Facile preparation and highly efficient photocatalytic hydrogen evolution of novel CuxNiy nanoalloy/graphene nanohybrids
Xiangqing
Li
*a,
Honglei
Xu
a,
Qiang
Luo
a,
Shizhao
Kang
a,
Lixia
Qin
a,
Guodong
Li
 c and
Jinghui
Yang
*b
c and
Jinghui
Yang
*b
aSchool of Chemical and Environmental Engineering, Center of Graphene Research, Shanghai Institute of Technology, 100 Haiquan Road, Shanghai 201418, China. E-mail: xqli@sit.edu.cn
bEast China University of Science and Technology, 130 Meilong Road, Shanghai 200237, China. E-mail: jhyang@ecust.edu.cn
cState Key Laboratory of Inorganic Synthesis and Preparative Chemistry, College of Chemistry, Jilin University, Changchun 130012, China
First published on 10th January 2017
Abstract
Graphene nanohybrids loaded with CuxNiy bimetallic nanoalloys (CuxNiy/G) were obtained via a facile co-reduction process. The composition and structure of the CuxNiy/G were characterized by X-ray diffraction, high resolution transmission electron microscopy and X-ray photoelectron spectroscopy. The results showed that, besides composition, the size and morphology of CuxNiy particles on the graphene were different from those of Cu particles or Ni particles on graphene by the same preparation process. Furthermore, with eosin Y and rose bengal (ER) as co-sensitizers, and the CuxNiy bimetallic nanoalloy as the co-catalyst, the photocatalytic activity of hydrogen evolution over the CuxNiy/G increased and reached 5.05 mmol g−1 h−1 when the molar ratio of Cu2+ to Ni2+ was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. Compared with that of pure graphene (G), its activity was enhanced by up to 8.2 times. It was also higher than those of Cu or Ni loaded G, and even comparable to that of Pt loaded graphene under the same conditions. The higher activity of the CuxNiy/G could be attributed to the small size effect and special morphology of the CuxNiy alloy, fast interfacial electron transfer and synergic interaction in the CuxNiy/G system. Therefore, the CuxNiy bimetallic nanoalloy could act as a cheap and highly efficient noble metal-free cocatalyst for enhancing photocatalytic activity for hydrogen production over graphene-based photocatalysts.
3. Compared with that of pure graphene (G), its activity was enhanced by up to 8.2 times. It was also higher than those of Cu or Ni loaded G, and even comparable to that of Pt loaded graphene under the same conditions. The higher activity of the CuxNiy/G could be attributed to the small size effect and special morphology of the CuxNiy alloy, fast interfacial electron transfer and synergic interaction in the CuxNiy/G system. Therefore, the CuxNiy bimetallic nanoalloy could act as a cheap and highly efficient noble metal-free cocatalyst for enhancing photocatalytic activity for hydrogen production over graphene-based photocatalysts.
Introduction
Photocatalytic hydrogen production by water reduction is one of the most promising routes for transforming solar energy into a clean fuel in the future.1–3 In order to utilize solar energy effectively, the catalyst should be able to absorb visible light fully, and the electron/hole pairs should be separated efficiently.4–6With a large specific surface area, quick electron transfer, high thermal stability and tunable surface properties, graphene is highly desirable as a two-dimensional catalyst support in dye-sensitized graphene-based (DSG) photocatalyst systems.7–12 However, the photocatalytic activity of DSG catalysts still needs to be improved because of the rapid reaction of photoexcited electrons and oxidized dye species.13 To solve this problem, it is vital to find a cheap and highly efficient co-catalyst to transfer photoproduced electrons quickly. In general, loading a small amount of noble metal (such as Pt, Au) on graphene is beneficial for photocatalytic hydrogen evolution because it can provide trapping sites for photogenerated charges to promote charge separation and to decrease the overpotential of hydrogen production from water reduction.14–16 However, the application of a Pt co-catalyst is limited because of its low storage capacity and high cost.
Therefore, it is very important to prepare cheaper co-catalysts with comparable or even better performance than Pt. It is found that transition metals,17 transition metal oxides,11 transition metal hydroxides,18 and transition metal sulfides6 are efficient co-catalysts for photocatalytic hydrogen evolution. In addition, introducing another metal into noble metal is also an efficient way to avoid large-scale use of noble metals.19 For example, the platinum–tin alloy decorated graphene nanohybrid prepared by in situ photoreduction showed a higher activity for hydrogen evolution.9 Enhanced catalytic activity was also observed in other Pt-based transition metal alloys (such as Pt–Cu,20 Pt–Ni21 and Pt–Mo22). Ni nanoparticles have been demonstrated to act as cheap cocatalysts for H2 evolution.23 However, its catalytic activity was lower than those of Pt. In order to improve the catalytic activity of Ni, concomitant use of other metals such as Cu is promising as the reported catalytic reactions.24 It is found that the close proximity of Ni and Cu is important to achieve the high catalytic activity.25 Cu–Ni alloy is cheaper and has been used in electrocatalysis, photocatalysis, nonenzymatic detection of glucose and fuel cell,26,27 which has important practical significance. In the reported literature,25,28 Ni–Cu alloy nanoparticles were prepared mainly by thermal decomposition of Ni(acac)2 and/or Cu(acac)2 in deaerated oleylamine and 1-octadecene at 230 °C, or by thermal decomposition combined with reduction with NaBH4. The process still needs to be simplified. In addition, Ni–Cu alloy nanoparticles are apt to be oxidized on the surface of metal oxides, which is unsuitable for proton reduction.
In this work, various CuxNiy bimetallic alloy decorated graphene nanohybrids (CuxNiy/G) are prepared by a facile process. With CuxNiy bimetallic alloys as co-catalysts, eosin Y and rose bengal (ER) as co-sensitizers, the visible photocatalytic activity of CuxNiy/G nanohybrids for hydrogen production from water reduction is investigated in detail.
Experimental
Materials
Graphite power with a purity of >95% and other chemical reagents (AR) were purchased from Shanghai Chemical Reagent Ltd. and were used without further purification. The graphene oxide (GO) used was synthesized from graphite power by using a modified Hummers method.29Preparation of CuxNiy/G nanohybrids
CuxNiy/G nanohybrids were prepared via a one-step in situ chemical reduction method with hydrazine hydrate as the reductant. In a typical experiment, firstly, 100 mg of GO was added into 150 mL of ethylene glycol. After ultrasound treatment for 1 h, 0.8 mL of 0.1 mol L−1 CuCl2 and 0.8 mL of 0.1 mol L−1 NiCl2 were added into the solution, and the mixture was treated by ultrasound for another 10 min to get a homogeneous suspension (the mass percentage of Cu2+ and Ni2+ in GO was 10%, and molar ratio of Cu2+ to Ni2+ was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). After that, the mixture was magnetically stirred at room temperature for 1 h, and the pH value was adjusted to about 11. Finally, under N2 protection, 10 mL of 85% hydrazine hydrate was slowly added, and the resulting mixture was refluxed at 110 °C for 4 h. The target product (Cu1Ni1/G) was collected by centrifugation, washed with deionized water and ethanol, respectively, and dried in a vacuum oven at 45 °C for 12 h. By using the same procedure, various CuxNiy/G nanohybrids could be obtained by adjusting the molar ratio of Cu2+ to Ni2+. The samples obtained are summarized in Table 1. For comparison, pure graphene (G), Cu1Ni3 alloy nanoparticles, Ni/G, Cu/G and Pt/G were also prepared under the same conditions.
1). After that, the mixture was magnetically stirred at room temperature for 1 h, and the pH value was adjusted to about 11. Finally, under N2 protection, 10 mL of 85% hydrazine hydrate was slowly added, and the resulting mixture was refluxed at 110 °C for 4 h. The target product (Cu1Ni1/G) was collected by centrifugation, washed with deionized water and ethanol, respectively, and dried in a vacuum oven at 45 °C for 12 h. By using the same procedure, various CuxNiy/G nanohybrids could be obtained by adjusting the molar ratio of Cu2+ to Ni2+. The samples obtained are summarized in Table 1. For comparison, pure graphene (G), Cu1Ni3 alloy nanoparticles, Ni/G, Cu/G and Pt/G were also prepared under the same conditions.
| Samples | Mass percentages of Cu2+ and/or Ni2+ in GO | Molar ratio of Cu2+ to Ni2+ |
|---|---|---|
| Cu/G | [Cu2+] = 10% | Only introducing Cu2+ |
| Cu1Ni1/G | [Cu2+] + [Ni2+] = 10% | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| Cu1Ni3/G | [Cu2+] + [Ni2+] = 10% | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
| Cu1Ni5/G | [Cu2+] + [Ni2+] = 10% | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
| Ni/G | [Ni2+] = 10% | Only introducing Ni2+ |
Measurement of photocatalytic activity
Photocatalytic water reduction for hydrogen evolution was performed using a CEL-SP2N water splitting system with an outer-irradiation type quartz cell. In a typical process, 10 mg of the catalyst was dispersed into 80 mL of aqueous solution containing 15 vol% triethanolamine (TEOA). The pH value of the solution was adjusted to 9. The reaction temperature was kept at about 25 °C by a circulating water jacket. The light source was a 300 W Xe lamp equipped with a 420 nm cutoff filter. Before irradiation, the reactor was evacuated by a vacuum pump to remove air, and the mixture was stirred for 30 min in the dark. The amount of hydrogen evolution was measured by a continuous online gas chromatography system (AULTT Co., Beijing) equipped with a thermal conductivity detector (TCD), and high-purity N2 (99.999%) was used as the carrier gas.Characterization
X-ray diffraction patterns (XRD) of the samples were collected on a PANalytical Xpert Pro MRD X-ray diffractometer operated at 40 kV voltage and 40 mA current with Cu-Kα radiation (λ = 0.154056 nm) (Netherlands). The transmission electron microscopy (TEM) and high resolution transmission electron microscopy (HRTEM) images were collected on a JEOL JEM-2100F microscope operated at 200 kV (Japan). X-ray photoelectron spectroscopy (XPS) analyses were performed using a Thermo ESCALAB 250X-ray photoelectron spectrometer with an Al (Kα) X-ray resource (USA). Raman spectra were recorded with a Thermo Scientific DXR Raman microscope with a laser frequency of 780 nm as an excitation source (USA). The size of the laser spot was about 1 μm, and the incident laser power was 1 mW in order to avoid laser induced heating. The fluorescence spectra were recorded using a HITACHI F-4600 fluorescence spectrophotometer (Japan).Electrochemical impedance measurement
Electrochemical impedance spectra (EIS) were measured on an electrochemical analyzer (CHI660E Instruments) with a standard three-electrode system. A platinum wire electrode was used as the counter electrode and an Ag/AgCl electrode was used as the reference electrode. The working electrodes were prepared by drop-coating the suspension of the samples onto the pre-cleaned fluorine-doped tin oxide glass (FTO glass) (2 cm × 1 cm). The resulting electrodes were dried in an oven and heated at 150 °C for 30 min in N2. 0.1 mol L−1 Na2SO4 aqueous solution was used as the supporting electrolyte. The impedance spectra were recorded over a frequency range of 0.1 to 106 Hz. The amplitude was 5 mV, and the bias potential was 0.5 V. The air in the solution was removed by purging N2 for 15 min.Results and discussion
The formation of the CuxNiy bimetallic alloy on graphene is confirmed by XRD (Fig. 1). As shown in Fig. 1A, the peak at 2θ = 26.5° is ascribed to the (002) crystal face of graphite, and the interlayer spacing is about 0.34 nm. In contrast, the diffraction peak for the GO is located at 2θ = 10.7°, which corresponds to an interlayer spacing of 0.78 nm. It could be ascribed to the introduction of a large amount of oxygen-containing functional groups on both sides and edges of graphite sheets after oxidation of graphite. The diffraction peak at 2θ = 42.5° is associated with the (100) crystal face of the hexagonal structure of carbon, which is similar to that reported the literature.30 In addition, the diffraction peak of GO at 2θ = 10.7° shifts towards a higher angle (at about 24°) after reduction. The peak at 2θ = 24° can be observed in the XRD patterns of CuxNiy/G nanohybrids (Fig. 1B), which reveals that the GO in the composite has been reduced by hydrazine hydrate.31 In the diffraction pattern of the Cu/G (Fig. 1B), the peaks at 2θ = 43.3°, 50.4° and 74.1° correspond to (111), (200) and (220) planes of a face centered cubic (fcc) phase Cu (JCPDS 04-0836), respectively. In the diffraction pattern of Ni/G, the peaks at 2θ = 44.5°, 51.8° and 76.4° correspond to the fcc Ni (JCPDS 04-0850). Based on the above results, it is found that all of the diffraction peaks of the CuxNiy/G (x/y = 1, 1/3 and 1/5) are located between the Cu/G and the Ni/G, and move towards those of the Ni/G with increasing Ni content, which indicates that lattice constants increase followed by increase in the Ni content (compared with Cu, Ni has a larger lattice constant). The result is in good agreement with the formation of the CuxNiy nanoalloy.32 It demonstrates that various CuxNiy bimetallic alloy decorated graphene nanohybrids (CuxNiy/G) have been achieved.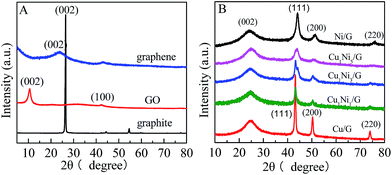 | ||
| Fig. 1 (A) XRD patterns of graphite, GO and graphene; (B) XRD patterns of CuxNiy/G nanohybrids with various molar ratios of Cu2+ to Ni2+. | ||
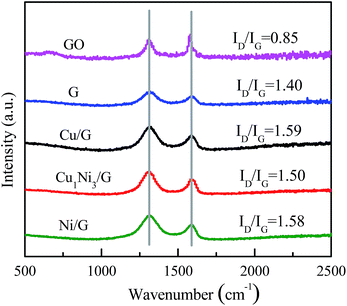
The Raman spectra of GO, graphene, Cu/G, Ni/G and Cu1Ni3/G are shown in Fig. 2. It can be seen that, two prominent peaks at about 1590 cm−1 and 1320 cm−1, corresponding to the well-documented G band and D band, respectively, are observed in the Raman spectra of these samples. It is well known that the G band is usually assigned to the E2g symmetry of sp2 carbon atoms, while D band is a breathing mode of the A1g symmetry.33 The D band is associated with structural defects and disorder, the intensity ratio of the D band to G band (ID/IG) is considered as an indicator of the disorder.34 As shown in Fig. 2, the intensity ratios for graphene (1.40), Cu/G (1.59), Ni/G (1.58) and Cu1Ni3/G (1.50) increase compared with that of GO (0.85), suggesting increased defect sites in the sp2 domains of Cu/G, Ni/G and Cu1Ni3/G nanohybrids. In addition, the intensity ratio (ID/IG) for Cu/G, Ni/G and Cu1Ni3/G nanohybrids are higher than that of graphene, which indicates that it becomes more disordered after introducing metals into graphene. Compared with those of Cu/G and Ni/G, the ID/IG of Cu1Ni3/G is slightly lower. It is demonstrated that the defect sites of Cu1Ni3/G are relatively fewer than those of Cu/G and Ni/G. It is deduced that the fewer defect sites in the Cu1Ni3/G could come from smaller deformation of graphene after the Ni–Cu alloy with smaller particle sizes are formed in the graphene as provided in the following TEM images (Fig. 4).
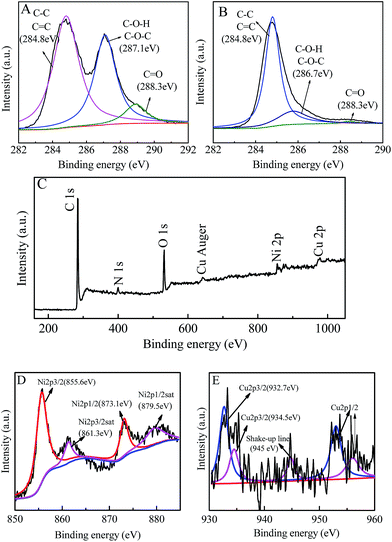
In the high resolution XPS spectra of C 1s for GO (Fig. 3A), the characteristic peaks of C–C, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (at ∼284.8 eV), C–O (at ∼287.1 eV) and C
C (at ∼284.8 eV), C–O (at ∼287.1 eV) and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (at ∼288.3 eV) can be observed, which indicate the presence of epoxide, hydroxyl and carboxyl groups in the GO.31 However, in Fig. 3B, the peaks corresponding to C–O–H, C–O–C and C–O show a significant decrease, which demonstrates that most of the epoxide, hydroxyl and carboxyl functional groups were removed and the GO in the nanohybrid had been reduced.31 Moreover, the peaks of Cu, Ni, O, C and N can be observed in the survey spectrum of the nanohybrid (Fig. 3C). C and O come from graphene, Cu and Ni come from the bimetallic alloy, and N may come from the C–N formed by graphene and hydrazine hydrate. Fig. 3D and E are the high resolution XPS of Ni 2p and Cu 2p for the Cu1Ni3/G nanohybrid. The Ni 2p1/2sat, Ni 2p1/2, Ni 2p3/2sat and Ni 2p3/2 peaks appeared at 879.5 eV, 873.1 eV, 861.3 eV and 855.6 eV, respectively. The result is similar to that reported in the literature.18,35 The slight difference for Ni 2p3/2 existed in Yeh's report and ours could come from the alloy Ni in our sample. Therefore, it is deduced that it is elemental Ni not Ni(II) in the sample. It is consistent with the XRD result (Fig. 1). In the high resolution XPS of Cu2p, the Cu2p3/2 peak (Fig. 3E) can be divided into two peaks with binding energies at 932.7 eV and 934.5 eV, assigned to Cu2O/Cu (Cu1+/Cu0) (it is difficult to differentiate Cu+ and Cu0 by the XPS feature of Cu2p3/2 because the binding energy of Cu is very close to that of Cu2O) and CuO/Cu(OH)2 (Cu2+), respectively.11,35 The weak shake-up satellite at 945 eV could be attributed to Cu2+ in CuO (or probably the Cu(OH)2 species).36 However, as mentioned above (Fig. 1), no characteristic diffraction peaks for CuO, Cu2O and Cu(OH)2 are found in the XRD patterns of the nanohybrids. It is deduced that the surface of copper nanoparticles was slightly oxidized when the nanohybrids were exposed in air,36 and its dispersion is good, but crystallinity was poor. It is reasonable that it could be detected by XPS, but not by XRD. The main reason is that the XPS technique could only detect signals within the upper 5 nm thickness on the surface, while XRD is a bulk characterization technique, and can detect signals in micro-order thickness.36
O (at ∼288.3 eV) can be observed, which indicate the presence of epoxide, hydroxyl and carboxyl groups in the GO.31 However, in Fig. 3B, the peaks corresponding to C–O–H, C–O–C and C–O show a significant decrease, which demonstrates that most of the epoxide, hydroxyl and carboxyl functional groups were removed and the GO in the nanohybrid had been reduced.31 Moreover, the peaks of Cu, Ni, O, C and N can be observed in the survey spectrum of the nanohybrid (Fig. 3C). C and O come from graphene, Cu and Ni come from the bimetallic alloy, and N may come from the C–N formed by graphene and hydrazine hydrate. Fig. 3D and E are the high resolution XPS of Ni 2p and Cu 2p for the Cu1Ni3/G nanohybrid. The Ni 2p1/2sat, Ni 2p1/2, Ni 2p3/2sat and Ni 2p3/2 peaks appeared at 879.5 eV, 873.1 eV, 861.3 eV and 855.6 eV, respectively. The result is similar to that reported in the literature.18,35 The slight difference for Ni 2p3/2 existed in Yeh's report and ours could come from the alloy Ni in our sample. Therefore, it is deduced that it is elemental Ni not Ni(II) in the sample. It is consistent with the XRD result (Fig. 1). In the high resolution XPS of Cu2p, the Cu2p3/2 peak (Fig. 3E) can be divided into two peaks with binding energies at 932.7 eV and 934.5 eV, assigned to Cu2O/Cu (Cu1+/Cu0) (it is difficult to differentiate Cu+ and Cu0 by the XPS feature of Cu2p3/2 because the binding energy of Cu is very close to that of Cu2O) and CuO/Cu(OH)2 (Cu2+), respectively.11,35 The weak shake-up satellite at 945 eV could be attributed to Cu2+ in CuO (or probably the Cu(OH)2 species).36 However, as mentioned above (Fig. 1), no characteristic diffraction peaks for CuO, Cu2O and Cu(OH)2 are found in the XRD patterns of the nanohybrids. It is deduced that the surface of copper nanoparticles was slightly oxidized when the nanohybrids were exposed in air,36 and its dispersion is good, but crystallinity was poor. It is reasonable that it could be detected by XPS, but not by XRD. The main reason is that the XPS technique could only detect signals within the upper 5 nm thickness on the surface, while XRD is a bulk characterization technique, and can detect signals in micro-order thickness.36
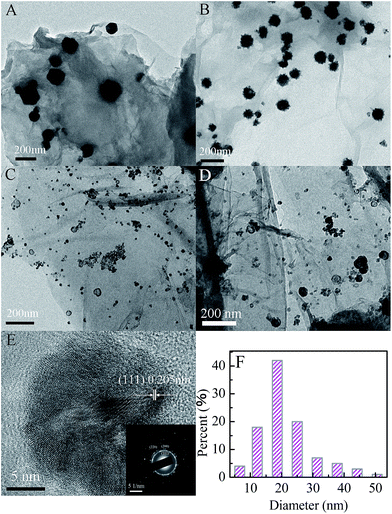
The TEM images of the Cu/G, Ni/G and CuxNiy/G nanohybrids are shown in Fig. 4. It can be clearly seen that some nanoparticles are well-dispersed on the surface of graphene nanosheets. This indicates that graphene nanosheets with large surface area can prevent the aggregation of nanoparticles. In addition, the morphology and the size of the nanoparticles in the three samples are different. It shows a sphere-like morphology for the nanoparticles in the Cu/G, and the size of the nanoparticles is about 90–160 nm (Fig. 4A). It exhibits a spinous sphere-like morphology for the nanoparticles in the Ni/G, and the size of the nanoparticles is about 60–106 nm (Fig. 4B).
However, it displays a capsule-like morphology in the TEM image of the CuxNiy/G nanohybrid. The size of the nanoparticles is focused on about 20 nm, which is smaller than those in the Ni/G and the Cu/G. The morphologies for the Cu1Ni3/G and the Cu1Ni5/G are similar, but the size of nanoparticles in the Cu1Ni5/G is slightly increased. The HRTEM image (Fig. 4E) of the Cu1Ni3/G nanohybrid shows a lattice fringe with a d-spacing of 2.05 Å, which corresponds to the (111) plane of the face-centered lattice of the Cu–Ni alloy.37 The selected area electron diffraction pattern (Fig. 4E, inset) displays a distinguishable ring-like feature, indicating that the as-obtained Cu1Ni3 bimetallic nanoparticles in the composite are polycrystalline. Moreover, the bimetallic nanoparticles are dispersed well and stuck onto the graphene. In the TEM images, though there is not a significant change in the dispersity of the metallic co-catalysts across the graphene sheets, dramatic changes in size and morphology were observed in the CuxNiy/G nanohybrids, which could have important influence on their catalytic activity.
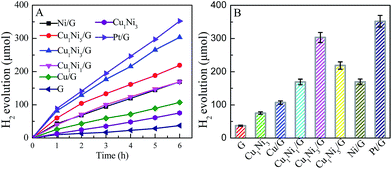
It has been reported that the co-sensitization of graphene sheets with multi-dyes can improve the photocatalytic performance for hydrogen evolution.31 Referenced to the literature, with eosin Y and rose bengal (ER) as the co-sensitizers, the photocatalytic activity of hydrogen evolution over various photocatalysts was investigated. Fig. 5A shows the time courses for hydrogen evolution catalyzed by various CuxNiy/G under visible light irradiation, together with those of pure graphene (G), Cu1Ni3 bimetallic nanoparticles and Pt/G for comparison. As can be seen in Fig. 5, the lower activity for hydrogen generated is observed over the G, Cu/G or Cu1Ni3. The H2-production rate is 0.62 mmol g−1 h−1 over the G, 1.26 mmol g−1 h−1 over the Cu1Ni3, and 1.79 mmol g−1 h−1 over the Cu/G. It is indicated that the G, the Cu1Ni3 and the Cu/G are not active for hydrogen evolution. After introducing Ni into the Cu/G, the photocatalytic activity is significantly enhanced. These results clearly indicate the importance of Ni–Cu alloy formation, in which the metal–hydrogen bond strength may be optimised, because the copper–hydrogen bond seems too weak for H2 evolution as predicted by the density functional theory (DFT) calculations.38 In addition, low mobility of oxygen in graphene may prevent CuxNiy from oxidation, which is favourable for proton reduction.
When the molar ratio of Cu to Ni is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, the H2-production rate can reach 5.05 mmol g−1 h−1. It is about 8.2 times higher than that of pure G. It is also higher than those of the Cu/G (1.78 mmol g−1 h−1), the Ni/G (2.83 mmol h−1 g−1) and the Cu1Ni3 (1.26 mmol g−1 h−1), and even is comparable to that of the Pt/G under the same conditions. A further increase of the Ni content in the CuxNiy/G leads to a decrease of the photocatalytic activity, and the H2-production rate for the Ni/G is 2.83 mmol h−1 g−1. The higher activity of the CuxNiy/G could be attributed to the small size effect and special morphology of the CuxNiy alloy in the composite.11,31 In addition, the interface in the CuxNiy/G system would be favourable for electron transfer, and the lattice mismatch between Cu and Ni could also lead to increasing of the active sites.39 Moreover, a synergic interaction exists between graphene and CuxNiy in the hybrid. These factors would result in high photocatalytic activity for the CuxNiy/G nanohybrids.
3, the H2-production rate can reach 5.05 mmol g−1 h−1. It is about 8.2 times higher than that of pure G. It is also higher than those of the Cu/G (1.78 mmol g−1 h−1), the Ni/G (2.83 mmol h−1 g−1) and the Cu1Ni3 (1.26 mmol g−1 h−1), and even is comparable to that of the Pt/G under the same conditions. A further increase of the Ni content in the CuxNiy/G leads to a decrease of the photocatalytic activity, and the H2-production rate for the Ni/G is 2.83 mmol h−1 g−1. The higher activity of the CuxNiy/G could be attributed to the small size effect and special morphology of the CuxNiy alloy in the composite.11,31 In addition, the interface in the CuxNiy/G system would be favourable for electron transfer, and the lattice mismatch between Cu and Ni could also lead to increasing of the active sites.39 Moreover, a synergic interaction exists between graphene and CuxNiy in the hybrid. These factors would result in high photocatalytic activity for the CuxNiy/G nanohybrids.
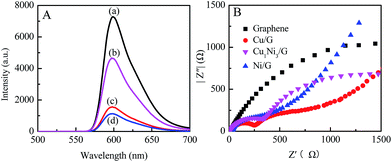
To investigate the roles of the CuxNiy and graphene in the photocatalyst system, fluorescence spectra and electrochemical impedance spectra of the photocatalysts are measured. As shown in Fig. 6A, the ER aqueous solution possesses a strong emission peak located at 599 nm, which is due to the recombination of excited charge pairs upon light excitation. After the introduction of graphene, Cu1Ni3 or Cu1Ni3/G, the emission peak shows a significant decrease. The quenching efficiency of Cu1Ni3/G (∼84.5%) is higher than that of graphene (∼78.5%) and significantly higher than that of Cu1Ni3 (∼36.2%). The strong fluorescence quenching might be caused by interfacial electron transfer from the excited ER molecules to graphene or Cu1Ni3 for the noncovalent π–π interaction of graphene or Cu1Ni3 with ER molecules.8 The result in Fig. 6A indicates that there exists a stronger interaction between ER and graphene in the Cu1Ni3/G nanohybrid. In addition, Cu1Ni3 nanoparticles onto the graphene can effectively prevent the aggregation of graphene sheets (Fig. 4C and D). These two factors are favourable for facilitating the transfer of photogenerated electrons and enhancing the photocatalytic activity of the Cu1Ni3/G.35
The important roles of CuxNiy in preventing electrons and holes from recombining and improving the photocatalytic activity of the graphene are further proved by electrochemical impedance spectra. The Nyquist spectra of graphene, Cu/G, Ni/G and Cu1Ni3/G are shown in Fig. 6B. Compared with that of the graphene, the semicircles of Cu/G, Ni/G and Cu1Ni3/G become smaller, indicating a lower resistance for the charge transfer in the solid state interface layer in the Cu/G, Ni/G and Cu1Ni3/G.11 However, there exists little difference in the semicircles of the Cu/G, the Ni/G and the Cu1Ni3/G. It is deduced that the higher photocatalytic activity over the Cu1Ni3/G nanohybrid could be mainly attributed to the small size effect of Cu1Ni3 bimetallic nanoparticles on the graphene sheets (Fig. 4). In addition, the interface in the CuxNiy/G system would be favourable for electron transfer. It is also helpful to improve the photocatalytic activity of the CuxNiy/G nanohybrids.
On the basis of the above results, a possible mechanism of hydrogen evolution over the CuxNiy/G photocatalyst with the help of ER is proposed and illustrated in Fig. 7. Under UV-vis light irradiation, ER molecules absorb visible light and turn into the excited state of ER (*ER).40 The photoproduced electrons released from *ER are transferred to graphene and CuxNiy nanoparticles loaded on the graphene nanosheets, where the water molecules accept the electrons to be reduced to H2. *ER moieties renew the ground state by accepting electrons from TEOA. Therefore, after loading with a certain amount of CuxNiy as the co-catalyst, dramatically enhanced hydrogen evolution activity is observed due to effective separation of photoproduced electron/hole pairs (Fig. 6) and the synergic interaction between graphene and CuxNiy in the hybrid (Fig. 5).
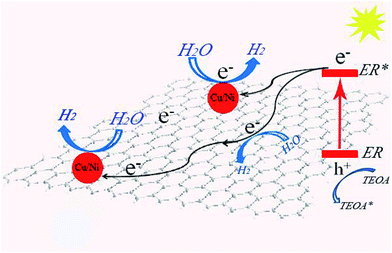 | ||
| Fig. 7 Proposed mechanism for photocatalytic hydrogen evolution over the ER sensitized CuxNiy/G photocatalyst. | ||
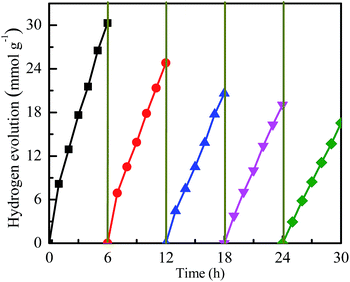
Furthermore, the photocatalytic stability of the Cu1Ni3/G photocatalyst is investigated. As shown in Fig. 8, the rate of hydrogen generation is 5.05 mmol h−1 g−1 in the first run, and then decreases slightly in the consecutive runs. It is probably due to the partial decomposition of ER or the consumption of TEOA.11 Although the photocatalytic activity of the Cu1Ni3/G photocatalyst has a slight decrease, the rate of hydrogen generation can still reach 2.76 mmol h−1 g−1 after 5 runs, which is also higher than the initial activity of the Cu/G, and almost amounts to that of the Ni/G. Subsequently, the CuxNiy alloy could be a cheap and highly efficient co-catalyst for hydrogen evolution in the dye-sensitized graphene-based photocatalyst systems, though its stability is still unsatisfactory.
Conclusions
The noble metal-free CuxNiy bimetallic nanoalloy decorated graphene nanohybrids were achieved via a facile one-step in situ chemical reduction method. It was found that the size and morphology of Cu nanoparticles can be modified by introducing Ni. Moreover, the dye-cosensitized CuxNiy/G nanohybrid exhibited higher photocatalytic activity for hydrogen production than that of Cu/G or Ni/G, and it was even comparable to that of Pt under the same conditions. Three main factors could result in high photocatalytic activity for the CuxNiy/G nanohybrids: the small size effect and special morphology of CuxNiy alloy, fast interfacial electron transfer and synergic interaction in the CuxNiy/G system. Therefore, the CuxNiy bimetallic nanoalloy was an excellent co-catalyst for hydrogen production in the graphene-based photocatalyst system. This study provides a new and cheaper noble-metal-free graphene-based photocatalyst system for visible light-driven photocatalytic hydrogen evolution.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 21301118, No. 21305092 and No. 21371070), and Innovation Program of Shanghai Municipal Education Commission (No. 15ZZ096).Notes and references
- T. H. Yang, Y. W. Harn, L. D. Huang, M. Y. Pan, W. C. Yen, M. C. Chen, C. C. Lin, P. K. Wei, Y. L. Chueh and J. M. Wu, J. Catal., 2015, 329, 167 CrossRef CAS.
- Y. Ma, X. L. Wang, Y. S. Jia, X. B. Chen, H. X. Han and C. Li, Chem. Rev., 2014, 114, 9987 CrossRef CAS PubMed.
- C. J. Chang, K. W. Chu, M. H. Hsu and C. Y. Chen, Int. J. Hydrogen Energy, 2015, 40, 14498 CrossRef CAS.
- R. Abe, K. Shinmei, N. Koumura, K. Hara and B. Ohtani, J. Am. Chem. Soc., 2013, 135, 16872 CrossRef CAS PubMed.
- M. Watanabe, H. Hagiwara, A. Iribe, Y. Ogata, K. Shiomi, A. Staykov, S. Ida, K. Tanaka and T. Ishihara, J. Mater. Chem. A, 2014, 2, 12952 CAS.
- L. K. Putri, W. J. Ong, W. S. Chang and S. P. Chai, Appl. Surf. Sci., 2015, 358, 2 CrossRef CAS.
- H. W. Jeong and H. Park, Catal. Today, 2014, 230, 15 CrossRef CAS.
- C. Kong, S. X. Min and G. X. Lu, ACS Catal., 2014, 4, 2763 CrossRef CAS.
- C. Kong, S. X. Min and G. X. Lu, Chem. Commun., 2014, 50, 9281 RSC.
- M. Latorre-Sánchez, I. Esteve-Adell, A. Primo and H. García, Carbon, 2015, 81, 587 CrossRef.
- H. L. Xu, X. Q. Li, S. Z. Kang, L. X. Qin, G. D. Li and J. Mu, Int. J. Hydrogen Energy, 2014, 39, 11578 CrossRef CAS.
- Q. Xiang, B. Cheng and J. Yu, Angew. Chem., Int. Ed., 2015, 54, 11350 CrossRef CAS PubMed.
- V. D. Dao, L. L. Larina, J. K. Lee, K. D. Jung, B. T. Huy and H. S. Choi, Carbon, 2015, 81, 710–719 CrossRef CAS.
- H. G. Yu, W. Y. Chen, X. F. Wang, Y. Xu and J. G. Yu, Appl. Catal., B, 2016, 187, 163–170 CrossRef CAS.
- G. Takao, J. J. Arockiam, H. Masanari, N. Toshiaki, T. Toyokazu, K. Shingo, M. Masahiro and M. Futoshi, Appl. Catal., B, 2016, 181, 475–480 CrossRef.
- L. C. Pop, V. Dracopoulos and P. Lianos, Appl. Surf. Sci., 2015, 333, 147 CrossRef CAS.
- Z. H. N. Al-Azri, W. T. Chen, A. Chan, V. Jovic, T. Ina, H. Idriss and G. I. N. Waterhouse, J. Catal., 2015, 329, 355–367 CrossRef CAS.
- Z. P. Yan, X. X. Yu, A. L. Han, P. Xu and P. W. Du, J. Phys. Chem. C, 2014, 118, 22896 CAS.
- A. P. Gaikwad, D. Tyagi, C. A. Betty and R. Sasikala, Appl. Catal., A, 2016, 517, 91–99 CrossRef CAS.
- C. X. Xu, H. Zhang, Q. Hao and H. M. Duan, ChemPlusChem, 2014, 79, 107–113 CrossRef CAS.
- B. M. Luo, S. Xu, X. B. Yan and Q. J. Xue, Electrochem. Commun., 2012, 23, 72–75 CrossRef CAS.
- A. Hassan, A. Carreras, J. Trincavelli and E. A. Ticianelli, J. Power Sources, 2014, 247, 712–720 CrossRef CAS.
- Y. Yamada, T. Miyahigashi, H. Kotani, K. Ohkubo and S. Fukuzumi, J. Am. Chem. Soc., 2011, 133, 16136–16145 CrossRef CAS PubMed.
- Q.-L. Zhu, J. Li and Q. Xu, J. Am. Chem. Soc., 2013, 135, 10210–10213 CrossRef CAS PubMed.
- Y. Yamada, S. Shikano, T. Akita and S. Fukuzumi, Catal. Sci. Technol., 2015, 5, 979–988 CAS.
- K. Xiao, X. Z. Qi, Z. H. Bao, X. X. Wang, L. S. Zhong, K. G. Fang, M. G. Lin and Y. H. Sun, Catal. Sci. Technol., 2013, 3, 1591–1602 CAS.
- A. Hornés, M. J. Escudero, L. Daza and A. Martínez–Arias, J. Power Sources, 2014, 249, 520–526 CrossRef.
- Y. Yamada, S. Shikano and S. Fukuzumi, RSC Adv., 2015, 5, 44912–44919 RSC.
- Q. X. Wu, L. D. L. Duchstein, G. L. Chiarello, J. M. Christensen, C. D. Damsgaard, C. F. Elkjær, J. B. Wagner, B. Temel, J. D. Grunwaldt and A. D. Jensen, ChemCatChem, 2014, 6, 301 CrossRef CAS.
- C. Nethravathi and M. Rajamathi, Carbon, 2008, 46, 1994–1998 CrossRef CAS.
- S. X. Min and G. X. Lu, Int. J. Hydrogen Energy, 2012, 37, 105644–110574 CrossRef.
- Q. X. Wu, L. D. L. Duchstein, G. L. Chiarello, J. M. Christensen, C. D. Damsgaard, C. F. Elkjær, J. B. Wagner, B. Temel, J. D. Grunwaldt and A. D. Jensen, ChemCatChem, 2014, 6, 301–310 CrossRef CAS.
- Y. J. Guo, S. J. Guo, J. T. Ren, Y. M. Zhai, S. J. Dong and E. Wang, ACS Nano, 2010, 4, 4001–4010 CrossRef CAS PubMed.
- R. F. Nie, J. H. Wang, L. N. Wang, Y. Qin, P. Chen and Z. Y. Hou, Carbon, 2012, 50, 586–596 CrossRef CAS.
- C. C. Yeh and D. H. Chen, Appl. Catal., B, 2014, 150–151, 298–304 CrossRef CAS.
- S. K. Movahed, M. Dabiri and A. Bazgir, Appl. Catal., A, 2014, 481, 79–88 CrossRef CAS.
- J. Ahmed, K. V. Ramanujachary, S. E. Lofland, A. Furiato, G. Gupta, S. M. Shivaprasad and A. K. Ganguli, Colloids Surf., A, 2008, 331, 206–212 CrossRef CAS.
- J. Greeley, T. F. Jaramillo, J. Bonde, I. Chrokendorff and J. K. Nørskov, Nat. Mater., 2006, 5, 909–913 CrossRef CAS PubMed.
- S. Z. Kang, L. L. Chen, X. Q. Li and J. Mu, Appl. Surf. Sci., 2012, 258, 6029 CrossRef CAS.
- Z. Mou, Y. Dong, S. Li, Y. Du, X. Wang, P. Yang and S. Wang, Int. J. Hydrogen Energy, 2011, 36, 8885–8893 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2017 |