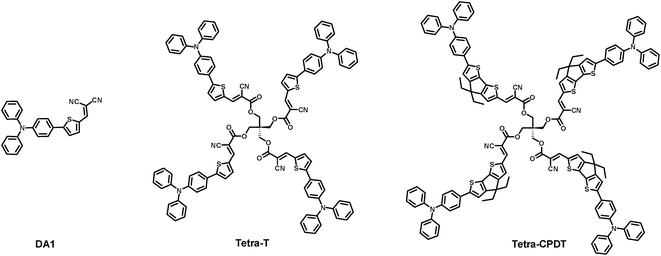
Pentaerythritol based push–pull tetramers for organic photovoltaics
Antoine
Labrunie
,
Pierre
Josse
,
Sylvie
Dabos-Seignon
,
Philippe
Blanchard
* and
Clément
Cabanetos
 *
*
CNRS UMR 6200, MOLTECH-Anjou, University of Angers, 2 Bd Lavoisier, 49045 Angers, France. E-mail: Philippe.blanchard@univ-angers.fr; clement.cabanetos@univ-angers.fr
First published on 13th September 2017
Abstract
The synthesis and characterization of two tetramers based on the functionalization of a central pentaerythritol σ-linker with push–pull chromophores is reported herein. Prepared in only few steps, these original molecules exhibit interesting optical and electrochemical properties. Moreover, once evaluated as donor materials, promising power conversion efficiencies of 4.5% were reached when blended with the [6,6]-phenyl-C71-butyric acid methyl ester (PC71BM) in bulk heterojunction solar cells.
Introduction
In the past few years, organic photovoltaics (OPVs) have experienced renewed growth thanks to impressive progress in the design and synthesis of high performance materials1–7 and lately to the development of non-fullerene acceptors (NFAs).8–17 As a result power conversion efficiencies (PCEs) exceeding 13% in fullerene-free single junction solar cells have recently been reported.18 However, even if this new class of materials is currently sparking an extraordinary level of energy and enthusiasm within the community, investigation of new complementary donor structures should not be overlooked. Moreover, besides record performances, one major focus must remain the cost-reduction through the simplicity and a synthetic accessibility of the active materials. Hence, molecular systems, prepared in few steps without batch-to-batch variations due to their well-defined chemical structures, appear to be an appealing option.19 In this context, our group has synthesized, over the last decade, various classes of simple and synthetically accessible arylamine based push–pull materials.20–28 Used as a reference, the DA1 compound (Fig. 1), afforded in only two steps at gram scale, shows promising PCEs either in planar and bulk heterojunction solar cells.23,29,30To further investigate the structure–properties relationships of such class of materials, we report herein the synthesis, characterization and preliminary evaluation of two original tetramers, built by grafting four push–pull molecules onto a central cheap and commercially available pentaerythritol core (Fig. 1). The main difference arises from the nature of the π-conjugated spacer separating the triphenylamine (TPA) block from the cyanoacrylic ester to assess their impact on the optical, electrochemical and therefore, photovoltaic properties. Hence, the simple thiophene linker (Tetra-T) is compared herein to the well-known π-conjugated a more electron-rich cyclopentadithiophene (Tetra-CPDT). Finally, it is noteworthy that amongst non-planar and/or 3D architectures already reported in the literature,5,31–39 only few examples deal with multimers based on non-conjugated (σ) cores.40
Results and discussion
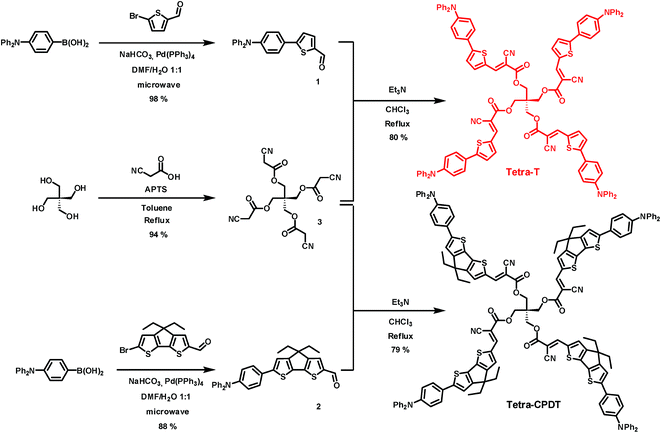
The synthetic route to the target tetramers is depicted in Scheme 1.Thus, the common and commercially available triphenylamine-4-boronic acid was first engaged in a Suzuki–Miyaura cross-coupling reaction in the presence of the 5-bromo-2-thiophenecarboxaldehyde or the 6-bromo-4,4-diethyl-4H-cyclopenta[1,2-b:5,4-b′]bisthiophene-2-carbaldehyde affording, in good yields, the corresponding intermediates, i.e.1 or 2 respectively. In parallel, the tetra functionalized connector 3 was synthesized by reacting pentaerythritol with an excess of cyanoacetic acid in acid-catalyzed conditions. Finally, a Knoevenagel polycondensation between the latter and the aldehyde derivatives 1 or 2 led to the target molecules namely Tetra-T and Tetra-CPDT in good yields.
To first assess the impact of the central linker on the thermal stability, thermogravimetric analyses were performed (Fig. 2).
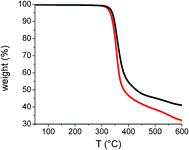 | ||
| Fig. 2 Thermogravimetric analysis of Tetra-T (red) and Tetra-CPDT (black) recorded at 5°C min−1 under N2. | ||
It turns out that both tetramers exhibit decomposition temperatures (Td) beyond 300 °C since the 5% weight loss were reached at 330 °C and 340 °C for Tetra-T and Tetra-CPDT respectively, corresponding to an increase in stability of more than 75 °C compared to the reference compound DA1 (Td = 255 °C).23
Once solubilized in chloroform, spectrums show an intense absorption band in the 400–600 nm range attributed to an internal charge transfer (ICT) from the electron rich TPA to the cyanoacrylic ester (Fig. 3).
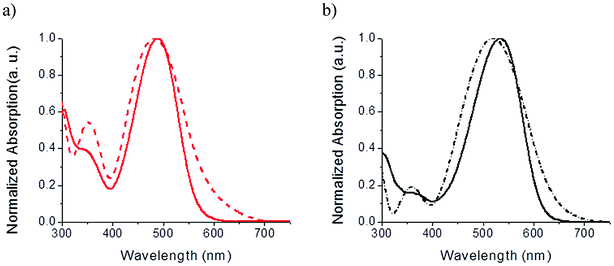 | ||
| Fig. 3 Normalized UV-vis absorption spectra of the Tetra-T (a) and Tetra-CPDT (b) in solution (solid) and as thin film on glass (dash). | ||
Compared to its thiophene counterpart, the Tetra-CPDT exhibits a slightly red-shifted but way more intense ICT band, corresponding to almost a two fold increase, in agreement with the stronger donor effect and extended π-conjugation of the cyclopentadithiophene connector.41–43 For comparative purposes, optical data are summarized in Table 1.
| Compound | λ max (nm) in chloroform | ε (M−1 cm−1) | λ max (nm) thin film | λ onset (nm) thin film | E g opt (eV) |
|---|---|---|---|---|---|
| Tetra-T | 490 | 132![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
483 | 624 | 1.98 |
| Tetra-CPDT | 533 | 222![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
520 | 661 | 1.87 |
When spun cast on glass-sheets, both spectra broaden and wide optical energy gaps (Egopt) of 1.98 and 1.87 eV were estimated for Tetra-T and Tetra-CPDT respectively from their absorption onsets. Although thin films of simple push–pull derivatives (e.g.DA1) typically show red shifted maximum absorption wavelengths (λmax), it is noteworthy that hypsochromic shifts were recorded for the tetramers, underscoring that the grafting on a central pentaerythritol σ-core clearly affect the overall packing properties.40
Cyclic voltammetry (CV) was then performed in dichloromethane using Bu4NPF6 as supporting electrolyte (Fig. 4).
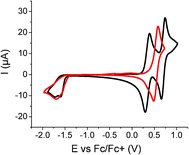 | ||
| Fig. 4 Cyclic voltammograms of Tetra-T (red) and Tetra-CDPT (black) at a concentration of 0.5 mM in 0.10 M Bu4NPF6/CH2Cl2, 100 mV s−1, Pt working electrode. | ||
The impact of the π-conjugated connector constituting the push–pull chromophore (i.e., T or CPDT) can be unambiguously assessed in the positive potentials region. Indeed, while the Tetra-T is characterized by a single reversible process at Epa = 0.59 V, its CPDT counterpart exhibits two reversible oxidation waves peaking at 0.39 V and 0.73 V respectively, assigned to the formation of a stable radical cation and dication per independent push–pull unit (Table 2).42 Regarding the negative potential region, the slight differences spotted in patterns and potential also suggest that the LUMO level might be partly located on the π-linker but essentially on the cyanoacrylic ester moieties. Thus, from the onsets of oxidation and reduction processes and in agreement with the optical data, a higher electrochemical band gap of ca. 0.1 eV was estimated for the Tetra-T derivative mainly due to a deeper HOMO level, which should be beneficial for the open-circuits voltages (Voc) of the solar devices.44
| Tetramer | E pa 1 (V) | E pc 1 (V) | E onset/Ox (V) | E onset/red (V) | HOMO (eV) | LUMO (eV) | ||
|---|---|---|---|---|---|---|---|---|
| Tetra-T | 0.59 | — | −1.69 | — | 0.43 | −1.41 | −5.23 | −3.39 |
| Tetra-CPDT | 0.39 | 0.73 | −1.60 | −1.80 | 0.26 | −1.49 | −5.06 | −3.31 |
Hence, to investigate the potential of the two tetramers as molecular donors (D), air processed bulk heterojunction (BHJ) solar cells were fabricated using the well-known [6,6]-phenyl-C71-butyric acid methyl ester (PC71BM) as the complementary accepting materials (A). Best devices were achieved from blends spun-cast at 2000 rpm from chloroform solutions in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 weight-to-weight D
3 weight-to-weight D![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A ratio. The corresponding density-voltage (J–V) curves and photovoltaic parameters are depicted and gathered in Fig. 5 and Table 3 respectively.
A ratio. The corresponding density-voltage (J–V) curves and photovoltaic parameters are depicted and gathered in Fig. 5 and Table 3 respectively.
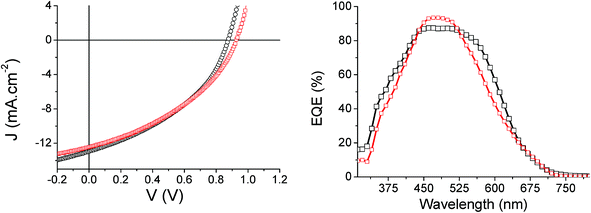 | ||
| Fig. 5 J–V (left) and EQE curves (right) of Tetra-T (red) and Tetra-CPDT (black) based best working OSCs. | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 w/w) and measured under an AM. 1.5 simulated solar illumination (100 mW cm−2). Average value recorded over 14 devices; maximum values in brackets
3 w/w) and measured under an AM. 1.5 simulated solar illumination (100 mW cm−2). Average value recorded over 14 devices; maximum values in brackets
| Tetramer | V oc (V) | J sc (mA cm−2) | FF (%) | PCE (%) |
|---|---|---|---|---|
| Tetra-T | 0.91 ± 0.02, (0.93) | 12.18 ± 0.31, (12.43) | 38.8 ± 1.9, (39.1) | 4.29 ± 0.20, (4.52) |
| Tetra-CPDT | 0.88 ± 0.03, (0.88) | 12.51 ± 0.30, (12.93) | 39.3 ± 1.8, (39.7) | 4.37 ± 0.35, (4.51) |
Interestingly, comparable and promising power conversion efficiencies of ca. 4.5% were reached for both materials. With similar fill-factors, the slightly higher short-circuit current (Jsc) recorded for the CPDT based device indeed compensates its lower Voc, which is in accordance with the shallower HOMO level of the Tetra-CPDT molecule.
To further rationalize the high values of Jsc measured for such wide band gap push–pull based materials, external quantum efficiencies (EQE) spectra were recorded on champion devices. Even though characterized by a narrow absorption ranges, especially for the thiophene derivative, it turns out that impressive photon-to-electron conversions, up to 87% at ∼462 nm and 93% at ∼475 nm were reached for the Tetra-CPDT and the Tetra-T based solar cells respectively.
Resulting from the synergistic and complementary contribution of both the donor (tetramers) and the fullerene acceptor, the nanoscale topography of the optimized blends has been finally investigated using atomic force microscopy (Fig. 6).
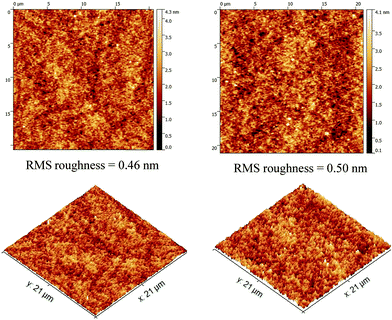 | ||
| Fig. 6 AFM surface topography images of the Tetra-T (left) and Tetra-CPDT (right) based optimized active layers (RMS = root mean square). | ||
The structural similarities between the two tetramers lead to smooth and comparable morphologies characterized by small and homogeneous nano-domains which appear to be, herein, beneficial for effective charge transports and collection to the electrodes.
Conclusion
To sum up, we report herein the simple and accessible synthesis of two tetramers via the Knoevenagel condensation of four triphenylamine based push–pull precursors on a functionalized pentaerythritol central core. This efficient strategy enabled us to afford original materials with interesting optical, electrochemical, self-assembling and therefore photovoltaic properties. Indeed, it is noteworthy that promising power conversion efficiencies of 4.5% were reached although both tetramers exhibit narrow and limited absorption ranges. Hence, this proof of concept opens doors for the design of new class of materials, namely the multimers, which are a powerful chemical platform for tuning and combining the properties of both the central core and/or the peripheral π-conjugated systems. Consequently, work is currently underway to improve specific interaction in the solid state as well as moving toward panchromatic materials.Experimental section
Materials
All reagents and chemicals from commercial sources were used without further purification. Reactions were carried out under nitrogen atmosphere unless otherwise stated. Solvents were dried and purified using standard techniques.Measurements and characterization
Flash chromatography was performed with analytical-grade solvents using Aldrich silica gel (technical grade, pore size 60 Å, 230–400 mesh particle size). Flexible plates ALUGRAM® Xtra SIL G UV254 from MACHEREY-NAGEL were used for TLC. Compounds were detected by UV irradiation (Bioblock Scientific) or staining with I2, unless stated otherwise. NMR spectra were recorded with a Bruker AVANCE III 300 (1H, 300 MHz and 13C, 75 MHz). Chemical shifts are given in ppm relative to TMS and coupling constants (J) in Hz. IR spectra were recorded on a Bruker spectrometer Vertex 70 and UV-vis spectra with a Shimadzu UV-1800. Matrix Assisted Laser Desorption/Ionization was performed on MALDI-TOF MS BIFLEX III Bruker Daltonics spectrometer using dithranol as matrix. Cyclic voltammetry was performed using a Biologic SP-150 potentiostat with positive feedback compensation in 0.10 M Bu4NPF6/CH2Cl2 (HPLC grade) under an inert atmosphere (Ar) using a glovebox. Experiments were carried out in a one-compartment cell equipped with a platinum working electrode (2 mm of diameter) and a platinum wire counter electrode. A silver wire immersed in 0.10 M Bu4NPF6/CH2Cl2 was used as pseudo-reference electrode and checked against the ferrocene/ferrocenium couple (Fc/Fc+) before and after each experiment. Atomic force microscopy (AFM) experiments were performed using the Nano-Observer device from CS Instrument. The topographic images were obtained at room temperature in tapping mode. Images were processed with the Gwyddion free SPM data analysis software.Synthetic procedures
The 6-bromo-4,4-diethyl-4H-cyclopenta[1,2-b:5,4-b′]bisthiophene-2-carbaldehyde41 and the 2,2-bis((2-cyanoacetoxy)methyl)propane-1,3-diyl bis(2-cyanoacetate) (3)45 were synthesized according to literature.![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N), ν = 1721 cm−1 (C
N), ν = 1721 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). HRMS (FAB): calculated for C109H76N8O8S4 1752.47, found 1752.4630. Tetra-CPDT (79%): 1H NMR (300 MHz, CDCl3): δ 8.29 (s, 1H), 7.63 (s, 1H), 7.46 (d, J = 9.1 Hz, 2H), 7.33–7.22 (m, 5H), 7.16–7.00 (m, 8H), 4.61 (s, 2H), 2.06–1.88 (m, 4H), 0.65 (t, J = 7.3 Hz, 6H). 13C NMR (76 MHz, CDCl3): δ 164.08, 163.29, 157.62, 151.27, 148.15, 147.47, 147.31, 136.14, 134.41, 129.54, 128.00, 126.59, 124.95, 123.63, 123.22, 117.04, 116.81, 92.38, 63.84, 55.31, 43.43, 30.37, 9.28. IR (neat): ν = 2212 cm−1 (C
O). HRMS (FAB): calculated for C109H76N8O8S4 1752.47, found 1752.4630. Tetra-CPDT (79%): 1H NMR (300 MHz, CDCl3): δ 8.29 (s, 1H), 7.63 (s, 1H), 7.46 (d, J = 9.1 Hz, 2H), 7.33–7.22 (m, 5H), 7.16–7.00 (m, 8H), 4.61 (s, 2H), 2.06–1.88 (m, 4H), 0.65 (t, J = 7.3 Hz, 6H). 13C NMR (76 MHz, CDCl3): δ 164.08, 163.29, 157.62, 151.27, 148.15, 147.47, 147.31, 136.14, 134.41, 129.54, 128.00, 126.59, 124.95, 123.63, 123.22, 117.04, 116.81, 92.38, 63.84, 55.31, 43.43, 30.37, 9.28. IR (neat): ν = 2212 cm−1 (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N), ν = 1717 cm−1 (C
N), ν = 1717 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). HRMS (MALDI-TOF): calculated for C145H116N8O8S4 2352.67, found 2352.6668.
O). HRMS (MALDI-TOF): calculated for C145H116N8O8S4 2352.67, found 2352.6668.
Device fabrication
Indium-tin oxide pre-coated glass slides of 24 × 25 × 1.1 mm with a sheet resistance of RS = 7 Ω □−1 were purchased from Visiontek Systems LTD. The substrates were washed with a diluted Deconex® 12 PA-x solution (2% in water) and scrubbed using dishwashing soap before being cleaned by a series of ultrasonic treatments in distilled water (15.3 MΩ cm−1), acetone and isopropanol for 15 min each. Once dried under a steam of nitrogen, a UV-ozone plasma treatment (UV/Ozone ProCleaner Plus, Bioforce Nanosciences) was performed for 15 min. An aqueous solution of poly(3,4-ethylenedioxy-thiophene)-poly(styrenesulfonate) (PEDOT:PSS; Clevios P VP. AI 4083), filtered through a 0.45 μm RC membrane (Millex®), was spun-cast onto the patterned ITO surface at 5000 rpm for 40 s before being baked at 140 °C for 30 min. Then, blends of molecular donor Tetra-T or Tetra-CPDT and PC71BM at different weight to weight ratios were dissolved in chloroform (10 mg mL−1), stirred at 35 °C for 30 minutes and spun-cast at different spin-speeds onto the PEDOT:PSS layer. Finally, devices were completed by the successive thermal deposition of lithium fluoride (1 nm) and aluminum (120 nm) at a pressure of 1.5 × 10−5 Torr through a shadow mask defining six cells of 27 mm2 each (13.5 mm × 2 mm). J vs. V curves were recorded using a Keithley 236 source-measure unit and a home-made acquisition program. The light source is an AM1.5 Solar Constant 575 PV simulator (Steuernagel Lichttecknik, equipped with a metal halogen lamp). The light intensity was measured by a broad-band power meter (13PEM001, Melles Griot). EQE were measured under ambient atmosphere using a halogen lamp (Osram) with an Action Spectra Pro 150 monochromator, a lock-in amplifier (Perkin-Elmer 7225) and a S2281 photodiode (Hamamatsu).Conflicts of interest
There are no conflicts to declare.Acknowledgements
The RFI LUMOMAT from the Région Pays de la Loire and the Ministère de la Recherche are acknowledged for the PhD grants of A. Labrunie and P. Josse respectively. Authors thank the PIAM (Plateforme d’Ingénierie et Analyses Moléculaires) of the University of Angers for the characterization of organic compounds.References
- L. Lu, T. Zheng, Q. Wu, A. M. Schneider, D. Zhao and L. Yu, Chem. Rev., 2015, 115, 12666–12731 CrossRef CAS PubMed.
- W. Cao and J. Xue, Energy Environ. Sci., 2014, 7, 2123–2144 CAS.
- K. R. Graham, C. Cabanetos, J. P. Jahnke, M. N. Idso, A. El Labban, G. O. Ngongang Ndjawa, T. Heumueller, K. Vandewal, A. Salleo, B. F. Chmelka, A. Amassian, P. M. Beaujuge and M. D. McGehee, J. Am. Chem. Soc., 2014, 136, 9608–9618 CrossRef CAS PubMed.
- S. Li, L. Ye, W. Zhao, S. Zhang, S. Mukherjee, H. Ade and J. Hou, Adv. Mater., 2016, 28, 9423–9429 CrossRef CAS PubMed.
- T. Aytun, P. J. Santos, C. J. Bruns, D. Huang, A. R. Koltonow, M. Olvera de la Cruz and S. I. Stupp, J. Phys. Chem. C, 2016, 120, 3602–3611 CAS.
- H. Yao, L. Ye, H. Zhang, S. Li, S. Zhang and J. Hou, Chem. Rev., 2016, 116, 7397–7457 CrossRef CAS PubMed.
- J. Roncali, P. Leriche and P. Blanchard, Adv. Mater., 2014, 26, 3821–3838 CrossRef CAS PubMed.
- A. A. F. Eftaiha, J.-P. Sun, I. G. Hill and G. C. Welch, J. Mater. Chem. A, 2014, 2, 1201–1213 CAS.
- P. Josse, C. Dalinot, Y. Jiang, S. Dabos-Seignon, J. Roncali, P. Blanchard and C. Cabanetos, J. Mater. Chem. A, 2016, 4, 250–256 CAS.
- P. Josse, A. Labrunie, C. Dalinot, S. M. McAfee, S. Dabos-Seignon, J. Roncali, G. C. Welch, P. Blanchard and C. Cabanetos, Org. Electron., 2016, 37, 479–484 CrossRef CAS.
- A. D. Hendsbee, J.-P. Sun, W. K. Law, H. Yan, I. G. Hill, D. M. Spasyuk and G. C. Welch, Chem. Mater., 2016, 28, 7098–7109 CrossRef CAS.
- N. Liang, W. Jiang, J. Hou and Z. Wang, Materials Chemistry Frontiers, 2017, 1, 1291–1303 RSC.
- C. Zhan, X. Zhang and J. Yao, RSC Adv., 2015, 5, 93002–93026 RSC.
- W. Chen and Q. Zhang, J. Mater. Chem. C, 2017, 5, 1275–1302 RSC.
- S. Holliday, R. S. Ashraf, A. Wadsworth, D. Baran, S. A. Yousaf, C. B. Nielsen, C.-H. Tan, S. D. Dimitrov, Z. Shang, N. Gasparini, M. Alamoudi, F. Laquai, C. J. Brabec, A. Salleo, J. R. Durrant and I. McCulloch, Nat. Commun., 2016, 7, 11585 CrossRef CAS PubMed.
- S. Chen, Y. Liu, L. Zhang, P. C. Y. Chow, Z. Wang, G. Zhang, W. Ma and H. Yan, J. Am. Chem. Soc., 2017, 139, 6298–6301 CrossRef CAS PubMed.
- P. Josse, L. Favereau, C. Shen, S. Dabos-Seignon, P. Blanchard, C. Cabanetos and J. Crassous, Chem.–Eur. J., 2017, 23, 6277–6281 CrossRef CAS PubMed.
- W. Zhao, S. Li, H. Yao, S. Zhang, Y. Zhang, B. Yang and J. Hou, J. Am. Chem. Soc., 2017, 139, 7148–7151 CrossRef CAS PubMed.
- R. Po and J. Roncali, J. Mater. Chem. C, 2016, 4, 3677–3685 RSC.
- A. Leliege, R. C.-H. Le, M. Allain, P. Blanchard and J. Roncali, Chem. Commun., 2012, 48, 8907–8909 RSC.
- S. Mohamed, D. Demeter, J.-A. Laffitte, P. Blanchard and J. Roncali, Sci. Rep., 2015, 5, 9031 CrossRef CAS PubMed.
- D. Demeter, S. Mohamed, A. Diac, I. Grosu and J. Roncali, ChemSusChem, 2014, 7, 1046–1050 CrossRef CAS PubMed.
- A. Labrunie, Y. Jiang, F. Baert, A. Leliege, J. Roncali, C. Cabanetos and P. Blanchard, RSC Adv., 2015, 5, 102550–102554 RSC.
- Y. Jiang, C. Cabanetos, M. Allain, P. Liu and J. Roncali, J. Mater. Chem. C, 2015, 3, 5145–5151 RSC.
- J. Grolleau, F. Gohier, M. Allain, S. Legoupy, C. Cabanetos and P. Frère, Org. Electron., 2017, 42, 322–328 CrossRef CAS.
- Y. Jiang, C. Cabanetos, M. Allain, S. Jungsuttiwong and J. Roncali, Org. Electron., 2016, 37, 294–304 CrossRef CAS.
- Y. Jiang, D. Gindre, M. Allain, P. Liu, C. Cabanetos and J. Roncali, Adv. Mater., 2015, 27, 4285–4289 CrossRef CAS PubMed.
- F. Baert, C. Cabanetos, M. Allain, V. Silvestre, P. Leriche and P. Blanchard, Org. Lett., 2016, 18, 1582–1585 CrossRef CAS PubMed.
- A. Leliege, P. Blanchard, T. Rousseau and J. Roncali, Org. Lett., 2011, 13, 3098–3101 CrossRef CAS PubMed.
- J. W. Choi, C.-H. Kim, J. Pison, A. Oyedele, D. Tondelier, A. Leliege, E. Kirchner, P. Blanchard, J. Roncali and B. Geffroy, RSC Adv., 2014, 4, 5236–5242 RSC.
- M. T. Lloyd, J. E. Anthony and G. G. Malliaras, Mater. Today, 2007, 10, 34–41 CrossRef CAS.
- B. L. Rupert, W. J. Mitchell, A. J. Ferguson, M. E. Kose, W. L. Rance, G. Rumbles, D. S. Ginley, S. E. Shaheen and N. Kopidakis, J. Mater. Chem., 2009, 19, 5311–5324 RSC.
- S. Roquet, R. de Bettignies, P. Leriche, A. Cravino and J. Roncali, J. Mater. Chem., 2006, 16, 3040–3045 RSC.
- S. Karpe, A. Cravino, P. Frère, M. Allain, G. Mabon and J. Roncali, Adv. Funct. Mater., 2007, 17, 1163–1171 CrossRef CAS.
- X. Sun, Y. Zhou, W. Wu, Y. Liu, W. Tian, G. Yu, W. Qiu, S. Chen and D. Zhu, J. Phys. Chem. B, 2006, 110, 7702–7707 CrossRef CAS PubMed.
- H. Shang, H. Fan, Y. Liu, W. Hu, Y. Li and X. Zhan, Adv. Mater., 2011, 23, 1554–1557 CrossRef CAS PubMed.
- S. Roquet, A. Cravino, P. Leriche, O. Alévêque, P. Frère and J. Roncali, J. Am. Chem. Soc., 2006, 128, 3459–3466 CrossRef CAS PubMed.
- D. Xia, D. Gehrig, X. Guo, M. Baumgarten, F. Laquai and K. Mullen, J. Mater. Chem. A, 2015, 3, 11086–11092 CAS.
- H. Sun, P. Sun, C. Zhang, Y. Yang, X. Gao, F. Chen, Z. Xu, Z.-K. Chen and W. Huang, Chem.–Asian J., 2017, 12, 721–725 CrossRef CAS PubMed.
- A. Zitzler-Kunkel, M. R. Lenze, K. Meerholz and F. Wurthner, Chem. Sci., 2013, 4, 2071–2075 RSC.
- X. Cheng, S. Sun, M. Liang, Y. Shi, Z. Sun and S. Xue, Dyes Pigm., 2012, 92, 1292–1299 CrossRef CAS.
- P. Josse, S. Dabos-Seignon, S. M. McAfee, G. C. Welch, P. Blanchard and C. Cabanetos, Dyes Pigm., 2017, 145, 7–11 CrossRef CAS.
- F. Baert, C. Cabanetos, A. Leliege, E. Kirchner, O. Segut, O. Aleveque, M. Allain, G. Seo, S. Jung, D. Tondelier, B. Geffroy, J. Roncali, P. Leriche and P. Blanchard, J. Mater. Chem. C, 2015, 3, 390–398 RSC.
- G. T. Mola and N. Abera, Phys. B, 2014, 445, 56–59 CrossRef.
- S. Nakanishi and M. Ueda, Chem. Lett., 2007, 36, 452–453 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2017 |