Ab initio characterization and experimental validation on the roles of oxygen-containing groups in graphene based formaldehyde sensors†
Liangping
Duan
,
Zheng
Bo
 *,
Xia
Chen
,
Hualei
Qi
,
Jianhua
Yan
and
Kefa
Cen
*,
Xia
Chen
,
Hualei
Qi
,
Jianhua
Yan
and
Kefa
Cen
State Key Laboratory of Clean Energy Utilization, Institute for Thermal Power Engineering, College of Energy Engineering, Zhejiang University, Hangzhou, Zhejiang Province 310027, China. E-mail: bozh@zju.edu.cn; Fax: +86 571 87951616; Tel: +86 571 87951369
First published on 4th September 2017
Abstract
The development of formaldehyde (HCHO) sensors employing reduced graphene oxide (rGO) as sensing materials calls for a profound, atomic level understanding on the roles of oxygen-containing groups. In this work, the performances of rGO-based HCHO sensors were investigated using ab initio calculations and experimental validation. Density functional theory (DFT) simulations were performed to calculate the adsorption energy (Eads) and charge transfer (ΔQ) for the adsorption of HCHO on pristine graphene, rGO with epoxides, rGO with hydroxyl groups, and rGO with carboxyl groups. The results show that the incorporation of oxygen-containing groups leads to an obvious increase of Eads and ΔQ values, with an order of carboxyl group > hydroxyl group > epoxides > pristine graphene. The increase of Eads and ΔQ values could increase the variation in the concentration of charge carriers, the change of conductance of the sensing materials, and hence the sensor response. The experimental measurements indicate that with a decrease in the C/O atomic ratio from 16.2 to 6.6, the sensor response to 1 ppm HCHO increases from 0.10% to 0.73%, confirming the DFT calculation results. Moreover, even with a certain C/O atomic ratio of ∼6.6, rGO with 6.80% carboxyl groups exhibits a distinctly larger response to 0.2–3 ppm HCHO, compared with the counterpart with 3.09% carboxyl groups. The as-obtained insights into the effects of oxygen-containing groups on the response of rGO to HCHO could be instructive for preparing rGO-based HCHO sensors for advanced performances.
Introduction
Formaldehyde (HCHO) is one of the most common indoor air pollutants and has been categorized as a human carcinogen by the U.S. Environment Protection Agency (EPA).1 Continuous endeavors have been directed towards high quality sensors for the rapid and effective detection of HCHO. Among various practices, the chemoresistive sensor has attracted great attention due to its long life-time, simple structure, low cost, inherent miniaturization, great compatibility with integrated circuit (IC) technology, etc.2,3The basic principle of a common chemoresistive sensor is the adsorption and desorption of gas molecules on sensing materials.4 It has been extensively demonstrated that sensing materials play a crucial role in determining the overall performance of sensors.2,5,6 The great interest in using graphene as a sensing material mainly stems from its unique two-dimensional morphology and a series of superior physical and chemical properties, such as ultrathin structure, huge specific surface area, high conductivity, and low electrical noise.4,7–9 For practical sensing applications, reduced graphene oxide (rGO), instead of pristine single-layer graphene, is preferred due to its low production cost and fine-tuning of properties (e.g., conductivity and dispersibility in water).10–14 Studies on HCHO detection utilizing rGO-based materials laid emphasis on the hybridization of rGO with nanoparticles (e.g., SnOx, ZnO, and TiO2) and polymers (e.g., PMMA and PMAA) to improve the sensing performances.4,6–9,15 Chu et al.7 reported that sensors based on rGO/SnO2 composites could detect 1 ppb HCHO with a fast response and recovery. Alizadeh et al.4 blended rGO with PMMA as sensing materials and found that the corresponding sensor exhibited a linear response to 0.05–0.5 ppm HCHO with a detection limit of 0.01 ppm.
On the other hand, the residual oxygen-containing groups in rGO16 could significantly influence the sensing behaviors (e.g., response, selectivity, and reversibility).17–22 Fowler et al.20 reported that the slow recovery of sensing signals could be attributed to the oxygen-containing groups. Robinson et al.19 found that the reduction level of rGO affects both the sensor response and noise. Phan et al.21 indicated that the response of the humidity sensor increased with the increase of the amount of oxygen-containing groups. Similar results were obtained with carbon nanotube based sensors, where the presence of oxygen-containing groups could lead to a manyfold increase in the sensor response.17,18
Basically, the response of the chemoresistive sensor is ascribed to the change of conductance, that is, the variation of the concentration of charge carriers (i.e., holes or electrons) with the adsorption of gas molecules.4,7,23,24 Oxygen-containing groups might influence the adsorption of gas molecules, for example, the adsorption energy (Eads) and charge transfer (ΔQ). The incorporation of oxygen-containing groups in graphene could increase the Eads and ΔQ values, thereby enhancing the variation of the concentration of charge carriers and hence the sensor response. The aforementioned effects of oxygen-containing groups may be mainly responsible for the observed improvement of the sensor response.17–21 Consequently, a profound understanding of the adsorption of HCHO on oxygen-containing groups, especially at an atomic level, is thus needed for the optimization of rGO-based HCHO sensors.
Density functional theory (DFT) calculation is a powerful tool to study the adsorption of gas molecules on a solid surface.25–27 It has been demonstrated that DFT calculations can provide reliable information on the molecular/electronic properties (e.g., energy and electron distribution) via iterative calculations of electron density (ρ(r)). The adsorption energy, distance above the surface, and charge transfer are generally investigated for the adsorption of gas molecules. Chi et al.28 reported that the Eads and ΔQ values for the adsorption of HCHO on Al-doped graphene are at least 8 times larger than those of HCHO on pristine graphene. Maaghoul et al.29 revealed that the decoration of Ni on a graphene surface could strengthen the adsorption of HCHO with a larger Eads and ΔQ value. While great efforts have been devoted to the calculation of adsorption of HCHO on graphene doped with heteroatoms (Al, Ti, Ni, B, N, and S),28–31 the understanding of the roles of oxygen-containing groups in HCHO sensing from DFT insight has not received the attention it deserves.
This work provides an ab initio characterization, in conjunction with the experimental validation of the roles of oxygen-containing groups in HCHO sensing. Much attention has been paid to determine the influence of carboxyl groups. DFT simulations were performed to calculate the Eads and ΔQ values for the adsorption of HCHO on pristine graphene and rGO with different oxygen-containing groups (i.e., epoxides, hydroxyl groups, and carboxyl groups). Experiments on the HCHO sensing performances of rGO with different reduction levels and carboxyl functionalized rGO were then performed to confirm the DFT calculation results. The as-obtained findings could facilitate the designing of rGO-based HCHO sensors for advanced performances.
Simulation and experimental methods
DFT calculations
All the DFT calculations were performed by using the OpenMX (Open source package for Material eXplorer) code.32 The pseudo atomic orbitals (PAOs) were used as the basis function for expanding the one-particle Kohn–Sham wave functions. The PAOs were specified by H7.0-s2p1, C7.0-s2p2d1, and O7.0-s2p2d1 for H, C, and O atoms, respectively. Specifically, 7.0 represents the cutoff atomic radius in Bohr, and s2p2d1 indicates that the basic functions are expanded by two primitive s, p orbitals and one primitive d orbital. The generalized gradient approximation (GGA) for the exchange–correlation functional described by Perdew–Burke–Ernzerhof (PBE) was employed for the calculations.33 The norm-conserving pseudopotential proposed by Morrison et al.34 was used to describe the core electrons. Moreover, the spin-polarization was set to be off for all the calculations. A 5 × 5 × 1 supercell with periodic boundary conditions on the x-axes and y-axes was used to calculate the adsorption of HCHO on the surface (i.e., graphene and rGO). A vacuum region of 20 Å was built to avoid the interaction between periodic images. The energy cutoff for the numerical calculations was finally set to 200 Ry according to the convergence test. Special point sampling integration over the Brillouin zone was employed using Monkhorst–Pack schemes35 with a 4 × 4 × 1 k-point mesh. The convergence criterion for the energy calculation was set to be 3.5 × 10−7 Ha. For geometry optimization, the force tolerance was 1.0 × 10−4 Ha per Bohr. Moreover, the van der Waals correction proposed by Grimme (DFT-D2)36 was incorporated to describe the long-range interaction.To evaluate the interaction between HCHO molecules and the surface (i.e., graphene and rGO), the adsorption energy is defined as follows:
| Eads = EHCHO + Eslab − EHCHO+slab | (1) |
| ΔQ = Qads − Qslab | (2) |
Synthesis of GO, rGO and carboxyl functionalized GO
GO was synthesized by a modified Hummer's method,38 which was mentioned in our previous report.39 Two kinds of rGO were prepared by reducing GO with hydrazine hydrate. One was obtained in a 95 °C oil bath for 2 h through a common process (labeled as ‘rGO-h95’),40 and the other was in a 20 °C oil bath for 24 h (labeled as ‘rGO-h20’).Carboxyl functionalized GO (GO-COOH) was synthesized through a reaction with chloroacetic acid under strong basic conditions.41–43 In a typical procedure, 100 mL GO suspension (1 mg mL−1) was bath-sonicated for 1.5 h to obtain a clear solution. Chloroacetic acid was dispersed in 4 M sodium hydroxide (NaOH) solution in an ice bath. Then GO aqueous solution was added to the above mixture, followed by sonication for 2 h at room temperature. The resulting GO-COOH solution was then rinsed with 4 M hydrochloric acid (HCl) solution and deionized water several times. Finally, the material was freeze-dried to obtain GO-COOH powder.
Material characterization
The morphologies of the samples were observed by using a SU-70 scanning electron microscope (SEM, Hitachi). Fourier transform infrared (FTIR) spectra were recorded on a Nicolet 5700 FTIR spectrometer. The surface chemical composition was characterized by X-ray photoelectron spectroscopy (XPS, VG Escalab Mark II) with a monochromatic Mg Kα X-ray source (1253.6 eV, West Sussex). The C/O atomic ratio is derived from XPS data. It is normally calculated as follows:| C/O = (AC 1s/AO 1s) × (0.63/0.205) | (3) |
pCOOH = (ACOOH × 2)/(ACOOH × 2 + AC–O × 1 + AC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O × 1)/(C/O). O × 1)/(C/O). | (4) |
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O represent the area of the fitted peaks of O
O represent the area of the fitted peaks of O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O, C–O, and C
C–O, C–O, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in the C 1s spectrum.
O in the C 1s spectrum.
Fabrication of sensors and sensing tests
The interdigital electrodes (IDEs) for the sensors were fabricated by a standard microfabrication procedure. Specifically, 30 nm Ti and 100 nm Au were deposited on the SiO2/Si substrates to form the IDEs with a gap distance of 2 μm.To obtain the sensing devices, GO, GO-COOH, and rGO samples were well dispersed in N,N-dimethylformamide (DMF), and 0.2 μL solution (0.003 mg mL−1) was then dropwise added onto the IDEs with a microsyringe. Finally, the IDEs were annealed at a certain temperature under an Ar flow.
The response of sensors was obtained using a homemade gas sensor measurement system (see Fig. 1). The as-prepared sensors were kept in an air-tight chamber, and a constant voltage (1 V) was applied to the IDEs. The real-time current was recorded using a Keithley 2602 source meter. Typically, the sensing tests included three steps. First, nitrogen (N2) was flowed into the chamber to record a baseline current. Then, HCHO was introduced to obtain the response signals. Finally, the sensor was recovered in N2. The total flow rate was maintained at 1 L min−1 all the time. The sensor response (R) in conductance is defined as R = (G1 − G0)/G0, where G0 and G1 are the conductance of the sensor in N2 and HCHO, respectively.
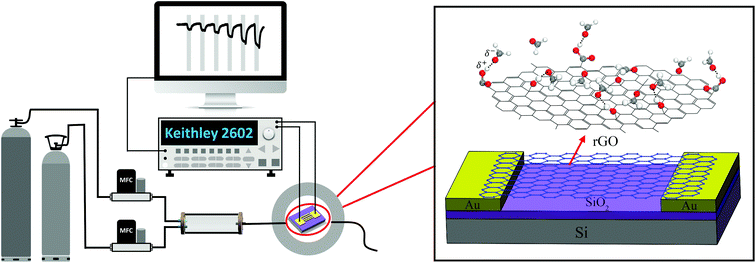 | ||
| Fig. 1 Schematic illustration of the gas sensor measurement system (the enlarged image shows the diagram of the rGO sensor device). | ||
Results and discussion
DFT calculations of HCHO adsorbed on pristine graphene
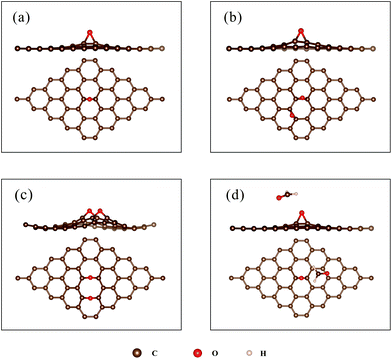
To verify the simulation methods, DFT calculations of HCHO adsorbed on pristine graphene were performed. Three initial configurations, corresponding to the structures with C, O, and H atoms close to the surface, respectively, were considered. The initial structures are shown in Fig. 2. Table 1 compares the calculated results (adsorption energy, charge transfer, and distance of HCHO above the surface) with previous studies.28 The distance listed in Table 1 is defined as the shortest length between the atom of HCHO and the graphene surface.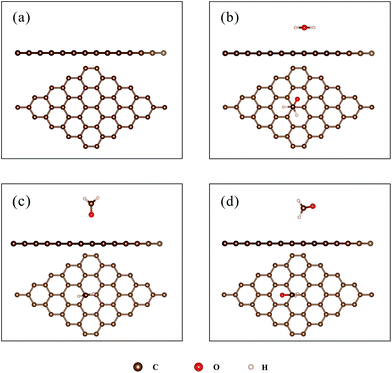 | ||
| Fig. 2 (a) The structure of pristine graphene; (b–d): the initial structure for the adsorption of HCHO on pristine graphene, (b) C-closed; (c) O-closed; (d) H-closed. | ||
| Configurations | This work | Ref. 28 | ||||
|---|---|---|---|---|---|---|
| E ads (meV) | ΔQ (e) | d (Å) | E ads (meV) | ΔQ (e) | d (Å) | |
| C-closed | 69 | −0.024 | 3.01 | 83 | −0.019 | 3.127 |
| O-closed | 34 | −0.008 | 3.17 | 32 | — | 3.327 |
| H-closed | 47 | −0.015 | 2.8 | 53 | — | 2.866 |
The adsorption energies are relatively small (34–69 meV), implying weak van der Waals interactions between the HCHO molecules and pristine graphene. The negative ΔQ indicates that HCHO grabs a few electrons (0.008–0.024e) from pristine graphene, which is in accordance with previous calculations.28,29,31,45 Moreover, the HCHO molecule is found floating above the graphene surface at about 3 Å for all adsorption configurations. As listed in Table 1, the adsorption energy, charge transfer, and the distance above the surface are in a similar range to those in previous studies,28,31 thus confirming the validity and reliability of the calculations. The as-obtained results were then compared with those of HCHO adsorbed on rGO to elucidate the role of oxygen-containing groups.
DFT calculations of HCHO adsorbed on rGO
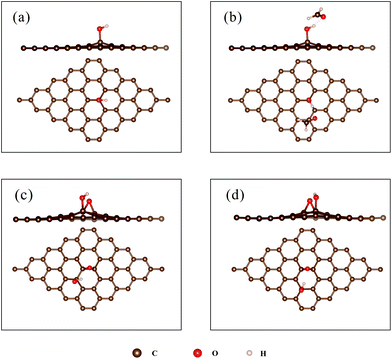
The largest Eads and ΔQ for the adsorption of HCHO on rGO with epoxides (denoted as ‘rGO-O’) are 119 meV and 0.025e, respectively. (For detailed results on each configuration, see ESI Table S1.†) The Eads and ΔQ values are larger than the corresponding values of HCHO on pristine graphene (69 meV and 0.024e). Furthermore, the Eads ranges from 47 meV to 110 meV, corresponding to HCHO adsorbed on ‘C–O__C–O’ with different initial structures (see ESI Fig. S2†). It implies that the Eads and ΔQ values are strongly related to the initial configuration. The adsorption of HCHO on epoxides could be promoted evidently with the H atom of HCHO towards O sites on the surface, whereas no obvious enhancement is observed with the O atom of HCHO close to the surface.
Furthermore, the possible influence of epoxides on the adsorption of HCHO on the hydroxyl group was studied. Two configurations of rGO with epoxides and hydroxyl groups in a carbon hexagon were considered (see Fig. 4c and d). The Eads and ΔQ values are within the range of 77–230 meV and 0.007–0.078e for different configurations (for detailed results for each configuration, see ESI Table S2†). The values are smaller than those of HCHO adsorbed on ‘rGO-OH’ (239 meV and 0.080e). The Eads and ΔQ values depend on the initial configuration. When the O atom of the HCHO molecule points to the H atom of the hydroxyl group, the Eads and ΔQ values are large due to the formation of O–H⋯O hydrogen bonds. Otherwise, the Eads and ΔQ values remain relatively small. The aforementioned results suggest that the incorporation of epoxides could not enhance the adsorption of HCHO on the hydroxyl group.
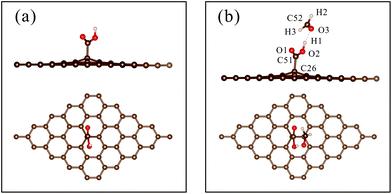 | ||
| Fig. 5 (a) The optimized structure of ‘rGO-COOH’; (b) the most stable configuration of HCHO adsorbed on ‘rGO-COOH’. | ||
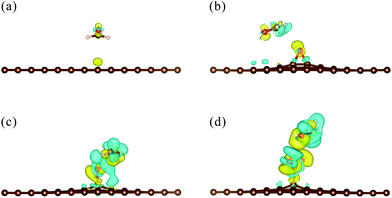
To evaluate the charge redistribution induced by HCHO molecule adsorption, Mulliken charge population analysis is summarized in Table 2. The charge of the H1 atom changes from −0189e to −0.09e, while that of the O3 atom changes from 0.193e to 0.157e. Moreover, the charge of the C52 atom decreases from −0.179e to −0.250e. The results indicate that the carboxyl group draws electrons from the HCHO molecule, leading to an increase in the number of electrons of ‘rGO-COOH’. In detail, the electropositive H1 atom attracted electrons from the electronegative O3 atom. The Pauling electronegativities of the O atom and C atom are 3.44 and 2.55,47 respectively, indicating that the O atom possesses a stronger ability to capture electrons. Thus, the O3 atom further seized electrons from the C52 atom, leading to a great decrease in the electrons of the C52 atom. Fig. 6 shows the isosurface map of the electron density difference of HCHO adsorbed on pristine graphene, ‘rGO-O’, ‘rGO-OH’, and ‘rGO-COOH’, with an isovalue of 5 × 10−4 e Å−3. The yellow and blue colors indicate electron accumulation and depletion, respectively. When the volume of the isosurface is large, the electron difference is large. The visible isosurface of HCHO adsorbed on ‘rGO-COOH’ exhibits the largest volume, indicating greatest electron transfer due to the adsorption process.
| Carboxyl group | HCHO molecule | |||||||
|---|---|---|---|---|---|---|---|---|
| C51 | O1 | O2 | H1 | O3 | C52 | H2 | H3 | |
| Before | −0.240 | 0.284 | 0.177 | −0.189 | 0.193 | −0.179 | −0.007 | −0.007 |
| After | −0.237 | 0.316 | 0.183 | −0.09 | 0.157 | −0.250 | −0.033 | −0.023 |
| Variation | 0.003 | 0.032 | 0.006 | 0.099 | −0.036 | −0.071 | −0.026 | −0.016 |
The Eads and ΔQ values for the adsorption of HCHO on ‘rGO-COOH’ are 436 meV and 0.115e, respectively. Compared with those of HCHO on pristine graphene, the Eads and ΔQ values increase by 6.3 times and 4.5 times, respectively. The enhancement of the carboxyl group is obviously larger than those of epoxides and hydroxyl groups. Similar to HCHO adsorbed on the hydroxyl group, the HCHO molecule could form a hydrogen bond with the carboxyl group (see Fig. 6d). The OH⋯O bond formed between HCHO and the carboxyl group is 1.74 Å, which is shorter than that formed between HCHO and the OH group (1.88 Å). Meanwhile, the O–H⋯O bond formed between HCHO and the carboxyl group is almost in a straight line, with an angle of 172°. The above findings imply that the O–H⋯O bond formed between HCHO and ‘rGO-COOH’ is stronger than that between HCHO and ‘rGO-OH’.48 The formation of the hydrogen bond between carboxyl groups and target gases (e.g., HCHO, NH3, and CH3OH) might be mainly responsible for the observed enhancement of the sensing performance.4,17,18,49 The hydrogen bond is commonly formed between an electropositive hydrogen (H) atom and a highly electronegative atom, such as nitrogen (N), oxygen (O), or fluorine (F). Moreover, as proposed by Eduardo Peris et al.,50 the increase of electropositivity (δ+) of the H atom or the electronegativity (δ−) of the electronegative atom could enhance the hydrogen bond strength. Before adsorption, the H atom in the carboxyl group has a positive charge of 0.189e, which is larger than that of the H atom in the hydroxyl group (0.129e). The more positively charged H atom thus contributes to the formation of a stronger hydrogen bond between HCHO and ‘rGO-COOH’, which finally leads to a greater enhancement of Eads and ΔQ values.
Experimental validation of DFT calculation results
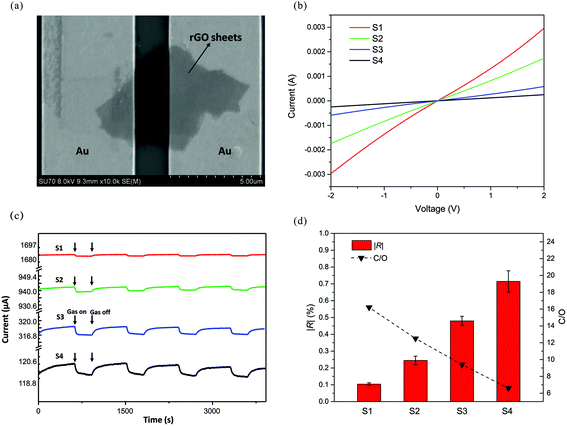
SEM was employed to observe the rGO sheets on IDEs. As shown in Fig. 7a, the rGO sheets bridge the neighbouring Au electrodes, functioning as a conducting channel. The linear current–voltage curves in Fig. 7b indicate an Ohmic contact between rGO sheets and Au electrodes. The work function of rGO varies from 4.3 to 4.7 eV,51,52 which is smaller than that of Au (5.1 eV).53 Thus, rGO forms an Ohmic contact with Au.54 The obtained sensors were then measured in the test chamber by applying a constant voltage (1 V). Fig. 7c shows the real-time current of sensors periodically exposed to 1 ppm HCHO. The current decreased when HCHO gas was introduced into the chamber. As is well known, rGO usually exhibits p-type behaviour, due to the residual oxygen-containing groups during the preparation and reduction process.10,11,55 Therefore, the positive charge carrier (holes) is the majority charge carrier in rGO and is mainly responsible for the electric current. Generally, HCHO is a reducing gas and usually functions as an electron donor. When HCHO was flowed into the test chamber, rGO adsorbed HCHO and attracted electrons from HCHO molecules, leading to the decrease of holes (majority charge carrier), and thereby the current of sensors. Fig. 7d summarizes the response and C/O atomic ratios of S1, S2, S3, and S4. The C/O atomic ratio was computed by considering the area of C 1s and O 1s peaks observed in the XPS survey spectrum, as shown in eqn (3) (for the XPS spectra of S1–S4, see ESI Fig. S7†). The highly reduced S1 exhibits a very small response to 1 ppm HCHO. With the C/O atomic ratio decreasing from 16.2 (S1) to 6.6 (S4), the response to 1 ppm HCHO increases from 0.10% to 0.73%.
To further explore the response mechanism of the rGO-based HCHO sensors, statistical thermodynamics adsorption analysis was performed. Chemoresistive sensors detect gas molecules based on the conductance change after target gas adsorption. The response in conductance (R) can be further expressed as:
| R = (σ − σ0)/σ0 | (5) |
| σ = neμ | (6) |
| R = (σ − σ0)/σ0 ≈ (n − n0)/n0 = Δn/n0 | (7) |
| θi = mi/Mi | (8) |
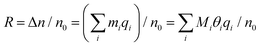 | (9) |
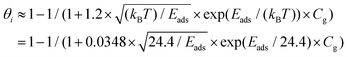 | (10) |
According to the DFT calculation results, oxygen-containing groups could significantly increase the Eads and ΔQ values for the adsorption of HCHO. Based on the above-mentioned statistical analysis, the increased Eads and ΔQ values could yield an improvement of the sensor response. Consequently, the poor response of highly reduced S1 might stem from the lack of oxygen-containing groups. The adsorption sites created by oxygen-containing groups are in small quantities and are easily saturated. Abundant HCHO molecules adsorb on vast areas of small sp2 domains with small Eads and ΔQ values, as listed in Table 1. With the C/O atomic ratio decreasing from 16.2 to 6.6, the adsorption sites provided by oxygen-containing groups were enriched. The HCHO molecules could adsorb on the oxygen-containing groups with enhanced Eads and ΔQ values, thereby leading to the increase of response to HCHO. As a consequence, the oxygen-containing groups play a crucial role in HCHO sensing. An appropriate amount of oxygen-containing groups could improve the response of rGO to HCHO.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of the carboxyl group, –C
O of the carboxyl group, –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–, –CH2–, –C–O of the epoxide, and –C–O of the alkoxyl, respectively.57 Additionally, the two peaks at 2923 and 2848 cm−1 are associated with the –CH2– groups.58 The oxidation of GO by chloroacetic acid is expected to replace the –OH with –O–CH2–COOH. The –C–O–C epoxide ring would also be firstly opened under basic conditions and subsequently replaced by –O–CH2–COOH. The peak at ∼1724 cm−1 of the GO FTIR spectrum increased apparently in the GO-COOH spectrum, indicating the increase in the number of carboxyl groups. Moreover, the –C–O peak (∼1226 cm−1) of the GO FTIR spectrum almost disappeared in the GO-COOH spectrum. The GO-COOH FTIR spectrum presents stronger peaks of –CH2– groups, compared with those of the GO FTIR spectrum. The above-mentioned evidence confirms the attachment of –O–CH2–COOH on the GO surface. To gain experimental insights into the effect of carboxyl groups, the GO–GOOH and GO without carboxyl functionalization were well dispersed in DMF solvent to form a homogeneous solution. Then 0.2 μL the above-mentioned solution was dropwise added onto the IDEs. The as-obtained IDEs were annealed at 200 °C to obtain sensors based on rGO-COOH and the counterpart rGO (labelled as S5 and S4, respectively).
C–, –CH2–, –C–O of the epoxide, and –C–O of the alkoxyl, respectively.57 Additionally, the two peaks at 2923 and 2848 cm−1 are associated with the –CH2– groups.58 The oxidation of GO by chloroacetic acid is expected to replace the –OH with –O–CH2–COOH. The –C–O–C epoxide ring would also be firstly opened under basic conditions and subsequently replaced by –O–CH2–COOH. The peak at ∼1724 cm−1 of the GO FTIR spectrum increased apparently in the GO-COOH spectrum, indicating the increase in the number of carboxyl groups. Moreover, the –C–O peak (∼1226 cm−1) of the GO FTIR spectrum almost disappeared in the GO-COOH spectrum. The GO-COOH FTIR spectrum presents stronger peaks of –CH2– groups, compared with those of the GO FTIR spectrum. The above-mentioned evidence confirms the attachment of –O–CH2–COOH on the GO surface. To gain experimental insights into the effect of carboxyl groups, the GO–GOOH and GO without carboxyl functionalization were well dispersed in DMF solvent to form a homogeneous solution. Then 0.2 μL the above-mentioned solution was dropwise added onto the IDEs. The as-obtained IDEs were annealed at 200 °C to obtain sensors based on rGO-COOH and the counterpart rGO (labelled as S5 and S4, respectively).
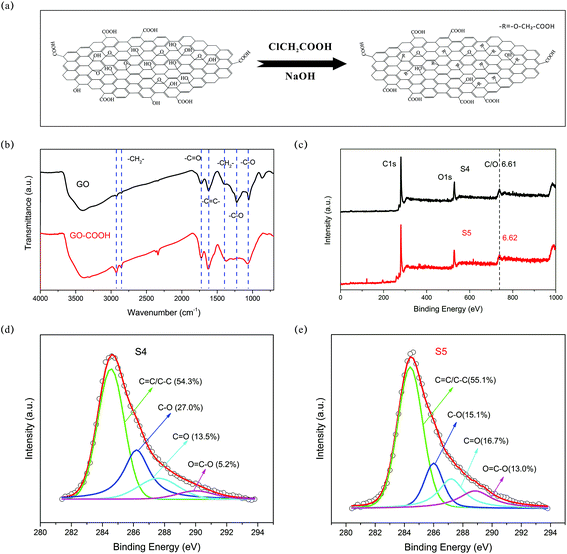 | ||
| Fig. 8 (a) Schematic illustration of the preparation of GO-COOH; (b) FTIR spectra of GO and GO-COOH; (c) XPS spectra of S4 and S5; (d) XPS C 1s spectrum of S4; (e) XPS C 1s spectrum of S5. | ||
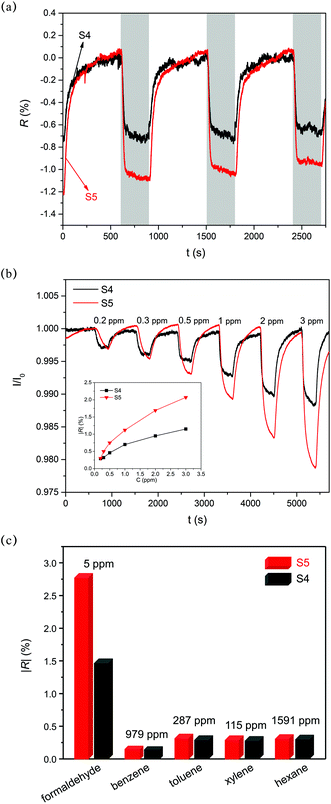
As shown in Fig. 8c, the C/O atomic ratios of S4 and S5 are 6.61 and 6.62, respectively, indicating similar reduction levels. Moreover, the percentage of different oxygen-containing groups is obtained by analysing the fitted peaks of the C 1s spectrum (further details can be found in eqn (4)). From Fig. 8d and e, it can be deduced that S5 has more carboxyl groups (6.80%) compared with S4 (3.09%). Fig. 9a shows three cycles of sensor response and recovery to 1 ppm HCHO. Sensors based on S4 and S5 show relatively good repeatability. However, S5 presented a more obvious response decay, which may be ascribed to the relatively strong adsorption induced by the carboxyl group. Fig. 9b shows the response dependence on the HCHO concentration of S4 and S5. The absolute values of the response (|R|) of S5 to 0.2–3 ppm HCHO are distinctly larger than those of S4. The response of S4 to 5 ppm HCHO is about 1.5%, which is in a similar range to those of bare rGO or pristine graphene (0.32–1.5% and 1–10 ppm).6,8,59,60 Surprisingly, the carboxyl functionalized reduced graphene (rGO-COOH) exhibits a response value of 2.75%, which is about 80% larger than those of common rGO and pristine graphene. As shown in the DFT calculation results, the carboxyl groups could evoke a strong enhancement of adsorption, with an Eads of 436 meV and a ΔQ of 0.115e. The values of Eads and ΔQ are several times as large as those of the hydroxyl group, epoxides and pristine graphene. As discussed in the former section, when Eads is larger than 13 meV, the response increases with the Eads and ΔQ values (more details can be inferred from eqn (5)–(10)). Therefore, the larger response of S5, compared with that of S4, might mainly result from the increase in the amount of carboxyl groups in S5. Moreover, the response and recovery time of S5 is relatively longer than that of S4 (see Fig. 9b). As indicated by Robinson et al.,19 the molecular interaction with high energy binding sites typically leads to a slower response. Thus, S5 with more high energy binding sites (carboxyl groups) exhibits a relatively slower response, as well as recovery. Fig. 9c summarizes the sensor selectivity to interfering gases, such as benzene, toluene, xylene, and hexane. The response of S5 to 5 ppm HCHO is 2.75%, while the responses to 979 ppm benzene, 287 ppm toluene, 115 ppm xylene, and 1591 ppm hexane are only 0.21%, 0.39%, 0.37%, and 0.38%, respectively. The response to target gas is about 7–13.1 times as large as the response to interfering gases. The ratio of selectivity (7–13.1) is as large as that in previous studies (∼1.2–17.9).4,6,8,61 The apparent selectivity of S5 might be ascribed to the difference of the analyte polarity.17,62,63 As a consequence, carboxyl groups play a significant role in HCHO sensing. Suitably functionalizing rGO with carboxyl groups could improve the response of rGO to HCHO.
Conclusions
In summary, the effects of oxygen-containing groups on the response of rGO-based HCHO sensors were investigated through a combination of DFT calculations and experimental research. DFT calculations were carried out to quantify the Eads and ΔQ values for the adsorption of HCHO on pristine graphene and rGO with different oxygen-containing groups (i.e., epoxides, hydroxyl groups, and carboxyl groups). The results reveal that HCHO adsorbs on pristine graphene via weak van der Waals interactions. The incorporation of oxygen-containing groups could promote the adsorption with enhanced Eads and ΔQ values. The Eads and ΔQ values decrease in the order of carboxyl group > hydroxyl group > epoxides > pristine graphene. The greater enhancement of the carboxyl group and hydroxyl group might mainly result from the formation of the hydrogen bond O–H⋯O with HCHO molecules. Generally, the increase of Eads and ΔQ values could improve the change in conductance of sensing materials, that is, the sensor response. Sensing experimental results show that with the decrease of the C/O atomic ratio from 16.2 to 6.6, the response to 1 ppm HCHO increases from 0.10% to 0.73%, validating the DFT calculation results. Furthermore, two kinds of rGO with a similar C/O atomic ratio (∼6.6), but different ratios of oxygen-containing groups, were prepared. The one with 6.80% carboxyl groups presents a distinctly larger response to 0.2–3 ppm HCHO, compared to the other with 3.09% carboxyl groups. The above-mentioned experimental findings, consistent with DFT calculation results, highlight the significant role of oxygen-containing groups, especially the carboxyl group, in HCHO sensing. The results in the current work provide a good understanding of the mechanism of rGO-based HCHO sensors and pave the way for the optimization of rGO-based HCHO sensors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the financial support from the National Natural Science Foundation of China (no. 51576175) and the Zhejiang Provincial Natural Science Foundation of China (no. LR17E060002).Notes and references
- R. E. Albert, Crit. Rev. Toxicol., 1994, 24, 75–85 CrossRef CAS PubMed.
- G. Neri, Chemosensors, 2015, 3, 1–20 CrossRef CAS.
- T. Wang, D. Huang, Z. Yang, S. Xu, G. He, X. Li, N. Hu, G. Yin, D. He and L. Zhang, Nano-Micro Lett., 2015, 8, 95–119 CrossRef.
- T. Alizadeh and L. H. Soltani, J. Hazard. Mater., 2013, 248–249, 401–406 CrossRef CAS PubMed.
- G. Eranna, B. C. Joshi, D. P. Runthala and R. P. Gupta, Crit. Rev. Solid State Mater. Sci., 2004, 29, 111–188 CrossRef CAS.
- Z. Ye, H. Tai, T. Xie, Z. Yuan, C. Liu and Y. Jiang, Sens. Actuators, B, 2016, 223, 149–156 CrossRef CAS.
- X. Chu, X. Zhu, Y. Dong, W. Zhang and L. Bai, J. Mater. Sci. Technol., 2015, 31, 913–917 Search PubMed.
- W. Y. Chuang, S. Y. Yang, W. J. Wu and C. T. Lin, Sensors, 2015, 15, 28842–28853 CrossRef CAS PubMed.
- X. Li, J. Wang, D. Xie, J. Xu, R. Dai, L. Xiang, H. Zhu and Y. Jiang, Sens. Actuators, B, 2015, 221, 1290–1298 CrossRef CAS.
- G. Lu, L. E. Ocola and J. Chen, Nanotechnology, 2009, 20, 445502 CrossRef PubMed.
- G. Lu, L. E. Ocola and J. Chen, Appl. Phys. Lett., 2009, 94, 083111 CrossRef.
- A. Lipatov, A. Varezhnikov, P. Wilson, V. Sysoev, A. Kolmakov and A. Sinitskii, Nanoscale, 2013, 5, 5426–5434 RSC.
- Y. Wang, L. Zhang, N. Hu, Y. Wang, Y. Zhang, Z. Zhou, Y. Liu, S. Shen and C. Peng, Nanoscale Res. Lett., 2014, 9, 1–12 CrossRef PubMed.
- S. S. Varghese, S. Lonkar, K. K. Singh, S. Swaminathan and A. Abdala, Sens. Actuators, B, 2015, 218, 160–183 CrossRef CAS.
- Q. Huang, D. Zeng, H. Li and C. Xie, Nanoscale, 2012, 4, 5651–5658 RSC.
- S. Pei and H.-M. Cheng, Carbon, 2012, 50, 3210–3228 CrossRef CAS.
- J. A. Robinson, E. S. Snow, Ş. C. Bǎdescu, T. L. Reinecke and F. K. Perkins, Nano Lett., 2006, 6, 1747–1751 CrossRef CAS PubMed.
- M. L. Y. Sin, G. C. T. Chow, G. M. K. Wong, W. J. Li, P. H. W. Leong and K. W. Wong, IEEE Trans. Nanotechnol., 2007, 6, 571–577 CrossRef.
- J. T. Robinson, F. K. Perkins, E. S. Snow, Z. Wei and P. E. Sheehan, Nano Lett., 2008, 8, 3137–3140 CrossRef CAS PubMed.
- J. D. Fowler, M. J. Allen, V. C. Tung, Y. Yang, R. B. Kaner and B. H. Weiller, ACS Nano, 2009, 3, 301–306 CrossRef CAS PubMed.
- D.-T. Phan and G.-S. Chung, Sensors 2012 IEEE, 2012, pp. 1–4 Search PubMed.
- X. Wang, X. Li, Y. Zhao, Y. Chen, J. Yu and J. Wang, RSC Adv., 2016, 6, 52339–52346 RSC.
- Y. Wang and J. T. W. Yeow, J. Sens., 2009, 2009, 1–24 CrossRef.
- U. Latif and F. L. Dickert, Sensors, 2015, 15, 30504–30524 CrossRef CAS PubMed.
- O. Leenaerts, B. Partoens and F. Peeters, Phys. Rev. B, 2008, 77, 125416 CrossRef.
- J. Dai, J. Yuan and P. Giannozzi, Appl. Phys. Lett., 2009, 95, 232105 CrossRef.
- H. H. Pu, S. H. Rhim, M. Gajdardziksa-Josifovska, C. J. Hirschmugl, M. Weinert and J. H. Chen, RSC Adv., 2014, 4, 47481–47487 RSC.
- M. Chi and Y.-P. Zhao, Comput. Mater. Sci., 2009, 46, 1085–1090 CrossRef CAS.
- Z. Maaghoul, F. Fazileh and J. Kakemam, Physica E, 2015, 66, 176–180 CrossRef CAS.
- H.-p. Zhang, X.-g. Luo, X.-y. Lin, X. Lu, Y. Leng and H.-t. Song, Appl. Surf. Sci., 2013, 283, 559–565 CrossRef CAS.
- Q. Zhou, L. Yuan, X. Yang, Z. Fu, Y. Tang, C. Wang and H. Zhang, Chem. Phys., 2014, 440, 80–86 CrossRef CAS.
- T. Ozaki, Phys. Rev. B: Condens. Matter, 2003, 67, 155108 CrossRef.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865 CrossRef CAS PubMed.
- I. Morrison, D. M. Bylander and L. Kleinman, Phys. Rev. B: Condens. Matter, 1993, 47, 6728–6731 CrossRef CAS.
- H. J. Monkhorst and J. D. Pack, Phys. Rev. B: Solid State, 1976, 13, 5188–5192 CrossRef.
- S. Grimme, J. Comput. Chem., 2006, 27, 1787–1799 CrossRef CAS PubMed.
- R. S. Mulliken, J. Chem. Phys., 1955, 23, 1833–1840 CrossRef CAS.
- W. S. Hummers Jr. and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339–1339 CrossRef.
- Z. Bo, X. Shuai, S. Mao, H. Yang, J. Qian, J. Chen, J. Yan and K. Cen, Sci. Rep., 2014, 4, 4684 CrossRef PubMed.
- S. Stankovich, D. A. Dikin, R. D. Piner, K. A. Kohlhaas, A. Kleinhammes, Y. Jia, Y. Wu, S. T. Nguyen and R. S. Ruoff, Carbon, 2007, 45, 1558–1565 CrossRef CAS.
- X. Sun, Z. Liu, K. Welsher, J. T. Robinson, A. Goodwin, S. Zaric and H. Dai, Nano Res., 2008, 1, 203–212 CrossRef CAS PubMed.
- A. Azadbakht, A. R. Abbasi, Z. Derikvand and Z. Karimi, Monatsh. Chem., 2015, 147, 705–717 CrossRef.
- R. Imani, S. H. Emami and S. Faghihi, J. Nanopart. Res., 2015, 17, 88 CrossRef.
- P. G. Ren, D. X. Yan, X. Ji, T. Chen and Z. M. Li, Nanotechnology, 2011, 22, 055705 CrossRef PubMed.
- X. Qin, Q. Meng and W. Zhao, Surf. Sci., 2011, 605, 930–933 CrossRef CAS.
- D. Shi, L. Wei, J. Wang, J. Zhao, C. Chen, D. Xu, H. Geng and Y. Zhang, Sens. Actuators, B, 2013, 177, 370–375 CrossRef CAS.
- L. Pauling, J. Am. Chem. Soc., 1932, 54, 3570–3582 CrossRef CAS.
- M. Chaplin, Water and Life: The unique properties of H2O, 2010, pp. 69–86 Search PubMed.
- Y. Jia, L. Chen, H. Yu, Y. Zhang and F. Dong, RSC Adv., 2015, 5, 40620–40627 RSC.
- E. Peris, J. C. Lee, J. R. Rambo, O. Eisenstein and R. H. Crabtree, J. Am. Chem. Soc., 1995, 117, 3485–3491 CrossRef CAS.
- Y. Lin, K. Zhang, W. Chen, Y. Liu, Z. Geng, J. Zeng, N. Pan, L. Yan, X. Wang and J. Hou, ACS Nano, 2010, 4, 3033–3038 CrossRef CAS PubMed.
- P. Matyba, H. Yamaguchi, G. Eda, M. Chhowalla, L. Edman and N. D. Robinson, ACS Nano, 2010, 4, 637–642 CrossRef CAS PubMed.
- Y. Zhou, C. Fuentes-Hernandez, J. Shim, J. Meyer, A. J. Giordano, H. Li, P. Winget, T. Papadopoulos, H. Cheun and J. Kim, Science, 2012, 336, 327–332 CrossRef CAS PubMed.
- M. R. Islam, D. Joung and S. I. Khondaker, New J. Phys., 2011, 13, 035021 CrossRef.
- I. Jung, D. A. Dikin, R. D. Piner and R. S. Ruoff, Nano Lett., 2008, 8, 4283–4287 CrossRef CAS PubMed.
- S. Cui, H. Pu, S. A. Wells, Z. Wen, S. Mao, J. Chang, M. C. Hersam and J. Chen, Nat. Commun., 2015, 6, 8632 CrossRef CAS PubMed.
- D. R. Dreyer, S. Park, C. W. Bielawski and R. S. Ruoff, Chem. Soc. Rev., 2010, 39, 228–240 RSC.
- T. Szabó, E. Tombácz, E. Illés and I. Dékány, Carbon, 2006, 44, 537–545 CrossRef.
- H. Mu, Z. Zhang, X. Zhao, F. Liu, K. Wang and H. Xie, Appl. Phys. Lett., 2014, 105, 033107 CrossRef.
- H. Mu, K. Wang, Z. Zhang and H. Xie, J. Phys. Chem. C, 2015, 119, 10102–10108 CAS.
- S. Chen, Y. Qiao, J. Huang, H. Yao, Y. Zhang, Y. Li, J. Du and W. Fan, RSC Adv., 2016, 6, 25198–25202 RSC.
- L. Zhang, C. Li, A. Liu and G. Shi, J. Mater. Chem., 2012, 22, 8438–8443 RSC.
- G. S. Kulkarni, K. Reddy, Z. Zhong and X. Fan, Nat. Commun., 2014, 5, 4376 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7an01051f |
| This journal is © The Royal Society of Chemistry 2018 |