A redox-responsive mesoporous silica based nanoplatform for in vitro tumor-specific fluorescence imaging and enhanced photodynamic therapy†
Fan
Yuan
,
Jiang-Lan
Li
,
Han
Cheng
,
Xuan
Zeng
* and
Xian-Zheng
Zhang
 *
*
Key Laboratory of Biomedical Polymers of Ministry of Education & Department of Chemistry, Wuhan University, Wuhan 430072, P. R. China. E-mail: zeng_xuan@163.com; xz-zhang@whu.edu.cn
First published on 22nd November 2017
Abstract
In order to obtain an optimal therapeutic effect with minimal systemic toxicity, a redox-responsive mesoporous silica nanoparticle (MSN)-based platform modified with protoporphyrin IX (PpIX)-multifunctional peptides was synthesized as an intelligent theranostic agent carrier. This redox-responsive nanoplatform could release the theranostic agent under a glutathione stimulus, produce fluorescence recovery for tumor-specific fluorescence imaging and provide tumor-enhanced photodynamic therapy.
Considering that theranostic systems aim to combine diagnosis and treatment to visualize the therapeutic effects of tumor treatment, the agents first should be equipped with excellent imaging function. At present, conventional diagnostic methods are widely applied in clinical applications, among which fluorescence imaging is especially valued in biodiagnostic research for its convenience, low cost, high sensitivity and various marked targets.1 In order to obtain an optimal therapeutic effect with minimal systemic toxicity, intelligent theranostic agents are highly required to achieve highly specific and selective therapies.
Photodynamic therapy (PDT) is considered as an ideal strategy for cancer therapy due to its non-invasive, low systemic toxicity, and light-controllable properties.2 Numerous photosensitizers have been developed for PDT and can be effectively activated by different intensities/wavelengths of light to destroy tumor cells.3 Among all photosensitizers, protoporphyrin IX (PpIX) is optimal for its good biocompatibility, obvious red fluorescence and excellent PDT efficacy.4 However, the weaknesses and constraints of PpIX, such as its non-selectivity for tumors and hydrophilic property, make it more difficult for use in clinical applications.5 Recent studies have shown that multifunctional peptides can improve the properties of PpIX.6 On the other hand, fluorescence imaging should emerge where PDT is needed to be carried out, making the treatment location more accurate. Mesoporous silica nanoparticles (MSNs) have been widely used as classical platforms for photosensitizer-mediated PDT in controlled drug delivery.7 Black hole quenchers (BHQs) doped on the inner walls of the mesopores endow MSN with quenching ability by closing the distance between the fluorescent probe and quencher.8
The micro environmental conditions of cancerous tissues are specific and dramatically different from those of the extracellular matrix and normal tissues.9 Glutathione (GSH) is an essential intracellular antioxidant and plays an important role in biochemical processes. It has been reported that the intracellular GSH level (about 10 mM) is considerably higher than that in the extracellular matrix (about 2 μM), and moreover, cancer cells contain higher levels of GSH compared with normal cells.10 Previous studies have shown that high intracellular GSH levels in tumor cells can effectively trigger drug release inside the cells, and the release rate speeds up with increasing concentrations of GSH.11
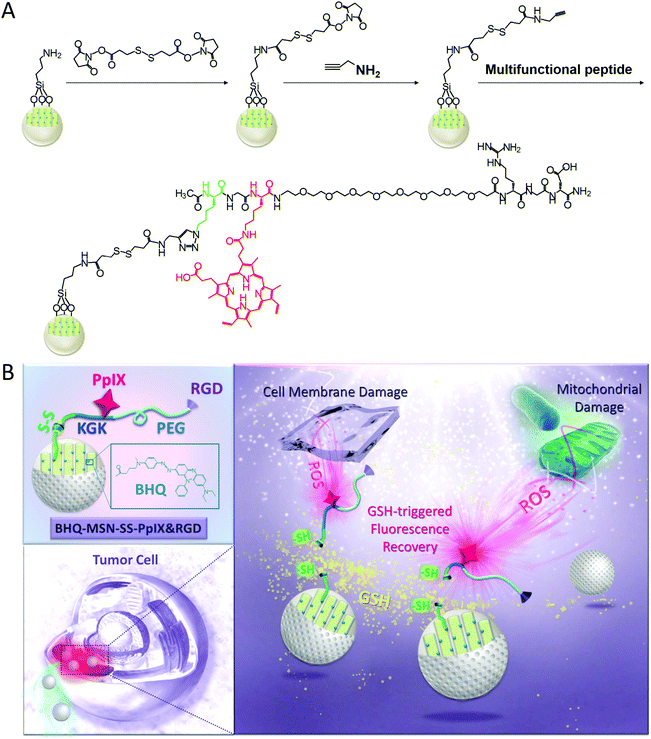
Here, we reported the design and synthesis of a theranostic anticancer drug delivery system based on photodynamic MSNs, BHQ-MSN-SS-PpIX&RGD (Fig. 1). The photosensitizer PpIX was PEGylated to enhance its long circulating property. Since a strong interaction between the tumor-targeted peptide sequence RGD with the αvβ3 integrin receptor (overexpressed on tumor cells) has been proven in previous studies, RGD was conjugated to the PEGylated PpIX, and the resulting PpIX-multifunctional peptides achieved tumor-triggered targeting ability.12 Accordingly, the PpIX-multifunctional peptide modified nanoparticles BHQ-MSN-SS-PpIX&RGD could specifically target tumor cells under the guidance of RGD. After receptor-mediated endocytosis, rapid fluorescence recovery of PpIX and tumor-enhanced photodynamic therapy took place due to the GSH-triggered transformation of the multifunctional BHQ-doped MSNs.
Detailed fabrication procedures for the BHQ-MSN-SS-PpIX&RGD platform are illustrated in Scheme S1.† The surface of the MSNs was first grafted with amino groups to obtain BHQ-MSN-NH2, which was confirmed by the zeta potential value (+24.0 mV) which belonged to the amino groups. The BHQ-doped MSNs maintained the absorption spectrum of the BHQ (550–650 nm) (Fig. S1†). Then, 3,3′-dithiodipropionic acid di(N-succinimidyl ester) was conjugated to obtain BHQ-MSN-SS-succinimidyl, resulting in the zeta potential changing to −13.4 mV. 2-Propynylamine was grafted onto the nanoparticles for further reaction. The increased zeta potential (from −13.4 to −2.30 mV) of the nanoparticles confirmed the accuracy of the structure. The PpIX-multifunctional peptide K(N3)GK(PpIX)-(PEG)8-RGD was synthesized using a standard Fmoc-based solid phase peptide synthesis (SPPS) protocol. The molecular weight of the peptide was determined by electrospray ionization mass spectrometry (ESI-MS) to be 1695.5 [M + H]+ (Fig. S2†). Finally, the peptide was clicked onto BHQ-MSN-SS-alkyne, and BHQ-MSN-SS-PpIX&RGD nanoparticles were obtained. The Fourier transform infrared (FT-IR) spectra of these nanoparticles exhibited an expected disappearance of the minor peak at ∼2200 cm−1 which was attributed to the alkynyl group (Fig. S3†). The zeta potential value of BHQ-MSN-SS-PpIX&RGD was −5.18 mV (Table S1†).
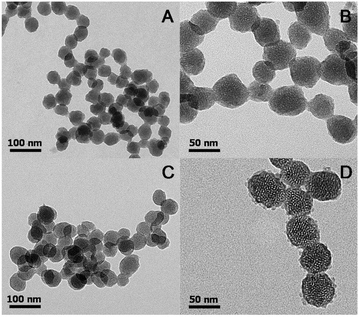
The structure and morphology of the synthesized mesoporous nanoparticles were investigated by transmission electron microscopy (TEM). As shown in Fig. 2, BHQ-MSN-NH2 and BHQ-MSN-SS-PpIX&RGD exhibited monodispersed spherical morphologies with diameters in the range of 30–50 nm (Fig. 2A) and 40–60 nm (Fig. 2C), respectively. Well-ordered mesoporous architectures can be found in both of the TEM images of the nanoparticles (Fig. 2B and D). Moreover, discontinuous and irregular bumps can be observed on the surfaces of the BHQ-doped MSNs, according to Fig. 2D, indicating that the MSNs were successfully coated with multifunctional peptides.
To investigate the GSH-triggered drug release behavior of the PpIX-loaded MSNs and the fluorescence recovery, the in vivo redox microenvironment of the tumor cytoplasm was mimicked in vitro and the fluorescence spectra of PpIX in the BHQ-MSN-SS-PpIX&RGD nanoparticles were measured after treatment with 10 mM GSH in pH 7.4 PBS buffer at 37 °C for different periods of time (from 15 min to 96 h). As shown in Fig. 3, after 96 h of incubation in the artificial redox microenvironment, the fluorescence intensity showed three-fold enhancement over the control (after 15 min of incubation). The fluorescence intensity steadily increased over time due to the sustained-release of PpIX-multifunctional peptides from the BHQ-doped MSNs, indicating the reducing ability of GSH and its efficient cleavage of disulfide bonds.
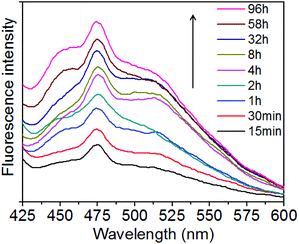 | ||
| Fig. 3 Fluorescence recovery of PpIX after BHQ-MSN-SS-PpIX&RGD was treated with GSH (10 mM) in PBS buffer (pH 7.4) at 37 °C for different periods of time. | ||
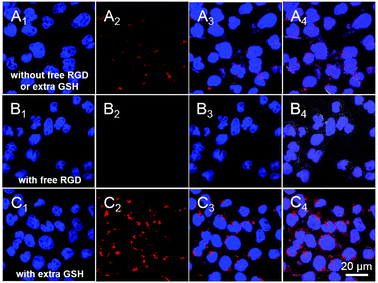
The active targeting ability of BHQ-MSN-SS-PpIX&RGD for integrin-positive tumor cells was confirmed by confocal laser scanning microscopy (CLSM). Cellular uptake of the nanoparticles was carried out in HeLa cells (cervical cancer cells) in the presence and absence of free RGD (5 μM). The HeLa cells treated with BHQ-MSN-SS-PpIX&RGD exhibited obvious red fluorescence (Fig. 4A), proving that the cellular uptake was efficient and that the quenched fluorescence of PpIX was recovered successfully. In contrast, Fig. 4B showed that fluorescence was hardly detected in the receptor blocked control group (the HeLa cells were pre-treated with free RGD). By blocking the αvβ3 integrin receptor with free RGD, the cellular uptake of nanoparticles in the HeLa cells was inhibited obviously. Consequently, the nanoplatform equipped with the RGD active targeting moiety could enhance the cellular uptake via receptor-mediated endocytosis. Moreover, in order to evaluate the potential of BHQ-MSN-SS-PpIX&RGD as an effective GSH-controlled photosensitizer delivery system, the cellular uptakes of nanoparticles were measured in HeLa cells with/without 5 μM GSH. But the phenomenon was different in another control group (Fig. 4C). After the cells were pre-treated with extra GSH, numerous red fluorescent spots of PpIX appeared in the cytoplasm, and most of them aggregated around the nucleus. Compared with that in Fig. 4A, the red fluorescence in Fig. 4C is brighter and more intense, which is an indication of abundant endocytosis and the fact that a much higher percentage of PpIX fluorescence was recovered.
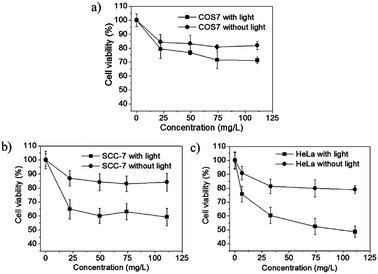
To further evaluate the PDT efficacy of the BHQ-MSN-SS-PpIX&RGD nanoplatform in vitro, an MTT assay was used to assess the cell viability after the cells were treated with nanoplatforms and visible light irradiation. Fig. 5 demonstrates that the BHQ-MSN-SS-PpIX&RGD nanoparticles displayed low cytotoxicity while light played a key role in the obvious anti-tumor activity of the HeLa cells. Both the tumor cells and the normal cells incubated with BHQ-MSN-SS-PpIX&RGD and treated without light irradiation exhibited slight dark cytotoxicity. In addition, the cell viabilities of the tumor cells (SCC-7 cells and HeLa cells) incubated with BHQ-MSN-SS-PpIX&RGD (Fig. 5b and c) under 30 min light irradiation were much lower than those of the normal cells (COS7 cells, Fig. 5a) under the same conditions. The half-inhibitory concentration (IC50) of BHQ-MSN-SS-PpIX&RGD with light against the HeLa cells was 85 μg mL−1 (Fig. 5c). These results indicate that the high selective light toxicity for the tumor cells was attributed to the tumor-targeted peptide RGD-mediated endocytosis and high intracellular GSH level.
In summary, a novel MSN-based nanoplatform with multiple functions and redox-responses has been successfully developed for tumor microenvironment-enhanced photodynamic therapy. The obtained nanoparticles can selectively target tumor cells with the help of RGD, release the photosensitizer for tumor-specific fluorescence imaging under the stimulus of high GSH levels, and exhibit high selective light toxicity on tumor cells. This redox-responsive theranostic system could locate the tumor cells efficiently and accurately, and the multifunctional peptide decorated MSNs proposed here may provide a new strategy for enhanced and safer PDT.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was supported by the National Natural Science Foundation of China (51303137 and 51573142) and the Chinese Postdoctoral Science Foundation (2014T70729 and 2016M590708).Notes and references
- (a) A. Kumar, S. Kim and J. M. Nam, J. Am. Chem. Soc., 2016, 138, 14509 CrossRef CAS PubMed; (b) V. Giannini, A. I. Fernández-Domínguez, S. C. Heck and S. A. Maier, Chem. Rev., 2011, 111, 3888 CrossRef CAS PubMed; (c) B. Tian, C. Wang, S. Zhang, L. Z. Feng and Z. Liu, ACS Nano, 2011, 5, 7000 CrossRef CAS PubMed.
- S. S. Lucky, K. C. Soo and Y. Zhang, Chem. Rev., 2015, 115, 1990 CrossRef CAS PubMed.
- (a) K. Liu, R. Xing, Q. Zou, G. Ma, H. Möhwald and X. Yan, Angew. Chem., Int. Ed., 2016, 55, 3036 CrossRef CAS PubMed; (b) D. W. Zheng, B. Li, C. X. Li, J. X. Fan, Q. Lei, C. Li, Z. Xu and X. Z. Zhang, ACS Nano, 2016, 10, 8715 CrossRef CAS PubMed; (c) H. Cheng, J. Y. Zhu, S. Y. Li, J. Y. Zeng, Q. Lei, C. Zhang and X. Z. Zhang, Adv. Funct. Mater., 2016, 26, 7847 CrossRef CAS; (d) S. Y. Li, W. X. Qiu, H. Cheng, F. Gao, F. Y. Cao and X. Z. Zhang, Adv. Funct. Mater., 2017, 27, 1604916 CrossRef; (e) W. H. Chen, G. F. Luo, W. X. Qiu, Q. Lei, L. H. Liu, S. B. Wang and X. Z. Zhang, Biomaterials, 2017, 117, 54 CrossRef CAS PubMed.
- (a) M. Ethirajan, Y. Chen, P. Joshi and R. K. Pandey, Chem. Soc. Rev., 2011, 40, 340 RSC; (b) L. H. Liu, W. X. Qiu, Y. H. Zhang, B. Li, C. Zhang, F. Gao, L. Zhang and X. Z. Zhang, Adv. Funct. Mater., 2017, 27, 1700220 CrossRef; (c) G. F. Luo, W. H. Chen, S. Hong, Q. Cheng, W. X. Qiu and X. Z. Zhang, Adv. Funct. Mater., 2017, 27, 1702122 CrossRef.
- R. Mahato, W. Tai and K. Cheng, Adv. Drug Delivery Rev., 2011, 63, 659 CrossRef CAS PubMed.
- (a) K. Han, S. B. Wang, Q. Lei, J. Y. Zhu and X. Z. Zhang, ACS Nano, 2015, 9, 10268 CrossRef CAS PubMed; (b) S. Y. Li, H. Cheng, B. R. Xie, W. X. Qiu, L. L. Song, R. X. Zhuo and X. Z. Zhang, Biomaterials, 2016, 104, 297 CrossRef CAS PubMed.
- (a) G. F. Luo, W. H. Chen, Q. Lei, W. X. Qiu, Y. X. Liu, Y. J. Cheng and X. Z. Zhang, Adv. Funct. Mater., 2016, 26, 4339 CrossRef CAS; (b) Y. J. Cheng, G. F. Luo, J. Y. Zhu, X. D. Xu, X. Zeng, D. B. Cheng, Y. M. Li, Y. Wu, X. Z. Zhang, R. X. Zhuo and F. He, ACS Appl. Mater. Interfaces, 2015, 7, 9078 CrossRef CAS PubMed.
- (a) X. L. Huang, M. Swierczewska, K. Y. Choi, L. Zhu, A. Bhirde, J. Park, K. Kim, J. Xie, G. Niu, K. C. Lee, S. Lee and X. Y. Chen, Angew. Chem., Int. Ed., 2012, 51, 1625 CrossRef CAS PubMed; (b) R. C. Qian, L. Ding and H. X. Ju, J. Am. Chem. Soc., 2013, 135, 13282 CrossRef CAS PubMed; (c) P. Y. Yuan, X. Mao, K. C. Chong, J. Q. Fu, S. J. Pan, S. Z. Wu, C. M. Yu and S. Q. Yao, Small, 2017, 13, 1700569 CrossRef PubMed.
- (a) D. F. Quail and J. A. Joyce, Nat. Med., 2013, 19, 1423 CrossRef CAS PubMed; (b) Y. L. Dai, C. Xu, X. L. Sun and X. Y. Chen, Chem. Soc. Rev., 2017, 46, 3830 RSC; (c) Y. J. Cheng, A. Q. Zhang, J. J. Hu, F. He, X. Zeng and X. Z. Zhang, ACS Appl. Mater. Interfaces, 2017, 9, 2093 CrossRef CAS PubMed.
- H. Wei, R. X. Zhuo and X. Z. Zhang, Prog. Polym. Sci., 2013, 38, 503 CrossRef CAS.
- (a) J. Zhang, Z. F. Yuan, Y. Wang, W. H. Chen, G. F. Luo, S. X. Cheng, R. X. Zhuo and X. Z. Zhang, J. Am. Chem. Soc., 2013, 135, 5068 CrossRef CAS PubMed; (b) D. W. Zheng, J. L. Chen, J. Y. Zhu, L. Rong, B. Li, Q. Lei, J. X. Fan, M. Z. Zou, C. Li, S. X. Cheng, Z. S. Xu and X. Z. Zhang, Nano Lett., 2016, 16, 4341 CrossRef CAS PubMed; (c) D. Xiao, J. J. Hu, J. Y. Zhu, S. B. Wang, R. X. Zhuo and X. Z. Zhang, Nanoscale, 2016, 8, 16702 RSC.
- (a) K. Han, Q. Lei, H. Z. Jia, S. B. Wang, W. N. Yin, W. H. Chen, S. X. Cheng and X. Z. Zhang, Adv. Funct. Mater., 2015, 25, 1248 CrossRef CAS; (b) W. Xiao, X. Zeng, H. Lin, K. Han, H. Z. Jia and X. Z. Zhang, Chem. Commun., 2015, 51, 1475 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis of BHQ-MSN-SS-PpIX&RGD and the peptide analogs; ESI of multifunctional peptides; UV-Vis spectrum of BHQ-MSN-NH2; FTIR spectra of BHQ-MSN-SS-alkyne and BHQ-MSN-SS-PpIX&RGD. See DOI: 10.1039/c7bm00793k |
| This journal is © The Royal Society of Chemistry 2018 |