Transforming waste newspapers into nitrogen-doped conducting interlayers for advanced Li–S batteries†
Chi-Hao
Chang
,
Sheng-Heng
Chung
and
Arumugam
Manthiram
 *
*
Materials Science and Engineering Program, Texas Materials Institute, The University of Texas at Austin, Austin, TX 78712, USA. E-mail: manth@austin.utexas.edu; Fax: +1-512-471-7681; Tel: +1-512-471-1791
First published on 6th March 2017
Abstract
Nitrogen-doped conducting (NC) interlayers, derived from waste newspapers, are inserted between a sulfur cathode and a separator to enhance the electrochemical performances of lithium–sulfur (Li–S) batteries. A simple strategy transforms wastes into valuable polysulfide trappers that improve the active-material utilization to 77% and accomplish good cycling stability in Li–S batteries.
The growing demand for energy has triggered great interest in developing affordable next-generation energy storage devices with high energy density. Lithium–sulfur (Li–S) batteries have drawn tremendous attention over the past few years mainly due to the low cost and high capacity of sulfur cathodes. Sulfur cathodes have a high theoretical charge-storage capacity of 1675 mA h g−1, offering advantages over conventional cathode materials.1–4 Moreover, sulfur is a by-product in the petroleum industry. Recycling the reductant by-product as the active material in high energy-density Li–S batteries satisfies the green demand.4 However, the practical development of Li–S batteries is stagnated by the intrinsic undesirable characteristics of sulfur.5 The insulating nature of sulfur and its end-discharge product (Li2S) leads to low electrochemical utilization. Moreover, the soluble polysulfides (Li2Sn, 4 < n ≤ 8) generated during intermediate charge and discharge states easily dissolve in the liquid electrolytes. The dissolved polysulfides freely diffuse out from sulfur cathodes, migrate through the porous separators, and reach the Li–metal anode during cell cycling, leading to an irreversible loss of the active material. The active-material loss from the sulfur cathode causes rapid capacity degradation.5,6 Furthermore, the conversion from sulfur to Li2S produces huge stress originating from the huge volume change of 80%, damaging the cathode integrity. The damaged cathode structure disconnects the insulating sulfur particles from the conductive carbon additives and the metal current collector, thereby increasing the cell polarization and aggravating the polysulfide diffusion and low utilization issues.5,7
To address these issues, extensive effort has been devoted to entrapping sulfur into various porous carbon materials to form sulfur/carbon (S/C) composite cathodes.2,8,9 Applying the composite cathodes in the Li–S cell demonstrates significant improvements in boosting the cell capacity and suppressing polysulfide migration. The improvement is attributed to the function of the porous carbon hosts to act simultaneously as a conducting agent to transport electrons and as a polysulfide container to confine the soluble polysulfides.8 Although the composite cathodes ameliorate the intrinsic undesirable characteristics of sulfur, the associated cathode fabrication might block industrial production. Moreover, a well-designed S/C composite cathode depends significantly on its elaborate synthesis routes and usually requires mature preparation processes.1,2 In recent years, an advanced Li–S battery configuration with a conducting interlayer inserted between a sulfur cathode and a porous separator has offered great improvement in cyclability.10–13 The inserted interlayer is bifunctional, working as not only a polysulfide trapper but also an upper current collector. As a polysulfide trapper, the interlayer effectively absorbs the diffusing polysulfides, and therefore stabilizes them within the cathode region of the cell.10,11 It also serves as an upper current collector to transfer electrons to reduce the cell polarization and to continuously utilize the trapped active material.10 As a result, the simple insertion of the bifunctional interlayer in Li–S cells effectively enhances the electrochemical utilization and cycling stability. In addition, in view of the engineering progress, the above-mentioned two advantages allow the direct use of a pure sulfur cathode that is easily prepared to be applied directly in Li–S cells.
Myriads of well-designed materials (e.g., carbon materials14–16 and conducting polymers17) have been employed as the interlayer in Li–S batteries. However, the complex preparation processes and the associated high costs might neutralize the advantages of Li–S batteries. Accordingly, waste materials possessing unique structures such as pores could offer a great opportunity (Table S1†).11,18,19 Moreover, it is appealing to recycle the waste materials into a component (interlayer) of Li–S batteries because these wastes are basically cost-free. Furthermore, the preparation is considered as a simple and sustainable process.
Paper wastes such as newspapers, wrapping papers, and books are still major wastes despite the digital era. Among all paper wastes, newspapers are one of the most common wastes due to their timeliness. In other words, yesterday's newspapers become today's trash. Therefore, how to recycle waste newspapers for other useful purposes is one of the most challenging issues. This paper focuses on transforming waste newspapers into a bifunctional interlayer (the nitrogen-doped, conducting (NC) interlayer) to effectively enhance the electrochemical performance of Li–S batteries. Unlike the synthesized interlayers, the fabrication process is neither complicated nor environmentally harmful. More importantly, the raw materials of the NC interlayers are cost-free, so they would greatly reduce the cost of the interlayers in Li–S batteries. Thus, this strategy successfully turns trash into treasure, achieving the purpose of sustainable development and the cost-reduction principle.
A commonly seen situation that waste newspapers are often utilized for absorbing water and drying wet articles illustrates their excellent liquid-absorption capability resulting from their unique porous structures formed by interconnected fibers (Fig. S1 in (ESI†)). Fig. 1 illustrates the conversion process of the waste newspapers to NC interlayers. The waste newspapers are recycled and soaked into a urea solution for 1 day. After the urea soaking, a carbonization process in an inert atmosphere at 950 °C for 3 h converts the waste newspaper into the NC paper (Fig. 1).20–22 The use of the resulting NC paper as an interlayer could not only facilitate fast electron transfer but also suppress the diffusion of polysulfides in Li–S cells. Therefore, the Li–S battery utilizing the NC interlayer (denoted as the advanced Li–S battery) is expected to outperform the Li–S battery without an NC interlayer (denoted as the conventional Li–S battery).
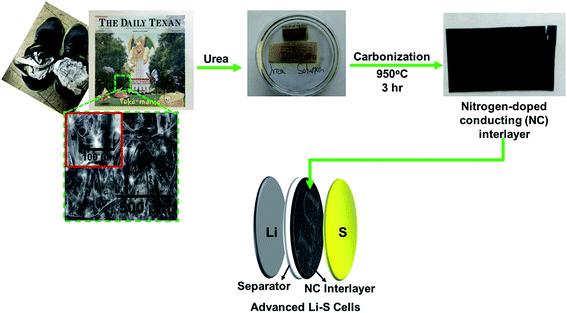 | ||
| Fig. 1 Schematic of transforming the waste newspaper into the NC interlayers of the advanced Li–S batteries. | ||
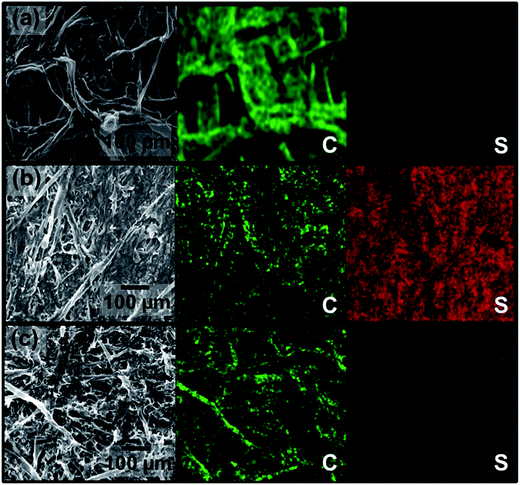
The microstructure of the fresh NC interlayer is observed by using a scanning electron microscope (SEM) and quantitatively analyzed by porosity measurements. Fig. 2a and S2† show that the carbonization treatment successfully converts the interconnected cellulose fibers of the newspaper (Fig. 1) into a long-range interconnected carbon framework, which creates numerous interspaces between each carbon fiber. Moreover, the interconnected conductive framework of the NC interlayer provides abundant and continuous macroporous channels for transporting the electrolyte. In addition to the microstructural inspection, the porosity of the NC interlayer is characterized by the nitrogen adsorption and desorption behaviors. Surprisingly, the NC interlayer possesses a high surface area of 420 m2 g−1 and a pore volume of 0.31 cm3 g−1 (Fig. S3†) that might be resulting from numerous plant veins. The high micropore area of 328 m2 g−1 and large micropore volume of 0.19 cm3 g−1 indicate that the micropores contribute to ∼80% of the surface area and more than 60% of the pore volume. Coupled with the high surface area, the abundant micropores enlarge the accessible reaction area for both trapping the dissolved polysulfides and reactivating the trapped sulfur-containing species.18,23 Of utmost importance is that such a high micropore volume offering a three-dimensional disordered porous carbon structure effectively increases the tortuosity of the polysulfide-migration routes, suppressing the movement of polysulfides from the sulfur cathode toward the Li anode.16,18
Chemically anchoring polysulfides is another merit of the NC interlayer. It has been demonstrated that carbon materials doped with more electronegative heteroatoms (e.g., N, S, P, B) have better absorption capability toward polysulfides due to stronger chemical interactions as compared to the pristine carbon materials.24,25 Therefore, in addition to physically retarding the migration of polysulfides, the nitrogen-doped sites allow the NC interlayer to chemically immobilize the polysulfide species. To identify the success in doping nitrogen within the carbon matrix of the NC interlayer, elemental mapping and Fourier transform infrared spectroscopy (FTIR) spectra were used to characterize the heteroatom doping effect (Fig. S4†). The elemental mapping results show a homogeneous distribution of elemental nitrogen signals, originating from the urea additive, within the NC interlayer (Fig. S4a†).20 On the other hand, no characteristic peaks of organic functional groups can be found in the FTIR spectrum of the NC interlayer, demonstrating the formation of nitrogen-doped carbon (Fig. S4b†).20,21 Therefore, the abundant micropores and the N-doped sites of the NC interlayer cooperatively sequester migrating polysulfides.
The microstructural and elemental inspections of the NC interlayer after 100 cycles demonstrate its excellent polysulfide-inhibiting capability. Much stronger sulfur signals on the cathode side of the NC interlayer (Fig. 2b) as compared to that on the anode side (Fig. 2c) imply that most of the active material is synergistically trapped within the cathode region rather than freely shuttling between the electrodes, as shown in Fig. 3. Moreover, the mapping results show no dense spots in the sulfur mapping but exhibit strong carbon signals in the carbon mapping. These demonstrate that the NC interlayer not only traps the active material but also transfers electrons to reactivate/reutilize the trapped active material. Additionally, the smooth Li surface and weak sulfur signals indicate that the NC interlayer indirectly prevents the Li anode from unfavorable reactions with polysulfides (Fig. S5†).26
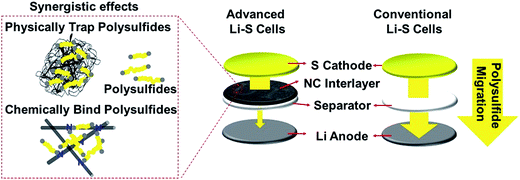 | ||
| Fig. 3 Comparison of the advanced Li–S cell with the NC interlayer and conventional Li–S cell without an interlayer. | ||
The enhanced electrochemical performances of the Li–S cells with the NC interlayer were evaluated with a series of electrochemical measurements. The sulfur loading in all cells including the advanced and conventional cells was 3 mg cm−2, which is higher than the loading commonly reported in the literature (<2 mg cm−2).27 The cyclic voltammetry (CV) curves of the advanced Li–S cell illustrate the reversible conversion reactions between sulfur and its reduction products, Li2S2/Li2S (Fig. S6†). Two cathodic peaks observed near 2.3 and 2.05 V represent, respectively, the reduction conversions from elemental sulfur to polysulfides and from polysulfides to Li2S2/Li2S.28 The single broad anodic peak at around 2.45 V is ascribed to the oxidation transformation from Li2S2/Li2S to polysulfides/S.29 The overlapping cathodic and anodic peaks show no obvious changes of the CV peaks and currents, indicating the improved electrochemical reversibility.27,29 The discharge/charge profiles (Fig. S7†) of the advanced Li–S cells are consistent with the CV results. The advanced Li–S cells show a typical two-plateau discharge curve that corresponds to the two-step reduction conversion from sulfur to sulfides. The overlapped upper-discharge plateaus that are attributed to the formation and existence of polysulfides,11 and the prolonged lower-discharge plateaus that involve a slow liquid-to-solid phase transformation13 indicate, respectively, the outstanding polysulfide retention and high redox accessibility of the advanced Li–S battery.16 In contrast, the voltage profile of the conventional cell exhibits a severe shrinkage for both upper- and lower-discharge plateaus (Fig. S8†). This is because the dissolved polysulfides diffuse out from the cathode, migrate across the separator, and deposit onto the Li anode (Fig. 3), causing the loss of active material and the degradation of both the electrodes.27
To better evaluate the electrochemical performance of the advanced Li–S cells with the NC interlayer, the upper- and lower-plateau discharge capacities (QH and QL) were used as semi-quantification indicators (Fig. S6d–f†).30–32 The upper-discharge plateau is related to the formation and migration of polysulfides, which is the key issue causing the electrochemical instability of the Li–S batteries.32 Therefore, the QH and its retention rate (RQH) reflect the polysulfide-trapping capability of the NC interlayers. The theoretical value of QH is 419 mA h g−1. The advanced Li–S cells show a high initial QH of 360 mA h g−1 and a high RQH (85%) at C/10 rate. However, the conventional cells show a relatively low QH of 315 mA h g−1, which drops quickly in the initial several cycles at the same cycling rate. The upper discharge plateau disappears after 10 cycles implying fast polysulfide migration towards the Li anode. As a result, the high QH and RQH values of the advanced Li–S cells evidence the capability of the NC interlayer to suppress the polysulfide migration.32 The lower discharge plateau, on the other hand, is attributed to the sluggish redox reactions regarding the change of liquid polysulfides to solid Li2S2/Li2S. The extended lower discharge plateau indicates that it dominates the main charge-storage capacity output.30,31 In this step, the formation/deposition of insoluble Li2S2/Li2S onto both the electrodes will compromise the Li-ion (Li+)/electron (e−) transport and may also lead to an increase in inactive sites.1,31 The high QL utilization of 44% (the theoretical value of QL: 1256 mA h g−1) and high retention rate of QL (RQL: 84%) indicate that the advanced Li–S cell with the NC interlayer has superior reaction kinetics with a fast Li+/e− transport during repeated cycling.32 As a result, the trapped active material could be reutilized/reactivated during cycling. Thus, the results of QH and QL analyses reconfirm that the NC interlayers significantly alleviate the unfavorable electrochemical issues of the Li–S batteries.
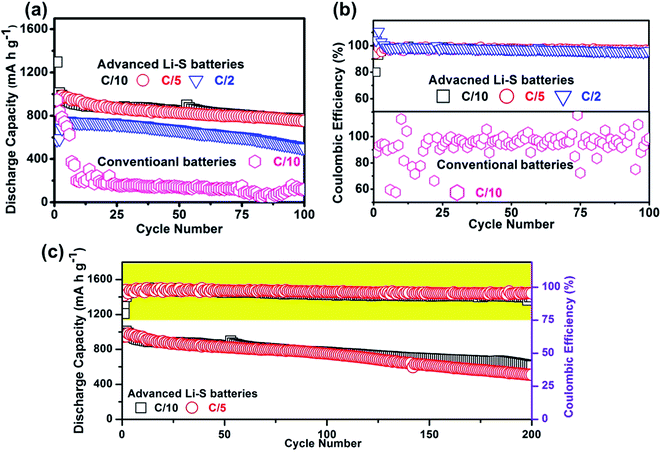
The electrochemical performances of the advanced and conventional Li–S cells were also examined at low cycling rates (C/10) (Fig. 4a). In this scenario, the polysulfide migration phenomenon will be magnified due to the longer discharge/charge period, providing more time for polysulfides to migrate from the sulfur cathode to the Li anode.32 As a result, the discharge capacity (active-material utilization in parentheses) of the conventional cells drops dramatically from 824 mA h g−1 (49%) to 175 mA h g−1 in the initial 10 cycles. After 100 cycles, the conventional cells barely offer a very low reversible discharge capacity of 118 mA h g−1. In sharp contrast, the advanced Li–S cell with the NC interlayer delivers a high discharge capacity of 1295 mA h g−1 (77%) at the C/10 rate. Even after 100 cycles, the advanced Li–S cell is still able to deliver a high reversible capacity of 770 mA h g−1. The high reversible capacity of the advanced Li–S cell corresponds to a significantly improved capacity-retention rate of 60% and a capacity-fade rate of 0.4% per cycle as compared to those of the conventional cell (capacity-retention rate: 14%; capacity-fade rate: 0.85% per cycle). In addition, the coulombic efficiency shown in Fig. 4b indicates that the NC interlayer cooperates with the LiNO3 electrolyte additive for improving the charge–discharge stability of the Li–S cells and also for suppressing the polysulfide shuttle effect.
Fig. 4a also shows that the advanced Li–S cells with the NC interlayer exhibit improved rate capability. Although high-loading sulfur cathodes increase the cathode resistance, the NC interlayer functions as an upper current collector, and therefore ensures stable high-rate cyclability at C/5 and C/2 rates. The initial discharge capacities (active-material utilization in parentheses) of the advanced Li–S cells with the NC interlayer are 990 (60%) and 750 (45%) mA h g−1 at, respectively, C/5 and C/2 rates. Different form the traditional rate-capability measurement that analyzes the high-rate performance of Li–S cells based on a short cycle number of 5–10 cycles, Fig. 4a demonstrates the enhanced rate capability for a long 100 cycles. After 100 cycles, the advanced cells maintain high reversible discharge capacities of 753 mA h g−1 at the C/5 rate and 504 mA h g−1 at the C/2 rate. In consideration of the electrochemical conversion reaction of the Li–S batteries, the long-term rate-capability test should illustrate the real high-rate performance of Li–S batteries. Thus, the enhanced long-term rate capability indicates the improved redox reaction capability of the advanced Li–S cell with the NC interlayer. The battery parameters and performances are summarized in Table 1.
| Sulfur loading (mg cm−2) | Sulfur content (%) | Sulfur contentd (%) | Peak capacity (mA h g−1) | Sulfur utilization (%) | 100th cycle (mA h g−1) | Capacity retention rate (%) | Capacity fade rate (% per cycle) | 200th cycle (mA h g−1) | |
|---|---|---|---|---|---|---|---|---|---|
| a C/10 rate. b C/5 rate. c C/2 rate. d Including the mass of the NC interlayer (∼2 mg cm−2). | |||||||||
| Conventional Li–S cella | 3 | 50 | 50 | 824 | 49% | 118 | 14% | 0.85 | — |
| Advanced Li–S cella | 3 | 75 | >50 | 1295 | 77% | 770 | 59% | 0.40 | 613 |
| Advanced Li–S cellb | 3 | 75 | >50 | 990 | 59% | 753 | 76% | 0.24 | 510 |
| Advanced Li–S cellc | 3 | 75 | >50 | 750 | 45% | 504 | 67% | 0.32 | — |
The long-term cycling performance is another criterion for building practical Li–S batteries. The advanced Li–S batteries with the NC interlayer have a great capability to cycle for a long life-span. In Fig. 4c, the reversible discharge capacity of the advanced Li–S cell after a long-term cycling (200 cycles) achieves 510 mA h g−1 at the C/5 rate. The corresponding capacity-retention and capacity-fade rates are, respectively, 52% and 0.24% per cycle. At a low cycling rate (C/10), the advanced Li–S cell with the NC interlayer delivers 613 mA h g−1, also reflecting a promising capacity-retention rate of 47% and a capacity-fade rate of 0.26% per cycle for 200 cycles. The enhanced long-term cyclability at both the high and low cycling rates demonstrates that the NC interlayer effectively suppresses the severe polysulfide diffusion, as evidenced in low-rate measurements, and also benefits the redox chemistry, as proved in high-rate measurements. Such long-term cycle life results originate from the ability to reutilize/reactivate the trapped active material in the porous interconnected fibrous framework within the NC interlayer.
Overall, the NC interlayers obtained from the waste newspaper exhibit great potential to improve the battery performances (e.g., high electrochemical utilization, long-term high-rate capability, and long cycle stability) of Li–S cells. First, the NC interlayer offers continuous macroporous channels for the electrolyte and additional transport routes for electrons, leading to high electrochemical utilization. Second, the complex interconnected fibrous structures of the NC interlayers have high tortuosity, retarding the migration of polysulfides. The intercepted polysulfides are subsequently trapped by the abundant micropores and immobilized by the nitrogen-doped sites of the NC interlayer. Collectively, the NC interlayers exhibit synergistic trapping ability toward polysulfides. Third, the NC interlayer facilitates electron transport to reutilize/reactivate the trapped active material during cell operation. The abundant microporous reaction sites that trap the polysulfides also improve the redox chemistry of the trapped active material. On the contrary, the conventional cells without an interlayer allow dissolved polysulfides to freely diffuse out from the cathode region to the Li anode. The irreversible polysulfide relocation results in a loss of active material and fast capacity fade.27 These undesirable electrochemical behaviors of the conventional Li–S battery configuration can be significantly mitigated by placing the NC interlayer between the sulfur cathode and the separator as in the advanced Li–S battery configuration presented here.
In conclusion, waste newspapers are recycled and animated to have a new life as a polysulfide inhibitor through a sustainable method. The NC interlayer functions as a polysulfide-trapping interface in the Li–S system. The NC interlayer possesses intertwined fibrous structures coupled with the numerous microporous spaces and the nitrogen-doped sites. As a result, the NC interlayer can synergistically intercept and trap/immobilize the migrating polysulfides, and indirectly protect the Li anode from the attack of polysulfides. The reduced polysulfide diffusion is reflected in the improved cycle stability and extended cycle life. Benefiting from the advantages of the NC interlayer, the advanced Li–S cells utilizing the high-loading cathodes with a sulfur loading of 3 mg cm−2 attain a high discharge capacity of 1295 mA h g−1 and a high reversible discharge capacity of 613 mA h g−1 after 200 cycles. The calculated electrochemical utilization rate and capacity-fade rate approach, respectively, 77% and 0.26% per cycle. The improved redox reaction capability and stability suggest that the NC interlayer paves a sustainable route to improve the Li–S battery system.
Acknowledgements
This work was supported by the U.S. Department of Energy, Office of Basic Energy Sciences, Division of Materials Science and Engineering under award number DE-SC0005397.Notes and references
- A. Manthiram, Y. Fu, S.-H. Chung, C. Zu and Y.-S. Su, Chem. Rev., 2014, 114, 11751–11787 CrossRef CAS PubMed.
- A. Manthiram, S.-H. Chung and C. Zu, Adv. Mater., 2015, 27, 1980–2006 CrossRef CAS PubMed.
- B. Li, S. Li, J. Xu and S. Yang, Energy Environ. Sci., 2016, 9, 2025–2030 CAS.
- W. J. Chung, J. J. Griebel, E. T. Kim, H. Yoon, A. G. Simmonds, H. J. Ji, P. T. Dirlam, R. S. Glass, J. J. Wie, N. A. Nguyen, B. W. Guralnick, J. Park, Á. Somogyi, P. Theato, M. E. Mackay, Y.-E. Sung, K. Char and J. Pyun, Nat. Chem., 2013, 5, 518–524 CrossRef CAS PubMed.
- L. Borchardt, M. Oschatz and S. Kaskel, Chem.–Eur. J., 2016, 22, 7324–7351 CrossRef CAS PubMed.
- A. Rosenman, E. Markevich, G. Salitra, D. Aurbach, A. Garsuch and F. F. Chesneau, Adv. Energy Mater., 2015, 5, 1500212 CrossRef.
- S.-E. Cheon, K.-S. Ko, J.-H. Cho, S.-W. Kim, E.-Y. Chin and H.-T. Kim, J. Electrochem. Soc., 2003, 150, A796–A799 CrossRef CAS.
- X. Ji, K. T. Lee and L. F. Nazar, Nat. Mater., 2009, 8, 500–506 CrossRef CAS PubMed.
- M. K. Rybarczyk, H.-J. Peng, C. Tang, M. Lieder, Q. Zhang and M.-M. Titirici, Green Chem., 2016, 18, 5169–5179 RSC.
- Y.-S. Su and A. Manthiram, Nat. Commun., 2012, 3, 1166 CrossRef PubMed.
- S.-H. Chung and A. Manthiram, Chem. Commun., 2014, 50, 4184–4187 Search PubMed.
- J.-Q. Huang, Q. Zhang and F. Wei, Energy Storage Mater., 2015, 1, 127–145 CrossRef.
- R. Singhal, S.-H. Chung, A. Manthiram and V. Kalra, J. Mater. Chem. A, 2015, 3, 4530–4538 CAS.
- Z. Xiao, Z. Yang, L. Wang, H. Nie, M. Zhong, Q. Lai, X. Xu, L. Zhang and S. Huang, Adv. Mater., 2015, 27, 2891–2898 CrossRef CAS PubMed.
- X. Wang, Z. Wang and L. Chen, J. Power Sources, 2013, 242, 65–69 CrossRef CAS.
- J. Balach, T. Jaumann, M. Klose, S. Oswald, J. Eckert and L. Giebeler, J. Phys. Chem. C, 2015, 119, 4580–4587 CAS.
- G. Ma, Z. Wen, Q. Wang, C. Shen, P. Peng, J. Jin and X. Wu, J. Power Sources, 2015, 273, 511–516 CrossRef CAS.
- S.-H. Chung and A. Manthiram, Adv. Mater., 2014, 26, 1360–1365 CrossRef CAS PubMed.
- S.-H. Chung and A. Manthiram, ChemSusChem, 2014, 7, 1655–1661 CrossRef CAS PubMed.
- T.-N. Ye, W.-J. Feng, B. Zhang, M. Xu, L.-B. Lv, J. Su, X. Wei, K.-X. Wang, X.-H. Li and J.-S. Chen, J. Mater. Chem. A, 2015, 3, 13926–13932 CAS.
- C. Wang, Y. Li, X. He, Y. Ding, Q. Peng, W. Zhao, E. Shi, S. Wu and A. Cao, Nanoscale, 2015, 7, 7550–7558 RSC.
- L. Zhang, Y. Wang, B. Peng, W. Yu, H. Wang, T. Wang, B. Deng, L. Chai, K. Zhang and J. Wang, Green Chem., 2014, 16, 3926–3934 RSC.
- S.-H. Chung and A. Manthiram, Adv. Mater., 2014, 26, 7352–7357 CrossRef CAS PubMed.
- X. Gu, C. Tong, C. Lai, J. Qiu, X. Huang, W. Yang, B. Wen, L. Liu, Y. Hou and S. Zhang, J. Mater. Chem. A, 2015, 3, 16670–16678 CAS.
- G. Zhou, E. Paek, G. S. Hwang and A. Manthiram, Nat. Commun., 2015, 6, 7760–7770 CrossRef CAS PubMed.
- C.-H. Chang, S.-H. Chung and A. Manthiram, Small, 2016, 12, 174–179 CrossRef CAS PubMed.
- C.-H. Chang, S.-H. Chung and A. Manthiram, Small, 2016, 12, 174–179 CrossRef CAS PubMed.
- C. Barchasz, F. Molton, C. Duboc, J.-C. Leprêtre, S. Patoux and F. Alloin, Anal. Chem., 2012, 84, 3973–3980 CrossRef CAS PubMed.
- X.-B. Cheng, H.-J. Peng, J.-Q. Huang, L. Zhu, S.-H. Yang, Y. Liu, H.-W. Zhang, W. Zhu, F. Wei and Q. Zhang, J. Power Sources, 2014, 261, 264–270 CrossRef CAS.
- C.-H. Chang, S.-H. Chung and A. Manthiram, J. Mater. Chem. A, 2015, 3, 18829–18834 CAS.
- X. Yang, N. Yan, W. Zhou, H. Zhang, X. Li and H. Zhang, J. Mater. Chem. A, 2015, 3, 15314–15323 CAS.
- S.-H. Chung, R. Singhal, V. Kalra and A. Manthiram, J. Phys. Chem. Lett., 2015, 6, 2163–2169 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental sections, SEM/EDX, BET, FTIR, discharge/charge curves. See DOI: 10.1039/c7se00014f |
| This journal is © The Royal Society of Chemistry 2017 |