A covalently cross-linked reduced functionalized graphene oxide/polyurethane composite based on Diels–Alder chemistry and its potential application in healable flexible electronics†
Jinhui
Li
ab,
Guoping
Zhang
*ac,
Rong
Sun
a and
Ching-Ping
Wong
cd
aShenzhen Institutes of Advanced Technology, University of Chinese Academy of Sciences, Shenzhen 518055, China. E-mail: gp.zhang@siat.ac.cn; Fax: +86-755-86392299; Tel: +86-755-86392104
bShenzhen College of Advanced Technology, University of Chinese Academy of Sciences, Shenzhen 518055, China
cDepartment of Electronic Engineering, Faculty of Engineering, The Chinese University of Hong Kong, Hong Kong, China
dSchool of Materials Science and Engineering, Georgia Institute of Technology, 771 Ferst Drive, Atlanta, GA 30332, USA
First published on 22nd November 2016
Abstract
A self-healing polymer with excellent mechanical properties, thermal stability, and multiple repairing has attracted increasing interest. However, very few works focused on the self-healing composites and investigated their applications in flexible electronics. Herein, covalently cross-linked reduced functionalized graphene oxide/polyurethane (RFGO/PU) composites based on Diels–Alder (DA) chemistry were developed and applied in healable flexible electronics. GO sheets were modified by furfurylamine followed by reduction resulting in RFGO sheets. Then RFGO was covalently cross-linked with furfuryl-contained linear PU and bismaleimide by DA chemistry. Benefiting from the successful modification of furfuryl, the good dispersion and excellent capability of microwave-to-heat conversion of RFGO, the synthesized composites exhibited not only enhanced mechanical properties and great thermal stability but also excellent electromagnetic wave healable properties. In addition, new composites were employed as the elastic matrix and introduced into the three-dimensional fragmentized graphene foam which served as the conductive path to fabricate flexible electronics. The as-prepared flexible electronics were successfully employed as flexible conductors and strain sensors and were able to detect the biosignals of finger bending. Besides all these, these flexible electronics could be healed efficiently using microwaves in 5 minutes showing great potential in healable flexible electronics.
1 Introduction
Flexible and deformable electronics have emerged as a very promising field and recently attracted significant attention.1,2 There are several approaches to fabricate flexible electronics in which the conductive filler/elastomer composite is one of the most facile and effective methods.3,4 Elastomers such as polydimethylsiloxane,5,6 polyurethane,7 natural rubber8,9 as well as poly(styrene-block-butadiene-block-styrene)10 have been widely employed. Most of the elastomers lack healing properties and cannot be healed after a fracture or damage, which leads to a sharp decrease in their sustainability and lifetime. And the healing of electrical connections has attracted substantial attention with advances in flexible electronics and robotics.4,11In this case, exploration of self-healing or healable polymeric materials that can repair themselves after mechanical damage is in high demand. In order to prepare self-healing or healable polymeric materials, microcontainers such as microcapsules,12,13 microvasculars14 and microchannels15 which store healing agents, are embedded in the polymer matrix to form self-healing polymeric composites. The microcontainers could heal themselves timely, but they can be healed only once at the same location because of exhaustion of the healing agents.16 Another main self-healing system is based on the reversible cross-linking of polymers which represent a promising way to heal microcracks due to their repetitive healing properties.17 Such reversibility of the reversible cross-linking of polymers can be triggered by supra-molecular interaction, photo, electrical or thermal activation.17,18
Diels–Alder (DA) chemistry, which is based on thermal reversible bonds that can be healed many times under mild conditions, has attained significant achievements recently. In 2002, Chen et al. reported the self-healing cross-linked epoxy resin based on DA chemistry for the first time.19 Later, many self-healing polymers of different architectures have been developed since this pioneering work.20–22 However, most of the self-healing processes based on DA chemistry require external heating across the materials and the healing efficiency is low with a long healing process.23,24 Therefore there is a need to develop new methods to heal the material with any cracks with high efficiency.25,26
Herein, graphene oxide (GO) sheets were modified by furfurylamine and then reduced, resulting in the reduced functionalized GO (RFGO) sheets. Then RFGO was covalently cross-linked with furfuryl-contained linear PU and bismaleimide by DA chemistry resulting in composites with self-healing properties using microwaves. It has been reported that microwaves could be used to heat graphene-related materials in a short time with high efficiency27,28 which could be used for the healing of the graphene-based composites based on DA reaction, and to the best of our knowledge, it has not been reported yet. In this work, benefiting from the good dispersion and excellent microwave absorption of RFGO, the synthesized composites exhibit not only enhanced mechanical properties and great thermal stability but also excellent electromagnetic wave healable properties with high efficiency. In addition, the promising composite was employed as the elastic matrix and introduced into conductive three-dimensional (3D) fragmentized graphene foam (FGF) to prepare self-healing flexible electronics. The as-prepared flexible electronics were successfully employed as flexible conductors and strain sensors and were able to detect the biosignals of finger bending and heal efficiently using microwaves in 5 minutes showing great potential in healable flexible electronics.
2 Experimental section
2.1 Materials
Graphite powder and ammonium sulfide solution (20% w/w aq.) were supplied by Aladdin. The chemicals including potassium permanganate (KMnO4), sodium nitrate (NaNO3), concentrated sulfuric acid (H2SO4, 98%), concentrated hydrochloric acid (HCl) and hydrazine hydrate (85%) were all of reagent grade purchased from Sinopharm Chemical Reagent Beijing Co., Ltd. Diglycidyl ether of bisphenol A (DGEBA) was obtained from Sigma-Aldrich Co. 4,4-Diphenylmethane diisocyanate, (MDI, 99%), furfurylamine (FA) and N,N′-(4,4′-diphenylmethane)bismaleimide (BMI) were supplied by Aladdin and used as received. Poly(tetramethylene glycol) (PTMEG, Mn = 2000 g mol−1) was purchased from Aladdin and used after 2 h drying under vacuum at 110 °C. Dimethyl formamide (DMF) was dried with molecular sieves for more than 24 h and freshly distilled before use.2.2 The preparation of reduced functionalized GO (RFGO)
RFGO was prepared according to the following procedure. First, GO was prepared by the modified Hummers' method.29,30 For the functionalization of GO, HCl was added to 300 mL of 1 mg mL−1 GO solution and the pH value was adjusted to 1. Then, 3 g of furfurylamine was added to the solution and stirred at room temperature for 1 h followed by heating at 60 °C for 12 h which resulted in furfurylamine functionalized GO. After that, 1.5 g of hydrazine hydrate was added to the system and heated at 60 °C for 24 h which resulted in a black solution. The resultant solution was filtered and washed with ultrapure water to remove the redundant hydrochloric acid and furfurylamine. Then RGOF was dried in an oven at 60 °C for 12 h. Finally, RFGO was dispersed in anhydrous DMF with a concentration of 5 mg mL−1 for further reactions.2.3 The synthesis of RFGO/PU composites
10 g of DGEBA were mixed and heated with 5.7 g of furfurylamine (in the stoichiometric molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and reacted in 20 mL methanol at 60 °C for 6 h. At the end of the reaction, the product (the component with bi-furan and bi-hydroxyl, 2F2OH) was obtained by drying the sample under vacuum until a constant weight was achieved.
2) and reacted in 20 mL methanol at 60 °C for 6 h. At the end of the reaction, the product (the component with bi-furan and bi-hydroxyl, 2F2OH) was obtained by drying the sample under vacuum until a constant weight was achieved.
In order to prepare the RFGO/PU composite, 1.0 g of MDI was dispersed in 10.0 g of anhydrous DMF and stirred at room temperature for 30 min, firstly. Then 4.0 g of PTMEG2000 in 20.0 g of anhydrous DMF was added dropwise to the above solution and reacted at 80 °C for 2 h in a N2 atmosphere. Afterwards, 1.07 g of 2F2OH dispersed in 5 g of anhydrous DMF was added and the resultant solution was reacted at 80 °C for another 2 h in a N2 atmosphere which resulted in a solution of furfuryl-containing linear PU (FLPU). And a covalently cross-linked composite containing 0.1 wt% RFGO was prepared by adding 1.21 mL of RFGO solution and 0.754 g of BMI to FLPU solution and reacted at 65 °C for 5 h. Other samples containing 0.5 wt% and 1.0 wt% RFGO were synthesized by the same procedure with an increase in the addition of RFGO solution and BMI. All these final composites with different RFGO contents were labeled RFGO–DAPU-1, RFGO–DAPU-2 and RFGO–DAPU-3, respectively. For comparison, the thermal healable cross-linked polyurethane was prepared with the same process but without the addition of GO and named DAPU. Yields were >95% in both cases.
2.4 The fabrication of healable flexible electronics
3D FGF was obtained by fragmenting the graphene foam (GF) which was prepared by the self-assembly process of GO and ammonium sulfide as described in our previous works.6,30 In a typical process, 10 mg of GF was placed in a 20 mL vial with 10 g of alcohol and fragmented by electromagnetic stirring with a stir bar for 3 h. The precipitation of the FGF was collected after standing for 24 h and the upper alcohol layer was removed. Then the FGF solution was added dropwise to a pattern with a size of 30 mm × 2 mm, and dried at 65 °C for 1 h. After that, the slice of FGF was connected with copper wires by silver paste and cured at 80 °C for 1 h. Then the solution of RFGO–DAPU-3 was added dropwise to the pattern and the solvent was dried at 65 °C for 3 h resulting in the formation of healable flexible electronics.2.5 Characterization
Scanning electronic micrographs (SEMs) were recorded with a Nova NanoSEM 450. Powder X-ray diffraction (XRD) patterns were recorded on an X-ray diffractometer (Rigaku D/Max 2500) with monochromated Cu Kα radiation (λ = 1.54 Å) at a scanning rate of 4° min−1. X-ray photoelectron spectroscopy (XPS) analyses were conducted with a XSAM800 system, where an Al Kα excitation source was used. Atomic Force Microscopy (AFM) images were recorded with a Dimension Icon (Bruker, USA) and operated in air in tapping mode. Fourier transform infrared (FTIR) spectra were recorded with a Bruker Vertex 70 spectrometer (Bruker Optik GmbH, Ettlingen, Germany) in the range of 4000–400 cm−1. Differential scanning calorimetry (DSC) was performed on a Q20-1173 DSC thermal system (TA instruments, New Castle, USA) with a heating rate of 10 °C min−1 and ranging from −45 °C to 180 °C. Nitrogen gas was purged at a flow rate of about 50 mL min−1. Thermal gravity analysis (TGA) was made on a TA SDTQ600 thermo-gravimetric analyzer; the microbalance has a precision of ±0.1 μg. Samples of about 10 mg were placed onto 70 μL alumina pans. The samples were heated from 30 °C to 800 °C under a nitrogen flow of 100 mL min−1. Tensile tests were conducted on a stretching machine (AG-X Plus100N, Japan) at room temperature with a cross-head speed of 10 mm min−1. The specimens were dumbbell-like (35 mm × 6mm × 0.1–0.3 mm).2.6 Self-healing test
The dumbbell-like samples were first torn using the tensile test machine with a head speed of 10 mm min−1. Then the two broken surfaces were immediately reunited, subjected to a gentle pressure and exposed them to a 800 W domestic microwave oven operating at 2.45 GHz for 5 min followed by 2 h at 70 °C without any continuous pressure. The healing efficiency was employed to evaluate the healing performance of the as-prepared DAPU and RFGO–DAPUs, which was calculated based on the ratio of the Young' modulus, breaking (tensile) strength as well as breaking strain of the healed sample to those of the virgin sample.3 Results and discussion
3.1 Characterization of RFGO, DAPU and RFGO–DAPUs
Graphene, which possesses good compatibility with polymer materials due to its large π-conjugated system, has been widely used as an efficient filler to enhance the composites because of their ultrahigh mechanical strength.31 However, the strong π–π reaction usually leads to the aggregation of graphene sheets which severely deteriorate the dispersion or the mechanical performance of the composites.32,33 In contrast to graphene, GO sheets with plenty of oxygen-containing groups exhibit good dispersion in water. However, its dispersion in organic solvents is poor and the defects of GO sheets which come from graphite during serious oxidation would affect the special physical properties.34 So it is necessary to modify GO and then reduce the sheets in order to improve its dispersion in organic solvents and take advantage of its physical properties, for example, the absorption of microwaves.35 So, in this work, we designed to employ GO sheets as raw materials which were modified by furfurylamine at first and then reduced by hydrazine hydrate to prepare the RFGO as shown in Scheme 1. The modification of GO by furfurylamine not only resulted in excellent dispersion but also introduced furfuryl into the GO sheets which would react with maleimide in the following steps forming a six-membered ring based on DA chemistry. Then the functionalized GO sheets were carefully reduced by hydrazine hydrate which would benefit for the absorption of microwaves. So, RFGO here served three purposes: firstly, the sheets served as fillers to reinforce the composites; secondly, the furfuryl groups on the surface of the graphene sheets would participate in the DA reactions in the following process; thirdly, the RFGO served as the absorption materials for the microwaves which could be used to achieve the microwave-to-heat process to heal the composites.As shown in Fig. 1a, the RFGO solutions possessed excellent stability and did not show any precipitation at the bottom of the bottle after placing for 30 days and the RFGO was tested by AFM which showed that the RFGO exhibited a similar sheet structure with GO (Fig. S1, ESI†) and the thickness was about 1.03 nm (Fig. 1a). The successful functionalization and reduction of GO were also examined using FTIR, XPS, XRD as well as TGA. Fig. 1b shows the FTIR spectrum of RFGO. The peak at 3329 cm−1 confirmed the presence of the –NH– group and the peaks at 2925 and 2852 cm−1 belongs to the –CH2– of furfurylamine. Besides, the peaks at 1495, 1413, and 1388 cm−1 should be assigned to the furfuryl, and the peaks at 1252 and 1059 cm−1 confirmed the presence of C–O–C of furfuryl of furfurylamine as well. In addition, the peak at 1726 cm−1 is ascribed to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of the reduced GO, which means most of the oxygen containing groups were removed. XPS spectra in Fig. 1c and d exhibited three characteristic peaks located at ca. 285, 399 and 532 eV, corresponding, respectively, to C 1s, N 1s, and O 1s, which means that the N atom was successfully introduced into the RFGO. The elemental content of C, N and O in RFGO was 81.98%, 9.11% and 8.91%, respectively. The high-resolution spectrum of C 1s in RFGO could be fitted by several peaks which corresponded to C–C (284.8 eV), C–O (286.7 eV), and C
O of the reduced GO, which means most of the oxygen containing groups were removed. XPS spectra in Fig. 1c and d exhibited three characteristic peaks located at ca. 285, 399 and 532 eV, corresponding, respectively, to C 1s, N 1s, and O 1s, which means that the N atom was successfully introduced into the RFGO. The elemental content of C, N and O in RFGO was 81.98%, 9.11% and 8.91%, respectively. The high-resolution spectrum of C 1s in RFGO could be fitted by several peaks which corresponded to C–C (284.8 eV), C–O (286.7 eV), and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (288.0 eV), while the particular peak located at 285.6 eV suggests the presence of C–N. In the XRD patterns (Fig. S2, ESI†), the peak of GO is at around 2θ = 11.00° (d-spacing = 8.10 Å). After functionalization and reduction of GO, a new broadened diffraction peak 2θ = 24.78° (d-spacing = 3.59 Å) appeared for RFGO, which is close to the d-spacing of 3.36 Å of graphite. Besides all the analysis above, the TGA curve of the RFGO displayed a slowly downward sloping line compared with that of GO and the char yields at 800 °C (68.23%) was much higher than that of GO (36.94%), which means that its thermal stability was enhanced due to the removal of oxygen-containing groups (Fig. S3, ESI†).
O (288.0 eV), while the particular peak located at 285.6 eV suggests the presence of C–N. In the XRD patterns (Fig. S2, ESI†), the peak of GO is at around 2θ = 11.00° (d-spacing = 8.10 Å). After functionalization and reduction of GO, a new broadened diffraction peak 2θ = 24.78° (d-spacing = 3.59 Å) appeared for RFGO, which is close to the d-spacing of 3.36 Å of graphite. Besides all the analysis above, the TGA curve of the RFGO displayed a slowly downward sloping line compared with that of GO and the char yields at 800 °C (68.23%) was much higher than that of GO (36.94%), which means that its thermal stability was enhanced due to the removal of oxygen-containing groups (Fig. S3, ESI†).
After the successful preparation of RFGO, the composites of RFGO–DAPUs were synthesized as shown in Scheme 2. At first, 2F2OH was prepared by the reaction between DGEBA and furfurylamine and then it was used to synthesize the FLPU with the pendant group of furfuryl. Finally, RFGO, FLPU and BMI were cross-linked together based on DA chemistry to obtain self-healing composites. Fig. S4 (ESI†) shows the 1H NMR spectra of 2F2OH. The successful synthesis of 2F2OH, FLPU and RFGO–DAPU was tested by FTIR and is shown in Fig. 2. The peak at 740 cm−1 which should be assigned to the furfuryl was obvious in 2F2OH and FLPU while it disappeared after the reaction with BMI resulting in DAPU. And two peaks appeared at 1713 cm−1 and 1778 cm−1 which should be ascribed to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of BMI and C
O of BMI and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C of the DA adduct.21–23
C of the DA adduct.21–23
As is well known, the distribution of nanofillers in the composite would significantly affect the mechanical properties. Before the investigation of the performance of the as-prepared RFGO–DAPUs, the distribution of RFGO sheets in the composites was observed, and the investigation of the distribution is carried out by using SEM on the cryogenic surface as shown in Fig. 3. It is seen that the RFGO sheets are well dispersed in the polymer matrix even at high content (1 wt%). Compared with our previous work on the preparation of GO/PU composites by in situ polymerization, the RFGO–DAPU composites showed much better dispersion in the polymer matrix,22 which can be ascribed to the good dispersion of RFGO in DMF and also to the modification of furfuryl on the RFGO which could react with BMI together with FLPU at the same time. In this case, the RFGO exhibited excellent dispersion in the polymer and improved the performance of the composites.
 | ||
| Fig. 3 The cryogenically fractured cross-section SEM images of (a) DAPU, (b) RFGO–DAPU-1, (c) RFGO–DAPU-2 and (d) RFGO–DAPU-3. | ||
The successfully occurred DA chemistry was detected by DSC in the following discussion which was verified by the characteristic peak of retro-DA (rDA) reaction as shown in Fig. 4a. The DSC curves of DAPU and RFGO–DAPUs exhibit significant endothermic peaks at 130–150 °C which was predominately attributed to the rDA reaction. Here, it should be noticed that the introduction of RFGO did not show a significant effect on the endothermic temperature of the DA chemistry which might be ascribed to the nature of the DA reaction.20,36 Therefore, the successfully synthesis of the RFGO–DAPU composites has been proved by the rDA reaction which provides thermal healing capability because of the thermal reversibility of the DA chemistry. And it is interesting that compared with DAPU, RFGO–DAPUs exhibited a melting point at about 15 °C. This phenomenon should be attributed to the introduction of RFGO and its good distribution in the composite improved the crystallinity of the RFGO–DAPUs.37
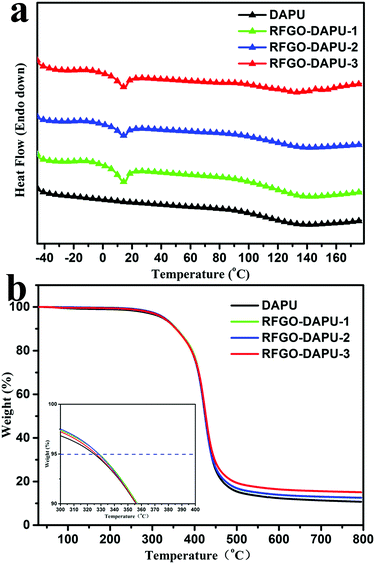 | ||
| Fig. 4 (a) DSC curves of DAPU and RFGO–DAPUs and (b) TGA curves of DAPU and RFGO–DAPUs (inset: the temperature at 5% weight loss (T5) of DAPU and RFGO–DAPUs). | ||
Besides, the thermal stability of DAPU and RFGO–DAPUs were assessed by TGA under a nitrogen atmosphere between 30 °C and 800 °C as shown in Fig. 4b. It can be seen that the decomposition of pure DAPU and RFGO–DAPUs took place in a single step which shows the uniformity of as-prepared materials. The decomposition all started at around 320 °C and from the inset figure we can see that the temperature at 5% weight loss (T5) decreased with increasing RFGO compared with pure DAPU and all these composites possess excellent thermal stability and maintain the T5 higher than 325 °C. In short, the temperature showed a slight increase with the addition of RFGO in the PU matrix ranging from 325 °C to 328 °C. Also, it can be seen that with the addition of RFGO the char yields of DAPU and RFGO–DAPUs at 800 °C increased slightly as well.
3.2 Mechanical properties of DAPU and RFGO–DAPU composites
With the good dispersion of RFGO in the composite, it was expected that the mechanical properties of the polymer would be enhanced due to the large aspect ratio of RFGO, and the covalent bonding between RFGO and the PU matrix. The mechanical properties of RFGO–DAPU composites are shown in Fig. 5 and summarized in Table 1. From the typical stress–strain curves, we can see that the Young's modulus of DAPU was about 29.27 ± 2.44 MPa which is much higher than that of the linear PU in our previous work.22 With the addition of RFGO, the Young’s modulus of the composites increased to 51.91 ± 3.28 MPa (RFGO–DAPU-3) and the increment was more than 177%, while the strain-at-break and the stress-at-break increased at first and then decreased. The average strain-at-break and stress-at-break of DAPU were 358 ± 12% and 19.92 ± 1.22 MPa and the values increased to more than 400% and 25 MPa and then decreased to 343 ± 21% and 24.22 ± 3.39 MPa for RFGO–DAPU-3. This can be ascribed to the fact that the composite with higher RFGO loading brings about the concentration of stress at RFGO sheets and leads to the breaking of the films during the stretching process. On the other hand, the RFGO sheets in the composites disturb the orientations of the polymer chain at high elongations at the same time.38| Sample | Young' modulus (MPa) | Strain-at-break (%) | Stress-at-break (MPa) |
|---|---|---|---|
| DAPU | 29.27 ± 2.44 | 358 ± 12 | 19.92 ± 1.22 |
| RFGO–DAPU-1 | 34.48 ± 0.68 | 420 ± 56 | 24.55 ± 3.11 |
| RFGO–DAPU-2 | 42.30 ± 3.18 | 410 ± 23 | 26.06 ± 2.59 |
| RFGO–DAPU-3 | 51.91 ± 3.28 | 343 ± 21 | 24.22 ± 3.39 |
3.3 The healing process using microwaves
Graphene materials also show excellent absorption of electromagnetic wave35,39 which could be used for heating and healing of self-healing materials. Chen’s group40 reported that graphene and commercially available thermoplastic polyurethane composites could be healed using microwaves. However, to the best of our knowledge, healable graphene and polymer composites based on DA chemistry using microwaves has not been reported before. Thus, we carefully investigated the microwave healing behaviors of the RFGO–DAPUs samples by exposing them to the waves of a domestic microwave oven. The original samples were first torn by the tensile test machine with a head speed of 10 mm min−1. Then the two broken surfaces were immediately reunited, subjected to a gentle pressure and stored in an 800 W domestic microwave oven operating at 2.45 GHz for 5 min. The detailed data of the mechanical properties of the healed films at different RFGO contents are shown in Table 2. After the microwave healing process the Young's modulus, breaking strain and breaking stress of the healed samples recovered significantly. The recovery of the mechanical properties should be ascribed to the recombination of the network formed by the Diels–Alder chemistry of the free furan–maleimide groups which were generated by the stretching process before as the interaction of the DA bond is much weaker than the other covalent bonds. The microwaves absorbed by RFGO turned into heat and then promoted the healing process of the composites based on DA chemistry. Fig. 6 shows the typical stress–strain curves before and after the healing tests of DAPU and RFGO–DAPUs films. Here, the healing efficiency is represented by the ratio of the Young’ modulus, breaking strength as well as breaking strain of the healed sample to those of the original samples. And the healing efficiencies of DAPU estimated from the experiments are presented in Fig. 7 which were 63.5 ± 2.5%, 51.0 ± 4.7% and 53.0 ± 4.5% for the Young’ modulus, breaking strength and breaking strain, respectively. With the increase of RFGO, the healing efficiencies increased which were 86.1 ± 4.5%, 70.0 ± 6.0% and 64.7 ± 4.5% for RFGO–DAPU-1, 86.8 ± 7.5%, 71.2 ± 5.6% and 70.3 ± 6.2% for RFGO–DAPU-2 and 83.8 ± 4.5%, 87.5 ± 8.7% and 93.2 ± 11.4% for RFGO–DAPU-3, respectively. The healing efficiencies were much higher than those of DAPU. The reason should be that the more RFGO in the composites the more microwaves could be absorbed and then more heat is transferred in the composites which resulted in higher healing efficiencies.40 And can be expected that with addition of a higher amount of RFGO, the healing time for the composite would be shorter for the healing process using microwaves.| Sample | Young' modulus (MPa) | Strain-at-break (%) | Stress-at-break (MPa) |
|---|---|---|---|
| DAPU | 18.6 ± 1.42 | 184 ± 17 | 10.57 ± 0.91 |
| RFGO–DAPU-1 | 29.67 ± 1.55 | 296 ± 65 | 15.89 ± 1.11 |
| RFGO–DAPU-2 | 36.7 ± 5.27 | 292 ± 23 | 18.33 ± 2.67 |
| RFGO–DAPU-3 | 43.5 ± 2.34 | 300 ± 30 | 22.56 ± 2.76 |
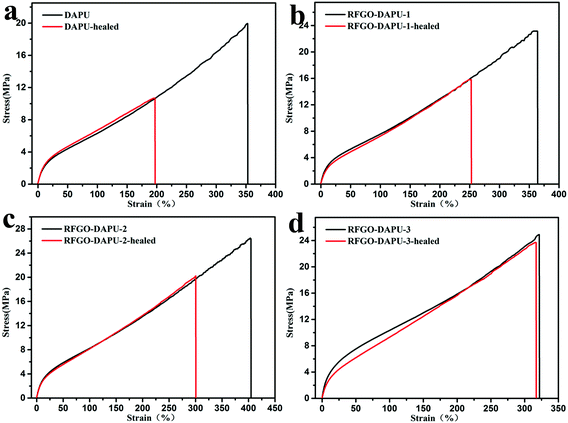 | ||
| Fig. 6 Stress–strain curves of DAPU and RFGO–DAPU films before and after microwave healing: (a) DAPU, (b) RFGO–DAPU-1, (c) RFGO–DAPU-2, and (d) RFGO–DAPU-3. | ||
 | ||
| Fig. 7 Healing efficiencies of DAPU and RFGO–DAPUs determined by the recovery of Young' modulus, breaking (tensile) strength as well as breaking strain. | ||
3.4 The application of healable composites for flexible conductors and strain sensors
Flexible conductors and sensors have been widely investigated for their growing demand in real-time healthcare monitoring, wearable displays and flexible consumer electronics.6 To prepare flexible electronics, flexible polymers and composites are essential.41 And most of the employed polymers for flexible electronics such as PDMS lack the healing properties.42,43 The possibility of introducing self-healing properties to flexible devices that compensate for incidental scratches or mechanical cuts shows great importance in future applications.44–46 As discussed before, RFGO–DAPU-3 exhibited high flexibility, great stretchability and excellent self-healing properties with the highest healing efficiency compared with DAPU and RFGO–DAPU-1 and RFGO–DAPU-2. In this case, RFGO–DAPU-3 was employed as the self-healing elastic matrix to prepare self-healing flexible conductors and strain sensors. In addition, 3D FGFs (Fig. S5, ESI†) obtained by the fragmentation of graphene foam, which were prepared by the self-assembly process of GO and ammonium sulfide as we reported before,30 were employed as conductive materials.The as-prepared flexible electronic material was made up of 3D FGFs, which formed the conductive path, and RFGO–DAPU-3, which offered flexibility and stretchability (as shown in Fig. 8a). Fig. 8b shows the cross-sectional image of an FGF/RFGO–DAPU-3 composite. The total thickness is about 165 μm and the thickness of the FGF layer is about 35 μm (Fig. S6, ESI†). The thin layer of the composite would significantly benefit its flexibility and sensitivity for strain sensors. The FGF/RFGO–DAPU-3 composite then was connected in the circuit and the unique structure of the film rendered it highly bendable and foldable while maintaining its original conductivity (Fig. 8c and Fig. S7, ESI†). In addition, the FGF/RFGO–DAPU-3 composite could be used as a flexible strain sensor as well and could detect biosignals, for example finger bending. Fig. 8d shows the relative change in resistance in several bending cycles demonstrating a favorable reproducibility. The results illustrated that the FGF/RFGO–DAPU-3 composite might be potentially applied as a wearable strain sensor material.
The healing ability of the FGF/RFGO–DAPU-3 composite using microwaves was investigated and is shown in Fig. 9. At first, the circuit was connected by the FGF/RFGO–DAPU-3 composite and the LED lamp was on. Then the FGF/RFGO–DAPU-3 composite were cut off by a clean blade, and the LED lamp was down and the microscope picture showed a clear crack of about 20 μm width. After that, the composite was pressed together and then put into the microwave oven for 5 min and then reconnected in the circuit. It was found that the LED lamp was on again and the microscope picture also proved that the crack almost disappeared. In this way, the FGF/RFGO–DAPU-3 composite exhibited its potential application in flexible electronics as well as its healing ability using microwaves.
4 Conclusions
In summary, we have successfully synthesized covalently cross-linked reduced functionalized graphene oxide/polyurethane composites based on the Diels–Alder chemistry and investigated the application and healing in flexible electronics. The RFGO sheets showed good dispersion in the polymer matrix and provided a significant enhancement of the mechanical properties, thermal stability, and the healing ability using microwaves. The as-prepared composites were successfully applied to the fabrication of self-healing flexible electronics with the combination of 3D FGF. The FGF/RFGO–DAPU-3 composites were employed as flexible conductors and strain sensors which successfully detected the biosignals of finger bending. In addition, these flexible electronics could be healed using microwaves in 5 minutes showing great potential in healable flexible electronics. In this case, the as-prepared RFGO–DAPUs not only serve as potential candidates for promising self-healing composites but also exhibit great applications in self-healing flexible electronics for next generation electronic devices.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant No. 21201175), the Guangdong and Shenzhen Innovative Research Team Program (No. 2011D052, KYPT20121228160843692), the R&D Funds for basic Research Program of Shenzhen (Grant No. JCYJ20150401145529012, JCYJ20150831154213681 and JCYJ20140418095735619), the Shenzhen Fundamental Research Program (JCYJ20160331191741738, JSGG20160229194437896), and the Key Deployment Project of Chinese Academy of Sciences (Grant No. KFZD-SW-202).References
- T. Cheng, Y. Zhang, W. Y. Lai and W. Huang, Adv. Mater., 2015, 27, 3349–3376 CrossRef CAS PubMed.
- Y. Tang, Z. Zhao, H. Hu, Y. Liu, X. Wang, S. Zhou and J. Qiu, ACS Appl. Mater. Interfaces, 2015, 7, 27432–27439 CAS.
- T. Yamada, Y. Hayamizu, Y. Yamamoto, Y. Yomogida, A. Izadi-Najafabadi, D. N. Futaba and K. Hata, Nat. Nanotechnol., 2011, 6, 296–301 CrossRef CAS PubMed.
- J. Y. Oh, S. Kim, H. K. Baik and U. Jeong, Adv. Mater., 2016, 28, 4455–4461 CrossRef CAS PubMed.
- Z. Chen, W. Ren, L. Gao, B. Liu, S. Pei and H.-M. Cheng, Nat. Mater., 2011, 10, 424–428 CrossRef CAS PubMed.
- J. Li, S. Zhao, X. Zeng, W. Huang, Z. Gong, G. Zhang, R. Sun and C.-P. Wong, ACS Appl. Mater. Interfaces, 2016, 8, 18954–18961 CAS.
- Y. Wei, S. Chen, X. Yuan, P. Wang and L. Liu, Adv. Funct. Mater., 2016, 26, 5078–5085 CrossRef CAS.
- Y. Lin, X. Dong, S. Liu, S. Chen, Y. Wei and L. Liu, ACS Appl. Mater. Interfaces, 2016, 8, 24143–24151 CAS.
- Y. Lin, S. Liu, S. Chen, Y. Wei and X. Dong, J. Mater. Chem. C, 2016, 4, 6345–6352 RSC.
- S. Zhao, J. Li, D. Cao, Y. Gao, W. Huang, G. Zhang, R. Sun and C.-P. Wong, J. Mater. Chem. C, 2016, 4, 6666–6674 RSC.
- S. J. Benight, C. Wang, J. B. Tok and Z. Bao, Prog. Polym. Sci., 2013, 38, 1961–1977 CrossRef CAS.
- H. Jin, C. L. Mangun, A. S. Griffin, J. S. Moore, N. R. Sottos and S. R. White, Adv. Mater., 2014, 26, 282–287 CrossRef CAS PubMed.
- D. S. Xiao, Y. C. Yuan, M. Z. Rong and M. Q. Zhang, Polymer, 2009, 50, 560–568 CrossRef CAS.
- C. J. Hansen, W. Wu, K. S. Toohey, N. R. Sottos, S. R. White and J. A. Lewis, Adv. Mater., 2009, 21, 4143–4147 CrossRef CAS.
- V. Vahedi, P. Pasbakhsh, C. S. Piao and C. E. Seng, J. Mater. Chem. A, 2015, 3, 16005–16012 CAS.
- S. R. White, N. Sottos, P. Geubelle, J. Moore, M. R. Kessler, S. Sriram, E. Brown and S. Viswanathan, Nature, 2001, 409, 794–797 CrossRef CAS PubMed.
- Y. Yang and M. W. Urban, Chem. Soc. Rev., 2013, 42, 7446–7467 RSC.
- E. B. Murphy and F. Wudl, Prog. Polym. Sci., 2010, 35, 223–251 CrossRef CAS.
- X. Chen, M. A. Dam, K. Ono, A. Mal, H. Shen, S. R. Nutt, K. Sheran and F. Wudl, Science, 2002, 295, 1698–1702 CrossRef CAS PubMed.
- Y.-L. Liu and T.-W. Chuo, Polym. Chem., 2013, 4, 2194–2205 RSC.
- J. Li, G. Zhang, L. Deng, K. Jiang, S. Zhao, Y. Gao, R. Sun and C. Wong, J. Appl. Polym. Sci., 2015, 132, 42167 Search PubMed.
- J. Li, G. Zhang, L. Deng, S. Zhao, Y. Gao, K. Jiang, R. Sun and C. Wong, J. Mater. Chem. A, 2014, 2, 20642–20649 CAS.
- G. Fu, L. Yuan, G. Liang and A. Gu, J. Mater. Chem. A, 2016, 4, 4232–4241 CAS.
- J. Wertz, J. Kuczynski and D. Boday, ACS Appl. Mater. Interfaces, 2016, 8, 13669–13672 CAS.
- K.-h. Pyo, D. H. Lee, Y. Kim and J.-W. Kim, J. Mater. Chem. C, 2016, 4, 972–977 RSC.
- B. Willocq, R. Bose, F. Khelifa, S. Garcia, P. Dubois and J.-M. Raquez, J. Mater. Chem. A, 2016, 4, 4089–4097 CAS.
- P. Tang, G. Hu, Y. Gao, W. Li, S. Yao, Z. Liu and D. Ma, Sci. Rep., 2014, 4, 5901 CAS.
- D. Voiry, J. Yang, J. Kupferberg, R. Fullon, C. Lee, H. Y. Jeong, H. S. Shin and M. Chhowalla, Science, 2016, 353, 1413–1416 CrossRef CAS PubMed.
- W. S. Hummers Jr and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef.
- J. Li, S. Zhao, G. Zhang, Y. Gao, L. Deng, R. Sun and C.-P. Wong, J. Mater. Chem. A, 2015, 3, 15482–15488 CAS.
- C. Lee, X. Wei, J. W. Kysar and J. Hone, Science, 2008, 321, 385–388 CrossRef CAS PubMed.
- M. Zhang, Y. Li, Z. Su and G. Wei, Polym. Chem., 2015, 6, 6107–6124 RSC.
- R. Verdejo, M. M. Bernal, L. J. Romasanta and M. A. Lopez-Manchado, J. Mater. Chem., 2011, 21, 3301–3310 RSC.
- D. R. Dreyer, S. Park, C. W. Bielawski and R. S. Ruoff, Chem. Soc. Rev., 2010, 39, 228–240 RSC.
- H. Hu, Z. Zhao, W. Wan, Y. Gogotsi and J. Qiu, Adv. Mater., 2013, 25, 2219–2223 CrossRef CAS PubMed.
- M. Scheiner, T. J. Dickens and O. Okoli, Polymer, 2016, 83, 260–282 CrossRef CAS.
- H. Xu, Z.-X. Feng, L. Xie and M. Hakkarainen, ACS Sustainable Chem. Eng., 2015, 4, 334–349 CrossRef.
- J. T. Kim, B. K. Kim, E. Y. Kim, S. H. Kwon and H. M. Jeong, Eur. Polym. J., 2013, 49, 3889–3896 CrossRef CAS.
- Y. Zhu, S. Murali, M. D. Stoller, A. Velamakanni, R. D. Piner and R. S. Ruoff, Carbon, 2010, 48, 2118–2122 CrossRef CAS.
- L. Huang, N. Yi, Y. Wu, Y. Zhang, Q. Zhang, Y. Huang, Y. Ma and Y. Chen, Adv. Mater., 2013, 25, 2224–2228 CrossRef CAS PubMed.
- M. Amjadi, K. U. Kyung, I. Park and M. Sitti, Adv. Funct. Mater., 2016, 26, 1678–1698 CrossRef CAS.
- Y. A. Samad, Y. Li, S. M. Alhassan and K. Liao, ACS Appl. Mater. Interfaces, 2015, 7, 9195–9202 CAS.
- R. Xu, Y. Lu, C. Jiang, J. Chen, P. Mao, G. Gao, L. Zhang and S. Wu, ACS Appl. Mater. Interfaces, 2014, 6, 13455–13460 CAS.
- T. P. Huynh and H. Haick, Adv. Mater., 2016, 28, 138–143 CrossRef CAS PubMed.
- J. Li, S. Qi, J. Liang, L. Li, Y. Xiong, W. Hu and Q. Pei, ACS Appl. Mater. Interfaces, 2015, 7, 14140–14149 CAS.
- Y. Yang, B. Zhu, D. Yin, J. Wei, Z. Wang, R. Xiong, J. Shi, Z. Liu and Q. Lei, Nano Energy, 2015, 17, 1–9 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6tc04715g |
| This journal is © The Royal Society of Chemistry 2017 |







