fig. 6 and 7†). We therefore hypothesize that the increase in the binding stability could be due to hydrogen bonding between the aromatic nitrogen and SA carboxyl group (rather than electrostatic interaction); a sterically hindered accessibility between the two groups may explain the above observation (Fig. 1E). We also looked into interactions between 5-boronopicolinic acid and the methyl ester derivative of SA as well as N-glycolyl neuraminic acid (Neu5Gc), a derivative implicated in metastatic tumor cells (ESI Fig. 10 and 11†).2d–f In accordance with the above hypothesis, in the former case where the SA carboxyl group is no longer available, the binding stability dramatically decreased, whereas in the latter case (Neu5Gc) with the carboxyl group still available in the structure it revealed still comparable boronate-binding stability to those observed for Neu5Ac. Furthermore, we confirmed that the interaction prevails across oligomers of SA (trimer and pentamer), where, interestingly, the binding strength was independent of the number of SA repeating units (ESI Fig. 12 and 13†), suggesting that the terminal SA with a sterically non-restricted carboxyl group is predominantly responsible for a sound interaction.
A question remains about the anomalous pH-dependent SA-binding profiles obtained in Fig. 1 and 2. The increased SA-binding stability at acidic pH values that are apparently lower than each boronic acid pKa implies that some fraction of boronic acid (as opposed to boronate) is also involved in the complex, which seems consistent with the observed B11 NMR chemical shifts (ESI Fig. 1–9†) as well as previous reports.4c,7c,f Those profiles might be interpreted in terms of the charge balance mechanism (“charge rule”), a theory taking into account the sum (“balance”) of charges between the complex and the free state species as a determinant for the abundance of each for a given pH in the system.11 Indeed, when boronic acid (as opposed to boronate) is involved in the complex, this theory predicts the occurrence of a maximal population of the complex at a pH lower than the boronic acid pKa, as is the case in Fig. 1 and 2. A steric factor between boronic acid (or boronate) and SA, due to their multisite binding nature, must also be considered to fully account for the relationship. However, the overarching mechanism remains elusive.
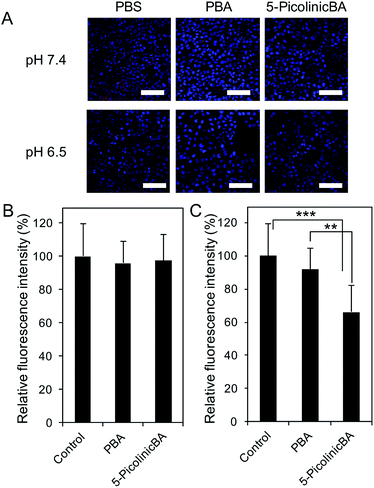
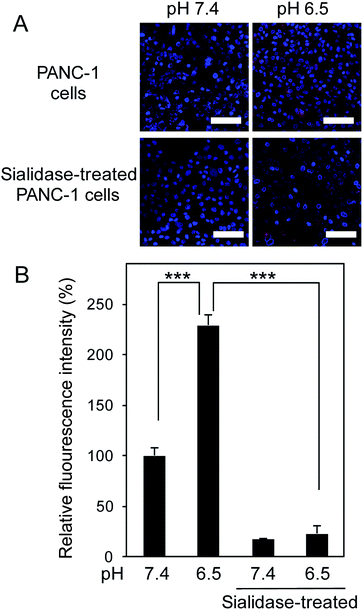
To translate our finding into significance for medical applications, we then assessed the ability of 5-boronopicolinic acid to bind with sialylated glycoconjugates, beyond the monosaccharide level recognition. For this purpose, human pancreatic epithelioid carcinoma cells (PANC 1) were utilized as a model for a SA-rich tumoral target (Fig. 3). The SA-binding ability of 5-boronopicolinic acid was compared to that of 3-propionamidophenylboronic acid, a gold-standard structure previously validated as an efficient SA-binder in a number of reports,3,4d–h in competition with SA-specific Sambucus Nigra (SNA) lectin (Fig. 3, see also ESI Fig. 15–17†). In the absence of boronic acids, the cell surface is well stained with the lectin (Fig. 3A, PBS, red). However, the staining is dramatically weakened when the cells are pre-incubated with these derivatives, presumably due to their SA-blocking effect, to a smaller and similar extent between the two derivatives at pH 7.4 (Fig. 3B) and to a greater extent when treated with 5-boronopicolinic acid at pH 6.5, i.e. intratumoral pH (Fig. 3C). The enhanced SA-blocking effect by 5-boronopicolinic acid under weakly acidic conditions is in accordance with the results shown in Fig. 1 and 2. This competitive blocking assay was reproducible even on a glycan array format installed with a variety of molecularly defined glycan structures (ESI Fig. 17†), further proving the ability of the boronate compound to bind with biologically relevant sialylated glycoconjugates, beyond the monosaccharide level recognition. For more direct evidence, we also prepared a rhodamine-modified version of 5-boronopicolinic acid (ESI Method†) and tested its capability for cell staining without the use of the lectin. As is evident in Fig. 4, a remarkably pH-dependent and dramatic silencing behaviour when treated by sialidase is achieved, providing direct evidence for the SA specificity. Taken together, these findings are promising for improved outcomes in all related tumor targeting and diagnostic applications.
Of note, we have previously reported a 3-propionamidophenylboronic acid installed (as a ligand to SA) polymeric micelle encapsulating anticancer drugs as a route to obtain tumor-specific chemotherapy.4e While achieving an effectively reduced growth rate of both orthotopic and lung metastasis models of melanoma (as compared to a non-ligand control), we also observed that the intravenously injected micelles retain prolonged blood circulation, with a striking similarity to the control (no ligand) system, indicative of the reduced interaction of the micelle with the red blood cells and endothelial cells in the vasculature. The reduced interaction was attributed to the interference by glucose in plasma (∼pH 7.4), normal glucose levels are ∼5 mM while the concentration of SA in erythrocytes is ∼0.2 μM (20 nmol/109 cells). Upon entering tumors, the glucose concentration decreases due to diffusion and its persistent metabolism into lactate in the cancer cells. Moreover, the metabolic products of this anaerobic glycolysis cause acidification of the intratumoral space (∼pH 6.5),5 which helps to decrease the binding constant for glucose and, conversely, increase that for SA. Thus, by virtue of such a reversible and pH-dependent binding nature between boronate and sugars, other competing sugars while in blood circulation (predominantly glucose) may not be a matter of concern for certain types of applications. With this in mind, the pattern of sugar-binding herein unveiled with the heterocyclic class of boronic acids should provide intriguing implications.
Conclusions
Relatively “weak” (as compared to lectins) but reversible (concentration-dependent) molecular recognition boronic acid chemistry is a special class of bio-application tool. Such dynamic modes of interaction can address otherwise difficult challenges such as continuous monitoring, and environmentally sensitive and bio-interactive applications, in which temporal, reversible, and oscillatory behaviour of biomolecules is of interest. In this study, we found that a group of heterocyclic boronic acids can undergo a remarkably SA specific interaction among other sugars, with stability constants orders of magnitude higher than those previously reported. Advantageously, both the binding strength and the SA specificity of these derivatives strengthen under weakly acidic pH conditions, a range closely associated with the tumoral microenvironment; this property should also help prevent nonspecific interactions with other glycan epitopes at healthy sites. The whole mechanism is still elusive, although hydrogen bonding between the aromatic nitrogen and SA carboxyl group is proposed to play an assistive role. The use of 5-boronopicolinic acid proved to be suitable for further chemical conjugation with a well-preserved SA-binding capability. In vitro assays validated its ability to bind with biologically relevant sialylated glycoconjugates, beyond the monosaccharide level recognition, and provided solid evidence for the SA-specificity. Also worth mentioning is that an excellent water solubility is generally found with these derivatives, which is otherwise (e.g., phenylboronic acid) often an issue limiting the range of medical applications.7a,b Taken together, these findings should offer an attractive alternative to a number of ongoing boronic acid chemistry methods aiming to achieve tumor-specific chemotherapies and diagnoses.Acknowledgements
The present work was supported by the Japan Society for the Promotion of Science (JSPS) through a Postdoctoral Fellowship for Overseas Researchers (14779), and, in part, by the Center of Innovation Program from the Japan Science and Technology Agency, JST.Notes and references
- (a) A. Varki and J. B. Lowe, Biological Roles of Glycans, in Essentials of Glycobiology, Cold Spring Harbor Laboratory Press, Cold Spring Harbor (NY), 2009 Search PubMed; (b) A. Varki, Trends Mol. Med., 2008, 14(8), 351 CrossRef CAS PubMed; (c) R. Schauer, Zool., 2004, 107(1), 49 CrossRef CAS PubMed; (d) M. Fukuda, Cancer Res., 1996, 56, 2237 CAS; (e) C. R. Bertozzi and L. L. Kiessling, Science, 2001, 291, 2357 CrossRef CAS PubMed; (f) R. Raman, S. Raguram, G. Venkataraman, J. C. Paulson and R. Sasisekharan, Nat. Methods, 2005, 2, 817 CrossRef CAS PubMed; (g) J. E. Scott, Conn’s Biological Stains. A Handbook of Dyes, Stains and Fluorochromes for Use in Biology and Medicine, J. Anat., 2003, 203(4), 433 CrossRef.
- (a) S. Hakomori, Cancer Res., 1996, 56, 5309 CAS; (b) D. H. Dube and C. R. Bertozzi, Nat. Rev. Drug Discovery, 2006, 4, 477 CrossRef PubMed; (c) Y. Xu, A. Sette, J. Sidney, S. J. Gendler and A. Franco, Immunol. Cell Biol., 2005, 83, 440 CrossRef CAS PubMed; (d) L. Daniel, J. Trouillas, W. Renaud, P. Chevallier, J. Gouvernet, G. Rougon and D. Figarella-Branger, Cancer Res., 2000, 60, 80 CAS; (e) L. Daniel, P. Durbec, E. Gautherot, E. Rouvier, G. Rougon and D. Figarella-Branger, Oncogene, 2001, 20, 997 CrossRef CAS PubMed; (f) S. M. Elkashef, S. J. Allison, M. Sadiq, H. A. Basheer, G. R. Morais, P. M. Loadman, K. Pors and R. A. Falconer, Sci. Rep., 2016, 6, 33026 CrossRef CAS PubMed.
- (a) A. Matsumoto, N. Sato, K. Kataoka and Y. Miyahara, J. Am. Chem. Soc., 2009, 131, 12022 CrossRef CAS PubMed; (b) A. Matsumoto, H. Cabral, N. Sato, K. Kataoka and Y. Miyahara, Angew. Chem., Int. Ed., 2010, 49, 5494 CrossRef CAS PubMed.
- (a) G. Lemieux, K. J. Yarema, C. L. Jacobs and C. R. Bertozzi, J. Am. Chem. Soc., 1999, 121, 4278 CrossRef CAS; (b) L. K. Mhal, N. W. Charter, K. Angata, M. Fukuda, D. E. Koshland Jr and C. R. A. Bertozzi, Science, 2001, 294, 380 CrossRef PubMed; (c) J. Frullano, J. S. Rohovec, T. Aime, T. Maschmeyer, M. I. Prata, J. J. P. D. Lima, C. F. G. C. Geraldes and J. A. Peters, Chem.–Eur. J., 2004, 10, 5205 CrossRef PubMed; (d) K. Djanashvili, G. A. Koning, A. J. G. M. van der Meer, H. T. Wolterbeek and J. A. Peters, Contrast Media Mol. Imaging, 2007, 2, 35 CrossRef CAS PubMed; (e) S. Deshayes, H. Cabral, T. Ishii, Y. Miura, S. Kobayashi, T. Yamashita, A. Matsumoto, Y. Miyahara, N. Nishiyama and K. Kataoka, J. Am. Chem. Soc., 2013, 135, 15501 CrossRef CAS PubMed; (f) A. Liu, S. Peng, J. C. Soo, M. Kuang, P. Chen and H. Duan, Anal. Chem., 2010, 83, 1124 CrossRef PubMed; (g) S. G. Crich, D. Alberti, I. Szabo, S. Aime and K. Djanashvili, Angew. Chem., Int. Ed., 2013, 52, 1161 CrossRef PubMed; (h) A. Matsumoto, K. Kataoka and Y. Miyahara, Polym. J., 2014, 46, 483 CrossRef CAS.
- (a) V. Estrella, T. A. Chen, M. Lloyd, J. Wojtkowiak, H. H. Cornnell, A. Ibrahim-Hashim, K. Bailey, Y. Balagurunathan, J. M. Rothberg, B. F. Sloane, J. Johnson, R. A. Gatenby and R. J. Gillies, Cancer Res., 2013, 73(5), 1524 CrossRef CAS PubMed; (b) Y. Kato, S. Ozawa, C. Miyamoto, Y. Maehata, A. Suzuki, T. Maeda and Y. Baba, Cancer Cell Int., 2013, 13, 89 CrossRef CAS PubMed; (c) R. A. Gatenby and R. J. Gillies, Nat. Rev. Cancer, 2004, 4(11), 891 CrossRef CAS PubMed.
- (a) J. P. Lorand and J. O. Edwards, J. Org. Chem., 1959, 24, 769 CrossRef CAS; (b) A. B. Foster, Adv. Carbohydr. Chem., 1957, 12, 81 CAS; (c) J. Boeseken, Adv. Carbohydr. Chem., 1949, 4, 189 CAS; (d) S. Aronoff, T. C. Chen and M. Cheveldayoff, Carbohydr. Res., 1975, 40, 299 CrossRef CAS; (e) T. D. James, K. R. A. S. Sandanayake and S. Shinkai, Angew. Chem., Int. Ed., 1996, 35(17), 1910 CrossRef; (f) R. Nishiyabu, Y. Kubo, T. D. James and J. S. Fossey, Chem. Commun., 2011, 47(4), 1106 RSC; (g) C. Lu, H. Li, H. Wang and Z. Liu, Anal. Chem., 2013, 85(4), 2361 CrossRef CAS PubMed.
- (a) M. Takeuchi, M. Yamamoto and S. Shinkai, Chem. Commun., 1997, 1731 RSC; (b) M. Yamamoto, M. Takeuchi and S. Shinkai, Tetrahedron, 1998, 54, 3125 CrossRef CAS; (c) H. Otsuka, E. Uchimura, H. Koshino, T. Okano and K. Kataoka, J. Am. Chem. Soc., 2003, 125, 3493 CrossRef CAS PubMed; (d) K. Djanashvili, L. Frullano and J. A. Peters, Chem.–Eur. J., 2005, 11, 4010 CrossRef CAS PubMed; (e) D. G. Hall, Boronic acids: preparation and applications in organic synthesis, medicine and materials, Wiley-VCH, Weinheim, 2nd completely rev edn, 2011 Search PubMed; (f) J. A. Peters, Coord. Chem. Rev., 2014, 268, 1 CrossRef CAS; (g) E. Uchimura, H. Otsuka, T. Okano, Y. Sakurai and K. Kataoka, Biotechnol. Bioeng., 2001, 72, 307 CrossRef CAS PubMed.
- (a) S. Iwatsuki, Y. Kanamitsu, H. Ohara, E. Watanabe and K. Ishihara, J. Phys. Org. Chem., 2012, 25(9), 760 CrossRef CAS; (b) Y. J. Huang, Y. B. Jiang, J. S. Fossey, T. D. James and F. Marken, J. Mater. Chem., 2010, 20(38), 8305 RSC; (c) M. Sanjoh, D. Iizuka, A. Matsumoto and Y. Miyahara, Org. Lett., 2015, 17(3), 588 CrossRef CAS PubMed.
- (a) G. Springsteen and B. H. Wang, Chem. Commun., 2001, 1608 RSC; (b) G. Springsteen and B. H. Wang, Tetrahedron, 2002, 58(26), 5291 CrossRef CAS.
- M. Regueiro-Figueroa, K. Djanashvili, D. Esteban-Gomez, T. Chauvin, E. Toth, A. de Blas, T. Rodriguez-Blas and C. Platas-Igleslas, Inorg. Chem., 2010, 49(9), 4212 CrossRef CAS PubMed.
- (a) M. van Duin, J. A. Peters, A. P. G. Kieboom and H. van Bekkum, Tetrahedron, 1984, 40, 2901 CrossRef CAS; (b) J. Yan, G. Springsteen, S. Deeter and B. Wang, Tetrahedron, 2004, 60, 11205 CrossRef CAS; (c) M. A. Martínez-Aguirre, R. Villamil-Ramos, J. A. Guerrero-Alvarez and A. K. Yatsimirsky, J. Org. Chem., 2013, 78, 4674 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7sc01905j |
| This journal is © The Royal Society of Chemistry 2017 |