Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A novel peptide stapling strategy enables the retention of ring-closing amino acid side chains for the Wnt/β-catenin signalling pathway†
Ye
Wu‡
ad,
Ye-Hua
Li‡
b,
Xiang
Li‡
a,
Yan
Zou
 a,
Hong-Li
Liao
a,
Hong-Li
Liao
 d,
Lei
Liu
b,
Ye-Guang
Chen
*b,
Donald
Bierer
*c and
Hong-Gang
Hu
d,
Lei
Liu
b,
Ye-Guang
Chen
*b,
Donald
Bierer
*c and
Hong-Gang
Hu
 *a
*a
aSchool of Pharmacy, Second Military Medical University, Shanghai 200433, China. E-mail: huhonggang_fox@msn.com
bTsinghua-Peking Center for Life Sciences, Tsinghua University, Beijing 100084, China. E-mail: ygchen@mail.tsinghua.edu.cn
cBayer AG, Department of Medicinal Chemistry, Aprather Weg 18A, Wuppertal 42096, Germany. E-mail: donald.bierer1@bayer.com
dSchool of Pharmacy, Chengdu Medical College, Chengdu 610083, China
First published on 29th August 2017
Abstract
The all-hydrocarbon peptide stapling strategy has recently been extensively explored in drug discovery. There remains the potential for improvement regarding the retention of the amino acid side chains at the stapled positions. Herein, we describe a new series of amino acids that not only contain the native side chains, but also carry the alkenyl arms that are needed for the ring-closing stapling chemistry. We incorporate the new amino acids into a β-catenin-binding domain of Axin (469–482) and develop a new category of stapled peptides with the retention of the native side chains. These stapled peptides exhibit high α-helicity, strong proteolytic stability and good cell permeability. Biochemical experiments demonstrate that these stapled peptides can activate β-catenin more efficiently than canonical stapled peptides due to the presence of extra side chains. We expect that the new side-chain-retention stapling method would expand the scope of the all-hydrocarbon stapled peptide strategy by retaining some important peripheral residues for protein–protein interactions or preserving key hydrophilic side chains to improve solubility.
Introduction
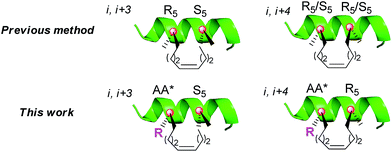
Peptide drugs offer the prospect of enhanced potency, high specificity and low toxicity because of their large interacting surfaces. These features are particularly attractive for the disruption of protein–protein interactions (PPIs). A large group of peptides bind their receptors in the α-helical conformation but the helix proportion of linear peptides is usually low in solution.1 The construction of peptide derivatives with a pre-organized stable α-helix topology is expected to favour receptor binding and therefore has been widely investigated in recent years.2–4 Generally, the stabilization of helices has been fulfilled through side-chain constraints by the covalent connection of two side-chain residues with disulfides,5 lactams,6 triazoles,7 and others.8 Among them, the all-hydrocarbon stapled peptide strategy developed by Verdine et al.9 has been considered as one of the most promising stapling strategies for PPIs (Fig. 1). This strategy has been successfully used to improve the in vivo stability of peptides and enhance their membrane penetration against many biological macromolecular receptors associated with cancer, atherosclerosis, HIV and other diseases.10–12To avoid the intrinsic helix-destabilizing effect of D-configured amino acids while capitalizing on the helix-stabilizing effect of α,α-disubstituted amino acids, Verdine and co-workers introduced an α-methyl group into Fmoc-R5-OH and Fmoc-S5-OH,9 which restricted the stapling position to Ala or mutated Ala. However, it is technically difficult to staple a peptide at the Ala position for every sequence, especially when each stapled peptide requires two stapling positions. Although reasonable stapled peptides can be rationally designed based on the Ala-scan and crystal structure of a peptide–protein complex, it remains interesting to explore whether the retention of the side chain of the stapling residue may bring about any extra benefit (Fig. 1). This may be especially important for the cases where we cannot ignore the role of peripheral residues around PPIs. Moreover, another often encountered drawback of the all-hydrocarbon stapling strategy is the solubility problem, especially in the cases where the native hydrophilic side chains of Ser, Lys or Arg are sacrificed due to the stapling.
To solve the above problems, several methods have been developed to stabilize α-helices by the replacement of an internal (i, i + 4) hydrogen bond with a covalent linkage, such as an ethylene bridge developed by Alewood et al.,13 a hydrazone bond proposed by Cabezas et al.,14 an alkyl linkage used by Arora et al.,15 a proline-derived transannular N-cap by Li et al.,16 or a pre-made diaminodiacid strategy.17 In this context, we are interested in developing a new alternative stapling approach in which the amino acid side chains are retained at the stapled positions.
Results and discussion
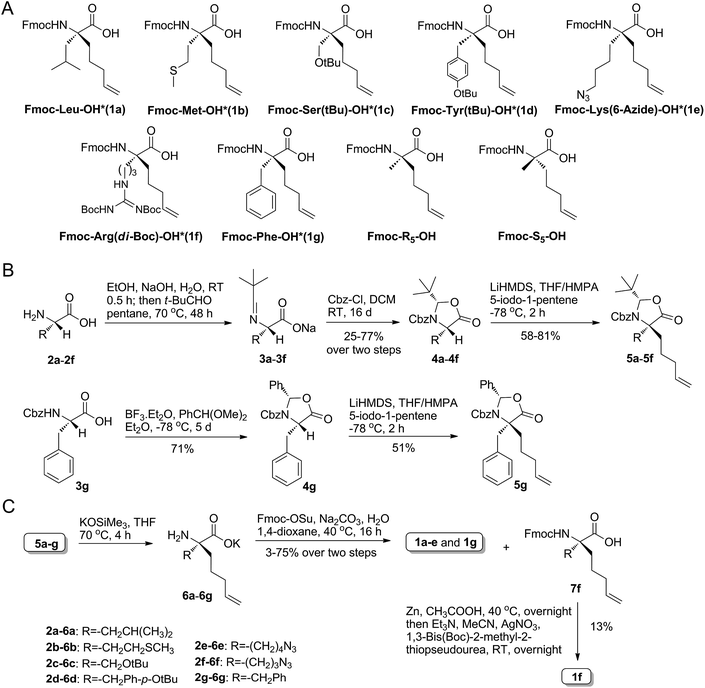
To achieve the above goal, we first need to make amino acids which contain both native side chains and stapling groups. For this purpose, seven classical N-Fmoc-α-pentene amino acids were tested as examples in the beginning, namely, Leu*, Met*, Ser*, Tyr*, Lys*, Arg* and Phe* (1a–g, as shown in Fig. 2A). A versatile de novo approach for the synthesis of these novel amino acids was developed by the optimization of a reported strategy for preparing Fmoc-R8-OH (Fig. 2B).18aThe configuration of the unnatural amino acids is critical and depends on a series of key intermediates 5a–g (Fig. 2B), oxazolidinone-like amino acid derivatives. In our study, steric hindrance caused by the N-benzyl protected group and oxazolidinone was exploited to acquire the product with correct stereochemistry. First, native α-amino acids (2a–f), which can be obtained easily from commercial resources or the reported methods (as shown in the ESI†), were protected at the nitrogen atom to provide N-t-Bu imide amino acids (3a–f).18b Second, 3a–f were converted to the corresponding oxazolidinones 4a–f by Cbz-Cl in DCM.19 Third, treatment of 4a–g with LiHMDS and 5-iodo-1-pentene in a THF/HMPA solution at −78 °C afforded the desired 5a–g in good yields. Particularly, the cyclization of 3g with BF3/Et2O and PhCH(OMe)2 generated 4g in 71% isolated yield. Hydrolysis of the alkylated oxazolidinones with KOSiMe3 afforded the free amino acids. Finally, the free amino groups of 6a–g were protected by a Fmoc group to give the key amino acids 1a–e,20 which were ready for use in standard Fmoc SPPS. The azide of 7f was reduced by Zn and CH3COOH, and then we used AgNO3 to promote the guanidinylation of the amino group, yielding 1f (Fig. 2C).21 To assume our novel amino acids would not racemize, we also synthesized D-configuration Phe* (1g), and then examined the enantiopurity of 1g. As anticipated, our synthesis route enabled 1g to reach 95.82% enantiopurity.
As a typical PPI model, previous studies have suggested that the Axin–β-catenin interaction is suitable for targeting by hydrocarbon-stapled peptides.22a Herein we incorporated our novel amino acids into a β-catenin-binding domain of Axin (469–482) and presented the first stapled peptides with the retention of the native side chains. The scaffolding protein Axin, glycogen synthase kinase-3β (GSK-3β) and the adenomatous polyposis coli protein (APC) constructed a cytoplasmic protein complex, which can catalyze the phosphorylation of β-catenin in the absence of a Wnt signal.23 It has been demonstrated that canonical Wnt/β-catenin signaling plays a critical role in the development of embryogenesis, the maintenance of adult tissue homeostasis and the control of tissue regeneration. A variety of human diseases, including osteoporosis, neurodegenerative diseases, diabetes and Joubert syndrome, are concluded to be closely related to the aberrant activation of the Wnt/β-catenin signalling pathway.24
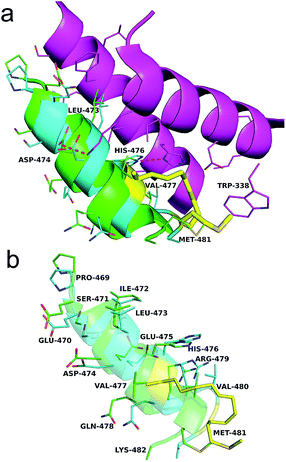
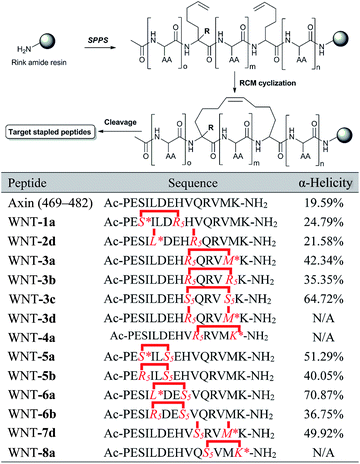
As shown in Fig. 4a, the Axin–β-catenin interface comprised both a rather hydrophobic interaction and some critical salt bridges and hydrogen bonds, of which Leu-473, Asp-474, and His-476 are critical residues and Ile-472, Val-477, Val-480 and Met-481 play moderately important roles.22b Each of these residues is more or less involved in the binding interaction. To evaluate the new stapling strategy, we incorporated the amino acids Leu*, Met*, Ser* and Lys* combined with S5 or R5 into the β-catenin-binding domain (Axin (469–482)) (Table 1). Solid-phase peptide synthesis25a and RCM25b were carried out as previously reported, and thirteen stapled peptides containing Leu*/Met*/Ser*/Lys* and S5/R5 were successfully synthesized (Table 1).
| a *: the synthetic amino acid with the retention of the native side chain. WNT-2d and -7d: the peptide failed to undergo RCM. N/A: not applicable. |
|---|
|
The linear peptide was first prepared using standard Fmoc SPPS procedures with Rink Amide MBHA resin as the solid support. The Fmoc protected amino acids were successively assembled onto the resin. After the peptide assembly was completed, the olefin-containing peptide was stapled using Grubb’s first-generation catalyst. The peptide was cleaved off from the resin and globally deprotected with reagent K (82.5% TFA, 5% H2O, 2.5% EDT, 5% thioanisole and 5% phenol). Ether precipitation gave the crude peptides, which were purified by semi-preparative RP-HPLC. WNT-2d and WNT-7d failed to undergo RCM, even under high temperature, suggesting that steric hindrance of these two sequences can result in non-productive conformations for the cyclization step.8d The amino acid side chain of Lys* was initially an azide group, which was reduced to an amine on the resin by a nickel catalyst in the presence of NaBH4 after RCM (ESI, 3.1†). To test the coupling efficiency of our unnatural amino acids, we monitored the coupling step of Met* and the subsequent elongation of Val in WNT-3a by analytical HPLC through micro-cleavage. According to the trace comparison of each intermediate in HPLC (Fig. S1†), the coupling efficiency was not obviously compromised in spite of the introduction of an extra long alkene linker.
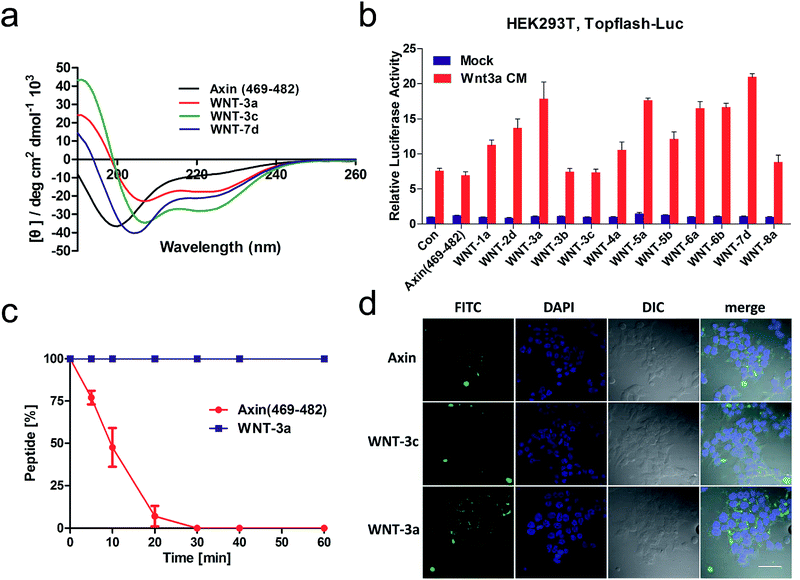
We next explored the effects of these peptides on Wnt/β-catenin dependent transcriptional activity. The Topflash reporter, in which firefly luciferase is transcriptionally activated by β-catenin, was employed. As shown in Fig. 3b, the new stapled peptides WNT-1a, -3a, -4a, -5a, -6a and -8a enhanced the Wnt3a-induced reporter expression more effectively than Axin (469–482), among which WNT-3a, -5a, and -6a treatment exhibited a three-fold enhancement. As expected, these stapled peptides promoted more Wnt3a-induced reporter expression than the corresponding regular stapled peptides WNT-3b, -3c, -5b, and -6b, demonstrating the benefit of maintaining the side chains of Met-481, Ser-471 and Leu-473. Among them, WNT-3a showed a more significant improvement than -3b and -3c, highlighting the influence of the side chain Met-481. As we expected, the activity of WNT-3d, the uncycled WNT-3a, showed less promotion when compared to Axin (469–482) owing to the remarkable weakness of linear peptides (Fig. S2†). All these results showcase the power of our new stapled peptides and the necessity of the retention of the specific side chain. It is worth noting that WNT-2d and -7d also significantly promoted Wnt3a-induced reporter expression as uncycled linear peptides, of which the most potent, WNT-7d, with a modified Met compared with WNT-3a, exhibited a three-fold increase in potency compared to the wild-type peptide. The failure of cyclizing WNT-7d makes us unable to evaluate the activity of WNT-7a, a potential potent activator of the Wnt signalling pathway.
Circular dichroism (CD) analysis of the peptides (Table 1 and Fig. 3a and S3†) indicates that the helicity of Axin (469–482) is only 20%, while the helicity of WNT-1a to WNT-7d ranges from 22% to 71% corresponding to a 1.1- to 3.6-fold increase. Consistent with the previous report,26 the “unstapled” analogue of WNT-2d and WNT-7d also displayed relatively high α-helicity, with an α-helical content value of 22% and 50%, respectively. These results demonstrate that our novel stapled peptide strategy can maintain the improvement of helicity when compared with the regular stapling strategy.
To test the protease stability of the stapled peptides with the retention of the side chains, we measured their susceptibility towards α-chymotrypsin-mediated degradation at room temperature in pH 7.4 PBS buffer containing 2 mM of CaCl2 as monitored by HPLC. α-Chymotrypsin is a protease that predominantly cleaves at the carboxyl side of positively charged amino acids such as methionine and leucine. Under these conditions, the half-life of Axin (469–482) is 9 min (Fig. 3c). In sharp contrast, WNT-3a is, as expected, completely stable and no degradation was observed even after 1 h. These results clearly demonstrate the inherent superiority of the new stapled peptides over linear peptides with respect to proteolytic stability.
We then assessed the cell permeability of the peptides by conjugating fluorescein isothiocyanate (FITC) to Axin (469–482), WNT-3a and WNT-3c (Fig. 3d). Our data show that FITC–β-Ala–WNT-3a and -3c efficiently entered into the cytoplasm in the HEK 293T/17 cells. Unexpectedly, FITC–β-Ala–Axin (469–482) also penetrated the cell membrane but showed weaker reporter expression in the presence of Wnt3a compared to WNT-3a, demonstrating the critical role of the native side chain of Met-481. To explore the detailed binding mode of WNT-3a against β-catenin, we conducted a Z-dock of the WNT-3a–β-catenin complex to predict structural models of the protein–protein complexes.27 As shown in Fig. 4a and b, subtle but important differences in the intermolecular interaction exist between WNT-3a and Axin (469–482). Owing to an introduction of the stapling group, Met-481 is closer to the Trp-338 of β-catenin, and thus forms a stronger interaction with that residue. In addition, a stronger salt bridge between the side chain carboxyl group of Asp-474 coordinated with the amino group of β-catenin Lys-292 and a more efficient hydrogen bond between the side chain of His-476 and β-catenin Asp-299 are constructed because of a closer distance geometrically. It is conceivable that these changes enhanced the conformational stability of WNT-3a in its bound state, thereby contributing to the improved binding activity.
Conclusions
In conclusion, we have developed a new series of stapling amino acids, which contain the native amino acid side chains and can be applied in the standard stapling chemistry for stabilized α-helical conformation peptides. In this study, we have made use of these amino acids and S5/R5, to prepare potent inhibitors of the Axin–β-catenin interaction. We have demonstrated that incorporation of AA* at the i position of an i, i + 3 or i, i + 4 stapled peptide is a tolerated modification that allows the all-hydrocarbon staple to effectively mimic Axin, and exhibit superior protease stability. In this regard, this methodology is a more flexible and alternative strategy for stapled peptides with potential impact for related PPIs.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful to the National Key R&D Program of China (No. 2017YFA0505200), National Natural Science Foundation of China (No. 21402235, 21532004, 81621002, and 21621003), and PLA Youth Medical Science and Technology Youth Development Program (No. 16QNP086), and to the Instrumental Analysis Center of our university for the NMR spectroscopic and mass spectrometric analyses.Notes and references
- (a) V. Azzarito, K. Long, N. S. Murphy and A. J. Wilson, Nat. Chem., 2013, 5, 161–173 CrossRef CAS PubMed; (b) M. Pazgier, M. Liu, G. Zou, W. Yuan, C. Li, C. Li, J. Li, J. Monbo, D. Zella, S. G. Tarasov and W. Lu, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 4665–4670 CrossRef CAS PubMed; (c) M. Liu, C. Li, M. Pazgier, C. Li, Y. Mao, Y. Lv, B. Gu, G. Wei, W. Yuan, C. Zhan, W. Y. Lu and W. Lu, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 14321–14326 CrossRef CAS PubMed; (d) E. Danelius, M. Pettersson, M. Bred, J. Min, M. B. Waddell, R. K. Guy, M. Groetli and M. Erdelyi, Org. Biomol. Chem., 2016, 14, 10386–10393 RSC.
- O. Keskin, A. Gursoy, B. Ma and R. Nussinov, Chem. Rev., 2008, 108, 1225–1244 CrossRef CAS PubMed.
- J. M. Mason, Future Med. Chem., 2010, 2, 1813–1822 CrossRef CAS PubMed.
- D. A. Guarracino, B. N. Bullock and P. S. Arora, Biopolymers, 2011, 95, 1–7 CrossRef CAS PubMed.
- M. Pellegrini, M. Royo, M. Chorev and D. F. Mierke, J. Pept. Res., 1997, 49, 404–414 CrossRef CAS PubMed.
- M. Chorev, E. Roubini, R. L. McKee, S. W. Gibbons, M. E. Goldman, M. P. Caulfield and M. Rosenblatt, Biochemistry, 1991, 30, 5968–5974 CrossRef CAS PubMed.
- S. Cantel, A. Le Chevalier Isaad, M. Scrima, J. J. Levy, R. D. DiMarchi, P. Rovero, J. A. Halperin, A. M. D. Ursi, A. M. Papini and M. Chorev, J. Org. Chem., 2008, 73, 5663–5674 CrossRef CAS PubMed.
- (a) G. Lautrette, F. Touti, H. G. Lee, P. Dai and B. L. Pentelute, J. Am. Chem. Soc., 2016, 138, 8340–8343 CrossRef CAS PubMed; (b) Y. Tian, J. X. Li, H. Zhao, X. Z. Zeng, D. Y. Wang, Q. S. Liu, X. G. Niu, X. H. Huang, N. H. Xu and Z. G. Li, Chem. Sci., 2016, 7, 3325–3330 RSC; (c) Y. Wu, L. B. Olsen, Y. H. Lau, C. H. Jensen, M. Rossmann, Y. R. Baker, H. F. Sore, S. Collins and D. R. Spring, ChemBioChem, 2016, 17, 689–692 CrossRef CAS PubMed; (d) T. E. Speltz, S. W. Fanning, C. G. Mayne, C. Fowler, E. Tajkhorshid, G. L. Greene and T. W. Moore, Angew. Chem. Int. Ed, 2016, 55, 4252–4255 CrossRef CAS PubMed; (e) Y. H. Lau, P. de Andrade, Y. Wu and D. R. Spring, Chem. Soc. Rev., 2015, 44, 91–102 RSC; (f) K. L. Keeling, O. Cho, D. B. Scanlon, G. W. Booker, A. D. Abell and K. L. Wegener, Org. Biomol. Chem., 2016, 14, 9731–9735 RSC; (g) H. Lin, Y. Jiang, H. Kuan, Q. Zhang, C. He, T. Wang and Z. Li, Org. Biomol. Chem., 2016, 14, 9993–9999 RSC; (h) C. M. Haney, H. M. Werner, J. J. McKay and W. S. Horne, Org. Biomol. Chem., 2016, 14, 5768–5773 RSC; (i) N. E. Shepherd, R. S. Harrison, G. Ruiz-Gomez, G. Abbenante, J. M. Mason and D. P. Fairlie, Org. Biomol. Chem., 2016, 14, 10939–10945 RSC.
- C. E. Schafmeister, J. Po and G. L. Verdine, J. Am. Chem. Soc., 2000, 122, 5891–5892 CrossRef CAS.
- L. D. Walensky, A. L. Kung, I. Escher, T. J. Malia, S. Barbuto, R. D. Wright, G. Wagner, G. L. Verdine and S. J. Korsmeyer, Science, 2004, 305, 1466–1470 CrossRef CAS PubMed.
- R. E. Moellering, M. Cornejo, T. N. Davis, B. C. Del, J. C. Aster, S. C. Blacklow, A. L. Kung, D. G. Gilliland, G. L. Verdine and J. E. Bradner, Nature, 2009, 462, 182–188 CrossRef CAS PubMed.
- S. Baek, P. S. Kutchukian, G. L. Verdine, R. Huber, T. A. Holak, K. W. Lee and G. M. Popowicz, J. Am. Chem. Soc., 2012, 134, 103–106 CrossRef CAS PubMed.
- A. J. Vernall, P. Cassidy and P. F. Alewood, Angew. Chem., Int. Ed., 2009, 48, 5675–5678 CrossRef CAS PubMed.
- E. Cabezas and A. C. Satterthwait, J. Am. Chem. Soc., 1999, 121, 3862–3875 CrossRef CAS.
- R. N. Chapman, G. Dimartino and P. S. Arora, J. Am. Chem. Soc., 2004, 126, 12252–12253 CrossRef CAS PubMed.
- Y. Tian, D. Y. Wang, J. X. Li, C. Shi, H. Zhao, X. G. Niu and Z. G. Li, Chem. Commun., 2016, 52, 9275–9278 RSC.
- (a) H. K. Cui, Y. Guo, Y. He, F. L. Wang, H. N. Chang, Y. J. Wang, F. M. Wu, C. L. Tian and L. Liu, Angew. Chem., Int. Ed., 2013, 52, 9558–9562 CrossRef CAS PubMed; (b) Y. Guo, D. M. Sun, F. L. Wang, Y. He, L. Liu and C. L. Tian, Angew. Chem., Int. Ed., 2015, 54, 14276–14281 CrossRef CAS PubMed; (c) F. L. Wang, Y. Guo, S. J. Li, Q. X. Guo, J. Shi and Y. M. Li, Org. Biomol. Chem., 2015, 13, 6286–6290 RSC.
- (a) G. H. Bird, W. C. Crannell and L. D. Walensky, Curr. Protoc. Chem. Biol., 2011, 3, 99–117 Search PubMed; (b) D. Seebach and A. Fadel, Helv. Chim. Acta, 1985, 68, 1243–1250 CrossRef CAS.
- M. W. Walter, N. Thaker, J. E. Baldwin, M. Mueller and C. J. Schofield, J. Chem. Res., 2000, 310–311 CrossRef CAS.
- B. Krawczyk, P. Ensle, W. M. Müller and R. D. Süssmuth, J. Am. Chem. Soc., 2012, 134, 9922–9925 CrossRef CAS PubMed.
- D. Ma, C. Xia, J. Jiang, J. Zhang and W. Tang, J. Org. Chem., 2003, 68, 442–451 CrossRef CAS PubMed.
- (a) H. K. Cui, B. Zhao, Y. H. Li, Y. Guo, H. Hu, L. Liu and Y. G. Chen, Cell Res., 2013, 23, 581–584 CrossRef CAS PubMed; (b) Y. Xing, W. K. Clements, D. Kimelman and W. Xu, Genes Dev., 2003, 17, 2753–2764 CrossRef CAS PubMed.
- S. Ikeda, S. Kishida, H. Yamamoto, H. Murai, S. Koyama and A. Kikuchi, EMBO J., 1998, 17, 1371–1384 CrossRef CAS PubMed.
- H. Clevers and R. Nusse, Cell, 2012, 149, 1192–1205 CrossRef CAS PubMed.
- (a) M. Pan, S. Gao, Y. Zheng, X. Tan, H. Lan, X. Tan, D. Sun, L. Lu, T. Wang, Q. Zheng, Y. Huang, J. Wang and L. Liu, J. Am. Chem. Soc., 2016, 138, 7429–7435 CrossRef CAS PubMed; (b) Y. W. Kim, T. N. Grossmann and G. L. Verdine, Nat. Protoc., 2011, 6, 761–771 CrossRef CAS PubMed.
- J. W. Checco, E. F. Lee, M. Evangelista, N. J. Sleebs, K. Rogers, A. Pettikiriarachchi, N. J. Kershaw, G. A. Eddinger, D. G. Belair, J. L. Wilson, C. H. Eller, R. T. Raines, W. L. Murphy, B. J. Smith, S. H. Gellman and W. D. Fairlie, J. Am. Chem. Soc., 2015, 137, 11365–11375 CrossRef CAS PubMed.
- B. G. Pierce, K. Wiehe, H. Hwang, B. H. Kim, T. Vreven and Z. Weng, Bioinformatics, 2014, 30, 1771–1773 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7sc02420g |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2017 |