Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Teixobactin analogues reveal enduracididine to be non-essential for highly potent antibacterial activity and lipid II binding†
Anish
Parmar‡
a,
Abhishek
Iyer‡
ab,
Stephen H.
Prior
c,
Daniel G.
Lloyd
d,
Eunice Tze
Leng Goh
e,
Charlotte S.
Vincent
d,
Timea
Palmai-Pallag
 d,
Csanad Z.
Bachrati
d,
Csanad Z.
Bachrati
 d,
Eefjan
Breukink
f,
Annemieke
Madder
d,
Eefjan
Breukink
f,
Annemieke
Madder
 b,
Rajamani
Lakshminarayanan
e,
Edward J.
Taylor
d and
Ishwar
Singh
b,
Rajamani
Lakshminarayanan
e,
Edward J.
Taylor
d and
Ishwar
Singh
 *a
*a
aSchool of Pharmacy, University of Lincoln, JBL Building, Beevor St., Lincoln LN67DL, UK. E-mail: isingh@lincoln.ac.uk
bOrganic and Biomimetic Chemistry Research Group, Department of Organic and Macromolecular Chemistry, Ghent University, Krijgslaan 281 (S4), B-9000 Ghent, Belgium
cSchool of Chemistry, University of Lincoln, JBL Building, Beevor St., Lincoln LN67DL, UK
dSchool of Life Sciences, University of Lincoln, JBL Building, Beevor St., Lincoln LN67DL, UK
eSingapore Eye Research Institute, The Academia, Discovery Tower Level 6, 20 College Road, Singapore 169857
fDepartment of Membrane Biochemistry and Biophysics, Institute of Biomembranes, Utrecht University, Padualaan 8, 3584 CH Utrecht, The Netherlands
First published on 5th October 2017
Abstract
Teixobactin is a highly promising antibacterial depsipeptide consisting of four D-amino acids and a rare L-allo-enduracididine amino acid. L-allo-Enduracididine is reported to be important for the highly potent antibacterial activity of teixobactin. However, it is also a key limiting factor in the development of potent teixobactin analogues due to several synthetic challenges such as it is not commercially available, requires a multistep synthesis, long and repetitive couplings (16–30 hours). Due to all these challenges, the total synthesis of teixobactin is laborious and low yielding (3.3%). In this work, we have identified a unique design and developed a rapid synthesis (10 min μwave assisted coupling per amino acid, 30 min cyclisation) of several highly potent analogues of teixobactin with yields of 10–24% by replacing the L-allo-enduracididine with commercially available non-polar residues such as leucine and isoleucine. Most importantly, the Leu10-teixobactin and Ile10-teixobactin analogues have shown highly potent antibacterial activity against a broader panel of MRSA and Enterococcus faecalis (VRE). Furthermore, these synthetic analogues displayed identical antibacterial activity to natural teixobactin (MIC 0.25 μg mL−1) against MRSA ATCC 33591 despite their simpler design and ease of synthesis. We have confirmed lipid II binding and measured the binding affinities of individual amino acid residues of Ala10-teixobactin towards geranyl pyrophosphate by NMR to understand the nature and strength of binding interactions. Contrary to current understanding, we have shown that a cationic amino acid at position 10 is not essential for target (lipid II) binding and potent antibacterial activity of teixobactin. We thus provide strong evidence contrary to the many assumptions made about the mechanism of action of this exciting new antibiotic. Introduction of a non-cationic residue at position 10 allows for tremendous diversification in the design and synthesis of highly potent teixobactin analogues and lays the foundations for the development of teixobactin analogues as new drug-like molecules to target MRSA and Mycobacterium tuberculosis.
Introduction
By 2050, antimicrobial resistance (AMR) is predicted to cause more mortality than cancer.1 To address the challenges of AMR, there is a continual need to develop new antibacterial compounds. The recently discovered molecule teixobactin is one such very promising antibacterial.2 Teixobactin kills bacteria such as Methicillin-resistant Staphylococcus aureus (MRSA) and Mycobacterium tuberculosis without detectable resistance.2 In 2015, Mycobacterium tuberculosis infected 10.4 million individuals and was responsible for 1.5 million deaths worldwide.3 Teixobactin kills bacteria through the inhibition of cell wall synthesis.2 The development of resistance against teixobactin is predicted to be low because it targets cell wall substrates from multiple biosynthetic pathways.4 Teixobactin, thus, does not target enzymes, but multiple bactoprenol-coupled cell wall building blocks such as lipid II (needed for peptidoglycan synthesis) and lipid III (a starting material for cell wall teichoic acid synthesis) leading to inhibition of cell wall synthesis.5 The targeting of building blocks (lipid II and lipid III) rather than enzymes makes it inherently more difficult for bacteria to develop resistance. The inhibition of cell wall synthesis via multiple pathways results in cell wall damage, lysis (due to delocalisation of autolysins, lytic enzymes) and cell death. The aforementioned reasons make teixobactin an outstanding molecule for antibiotic development.6Teixobactin is an undecapeptide containing four D amino acids namely N-Me-D-Phe1, D-Gln4, D-allo-Ile5 and D-Thr8 (Fig. 1, marked in red) and the rare L-allo-enduracididine amino acid7 (Fig. 1A, marked in blue). In the past year, articles have been published which describe the total synthesis of teixobactin,8,9 and the syntheses and biological activities of teixobactin analogues.10–12 The synthesis of Arg10-teixobactin analogues was reported by us11 and others.10,12 Arg10-teixobactin was obtained by replacing the L-allo-enduracididine amino acid at position 10 with the structurally similar, commercially available arginine. Arg10-teixobactin showed a similar trend in terms of antibacterial activity as teixobactin. Our previous work13 established the importance of D amino acids via the total syntheses and biological evaluation of the D and L analogues of Arg10-teixobactin. Changing the amino acid configuration of any one of the four D amino acids (N-Me-D-Phe1, D-Gln4, D-allo-Ile5 and D-Thr8) from D to L leads to significant loss in antibacterial activity. This work also defined the 3D molecular structure of seven teixobactin analogues whereby (1) the disordered structure of these analogues were found to be vital for biological activity, (2) D-Gln4 is essential and (3) D-allo-Ile5 is important to maintain the disordered structure.13
Nowick et al.12 reported a minimum pharmacophore of teixobactin coined lipobactin by replacing the N-terminal residues 1–5 with a dodecanoyl chain. They described that any change to D-Thr8 or modification of configuration of any of the residues in the core ring structure of teixobactin results in a significant decrease in activity. The enantiomeric Arg10-teixobactin reported by them shows similar biological activity as Arg10-teixobactin. These results suggest that, in order to maintain biological activity, only the relative configurations of amino acids are important and not their absolute configurations. Very recently, Nowick et al. also reported the X-ray crystallographic structure of a teixobactin analogue and described its hydrophobic interactions, as well as a hydrogen bonding cavity featuring a chloride ion.14 The Albericio group has reported a lysine scan of Arg10-teixobactin.15 They concluded that the replacement of any one of the four isoleucine residues with lysine led to complete loss of activity. However, replacement of the polar, non-charged residues Ser3, Gln4 and non-polar alanine by lysine resulted in analogues with comparable biological activities to that of Arg10-teixobactin. Wu C. et al. have reported that the guanidine of arginine or amine of lysine at position 10, Ser7 and the NH group of N terminal phenylalanine are all critical for the biological activity of teixobactin analogues.16 The replacement of the arginine or lysine at position 10 by histidine, Ser7 by alanine (including analogue 7) and N-methyl phenylalanine1 by N,N-dimethyl phenylalanine leads to less active teixobactin analogues compared to the Arg10-teixobactin. Recently, we have reported the synthesis of teixobactin analogues by replacing L-allo-enduracididine with isosteres such as homoarginine10-teixobactin, norarginine10-teixobactin. These analogues showed promising activity against MRSA. However these were less active than natural teixobactin.17
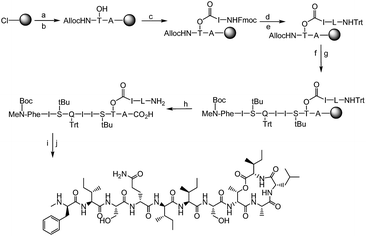
In this work, we describe a unique design and rapid synthesis of several highly potent analogues of teixobactin against Staphylococcus aureus (MSSA), methicillin-resistant Staphylococcus aureus (MRSA) and Enterococcus faecalis (vancomycin-resistant Enterococci, VRE) by replacing the synthetically challenging L-allo-enduracididine with commercially available non-polar residues such as alanine, leucine and isoleucine. This study aims to answer two important questions. Firstly, is it essential to incorporate a residue with a positively charged side chain at position 10 for maintaining target binding (lipid II) and biological activity of teixobactin and its analogues? Secondly, what are the key residues involved and what are the target binding contributions of the individual amino acid residues in the teixobactin analogues? To evaluate both these questions and identify the key residues particularly with respect to position 10, an alanine scan was performed on Arg10-teixobactin (Fig. 2, 1–8). The alanine scanning technique has been used earlier on other antimicrobial peptides with success18 but has not yet been performed on teixobactin or its analogues. In order to further improve the antibacterial activity of Arg10-teixobactin by modifying the amino acid at position 10, new analogues of teixobactin were prepared by systematic replacement of Arg10 with D-Ala (D-Ala10-teixobactin, 9), Gly (Gly10-teixobactin, 10), Val (Val10-teixobactin 11), Ile (Ile10-teixobactin, 12), Leu (Leu10-teixobactin, 13), Ser (Ser10-teixobactin, 14) and Phe (Phe10-teixobactin, 15) (Fig. 2, 9–15). We thus synthesised 15 analogues (Fig. 2) of teixobactin using the conditions described in Fig. 3, Page S3.†
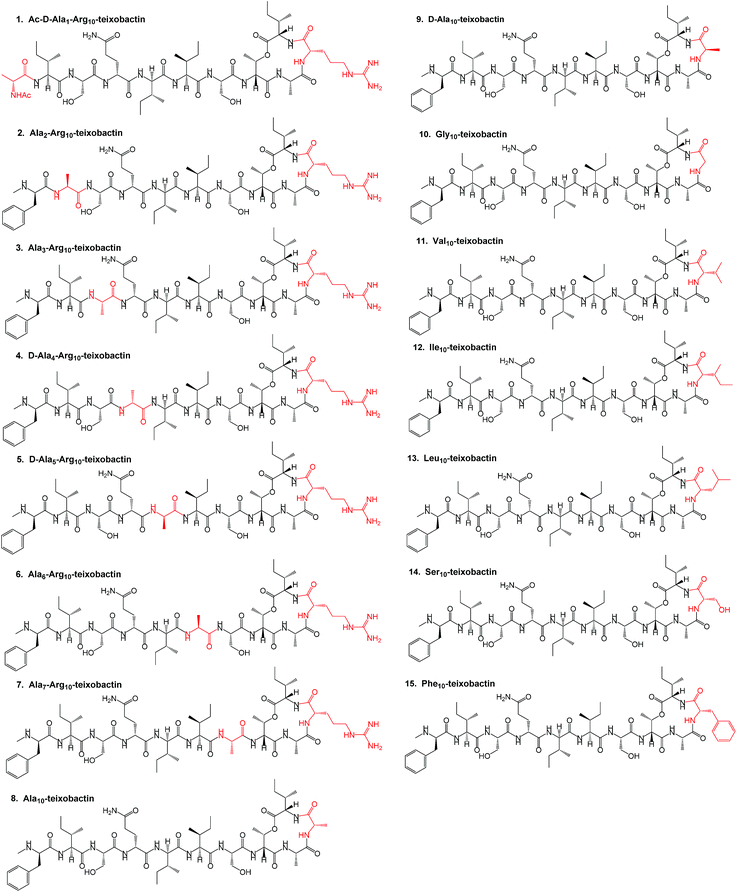 | ||
| Fig. 2 Structure of teixobactin analogues 1–15 synthesised with the replaced amino acids highlighted in red. | ||
Results and discussion
Design and syntheses
In our ongoing research to develop potent teixobactin analogues against resistant bacteria, we are particularly interested in understanding the role of polar amino acid residues at position 10, namely; L-allo-enduracididine, arginine and lysine. L-allo-Enduracididine is important for the biological activity of teixobactin and its replacement by Arg results in decreased biological activity (Table 1). However, it is also a key limiting factor in the development of potent teixobactins due to several synthetic challenges such as (1) it is not commercially available, (2) it requires a multistep synthesis (7 steps, 17% yield),8 (3) a suitably protected L-allo-enduracididine is difficult to incorporate into teixobactin and (4) it requires long and multiple couplings (16–30 hours).8,9 Due to all of these challenges, the synthesis of teixobactin is laborious and low yielding (3.3%).| Compound | Name | MICa (μg mL−1) |
|---|---|---|
| a MIC: minimum inhibitory concentration. MRSA ATCC 33591 used. Culture media: Mueller Hinton broth (Oxoid). b 10% volume with human serum (SIGMA, H4522). | ||
| 1 | Ac-D-Ala1-Arg10-teixobactin | >128 |
| 2 | Ala2-Arg10-teixobactin | >128 |
| 3 | Ala3-Arg10-teixobactin | 1–2 |
| 4 | D-Ala4-Arg10-teixobactin | 2–4 |
| 5 | D-Ala5-Arg10-teixobactin | 64–128 |
| 6 | Ala6-Arg10-teixobactin | >128 |
| 7 | Ala7-Arg10-teixobactin | 16–32 |
| 8 | Ala10-teixobactin | 1–2 |
| 9 | D-Ala10-teixobactin | 32 |
| 10 | Gly10-teixobactin | 2 |
| 11 | Val10-teixobactin | 0.5 |
| 12 | Ile10-teixobactin | 0.25 |
| 12a | Ile10-teixobactin + 10% human serumb | 0.25 |
| 13 | Leu10-teixobactin | 0.25 |
| 13a | Leu10-teixobactin + 10% human serumb | 0.25 |
| 14 | Ser10-teixobactin | 16 |
| 15 | Phe10-teixobactin | 2 |
| 16 | Arg10-teixobactin | 2 |
| 17 | Teixobactin | 0.25 |
| 18 | Vancomycin | 2 |
The synthesis of all our analogues involved loading Fmoc-alanine on the 2-chlorotritylchloride resin, followed by amide coupling with alloc-NH-D-Thr-OH, resin esterification with 10 eq. Fmoc-Ile-OH, and adding 10 eq. DIC and 5 mol% DMAP for 2 h. The next amino acid (AA) was then coupled using 4 eq. AA with 4 eq. HATU/8 eq. DIPEA in DMF for 1 h followed by Fmoc deprotection and trityl protection. Next, the N-terminal alloc protecting group was removed using Pd(PPh3)4 and phenylsilane (Fig. 3). All other amino acids were coupled using 4 eq. AA with 4 eq. DIC/Oxyma using an automated microwave peptide synthesiser (coupling time of 10 min each). Fmoc deprotection was performed using 20% piperidine in DMF (Fig. 3, Page S3†). Cyclisation was performed using 1 eq. HATU and 10 eq. DIPEA and was found to be complete within 30 min with complete conversion of the linear product into its cyclised counterpart (Pages S5–S32†). Yields after HPLC purification were found to be 10–24% (Table S1†). We have identified a unique design in which the introduction of hydrophobic residues such as leucine at position 10 (Fig. 1) has several advantages over the lengthy low-yielding (3.3%) synthesis of teixobactin, including overall yields of up to 24%, faster automated syntheses, and use of commercially available building blocks.
Teixobactin and its active analogues such as Arg10-teixobactin and Lys10-teixobactin contain two positive charges. However, the analogues 1, 8–15 contain only one positive charge and were therefore found to be more hydrophobic than the analogues 2–7. All the compounds were found to be completely soluble in DMSO. Therefore, stock solutions of these compounds were prepared in DMSO for MIC testing. Upon dilution in the Mueller Hinton broth (Oxoid) culture media in which bacteria were grown (concentration ∼256 μg mL−1), no turbidity or precipitation was observed indicating that the compounds were soluble in the culture media.
Antibacterial studies
Analogues of teixobactin derived through an alanine scanning of teixobactin reveal that residues N-Me-Phe1, Ile2D-allo-Ile5, L-Ile6 and Ser7 are important for antibacterial activity and their replacement by L-Ala or D-Ala results in decrease in biological activity (Table 1). Interestingly, replacement of L-Ser3 and D-Gln4 by L-Ala and D-Ala has no effect on antibacterial activity. Thus, the two residues L-Ser3 & D-Gln4 are ideal candidates for replacement in the case of teixobactin due to their more facile synthesis and minimal impact on biological activity. It has been suggested that replacement of any of the residues in the core ring structure of teixobactin negates all biological activity of the molecule.12 In our case, however, the most interesting result was obtained through the replacement of L-Arg10 by L-Ala (8).The design and syntheses of potent teixobactin analogues published in the literature has thus far been limited to the substitution of L-allo-enduracididine with amino acids such as Arg,10,11 Lys12 and Orn,9 all of which possess a cationic side chain. A positive charge is a common structural characteristic of depsipeptides which bind to lipid II.4L-allo-Enduracididine is thus reported to be important for potent antibacterial activity of teixobactin.8 Therefore, it was expected that replacement of this residue with alanine, which is non-polar and uncharged, would completely abolish the biological activity of the molecule. Contrary to this, we observed that Ala10-teixobactin was highly active against MRSA (Table 1) with an MIC of 1–2 μg mL−1.
A plausible explanation could be that Ala10-teixobactin binds to the pyrophosphate motif of lipid II using the amide backbone in a similar way to that proposed for the binding of nisin.19 Superior results were obtained with Ile10-teixobactin (12) and Leu10-teixobactin (13), which consistently gave identical MIC values of 0.25 μg mL−1 as compared to the teixobactin against MRSA (Table 1). Leu has a very similar hydrocarbon framework to L-allo-enduracididine (Fig. 1), followed very closely by Ile, which could explain the identical MIC value of these analogues (12 & 13) to teixobactin (17) against MRSA. In order to determine the effect of serum on antibacterial activity, the MIC of compounds 12 and 13 were measured in presence of 10% human serum (Page S53†). In both cases no change was observed in the MIC (Table 1) indicating that 10% human serum has no effect on the antibacterial activity.
The fact that a cationic residue at position 10 is not essential for antibacterial activity represents a significant breakthrough in teixobactin research given the earlier stated importance of the L-allo-enduracididine amino acid in the total synthesis of teixobactin.8 Our design has considerably improved not only the antibacterial activity of teixobactin analogues but also the ease of synthesis. Our findings are of particular importance as MRSA is responsible for many infections worldwide.20
D-Ala10-teixobactin shows 16-times lower antibacterial activity than Arg10-teixobactin which would be expected, as inversion of configuration of even a single amino acid in the core ring structure can significantly lower the MIC value of a teixobactin analogue.12 Surprisingly, Gly10-teixobactin (10) shows identical activity to Arg10-teixobactin (16) showing that complete removal of the chiral center at position 10 is tolerated provided the configuration of the remaining residues is intact. Val10-teixobactin (11) shows 4-times better antibacterial activity than Arg10-teixobactin but Ser10-teixobactin (14) shows 8-times lower activity, indicating that Ser at position 10 probably interferes with hydrogen bonding between the core ring structure of teixobactin and lipid II. Phe10-teixobactin (15) gave an MIC of 2 μg mL−1 against MRSA indicating that an aromatic amino acid such as phenylalanine at position 10 is also tolerated. Overall, from our work it appears that the claimed importance of a charged residue at position 10 in the form of an amine or guanidine group in teixobactin has been overstated in the literature given that the most potent analogues obtained thus far are the Leu10-teixobactin and Ile10-teixobactin both of which are non-polar and non-charged. This unexpected result facilitates the development of several highly potent teixobactin analogues against a broader panel of MRSA, MSSA and Enterococcus faecalis (VRE) including Mycobacterium smegmatis (Table 2) but with significantly higher yields compared to teixobactin. Although analogues of teixobactin with improved yields have been synthesised previously,10–16 none possess comparable activity to teixobactin and therefore the yields obtained for Ile10-teixobactin and Leu10-teixobactin (Table S1, page S4 and Table S4, page S54,† 10–20%) cannot be compared to those of the other less potent analogues of teixobactin described in literature.10–16 Based on the initial MIC results (Table 1), we identified Ala10-teixobactin (8), Val10-teixobactin (11), Ile10-teixobactin (12) and Leu10-teixobactin (13) as our lead compounds. These compounds along with Arg10-teixobactin (16), and vancomycin/daptomycin as controls, were tested against an extended panel of Gram positive bacteria (Table 2) to provide a more comprehensive overview of the biological activity of these molecules. A significant difference in MIC was observed in the presence and absence of polysorbate 80 (Table S4, page S54†).2 Leu10-teixobactin (13) showed potent activity against M. smegmatis (MIC ∼1 μg mL−1). Ala10-teixobactin (8) and Arg10-teixobactin (16) showed comparable activity against M. smegmatis with MICs in the range of 1–2 μg mL−1. In general, the MBCs of all compounds were found to be 2–4 times the MIC value. Ile10-teixobactin (12) and Leu10-teixobactin (13) were found to be the most potent compounds showing MICs ≤ 0.25 μg mL−1 in all strains. Ile10-teixobactin (12) in particular was found to be highly active against both VRE strains with MICs ≤ 0.0625 μg mL−1 and also an MIC 0.5 of μg mL−1 against M. smegmatis. We thus report, for the first time, two analogues of teixobactin showing highly potent antibacterial activity against a broader panel of resistant Gram positive bacteria. This is a very significant advancement in terms of teixobactin research and allows for the synthesis of a library of teixobactin derivatives based on Ile10-teixobactin and Leu10-teixobactin which can be simpler, highly potent and significantly more cost effective than the synthesis of teixobactin.
| Strain | Compound | |||||||
|---|---|---|---|---|---|---|---|---|
| (8) | (11) | (12) | (13) | (16) | Vancomycin | Daptomycin | ||
| MRSA 1 | MIC | 4 | 1 | 0.25 | 0.25 | 1 | 2 | 0.5 |
| MBC | 16 | 4 | 1 | 2 | 2 | — | — | |
| MRSA 2 | MIC | 1 | 0.5 | ≤0.0625 | ≤0.0625 | 0.125 | 2 | 0.5 |
| MBC | 4 | 4 | ≤0.0625 | ≤0.0625 | 0.5 | — | — | |
| MRSA 3 | MIC | 1 | 0.25 | ≤0.0625 | ≤0.0625 | 0.5 | 2 | 0.5 |
| MBC | 2 | 2 | 0.125 | ≤0.0625 | 1 | — | — | |
| Staphylococcus aureus | MIC | 1 | 0.25 | ≤0.0625 | ≤0.0625 | 0.25 | 4 | 0.25 |
| MBC | 2 | 1 | 0.125 | 0.125 | 1 | — | ||
| VRE 1 | MIC | 4 | 0.5 | ≤0.0625 | 0.25 | 2 | >4 | 0.5 |
| VRE 2 | MIC | 4 | 0.5 | ≤0.0625 | 0.25 | 2 | >4 | 0.5 |
| M. smegmatis | MIC | 1–2 | — | 0.5 | 1 | 1–2 | >64 | — |
Time dependent killing of bacteria using teixobactin analogues 12 and 13
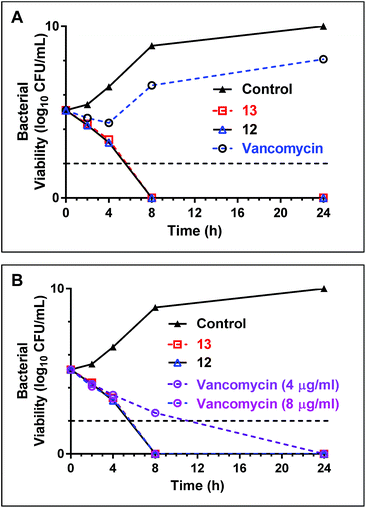
Early stage time-kill kinetics for Ile10-teixobactin (12) and Leu10-teixobactin (13) against MRSA ATCC 21455 using vancomycin as a control were carried out as described (Page S53†).2 At 0.5 μg mL−1, both Ile10-teixobactin (12) and Leu10-teixobactin (13) were found to elicit complete bactericidal activity within 8 h whereas substantial growth was observed in the presence of vancomycin (0.5 μg mL−1, Fig. 4A). The concentration of vancomycin needs to be increased to 8 μg mL−1 in order to have similar effects as the teixobactin analogues 12 & 13 (Fig. 4B).Toxicity studies and haemolysis assay
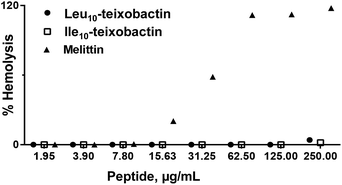
The analogues Ala10-teixobactin (8), Val10-teixobactin (11) and Leu10-teixobactin (13) were tested on HeLa cell cultures and no significant toxicity was observed (relative survival 90–100%) up to a concentration of 100 μM (Fig. S79†) which is well above the MIC values (0.2–0.8 μM, 125–500 times). Additionally, a haemolytic assay using Leu10-teixobactin and Ile10-teixobactin against rabbit erythrocytes using melittin as a control (Fig. 5) indicated that peptides Leu10-teixobactin (13) and Ile10-teixobactin (12) did not show any discernible haemolytic activity, even at concentrations that exceed >500× the mean MIC values whereas substantial haemolytic activity was observed for melittin (Fig. 5). These results establish the non-haemolytic properties of the designed teixobactin analogues.Lipid II binding assay
To better understand the potent antibacterial activity of Ala10-teixobactin we have performed the lipid II TLC binding assay as reported earlier for teixobactin.2 Teixobactin and Arg10-teixobactin bind to lipid II in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio resulting in the complete disappearance of the lipid II spot on TLC (Fig. S76†). Although Ala10-teixobactin also shows binding with lipid II in a 2
1 ratio resulting in the complete disappearance of the lipid II spot on TLC (Fig. S76†). Although Ala10-teixobactin also shows binding with lipid II in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, a small amount of lipid II was still visible on TLC. The lipid II spot, however, completely disappears by increasing the concentration of Ala10-teixobactin (Fig. S76†). TLC binding studies with Leu10-teixobactin also showed complete disappearance of the lipid II spot when a ratio of 2
1 ratio, a small amount of lipid II was still visible on TLC. The lipid II spot, however, completely disappears by increasing the concentration of Ala10-teixobactin (Fig. S76†). TLC binding studies with Leu10-teixobactin also showed complete disappearance of the lipid II spot when a ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was used (Fig. S78†). It is very interesting that Ala10-teixobactin and Leu10-teixobactin were able to bind to lipid II without having a cationic amino acid residue like L-allo-enduracididine/arginine off the cyclic peptide ring.
1 was used (Fig. S78†). It is very interesting that Ala10-teixobactin and Leu10-teixobactin were able to bind to lipid II without having a cationic amino acid residue like L-allo-enduracididine/arginine off the cyclic peptide ring.
Geranyl pyrophosphate (lipid II mimic) binding studies
In order to evaluate target binding, we have performed the lipid II TLC binding assay2 with Ala10-teixobactin. This assay provides qualitative binding data of Ala10-teixobactin with lipid II. Although the technique is fast and effective, the results obtained via this method do not necessarily reflect whole cell activities. This has been reported previously by us where both D and L derivatives of teixobactin were found to bind to lipid II but only the former was biologically active.13 Therefore, in order better understand the target binding of teixobactin analogues in a quantitative manner, extensive NMR studies (Fig. 6) on Ala10-teixobactin and geranyl pyrophosphate were performed. Geranyl pyrophosphate possesses a pyrophosphate and isoprenyl chain similar to lipid II making it suitable for solution phase NMR studies.Both TLC (Fig. S77†) and NMR (Fig. 6, Table 3) suggest a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding between Ala10-teixobactin and geranyl pyrophosphate. When titrating geranyl pyrophosphate into Ala10-teixobactin certain residues such as Ser7 were found to bind with classic Michaelis–Menton binding kinetics (Fig. 6B). However, some isotherms exhibited a sigmoidal shape (Fig. 6A). This can occur due to intermediate exchange on the NMR time-scale, and therefore cooperative binding is not an obvious choice. However, given our initial TLC data which shows a binding of 2
1 binding between Ala10-teixobactin and geranyl pyrophosphate. When titrating geranyl pyrophosphate into Ala10-teixobactin certain residues such as Ser7 were found to bind with classic Michaelis–Menton binding kinetics (Fig. 6B). However, some isotherms exhibited a sigmoidal shape (Fig. 6A). This can occur due to intermediate exchange on the NMR time-scale, and therefore cooperative binding is not an obvious choice. However, given our initial TLC data which shows a binding of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
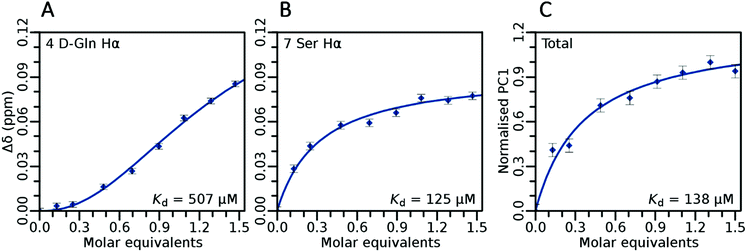
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 we have fitted the sigmoidal data using the Hill coefficient. It was found that all N-terminal residues weakly bound (KD ∼ 0.5 mM) geranyl pyrophosphate in a highly cooperative (Hill coefficient ∼ 2) manner, whereas ring-proximal residues bound significantly tighter but less cooperatively. Tightest binding was observed for Ser7 (KD ∼ 125 μM), which in a recently published teixobactin X-ray structure12 points its hydroxyl directly towards a bound anion. Analysing the overall binding using PCA (Fig. 6C), which removes any influence of intermediate exchange from the isotherms,21 gave a net KD of ∼138 μM.
1 we have fitted the sigmoidal data using the Hill coefficient. It was found that all N-terminal residues weakly bound (KD ∼ 0.5 mM) geranyl pyrophosphate in a highly cooperative (Hill coefficient ∼ 2) manner, whereas ring-proximal residues bound significantly tighter but less cooperatively. Tightest binding was observed for Ser7 (KD ∼ 125 μM), which in a recently published teixobactin X-ray structure12 points its hydroxyl directly towards a bound anion. Analysing the overall binding using PCA (Fig. 6C), which removes any influence of intermediate exchange from the isotherms,21 gave a net KD of ∼138 μM.
| Entry | K D (μM) | Hill coefficient |
|---|---|---|
| 1 Me-D-Phe Hα | n.d. | n.d. |
| 2 Ile Hα | 348 ± 18 | 2.1 |
| 3 Ser Hα | 503 ± 8 | 2.0 |
| 4 D-Gln Hα | 507 ± 2 | 2.2 |
| 5 D-Ile Hα | 483 ± 4 | 1.7 |
| 6 Ile Hα | n.d. | n.d. |
| 7 Ser Hα | 125 ± 3 | |
| 8 D-Thr Hα | 204 ± 3 | |
| 9 Ala Hα | 394 ± 4 | 2.1 |
| 10 Ala Hα | 314 ± 3 | |
| 11 Ile Hα | 391 ± 4 | 1.5 |
| Net | 138 ± 5 |
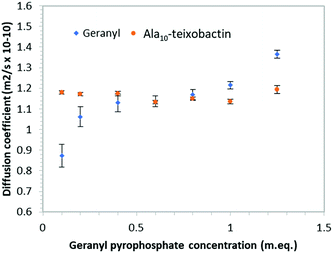
In order to determine if teixobactin aggregates in the presence of geranyl pyrophosphate 1H DOSY (diffusion ordered spectroscopy) spectra were recorded at each titration point and the diffusion coefficients calculated for both geranyl pyrophosphate and teixobactin (Fig. 7). Over the course of the titration the diffusion coefficient obtained from Ala10-teixobactin remained constant, indicating no aggregation occurred. The diffusion coefficient observed for geranyl pyrophosphate increased slightly over the course of the titration, indicating that it may have adopted a more compact structure upon association with the teixobactin.
Antagonization assay
In order to further prove a lipid II mediated mode of action, an antagonization assay was performed using Leu10-teixobactin (13) with lipid II as described in literature.2 The ratios of Leu10-teixobactin to lipid II tested were 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5, 1
0.5, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 1
2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 and growth was observed. These results are consistent with the 2
5 and growth was observed. These results are consistent with the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding ratio observed using the TLC assay (Table S3†). However in case of Leu10-teixobactin (control), no growth was observed.
1 binding ratio observed using the TLC assay (Table S3†). However in case of Leu10-teixobactin (control), no growth was observed.
Structural studies using NMR
NMR analysis of teixobactin analogues (Fig. 8) reveals common structural characteristics between those analogues which retain some residual antibacterial activity. Ala10-teixobactin was chosen for NMR studies as it provides the most direct comparison with other analogues. All analogues retain most of the NOEs observed in the Arg10-teixobactin, despite some differences in amide chemical shift (Fig. 8A). From Fig. 8B and C it can be observed that α proton chemical shifts show little variation between analogues at both termini: N-terminal similarities are likely due to these residues existing in a random coil environment; C-terminal similarities are likely due to the restraints placed upon these residues by the ring structure. Amide chemical shifts are more variable, particularly for residues 7 and 8, in which the chemical shift of these protons is ∼1 ppm downfield in Ala10-teixobactin (8). This is likely due to the loss of the guanidinium group, and suggests proximity between these residues and Arg10. The N-terminus again shows little variation, characteristic of a random coil. The mutated residue chemical shifts were excluded from the statistics. Fig. 8D shows that in all three mutants Ala3-Arg10-teixobactin (3), Ala4-Arg10-teixobactin (4) and Ala10-teixobactin (8) the N-termini were unstructured, but were showing evidence of structure starting approximately from residue 5, where in all cases the RMSD had dropped by ∼50% from that observed at their termini. The RMSDs observed at the C termini are low, as this area is highly constrained in structure by the ring.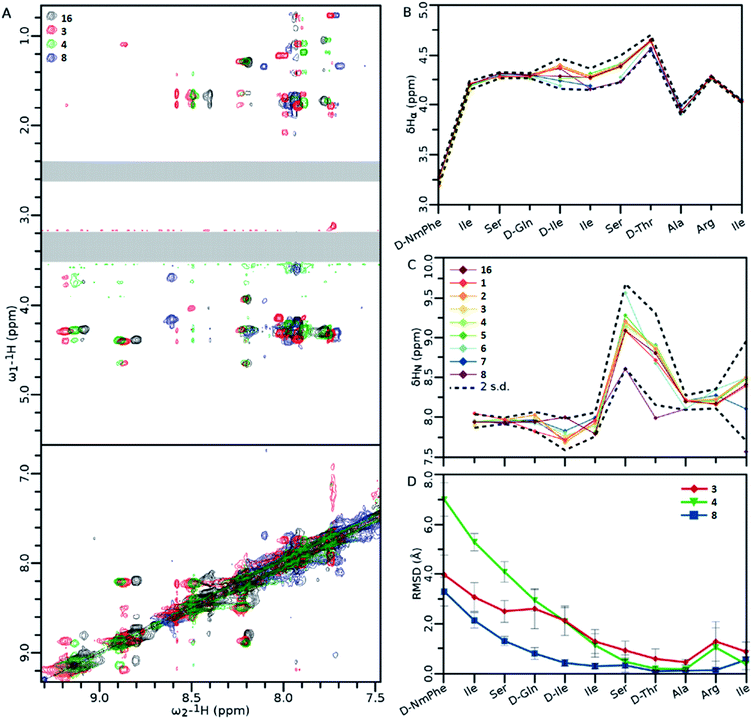 | ||
Fig. 8 (A) Overlay of the amide fingerprint regions of the 1H–1H NOESY spectra of wild type (Arg10-teixobactin) and active teixobactin analogues. (B and C) Chemical shift data obtained from α (B) and amide (C) protons. (D) Statistics of structures calculated using the NOEs obtained from panel (A). For clarity, the DMSO (∼3.3 ppm) and residual water (∼2.5 ppm) signals have been obscured with grey boxes. Data show the average RMSD of each atom in the residue from all 20 members of the ensemble. Error bars are standard deviation in the RMSDs of each residue's atoms. Standard deviations were calculated including the chemical shifts for 16.11 Ensembles of 20 structures generated by Cyana 2.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22 and refined in Gromacs.23 Error bars indicate standard deviation amongst all the atoms of that residue. Spectra were recorded on 1 mM teixobactin samples dissolved in DMSO-d6 on a 500 MHz spectrometer at 300 K. Legends for 8B and C are the same, and are shown in panel (C). 22 and refined in Gromacs.23 Error bars indicate standard deviation amongst all the atoms of that residue. Spectra were recorded on 1 mM teixobactin samples dissolved in DMSO-d6 on a 500 MHz spectrometer at 300 K. Legends for 8B and C are the same, and are shown in panel (C). | ||
Conclusion
In conclusion, we have described a unique design and rapid synthesis of several highly potent teixobactin analogues by replacing the synthetically challenging amino acid L-allo-enduracididine with commercially available non-polar residues such as leucine (13) and isoleucine (12). The teixobactin analogues from this work have shown highly potent antibacterial activity against a broad panel of MRSA, MSSA and VRE, despite their simpler design. Early stage kill kinetics data suggests Leu10-teixobactin and Ile10-teixobactin to be superior to vancomycin against MRSA. An antagonization assay suggests a lipid II mediated mode of action for Leu10-teixobactin. Most importantly, contrary to the current understanding we have demonstrated that cationic amino acids such as L-allo-enduracididine, arginine or lysine at position 10 are not essential for target (lipid II) binding and antibacterial activity. This surprising finding opens the door to the design and synthesis of several highly simplified potent teixobactin analogues and challenges many of the current assumptions about the mechanism of action of teixobactin. Our design of highly potent teixobactin analogues has several advantages such as improved yields ∼10–20%, ease of synthesis (including 10 min μwave assisted coupling steps and a 30 min cyclisation step) and uses commercially available building blocks.NMR studies reveal that the analogues Ala3-Arg10-teixobactin (3), Ala4-Arg10-teixobactin (4) and Ala10-teixobactin (8) are more unstructured towards the N-termini but highly structured towards the C termini due to the close-by ring. We have performed qualitative lipid II binding experiments and measured the binding affinities of individual amino acid residues of Ala10-teixobactin and geranyl pyrophosphate (lipid II mimic) by NMR to understand the role of amino acid residues in binding. Ser7 was found to have the tightest binding with an experimental KD of 125 μM.
To the best of our knowledge, Ile10-teixobactin (12) and Leu10-teixobactin (13) are the only reported teixobactin analogues which have shown superior potency against resistant Gram positive bacteria. The results from this work represent a significant advancement in our current understanding of the residues critical to the biological activity of teixobactin and associated analogues. We anticipate that our design and relatively rapid synthesis will help overcome current challenges in the field. As it stands, our work herein provides ready access to highly potent teixobactin analogues and will enable the development of teixobactin analogues with drug like properties against resistant bacterial strains. The findings presented in this work have broad implications and are expected to facilitate the development of peptide based antibiotics for combatting the serious global challenges posed by AMR.
Funding sources
Anish Parmar, Abhishek Iyer and Charlotte S. Vincent would like to thank the University of Lincoln for funding. Edward Taylor would like to thank the Royal Society for their kind support (grant number UF100116). Ishwar Singh would like to acknowledge the Royal Society for their kind support (grant number (RG130163) and Horizon 2020 (645684)). Daniel Lloyd would like to acknowledge the Rosetrees trust for their kind support (grant number JS16/M583). Timea Palmai-Pallag and Csanad Bachrati are funded by the BBSRC grant BB/K019597/1.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
We thank Novobiotic Pharmaceuticals, LLC for generously providing natural teixobactin, which was used as a positive control in MIC assays. We thank Dr Martin J. Lear for helpful discussions on preparation of the manuscript. Jan Goeman and Jos Van den Begin from Ghent University are thanked for LC-MS analyses. Nicholas Riess from University of Lincoln is thanked for the HRMS analysis.References
- http://amr-review.org/, 2015.
- L. L. Ling, T. Schneider, A. J. Peoples, A. L. Spoering, I. Engels, B. P. Conlon, A. Mueller, D. E. Hughes, S. Epstein, M. Jones, L. Lazarides, V. A. Steadman, D. R. Cohen, C. R. Felix, K. A. Fetterman, W. P. Millett, A. G. Nitti, A. M. Zullo, C. Chen and K. Lewis, Nature, 2015, 517, 455–459 CrossRef CAS PubMed.
- WHO, Global Tuberculosis Report, 2016 Search PubMed.
- F. von Nussbaum and R. D. Süssmuth, Angew. Chem., Int. Ed., 2015, 54, 6684–6686 CrossRef CAS PubMed.
- T. Homma, A. Nuxoll, A. B. Gandt, P. Ebner, I. Engels, T. Schneider, F. Götz, K. Lewis and B. P. Conlon, Antimicrob. Agents Chemother., 2016, 60, 6510–6517 CrossRef CAS PubMed.
- W. Lee, K. Schaefer, Y. Qiao, V. Srisuknimit, H. Steinmetz, R. Müller, D. Kahne and S. Walker, J. Am. Chem. Soc., 2016, 138, 100–103 CrossRef CAS PubMed.
- D. J. Atkinson, B. J. Naysmith, D. P. Furkert and M. A. Brimble, Beilstein J. Org. Chem., 2016, 12, 2325–2342 CrossRef CAS PubMed.
- A. M. Giltrap, L. J. Dowman, G. Nagalingam, J. L. Ochoa, R. G. Linington, W. J. Britton and R. J. Payne, Org. Lett., 2016, 18, 2788–2791 CrossRef CAS PubMed.
- K. Jin, I. H. Sam, K. H. L. Po, D. Lin, E. H. Ghazvini Zadeh, S. Chen, Y. Yuan and X. Li, Nat. Commun., 2016, 7, 12394 CrossRef CAS PubMed.
- Y. E. Jad, G. A. Acosta, T. Naicker, M. Ramtahal, A. El-Faham, T. Govender, H. G. Kruger, B. G. De La Torre and F. Albericio, Org. Lett., 2015, 17, 6182–6185 CrossRef CAS PubMed.
- A. Parmar, A. Iyer, C. S. Vincent, D. Van Lysebetten, S. H. Prior, A. Madder, E. J. Taylor and I. Singh, Chem. Commun., 2016, 52, 6060–6063 RSC.
- H. Yang, K. H. Chen and J. S. Nowick, ACS Chem. Biol., 2016, 11, 1823–1826 CrossRef CAS PubMed.
- A. Parmar, S. H. Prior, A. Iyer, C. S. Vincent, D. Van Lysebetten, E. Breukink, A. Madder, E. J. Taylor and I. Singh, Chem. Commun., 2017, 53, 2016–2019 RSC.
- H. Yang, D. R. Du Bois, J. W. Ziller and J. S. Nowick, Chem. Commun., 2017, 53, 2772–2775 RSC.
- S. A. H. Abdel Monaim, Y. E. Jad, E. J. Ramchuran, A. El-Faham, T. Govender, H. G. Kruger, B. G. de la Torre and F. Albericio, ACS Omega, 2016, 1, 1262–1265 CrossRef CAS.
- C. Wu, Z. Pan, G. Yao, W. Wang, L. Fang and W. Su, RSC Adv., 2017, 7, 1923–1926 RSC.
- A. Parmar, A. Iyer, D. G. Lloyd, C. S. Vincent, S. H. Prior, A. Madder, E. J. Taylor and I. Singh, Chem. Commun., 2017, 53, 7788–7791 RSC.
- S. M. Simonsen, L. Sando, K. J. Rosengren, C. K. Wang, M. L. Colgrave, N. L. Daly and D. J. Craik, J. Biol. Chem., 2008, 283, 9805–9813 CrossRef CAS PubMed.
- S.-T. D. Hsu, E. Breukink, E. Tischenko, M. A. G. Lutters, B. de Kruijff, R. Kaptein, A. M. J. J. Bonvin and N. A. J. van Nuland, Nat. Struct. Mol. Biol., 2004, 11, 963–967 CAS.
- R. V Rasmussen, V. G. Fowler Jr, R. Skov and N. E. Bruun, Future Microbiol., 2011, 6, 43–56 CrossRef PubMed.
- J. Xu and D. Van, Biophys. J., 2017, 112, 224–233 CrossRef CAS PubMed.
- P. Guntert and L. Buchner, J. Biomol. NMR, 2015, 62, 453–471 CrossRef PubMed.
- M. J. Abraham, T. Murtola, R. Schulz, S. Pall, J. C. Smith, B. Hess and E. Lindah, SoftwareX, 2015, 1–2, 19–25 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available: Peptide synthesis, HPLC, LC-MS analysis, NMR spectra, microbiological data (MIC, MBC, time kill kinetics), lipid II and geranyl pyrophosphate binding, cytotoxicity assay. See DOI: 10.1039/c7sc03241b |
| ‡ These authors have contributed equally to this work. The project was conceived and designed by Ishwar Singh. The manuscript was written by Abhishek Iyer, Stephen H. Prior and Ishwar Singh through contributions from all authors. Anish Parmar carried out the teixobactin analogues syntheses. Stephen H. Prior was responsible for the NMR analyses. Charlotte S. Vincent, Daniel G. Lloyd, and Edward J. Taylor have contributed to the antibacterial studies. Timea Palmai-Pallag and Csanad Z. Bachrati carried out the toxicity studies. Annemieke Madder was responsible for the LC-MS analyses. Eefjan Breukink was responsible for the syntheses and characterisation of lipid II. Eunice Tze Leng Goh and Rajamani Lakshminarayanan were responsible for the antibacterial studies and haemolytic assay. All authors have given approval to the final version of the manuscript. |
| This journal is © The Royal Society of Chemistry 2017 |