Iron oxide deposited on metallic nickel for water oxidation†
Mohammad Mahdi
Najafpour
 *ab and
Navid Jameei
Moghaddam
a
*ab and
Navid Jameei
Moghaddam
a
aDepartment of Chemistry, Institute for Advanced Studies in Basic Sciences (IASBS), Zanjan, 45137-66731, Iran. E-mail: mmnajafpour@iasbs.ac.ir; Tel: +98 24 3315 3201
bCenter of Climate Change and Global Warming, Institute for Advanced Studies in Basic Sciences (IASBS), Zanjan, 45137-66731, Iran
First published on 10th March 2017
Abstract
Solar energy is too intermittent to be directly used on a large scale, and thus, a large energy storage system should be developed. One interesting approach is the use of sunlight to perform water splitting for hydrogen production. For such systems, developing an efficient and stable water-oxidizing catalyst is an essential task. Using K2FeO4, we report a simple method to deposit iron oxide on metallic nickel for water oxidation. The Fe/Ni-based electrode, at pH = 13, shows a current density of 1.9 mA cm−2 at an overpotential of 250.0 mV, making it a promising anode for use in water-splitting systems. At a higher overpotential (700.0 mV), a current density of 23.5 mA cm−2 was observed.
1. Introduction
Finding a suitable method to store energy from abundant and sustainable sources is important.1 Hydrogen is a reliable, independent, renewable and efficient chemical for energy storage, which can be used directly as a fuel or used to generate electricity in fuel cells.2Among different methods, water electrolysis is a promising approach for hydrogen production.2–4 In this reaction, low-cost electrons for water reduction are provided by the water-oxidation reaction. Thus, an efficient water-oxidizing catalyst is very important for water electrolyzers.5–7 Au, Pt and Ir have been long known to be efficient catalysts for water electrolysis,8 but they are not easy to be used in large-scale production due to their high costs.
Mn, Fe, Co and Cu compounds are significantly considered as water-oxidizing catalysts because of the low cost, high availability and low toxicity of these metals.9–14 Iron is the most abundant transition metal in the earth's crust, and it is less toxic than many metal ions. However, the preparation of iron-based films by electro-deposition is not straightforward and Fe(III) ions can readily precipitate under neutral conditions.15,16
Lyons and Doyle indicated that the water-oxidation reaction depended strongly on conditions, under which the iron oxyhydroxide film was generated.15,16 For an iron film, Tafel slopes of ca. 40–60 mV per decade and ca. 120 mV per decade were calculated at low and high overpotentials, respectively.16 An ultrathin Fe-based film was obtained using the electrodeposition of an Fe(II) solution through a cyclic voltammetry method.17 In this case, the very low Fe loading on electrodes was critical for high atom efficiency and transparency of the obtained film.17 In 34 hours, the film showed controlled potential electrolysis at 1.45 V (vs. the NHE), a turnover number of 5.2 × 104 and a turnover frequency of 1528 h−1.
Recently, we reported an Fe(VI) to Fe(III) reduction method to synthesize a unique Fe oxide on the surface of a fluorine doped tin oxide electrode.18 This electrode was used as a stable water-oxidizing anode at pH = 13 to yield a current density of 1 mA cm−2 at an overpotential of 550 mV.18
Herein, using K2FeO4 and Ni foam, we report a simple method to deposit iron oxide on metallic nickel. The electrode at pH = 13 showed a current density of 1.9 mA cm−2 at an overpotential of 250 mV, making it a promising anode for use in water-splitting systems. At a higher overpotential (700 mV), a current density of 23.5 mA cm−2 was observed.
2. Experimental
2.1. Materials
All reagents and solvents were purchased from commercial sources and used without further purification. Ni foam and K2FeO4 were purchased from Sigma-Aldrich Company.2.2. Synthesis
The Ni foam (1.5 cm2, Sigma-Aldrich ≥ 99.9%) was immersed in acetone for 5 min, ultra-sonicated in deionized water, and then thoroughly washed with deionized water. The clean foam was then etched in 0.25 M HCl solution. To synthesize the catalyst, a solution of K2FeO4 (5.0 mg (A5), 10.0 mg (A10), 100.0 mg (A100), 200.0 mg (A200), 300.0 mg (A300), 400.0 mg (A400) or 500.0 mg (A500)) in water (70.0 mL) was added to the Ni foam (1.5 × 1 cm2) in a hydrothermal container at 120 °C for 12 h. Then, the foam was carefully washed with water.2.3. Characterization
SEM was carried out with a Philips CM120. For HRTEM and TEM, the samples were studied using a LEO 1430VP. X-ray powder diffraction patterns were recorded with a Bruker D8 ADVANCE (Germany) diffractometer (CuKα radiation). X-ray photoelectron spectroscopy (XPS) measurements were done on an X-ray BesTec XPS system (Germany) with an Al Kα X-ray source (hν = 1486.6 eV). EDX analysis/mapping was carried out with the scanning electron microscope CamScan 4DV (CamScan UK). The Fourier transform infrared spectroscopy (FTIR) spectra of the KBr pellets of the investigated compounds were recorded on a Bruker Vector 22 device in the range between 400 and 4000 cm−1. Electrochemical experiments were performed using an EmStat3+ from PalmSens (the Netherlands). Mössbauer spectroscopy was performed with an SM 1201 model (Russia). The distance between two opposite sides of the foam electrode was measured using a digital caliper MarCal 16 ER model (Mahr, Germany). The temperature was measured using a Laserliner 082 (Germany). Visible spectra were recorded on a mini spectrophotometer (Pooyesh Tadbir Karaneh (Phystec), Iran).3. Results and discussion
The idea of the electrode synthesis was based on the reaction of FeO42− ions as a strong oxidant (E0 = FeO42−/Fe(III) = 2.2 V) with nickel foam due to the fact that both Fe ions19,20 and oxidants21 improve the water-oxidizing activity of metallic nickel. However, the decomposition of FeO42− occurred on the surface of the electrode under hydrothermal conditions:| 4FeO42− + 8H+ → 2Fe2O3 + 3O2 + 4H2O |
Under these conditions (pH ∼7), FeO42− was reduced to form Fe oxide on the surface of Ni. Although such a reaction can occur in different forms of metallic nickel, we used nickel foam to obtain a high surface electrode. Such foam has a cellular shape with a very high porosity and a large volume fraction of pores, which can be sealed, or form an interconnected network.22
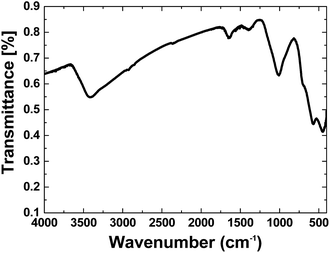
FTIR spectroscopy is a reliable method to identify Fe oxides. The FTIR spectrum of separated iron oxide on nickel foam is shown in Fig. 1. The spectrum showed the stretching vibration of the Fe–O octahedra at ∼450 and 570 cm−1, which is attributed to Goethite.23a The FTIR spectrum of the composites showed a broad peak at ∼3200–3500 cm−1 related to antisymmetric and symmetric O–H stretching modes.23 The peak at 1630 cm−1 was also attributed to H2O.23b
The scanning electron microscopy (SEM) images of the electrode showed a cellular structure attributed to the nickel foam. After the reaction with K2FeO4, in addition to the cellular structure, which is stable under our experimental conditions, the electrode also showed small particles (ca. 20–50 nm) with no defined morphology, which were located on the surface of the nickel foam (Fig. 2 and S1–S12, ESI†).
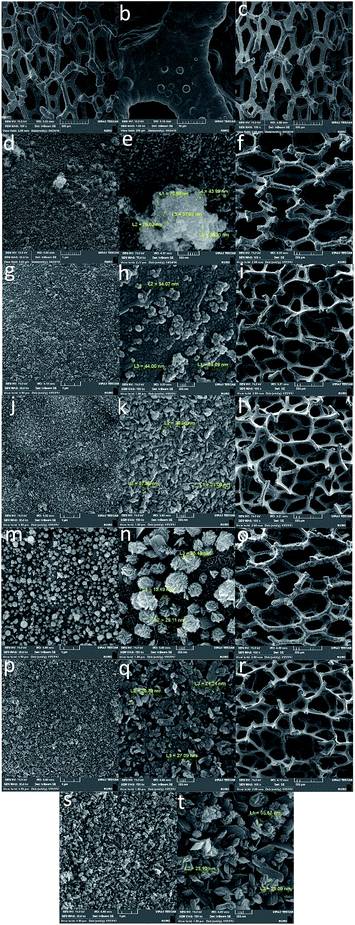 | ||
| Fig. 2 SEM images of the Ni foam (a and b) and Ni foam treated with K2FeO4 (A5: c–e; A10: f–h; A100: i–k; A200: l–n; A300: o–q; A500: r–t). | ||
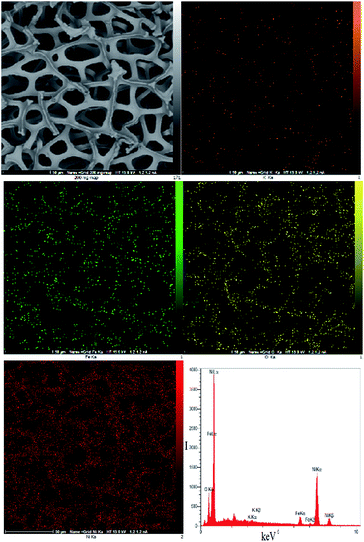
EDX–SEM showed Fe ions on the surface of the Ni foam. Small amounts of K were also observed on the surface of the Ni foam (K/Fe: 3.44%; Fig. 3).
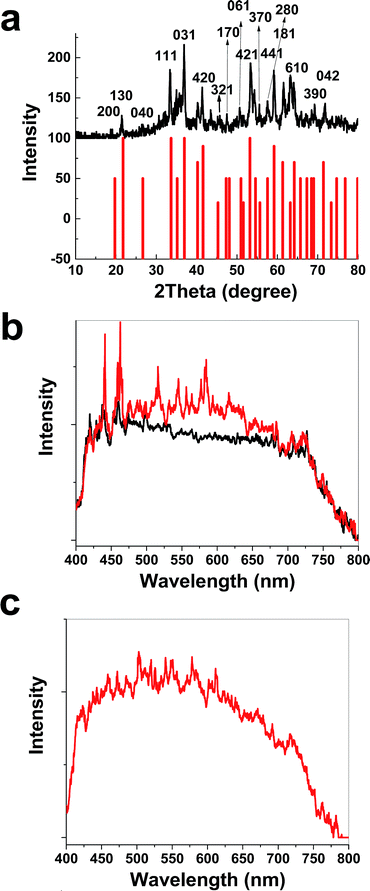
The XRD patterns before and after treatment with K2FeO4 were very similar and no additional phase other than the metallic nickel was detected (Fig. S13, ESI†). The XRD patterns of the Fe oxide powder scraped from the nickel foam surface showed a hydrated α-FeO(OH) phase (Goethite, reference code: 00-002-0281) (Fig. 4a). Visible spectroscopy showed changes on the surface of K2FeO4 treated Ni foam (Fig. 4b and c).
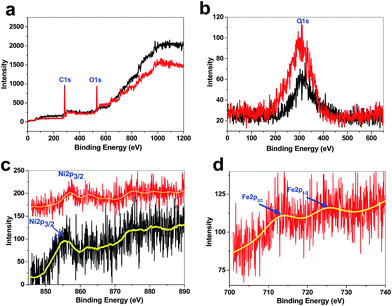
XPS showed a large contribution of oxygen on the surface of the treated Ni foam, indicating that metal (Ni and Fe) oxides were formed on the surface of the electrode (Fig. 5). Ni2p3/2 of the treated Ni foam showed a peak at 857 eV, which is attributed to Ni(OH)2 (Fig. 5). Ni2p3/2 of the nickel foam was observed at 855.7 eV, and thus, the surface of the treated Ni foam has higher amounts of nickel hydroxide.24 However, only low amounts of Fe were found on the surface of the Ni foam. The small peaks of Fe were observed at ∼712 and 725 eV, which are related to Fe(III).25 Such low amounts of Fe were observed in EDX–SEM.
Mössbauer spectroscopy was also used to study the iron oxide (Fig. 6). The Mössbauer effect was discovered by Rudolf L. Mössbauer in 1958 and is significantly useful to find the details of the structure of iron compounds.26,27 In this process a nucleus emits or absorbs gamma rays without loss of energy to nuclear recoil. Two important factors in the spectroscopy are isomer shift and quadrupole splitting. The isomer shift describes a shift in the resonance energy of a nucleus of iron due to the transition of electrons within its s orbital. On the other hand, the quadrupole splitting describes the interaction between the nuclear energy levels and surrounding electric field gradient.26,27 These parameters can be related to α-FeO(OH).27 A doublet with an isomer shift = 0.39 mm s−1 (relative to metallic iron) and quadrupole splitting ΔEQ = 0.54 mm s−1 at room temperature was present in the spectrum, which is attributed to high spin Fe(III) ions (Fig. 6).
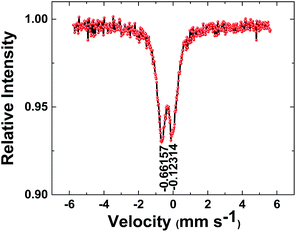 | ||
| Fig. 6 57Fe Mössbauer spectrum of the iron oxide. The iron oxide was mechanically separated from the electrode. The solid black lines are the fitting curves. | ||
3.1. Water oxidation
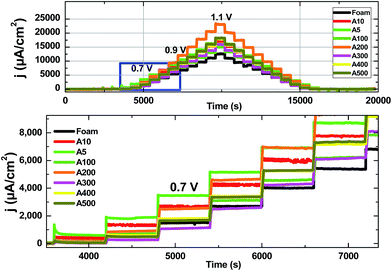
To study the heterogeneous catalyst for water oxidation, the current density vs. overpotential profiles were considered, and then compared with the overpotential required to reach the current density of 1 or 10 mA cm−2 for the other materials.31 The electrodes prepared as anodes were used in a three-electrode set-up with Hg|HgO, a Pt rod, and the Ni foam as the reference, counter and working electrodes, respectively. 20 mL of an argon saturated KOH (0.1 M) solution at room temperature was also used as an electrolyte solution.To obtain the Tafel plots of the electrode for water oxidation, chronoamperometric experiments (Fig. 7) were performed.18,21,28 Thus, the applied potential was increased stepwise from +0.3 to +1.1 V in 50 mV steps and held for 600 s at each step (Fig. 7). At higher concentrations of Fe, the activity of the electrode was decreased, most probably because of the covering of the Ni-based active sites or the agglomeration of Fe oxide (Fig. 2).
Among the different electrodes, A200 was an optimal electrode for water oxidation at high overpotentials and showed a current density of 23.5 mA cm−2 at the overpotential of 700.0 mV. On the other hand, A5 was the best electrode at low overpotentials and a current density of 1.9 mA cm−2 at an overpotential of 250 mV was obtained by the electrode (Fig. 7).
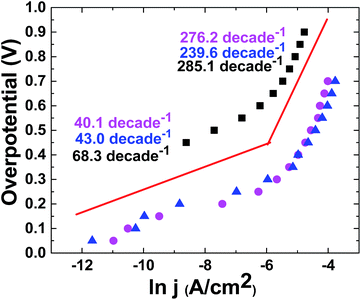
In the next step, ln(A cm−2)/overpotential plots were recorded for these electrodes in KOH (0.1 M), which showed the Tafel behavior of the electrodes (Fig. 8).
The current/voltage relation and Tafel plot of the catalyst at pH = 13 in KOH solution (0.1 M) showed the linearity of the log(j) vs. potential with two slopes related to both relatively low (40 mV per decade) and high overpotentials (240–270 mV per decade). For molecular catalysts, a slope of about 59 mV per decade is predicted. Such a low Tafel slope (40 mV per decade) at a low overpotential for the catalyst indicates its more favorable kinetics relative to many catalysts.15,16 On the other hand, since the Tafel slope is often influenced by electron and mass transports, we conclude that electron and mass transports are easily performed for the catalyst.
An electrode at the overpotential of 400 mV was studied for 5.0 hours to test its stability (Fig. 9). The electrode was relatively stable under the experimental conditions.
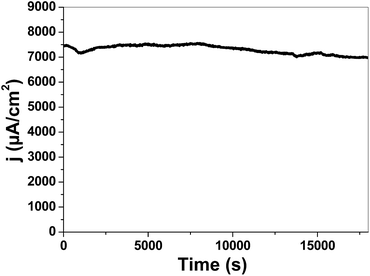 | ||
| Fig. 9 Chronoamperometric electrolysis of Ni 200 (0.1 M KOH, pH 13) at room temperature and 400 mV overpotential. | ||
Compared with other metal oxides, the iron oxide on nickel foam is an efficient (Table 1), simple and low-cost electrode for water oxidation.
| Compound | η [mV] | η [mV] | pH |
|---|---|---|---|
| a Onset overpotential. b @1 mA cm−2. c Layered double hydroxides. | |||
| Fe oxide deposited on Ni foam | <200 | <250 | 13 |
| α-Fe2O3 fluorine-doped tin oxide electrode | 450 | <550 | 13 |
| NiFeOx | — | 297 | 14 |
| NiOx | >400 | >1000 | 14 |
| NiOx | — | 300 | 14 |
| CoOx | — | 381 | 14 |
| NiCoOx | — | 312 | 14 |
| FeOx | 345 | 445 | 14 |
| FeOx | — | 405 | 14 |
| Fe2O3 | <350 | 430 | 14 |
| MnOx | 320 | 514 | 14 |
| Fe3Ni2Ox | 270 | — | 13 |
| FeNiOx | 211 | — | 13 |
| Fe2Ni3Ox | 190 | 250 | 13 |
| NiOx | 191 | 280 | 13 |
| NiOx | 295 | — | 13 |
| CoFeOxc | 397 | — | 13 |
| CoOx | <200 | <250 | 13 |
| FeOx | 320 | 410 | 13 |
| CoOx | 210 | 270 | 13 |
| CoOx | 295 | — | 13 |
| FeCoOx | 181 | — | 13 |
| FeCoNiOx | 191 | — | 13 |
| Ni2FeAlOx | 270 | — | 13 |
| NiFeMo3Ox | 250 | — | 13 |
| Ni2FeCr2Ox | 240 | — | 13 |
| NiFeGa3Ox | 240 | — | 13 |
| CoSe2 | 373 | 380 | 13 |
| NG-CoSe2 | 294 | 320 | 13 |
| MnOx | <300 | >1000 | >11.5 |
| FeOOH | 300 | 420 | 11 |
| NiBi | 300 | 425 | 9.2 |
| MnOx | <300 | >1000 | 8.5–5.5 |
| CoOx | <200 | <300 | 7 |
| MnOx | 390 | 590 | 7 |
| MnOx | 441 | 600 | 7 |
| CoFePBA | 291 | >600 | 7 |
| MnOx | 150 | >1000 | 7 |
| CoPi | 281 | 410 | 7 |
| MnOx | >700 | >1000 | 7 |
| LixMnP2O7 | 500 | — | 7 |
| Co(PO3)2 | 313 | 320 | 6.4 |
| MnOx | <300 | >1000 | 3.5 |
| Co2+ (1 M) | <580 | 600 | 1 |
As iron and nickel ions are among the cost effective and environmentally friendly ions, such an electrode is promising for use in water-splitting systems. The effect of iron in the composite is not known, but for various water-oxidizing oxides of monometallic first-row transition metals, the metal reduction is the rate-limiting step for water oxidation. The presence of Fe ions in nickel oxide stabilizes the low oxidation state of the Ni ions, and thus facilitates water oxidation.29 In comparison with the electrode previously reported by our group,18 this strategy significantly decreases the overpotential (250–300 mV, Table 1) for water oxidation.
In our procedure, we used FeO42− which is both an oxidant and a source of iron. On the other hand, some reports showed the positive effect of oxidant on the water-oxidizing activity of Ni foams toward water oxidation.20 Thus, both the presence of iron ions and the oxidation of the nickel surface can contribute to improving the water-oxidizing activity of the Ni foam. In addition to iron oxide, three-dimensional (3D) electrodes such as metal foams, compared to two-dimensional (2D) electrodes such as fluorine-doped tin oxide (FTO), indium tin oxide (ITO), and aluminium-doped zinc-oxide (AZO) seem to be more promising for water-splitting due to their larger surface area.30–32
4. Conclusions
In conclusion, we reported the deposition of iron oxide on a nickel foam by a promising method using K2FeO4. Such an electrode was characterized by some methods. The experiments showed that a nanosized α-FeO(OH) phase was formed under these conditions. It was relatively stable and showed a low Tafel slope (∼40 mV per decade) at a low overpotential. The electrode at pH = 13 showed a current density of 1.9 mA cm−2 at an overpotential of 250 mV, making it a promising anode for use in water-splitting systems. At a 700 mV overpotential, a current density of 23.5 mA cm−2 was observed.Acknowledgements
The authors are grateful to the Institute for Advanced Studies in Basic Sciences, the National Elite Foundation and the Iran Science Elites Federation for financial support. The authors also thank Professor Philipp Kurz for his help, support and consideration about electrochemical experiments.Notes and references
- J. A. Turner, Science, 2004, 305, 5686 CrossRef PubMed.
- J. A. Turner, Science, 2014, 344, 6183 Search PubMed.
- N. S. Lewis, Science, 2016, 351, 1920 CrossRef PubMed.
- Q. Wang, T. Hisatomi, Q. Jia, H. Tokudome, M. Zhong, C. Wang, Z. Pan, T. Takata, M. Nakabayashi, N. Shibata and Y. Li, Nat. Mater., 2016, 15, 611 CrossRef CAS PubMed.
- R. D. Smith, M. S. Prévot, R. D. Fagan, Z. Zhang, P. A. Sedach, M. K. Siu, S. Trudel and C. P. Berlinguette, Science, 2013, 340, 6128 Search PubMed.
- M. J. Kenney, M. Gong, Y. Li, J. Z. Wu, J. Feng, M. Lanza and H. Dai, Science, 2013, 342, 836 CrossRef CAS PubMed.
- J. Liu, Y. Liu, N. Liu, Y. Han, X. Zhang, H. Huang, Y. Lifshitz, S.-T. Lee, J. Zhong and Z. Kang, Science, 2015, 347, 970 CrossRef CAS PubMed.
- L. C. Seitz, C. F. Dickens, K. Nishio, Y. Hikita, J. Montoya, A. Doyle, C. Kirk, A. Vojvodic, H. Y. Hwang, J. K. Norskov and T. F. Jaramillo, Science, 2016, 353, 1011 CrossRef CAS PubMed.
- J. D. Blakemore, R. H. Crabtree and G. W. Brudvig, Chem. Rev., 2015, 115, 12974 CrossRef CAS PubMed.
- M. D. Kärkäs, O. Verho, E. V. Johnston and B. Åkermark, Chem. Rev., 2014, 114, 11863 CrossRef PubMed.
- M. M. Najafpour, G. Renger, M. Hołyńska, A. Nemati Moghaddam, E. M. Aro, R. Carpentier, H. Nishihara, J. J. Eaton-Rye, J. R. Shen and S. I. Allakhverdiev, Chem. Rev., 2016, 116, 2886 CrossRef CAS PubMed.
- K. J. Young, B. J. Brennan, R. Tagore and G. W. Brudvig, Acc. Chem. Res., 2015, 48, 567 CrossRef CAS PubMed.
- W. Ruttinger and G. C. Dismukes, Chem. Rev., 1997, 97, 1 CrossRef PubMed.
- M. Yagi and M. Kaneko, Chem. Rev., 2001, 101, 21 CrossRef CAS PubMed.
- M. E. G. Lyons and R. L. Doyle, Int. J. Electrochem. Sci., 2012, 7, 9488 CAS.
- R. L. Doylez and M. E. G. Lyons, J. Electrochem. Soc., 2013, 160, H142 CrossRef.
- M. Chen, Y. Wu, Y. Han, X. Lin, J. Sun, W. Zhang and R. Cao, ACS Appl. Mater. Interfaces, 2015, 7, 21852 CAS.
- M. M. Najafpour and S. M. Hosseini, Int. J. Hydrogen Energy, 2016, 41, 22635 CrossRef CAS.
- X. Lu X and C. Zhao, Nat. Commun., 2015, 6, 6616 CrossRef PubMed.
- M. Balasubramanian, C. A. Melendres and S. Mini, J. Phys. Chem. B, 2000, 104, 4300 CrossRef CAS.
- Z. Wei, Q. Jing, L. Kaiqiang and C. Rui, Adv. Energy Mater., 2016, 6, 150248 Search PubMed; M. M. Najafpour and N. Jameei Moghaddam, New J. Chem., 2017, 41, 1909 RSC.
- M. F. Ashby, T. Evans, N. A. Fleck, J. W. Hutchinson and H. N. G. Wadley, Metal foams: a design guide, Elsevier, 2000 Search PubMed.
- (a) R. M. Cornell and U. Schwertmann, The iron oxides: structure, properties, reactions, occurrences and uses, John Wiley & Sons, 2003 Search PubMed; (b) K. Nakamoto, Infrared and Raman spectra of inorganic and coordination compounds, Wiley-Interscience, 2009 Search PubMed.
- X. Lu and C. Zhao, Nat. Commun., 2015, 6616 CrossRef CAS PubMed.
- T. Yamashita and P. Hayes, Appl. Surf. Sci., 2008, 254(8), 2441 CrossRef CAS.
- G. Zacek, F. V. Feilitzsch, R. L. Mössbauer, L. Oberauer, V. Zacek, F. Boehm, F. Boehm, P. H. Fisher, J. L. Gimlett, A. A. Hahn, H. E. Henrikson, H. Kwon, J. L. Vuilleumier and K. Gabathuler, Phys. Rev. D: Part. Fields, 1986, 34, 2621 CrossRef CAS; J. Madarasz, R. Zboril, Z. Homonnay, V. K. Sharma and G. Pokol, J. Solid State Chem., 2006, 179, 1426 CrossRef.
- Mössbauer Spectroscopy: Applications in Chemistry, Biology, and Nanotechnology, ed. V. K. Sharma, G. Klingelhofer and T. Nishida, John Wiley & Sons, 2013 Search PubMed.
- S. Y. Lee, D. González-Flores, J. Ohms, T. Trost, H. Dau, I. Zaharieva and P. Kurz, ChemSusChem, 2014, 7(12), 3442 CrossRef CAS PubMed.
- M. Görlin, P. Chernev, J. Ferreira de Araújo, T. Reier, S. Dresp, B. Paul, R. Krähnert, H. Dau and P. Strasser, J. Am. Chem. Soc., 2016, 138(17), 5603 CrossRef PubMed.
- D. Gangasingh and J. B. Talbot, Chem. Commun., 2013, 49, 9323 RSC.
- F. J. Perez-Alonso, C. Adan, S. Rojas, M. A. Pena and J. L. G. Fierro, Int. J. Hydrogen Energy, 2014, 39, 5204 CrossRef CAS.
- C. Tang, N. Cheng, Z. Pu, W. Xing and X. Sun, Angew. Chem., Int. Ed., 2015, 127, 9483 CrossRef.
- J. R. Galan-Mascaros, ChemElectroChem, 2015, 2, 37 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7se00064b |
| This journal is © The Royal Society of Chemistry 2017 |