Toward high practical capacitance of Ni(OH)2 using highly conductive CoB nanochain supports†
Hui
Wang‡
a,
Jingjing
Yan‡
a,
Rongfang
Wang
*a,
Shunxi
Li
a,
Dan J. L.
Brett
b,
Julian
Key
c and
Shan
Ji
*ab
aCollege of Chemistry and Chemical Engineering, Northwest Normal University, Lanzhou, 730070, China. E-mail: wangrf@nwnu.edu.cn
bEIL, Dept. Chemical Engineering, University College London, London WC1E 7JE, UK. E-mail: shan.ji@ucl.ac.uk
cCollaborative Innovation Center of Renewable Energy Materials (CICREM), Guangxi University Guangxi Univerisity, Nanning, 530004, China
First published on 23rd November 2016
Abstract
Ultrathin porous Ni(OH)2 sheets were grown on the surface of nano-chain CoB as cores via a facile two-step solution-based method at ambient conditions. The resultant CoB@Ni(OH)2 of 27.89 wt% Ni(OH)2 loading has a high specific capacitance of 1504.4 F g−1 at 0.5 A g−1, 1293.7 F g−1 at 2 A g−1 and 746.8 F g−1 at 6 A g−1.
High-performance supercapacitors are widely applied in the fields of mobile electronics, backup power supplies, and electric vehicles due to their high power density, fast charge/discharge, and long cycle life.1,2 Transition metal oxides and hydroxides, such as RuO2, MnO2, Co3O4, NiO and Ni(OH)2, are examples of pseudocapacitive materials characterized by high reversible redox-based capacitance via oxidation state transitions.3,4 However, due to their low electrical conductivity, their observed specific capacitance is much lower than that might be expected from their high theoretical specific capacities, especially at high current densities required for supercapacitor performance. As a result, high specific capacitance in pseudocapacitive oxide material is usually limited to very thin films.5 Such films are impractical for commercial cells due to the much larger relative mass and volume contribution of the current collectors the films are formed on.
Physically attaching pseudocapacitive oxide materials to materials such as particulate carbon6–8 and conducting polymers,9–11 either at the nano or micrometer scale of particle size, is frequently employed to improve oxide conductivity. Some reports have also shown that the practical capacitance of oxides is significantly improved using metal and metal alloys as conductive supports.12–17 Chen and co-workers showed that RuO2 and MnO2 supported on nanoporous Au or NiMn alloy, could reach high specific capacitances close to their theoretical values due to the provision of fast ionic conduction and excellent electron–proton transport.14–16 However, both the high material cost of nanoporous Au and elaborate fabrication process of electrochemical polarization narrows the practical applicability of these oxide@porous metal/alloy electrodes.16 An oxy/hydroxide@ low-cost metal/alloy combination achieving the same high specific capacitance and cyclic stability, particularly through a facile large-scale production method, is therefore of obvious interest.
Various reports show that transition metal borides, particularly CoB alloys, exhibit high specific capacity and cycling stability as anode materials for aqueous batteries in alkaline electrolyte.18–21 In contrast, their performance as supercapacitor material has received surprisingly little attention,21 with CoB being no exception. However, CoB alloys have high electronic conductivity in the order of 103 S cm−1 (similar to Co metal), which is around ten orders of magnitude higher than that of oxides/hydroxides (e.g. Ni(OH)2 in the order of 10−15 S cm−1).20,21 This suggests CoB should either have high rate capability as a capacitance material and/or be useful as a conductive support for other capacitance material. In particular, Ni(OH)2, while low in conductivity, has high theoretical specific capacity (289.1 mA h g−1), as well as well-defined redox behavior in alkaline electrolyte, and can form layered structures with large interlayer spacing theoretically supportive of high capacitance.22–26 In the present study, we scrupulously aimed for a particle architecture that combines Ni(OH)2 nanoflakes supported on CoB nanochains. Through this novel structural design, the material has both high rate capability and high specific capacitance.
Fig. S1† shows the powder X-ray diffraction (XRD) patterns of CoB and CoB@Ni(OH)2. The XRD pattern of CoB has wide peak dispersion indicative of amorphous structure. In the case of CoB@Ni(OH)2, beside the dispersion peak, two new small peaks appear at 2θ ∼34.0 and 60.5° corresponding to the (100) and (111) planes of hexagonal phase β-Ni(OH)2 (JCPDS card no. 1-1047), thus indicating hybrid particle structure was obtained. Bulk composition analyses of the two samples by inductively coupled plasma (ICP), found the Co![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B atomic ratio in the CoB sample to be ca. 2.2
B atomic ratio in the CoB sample to be ca. 2.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and the Co
1, and the Co![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B
B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni atomic ratio in the CoB@Ni(OH)2 sample to be ca. 10.43
Ni atomic ratio in the CoB@Ni(OH)2 sample to be ca. 10.43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.74
4.74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The estimated mass percentage of Ni(OH)2 in the CoB@Ni(OH)2 sample is ca. 12.26%.
1. The estimated mass percentage of Ni(OH)2 in the CoB@Ni(OH)2 sample is ca. 12.26%.
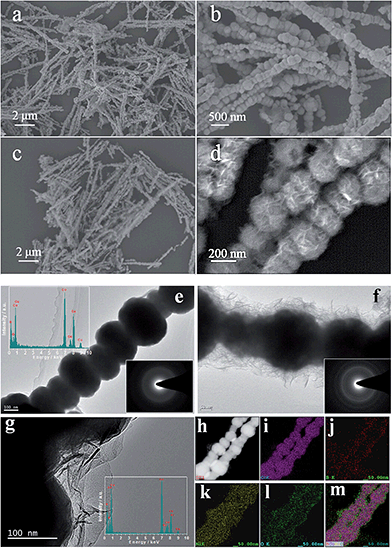
Fig. 1a–d shows scanning electron microscopy (SEM) images of CoB and CoB@Ni(OH)2. CoB formed uniform bead-like particles of ∼50 to 250 nm that joined together as long chains (Fig. 1a). This underlying chain structure was clearly maintained after Ni(OH)2 deposition, which formed a continuous covering on the CoB surface as nanoflakes (Fig. 1c and b). The nanoflake structure is clearly visible in the transmission electron microscopy (TEM) images (Fig. 1f and g). Energy dispersive spectroscopy (EDS) of CoB (upper left corner inset in Fig. 1e) revealed the presence of Co and B elements, and its selected area electron diffraction (SAED) pattern (lower right corner inset) formed a continuous hollow ring diffraction pattern typical of amorphous material,27 which correlates with the XRD result. The enlarged TEM image of CoB@Ni(OH)2 (Fig. 1g) shows the Ni(OH)2 flocculent layer comprised ultrathin sheets, and the SAED pattern (lower right corner inset in Fig. 1f) displays several rings indicating Ni(OH)2 had polycrystalline structure. The EDS pattern of CoB@Ni(OH)2 (lower right corner inset in Fig. 1g) revealed the presence of Co, B, Ni and O elements, and the scanning TEM (STEM) image (Fig. 1h) and electron energy loss spectroscopic mapping (Fig. 1i–l) shows uniform distribution of Co, B, Ni and O elements, which with overlapping (Fig. 1m) reveals the core–shell structure distribution of these elements.
X-ray photoelectron spectroscopy (XPS) analysis was carried out to identify the elemental composition and chemical states of the elements on the surface of CoB@Ni(OH)2. The survey spectra of CoB and CoB@Ni(OH)2 (Fig. S2a†) revealed the presence of Co and B elements in CoB and Co, B, Ni, O in CoB@Ni(OH)2. Fig. S2b† shows the Co 2p XPS spectrum of CoB contains the two characteristic peaks of Co 2p3/2 (781.8 eV) and Co 2p1/2 (797.8 eV) along with shake-up satellites. The two peaks clearly shift to higher binding energy compared to that of metallic Co or Co hydro/oxides,28,29 resulting possibly from electron transfer from Co to B atoms. In contrast, shake-up satellites in the Co 2p XPS spectrum of CoB@Ni(OH)2 can hardly be observed, most likely due to signal suppression by the Ni(OH)2 shell, which offers further evidence of the CoB@Ni(OH)2 core–shell structure. Similarly, the B 1s XPS signal (Fig. S2c†) clearly appears in the CoB plot, but is absent in the CoB@Ni(OH)2 plot. The B 1s XPS spectrum of CoB displayed two peaks, one at low binding energy (188.0 eV) that may correlate to the free form of elemental B, and the other at high binding energy (192.0 eV) to boron oxide.30 The Ni XPS signal (Fig. S2d†) for CoB@Ni(OH)2 has two intense peaks centered at 855.8 eV and 873.7 eV corresponding to Ni 2p3/2 and Ni 2p1/2 respectively, followed by clear satellite signals at high binding energy. These bands are characteristic of the Ni(OH)2 phase and are consistent with previous reports.31,32 In the O 1s spectrum of the Ni(OH)2 (Fig. S2e†), the main peak of 529.4 eV corresponds to metal–O bonds.33,34
N2 isotherm analysis (Fig. S5†) was used to evaluate the porosity of CoB and CoB@Ni(OH)2. With increase of relative pressure, the adsorbed N2 volume by CoB was much less than that of CoB@Ni(OH)2, indicating that CoB@Ni(OH)2 has higher porosity than CoB. The N2 isotherm of CoB forms the typical I isotherm shape of IUPAC classification, and the absence of uptakes and hysteresis loop suggests limited presence of pores.35 In contrast, CoB@Ni(OH)2 produced a type II isotherm with an hysteresis loop starting at 0.47 relative pressure, indicating the presence of mesopores. The overall pore size distribution for CoB@Ni(OH)2, was thus in the mesopore size range with few micropores present (inset Fig. S6†). Furthermore, the BET surface area of CoB and CoB@Ni(OH)2 was 6.6 and 42.5 m2 g−1 respectively, showing considerable surface area increase after Ni(OH)2 deposition due to the porous structure of the Ni(OH)2 shell.
To evaluate the capacitive influence of Ni(OH)2 mass loading on CoB, NiCl2 concentration was varied during synthesis to form different CoB@Ni(OH)2 hybrids of varying Ni(OH)2 wt%. Their bulk composition, physical characterization including XRD, SEM, N2 and isotherms are provided in Table S1 and Fig. S3–S6.† As seen in Table S1,† the maximum mass percentage of Ni(OH)2 that can be achieved was 27.98 wt% at an NiCl2 concentration of 0.2 mmol, while retaining morphology and porous structure (Fig. S3–S6†).
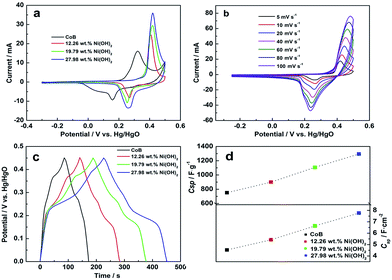
The electrochemical behavior of CoB and CoB@Ni(OH)2 was studied using cyclic voltammetry (CV) and galvanostatic cycling, both using three-electrode cell configurations in 6 mol L−1 KOH solution. Fig. 2a shows the CV plots of the CoB and CoB@Ni(OH)2 electrodes after activation at a scan rate of 5 mV s−1. It should be noted that Co(OH)2 forms on the CoB surface during the activation process in alkaline solution through reaction (1).22,36 The CV plot of CoB produced an oxidation peak at 0.347 V and a corresponding reduction peak at 0.151 V,37,38 whereas CoB@Ni(OH)2 produced an oxidation peak at ca. 0.430 V and a reduction peak at 0.265 V. The corresponding reversible reactions associated with these peaks respectively were considered to be reaction (2) and reaction (3):
| Co + 2OH− → Co(OH)2 + 2e− | (1) |
| Co(OH)2 + OH− → CoOOH + H2O + e− | (2) |
| Ni(OH)2 + OH− → NiOOH + H2O + e− | (3) |
Notably, all the CV curves of CoB@Ni(OH)2 show larger voltammetric current than that of CoB, suggesting strong capacitive contribution from the Ni(OH)2 shell. Voltammetric current increase occurred with increase of Ni(OH)2 mass content, indicating CoB@Ni(OH)2 containing the highest Ni(OH)2 content of 27.98 wt% would also have the highest capacitance. Moreover, the high current response from the 27.98 wt% electrode (Fig. 2b) maintained the same CV peak shape and potential gap between oxidation and reduction peaks as scan rate increased, indicating CoB@Ni(OH)2 had good capacitive reversibility.38,39
Fig. 2c shows the galvanostatic charge–discharge plots of CoB and CoB@Ni(OH)2 at a current density of 2 A g−1. In all cases, the nonlinear charge curves are asymmetric to their corresponding discharge curves, which is commonly observed for pseudocapacitive materials.40,41 Compared to the pristine CoB nanochains, all CoB@Ni(OH)2 hybrids produced high capacitance discharge curves, which correlates to the high current responses of their CV curves. Specific capacitance (Csp) was calculated from the discharge curves considering total CoB and Ni(OH)2 mass content, and is shown in Fig. 2d. All the CoB@Ni(OH)2 hybrids produced higher Csp than that of CoB, which demonstrates the high capacitance contribution of the Ni(OH)2 shell. Specific capacitance increased with increased Ni(OH)2 loading from 12.26 wt% to 27.98 wt% (at 1293.7 F g−1); equating to 7.76 F cm−2 with respect to relative electrode surface area (Cap, Fig. 2d). The specific capacitance for CoB at 2 A g−1 was 750 F g−1, and thus considerably smaller than that of the CoB@Ni(OH)2 containing the highest mass percentage of Ni(OH)2. Fig. S7† compares the electrochemical impedance spectra (EIS) of the two materials. Here, it can be seen the CoB@Ni(OH)2 hybrid clearly has decreased overall equivalent series resistance (Rs, the real axis intercept) than CoB due to its higher specific capacitance.42,43
The specific capacitance of CoB@Ni(OH)2 hybrid with 27.98 wt% Ni(OH)2 is quite attractive when compared with previously reported values of other core/Ni(OH)2–shell-based electrodes31,44–46 (Table S2†). In particular, although some binder-free electrodes based on other core/Ni(OH)2–shell hybrid materials such as TiN@Ni(OH)2,13 ZnO@Ni(OH)2,47 NiCo2S4@Ni(OH)2,48 NiMoO4@Ni(OH)2,49 ZnCo2O4@Ni(OH)2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 have high specific capacitance, in general, complex synthesis steps and the limited loading density of the electrodes have predictably high manufacturing cost, thus hindering large-scale application possibilities. Therefore among the particle-based electrodes materials thus far produced, the present study's material of 1293.7 F g−1 at 2 A g−1 is a significant achievement within the cost affective category considering its simple synthesis and high specific capacitance at high current density.
50 have high specific capacitance, in general, complex synthesis steps and the limited loading density of the electrodes have predictably high manufacturing cost, thus hindering large-scale application possibilities. Therefore among the particle-based electrodes materials thus far produced, the present study's material of 1293.7 F g−1 at 2 A g−1 is a significant achievement within the cost affective category considering its simple synthesis and high specific capacitance at high current density.
Three physically properties of the Ni(OH)2/metal alloy combination, owing to its unique morphology and composition, may account for its overall high specific capacitance. Firstly, the Ni(OH)2 nanoflakes grown in situ on the CoB nanochains are well separated. This provides unhindered access for surface absorption and movement of OH− electrolyte ions involved in capacitance. Secondly, as illustrated in Fig. S8,† the electrolyte can theoretically reach the surface of the CoB core through the many mesopores in the Ni(OH)2 shell, as indicated by its pore size distribution (Fig. 2 inset). Here, since Co(OH)2 forms on the CoB surface during activation in alkaline solution,22,36 mesopores in Ni(OH)2 could allow OH− electrolyte ions access to Co(OH)2 to participate in capacitance through reaction (2), which suggests that the specific capacitance of CoB@Ni(OH)2 may derive from both CoB and Ni(OH)2. Thirdly, CoB provides a highly conductive support in CoB@Ni(OH)2 for rapid electron transport.
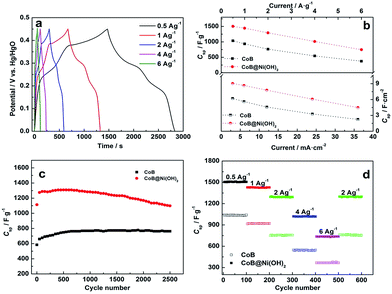
Fig. 3a and S9† compare the rate capabilities of CoB and CoB@Ni(OH)2 (27.98 wt%) at increasing current densities. Drop in specific capacitance occurred on both materials with current density increase, correlating with literature on other core/Ni(OH)2–shell-based materials.31,44–50 Within the current range of 0.5 A g−1 to 6 A g−1 (i.e. 3 mA cm−2 to 36 mA cm−2), CoB@Ni(OH)2 maintained a consistently higher specific capacitance than the CoB (Fig. 3b). At 6 A g−1, the specific capacitance of CoB@Ni(OH)2 (746.8 F g−1) was 49.7% of that measured at 0.5 A g−1. Therefore, at 6 A g−1 the specific capacity of CoB@Ni(OH)2 was still ∼2.0 times as much as that of CoB (373.5 F g−1 at 6 A g−1). The overall ∼50% drop in specific capacitance for both materials over the 12-fold increase from 0.5 to 6 A g−1 reveals both materials have high conductivity.
The cycle life of CoB and CoB@Ni(OH)2 (27.98 wt%) was evaluated over 2500 continuous charge–discharge cycles at a current density of 2 A g−1 (Fig. 3c). Interestingly, the specific capacitance of both electrodes gradually increased during the first 200 cycles, which is likely due in part to the activation processes involved the formation of Co(OH)2.22,51 Both CoB and CoB@Ni(OH)2 hybrid exhibit good long-term electrochemical stability, as further evident from their very stable charge–discharge curves for the comparison between the first and last 8 cycles (Fig. S10†). Both materials retained ∼99% coulombic efficiency. The specific capacitance loss for CoB@Ni(OH)2 after 2500 cycles was 15%. By contrast, 100% specific capacitance was retained for CoB, and this remarkable cycling stability appears to derive from the stability of the capacitive Co(OH)2 passivation layer, which interestingly also appears to prevent B dissolving (reaction (4)), which would no doubt result in capacitance loss:
| B + 4OH− → H2BO3− + H2O + 3e− | (4) |
Fig. S11a and b† shows SEM images before and after charge–discharge cycling tests of the CoB electrode. CoB nanochains were transformed into nanosheet-like shape after 2500-cycle test. The above change in surface morphologies should be caused by the dissolution of B and transformation of Co to Co(OH)2, resulting in a better cycle stability. Meanwhile, SEM images before and after charge–discharge cycling tests (Fig. S11c and d†) of CoB@Ni(OH)2 electrode show that the surface morphology were transformed into nanochains composed by nanoflower-like shape. The results demonstrate that CoB played an important role as a supporting skeleton and conductor successfully in the process of charge–discharge cycling tests. In order to further illustrate the role of the core, the cycle life of CoB@Ni(OH)2 and CoB@Ni(OH)2 without carbon electrodes was evaluated over 2500 continuous charge–discharge cycles at a current density of 2 A g−1 (Fig. S12†). The specific capacitance and cycle life testing confirm the role of CoB as supporting and conductive materials.
The stability of CoB@Ni(OH)2 hybrid is also much greater than that of other reported core/Ni(OH)2–shell hybrid materials such as TiN@Ni(OH)2,13 NiCo2S4@Ni(OH)2,48 NiMoO4@Ni(OH)2,49 ZnCo2O4@Ni(OH)2,50 and NiCo2O4@Ni(OH)2 (ref. 52) as listed in Table S1.† Therefore, CoB as the core in the core–shell CoB@Ni(OH)2 hybrid clearly promotes stability during cycling. To further explore the durability of CoB@Ni(OH)2 and CoB, cycling was carried out at progressively increased current densities (Fig. 3d) and then returned to 2 A g−1. Remarkably, after 500 cycles at different current densities, and despite the abrupt changes in current density, the capacitance of CoB@Ni(OH)2 hybrid returned to 1276.5 F g−1 at 2 A g−1, equating to ∼99% of the initial capacitance (1293.7, F g−1). Furthermore, this capacitance maintained its level for another 100 cycles without noticeable decrease. Therefore, this final result indicates CoB@Ni(OH)2 has excellent durability at different current densities, which is an important merit for practical energy storage devices.
Conclusions
A facile, in situ synthesis method at room temperature was achieved to directly grow porous Ni(OH)2 nano-sheets on the surface of nano-chain CoB alloy for use as capacitive supercapacitor material. In this particular architecture, both the Ni(OH)2 shell and CoB core may act in capacitive charge storage, while the CoB core also serves as a conductive backbone for electron transport. Electrochemical evaluation revealed that the optimized CoB@Ni(OH)2 electrode delivers a high specific capacitance (1504.4 F g−1) at 0.5 A g−1 with good rate capability and cyclic stability. The excellent performance is attributed to synergetic contribution from the CoB core and the porous Ni(OH)2 shell. In conclusion, the uncomplicated synthesis method could be readily scalable, thus offering a means to produce material of high specific capacitance and high stability by a practical and cost effective means.Acknowledgements
The authors would like to thank the National Natural Science Foundation of China (21363022, 51362027 and 51661008) for financially supporting this work.Notes and references
- Y. Q. Zhang, L. Li, S. J. Shi, Q. Q. Xiong, X. Y. Zhao, X. L. Wang, C. D. Gu and J. P. Tu, J. Power Sources, 2014, 256, 200–205 CrossRef CAS.
- Y. Du, X. Zhao, Z. Huang, Y. Li and Q. Zhang, RSC Adv., 2014, 4, 39087–39094 RSC.
- J. Liu, J. Jiang, C. Cheng, H. Li, J. Zhang, H. Gong and H. J. Fan, Adv. Mater., 2011, 23, 2076–2081 CrossRef CAS PubMed.
- H. Jiang, C. Li, T. Sun and J. Ma, Chem. Commun., 2012, 48, 2606–2608 RSC.
- C. Zhao and W. Zheng, Front. Energy Res., 2015, 3, 1–11 Search PubMed.
- Y. Zou, I. A. Kinloch and R. A. Dryfe, ACS Appl. Mater. Interfaces, 2015, 7, 22831–22838 Search PubMed.
- Y. Zhao, Y. Meng and P. Jiang, J. Power Sources, 2014, 259, 219–226 CrossRef CAS.
- R. Kumar, R. K. Singh, P. K. Dubey, D. P. Singh and R. M. Yadav, ACS Appl. Mater. Interfaces, 2015, 7, 15042–15051 Search PubMed.
- X. Zhang, X. Zeng, M. Yang and Y. Qi, ACS Appl. Mater. Interfaces, 2014, 6, 1125–1130 Search PubMed.
- X. Zhang, L. Ji, S. Zhang and W. Yang, J. Power Sources, 2007, 173, 1017–1023 CrossRef CAS.
- J.-G. Wang, Y. Yang, Z.-H. Huang and F. Kang, J. Power Sources, 2012, 204, 236–243 CrossRef CAS.
- Q. Lu, M. W. Lattanzi, Y. Chen, X. Kou, W. Li, X. Fan, K. M. Unruh, J. G. Chen and J. Q. Xiao, Angew. Chem., 2011, 123, 6979–6982 CrossRef.
- H. Yi, X. Chen, H. Wang and X. Wang, Electrochim. Acta, 2014, 116, 372–378 CrossRef CAS.
- X. Lang, A. Hirata, T. Fujita and M. Chen, Nat. Nanotechnol., 2011, 6, 232–236 CrossRef CAS PubMed.
- X. Xu, Z. Y. Fan, X. Y. Yu, S. Y. Ding, D. M. Yu and X. W. Lou, Adv. Energy Mater., 2014, 1400902 CrossRef.
- J. Kang, A. Hirata, H. J. Qiu, L. Chen, X. Ge, T. Fujita and M. Chen, Adv. Mater., 2014, 26, 269–272 CrossRef CAS PubMed.
- L. Y. Chen, Y. Hou, J. L. Kang, A. Hirata, T. Fujita and M. W. Chen, Adv. Energy Mater., 2013, 3, 851–856 CrossRef CAS.
- W. Ni, B. Wang, J. Cheng, X. Li, Q. Guan, G. Gu and L. Huang, Nanoscale, 2014, 6, 2618–2623 RSC.
- Y. D. Wang, X. P. Ai, Y. L. Cao and H. X. Yang, Electrochem. Commun., 2004, 6, 780–784 CrossRef CAS.
- H. X. Yang, Y. D. Wang, X. P. Ai and C. S. Cha, Electrochem. Solid-State Lett., 2004, 7, A212 CrossRef CAS.
- J. Liang, B. T. Dong, S. J. Ding, C. P. Li, B. Q. Li, J. Li and G. Yang, J. Mater. Chem. A, 2014, 2, 11299–11304 RSC.
- Y. D. Wang, X. P. Ai and H. X. Yang, Chem. Mater., 2004, 16, 5194–5197 CrossRef CAS.
- W. Zhang, Y. Tan, Y. Gao, J. Wu, B. Tang and J. Zhao, RSC Adv., 2014, 4, 27800 RSC.
- Z. Wang, X. Wang, Y. X. Zhao, C. M. Zhao and W. T. Zheng, J. Nano Res., 2012, 20, 53–60 CrossRef CAS.
- S.-i. Yamazaki, T. Ioroi, K. Tanimoto, K. Yasuda, K. Asazawa, S. Yamaguchi and H. Tanaka, J. Power Sources, 2012, 204, 79–84 CrossRef CAS.
- B. T. Dong, H. Zhou, J. Liang, L. S. Zhang, G. X. Gao and S. J. Ding, Nanotechnology, 2014, 25, 435403 CrossRef PubMed.
- Y. Ma, R. Wang, H. Wang, V. Linkov and S. Ji, Phys. Chem. Chem. Phys., 2014, 16, 3593–3602 RSC.
- X. T. Zhang, H. Wang, J. L. Key, V. Linkov, S. Ji, X. L. Wang, Z. Q. Lei and R. F. Wang, J. Electrochem. Soc., 2012, 159, B270–B276 CrossRef CAS.
- W. Huang, Z. Zuo, P. Han, Z. Li and T. Zhao, J. Electron Spectrosc. Relat. Phenom., 2009, 173, 88–95 CrossRef CAS.
- B. Crist, Handbook of the Elements and Native Oxides, XPS International, Inc, Mountain View, CA, 1999 Search PubMed.
- Q. Ke, C. Guan, M. Zheng, Y. Hu, K.-h. Ho and J. Wang, J. Mater. Chem. A, 2015, 3, 9538–9542 RSC.
- J.-H. Zhong, A.-L. Wang, G.-R. Li, J.-W. Wang, Y.-N. Ou and Y.-X. Tong, J. Mater. Chem. A, 2012, 22, 5656 RSC.
- Y. Ma, R. Wang, H. Wang, J. Key and S. Ji, J. Power Sources, 2015, 280, 526–532 CrossRef CAS.
- P. Tao, M. Shao, C. Song, S. Wu, M. Cheng and Z. Cui, J. Ind. Eng. Chem., 2014, 20, 3128–3133 CrossRef CAS.
- Y. Ma, H. Wang, W. Lv, S. Ji, B. G. Pollet, S. Li and R. Wang, RSC Adv., 2015, 5, 68655–68661 RSC.
- Y. Liu, Y. Wang, L. Xiao, D. Song, Y. Wang, L. Jiao and H. Yuan, Electrochim. Acta, 2008, 53, 2265–2271 CrossRef CAS.
- L. Ma, H. Zhou, X. Shen, Q. Chen, G. Zhu and Z. Ji, RSC Adv., 2014, 4, 53180–53187 RSC.
- C. Yuan, X. Zhang, L. Hou, L. Shen, D. Li, F. Zhang, C. Fan and J. Li, J. Mater. Chem. A, 2010, 20, 10809 RSC.
- R. Wang, Y. Ma, H. Wang, J. Key, D. Brett, S. Ji, S. Yin and P. K. Shen, J. Mater. Chem. A, 2016, 4, 5390–5394 RSC.
- K. Wang, C. Zhao, S. Min and X. Qian, Electrochim. Acta, 2015, 165, 314–322 CrossRef CAS.
- W. Sun, X. Rui, M. Ulaganathan, S. Madhavi and Q. Yan, J. Power Sources, 2015, 295, 323–328 CrossRef CAS.
- H. Chen, S. Zhou and L. Wu, ACS Appl. Mater. Interfaces, 2014, 6, 8621–8630 Search PubMed.
- H. Chen, L. Hu, Y. Yan, R. Che, M. Chen and L. Wu, Adv. Energy Mater., 2013, 3, 1636–1646 CrossRef CAS.
- C. H. Tang, X. Yin and H. Gong, ACS Appl. Mater. Interfaces, 2013, 5, 10574–10582 Search PubMed.
- W. Tian, X. Wang, C. Zhi, T. Zhai, D. Liu, C. Zhang, D. Golberg and Y. Bando, Nano Energy, 2013, 2, 754–763 CrossRef CAS.
- W. Zhou, X. Cao, Z. Zeng, W. Shi, Y. Zhu, Q. Yan, H. Liu, J. Wang and H. Zhang, Energy Environ. Sci., 2013, 6, 2216–2221 Search PubMed.
- I. Shakir, Z. Ali, J. Bae, J. Park and D. J. Kang, RSC Adv., 2014, 4, 6324 RSC.
- J. Zhang, H. Gao, M. Y. Zhang, Q. Yang and H. X. Chuo, Appl. Surf. Sci., 2015, 349, 870–875 CrossRef CAS.
- G. Jiang, M. Zhang, X. Li and H. Gao, RSC Adv., 2015, 5, 69365–69370 RSC.
- H. X. Chuo, H. Gao, Q. Yang, N. Zhang, W. B. Bu and X. T. Zhang, J. Mater. Chem. A, 2014, 2, 20462–20469 RSC.
- Y. Liu, Y. Wang, L. Xiao, D. Song, L. Jiao and H. Yuan, Electrochem. Commun., 2007, 9, 925–929 CrossRef CAS.
- W. Li, L. Xin, X. Xu, Q. Liu, M. Zhang, S. Ding, M. Zhao and X. Lou, Sci. Rep., 2015, 5, 9277 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental detail; XRD patterns; SEM and TEM images; elemental mapping images; XPS survey; N2 isotherms; CV curves; galvanic charge–discharge. See DOI: 10.1039/c6ta08796e |
| ‡ Hui Wang and Jingjing Yan contributed equally. |
| This journal is © The Royal Society of Chemistry 2017 |