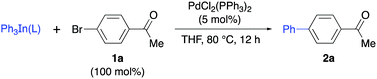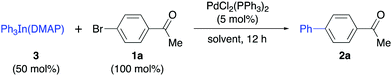Synthesis of bench-stable solid triorganoindium reagents and reactivity in palladium-catalyzed cross-coupling reactions†
José M.
Gil-Negrete
 ,
José
Pérez Sestelo
,
José
Pérez Sestelo
 * and
Luis A.
Sarandeses
* and
Luis A.
Sarandeses
 *
*
Centro de Investigaciones Científicas Avanzadas (CICA) and Departamento de Química, Universidade da Coruña, E-15071 A Coruña, Spain. E-mail: sestelo@udc.es; qfsarand@udc.es
First published on 12th January 2018
Abstract
Bench-stable solid triorganoindium compounds have been prepared by coordination with 4-(dimethylamino)pyridine (DMAP). The solid R3In(DMAP) complexes are obtained from the corresponding solution of R3In in quantitative yield and can be stored for up to several weeks. These reagents show excellent reactivity in palladium-catalyzed cross-coupling reactions with organic electrophiles.
In recent years, indium(III) organometallics have become an attractive alternative to classical organometallic species in organic synthesis.1 In 1999, we discovered the palladium-catalyzed cross-coupling reactions of triorganoindium reagents R3In,2 which is probably the most relevant reaction and whose utility in organic synthesis is continuously increasing.3 In this reaction the R3In species show high atom efficiency as all three organic groups are transferred from indium to the electrophile with excellent selectivity, high versatility and, in general, low toxicity.4 Triorganoindium reagents are commonly prepared by transmetallation from the corresponding organolithium or organomagnesium reagents, although other protocols have also been developed.5 A significant limitation for the use of these organometallic species is associated with their preparation and use as solutions, since attempts to isolate and store these reagents led to decomposition.6
The preparation of solid-salt stabilized reagents is an interesting research topic that allows the use of organometallic and other nucleophilic species as solid reagents. Organotrifluoroborates,7 MIDA-boronates8 and stable siloxanes9 are useful examples of solid-salt stabilized reagents, whereas among the main-group metal derivatives, organozinc pivalates have emerged as interesting reactive solid species.10
In the field of indium chemistry, Schumann and co-workers reported an extensive study on the characterization of indium organometallics stabilized by intramolecular coordination using nitrogen or phosphorus atoms.11 The isolation and characterization of allyl and 1-butenylindium halides stabilized by complexation with phosphine and pyridine ligands were also reported by Baba.6,12 Trimethylindium complexes have also been characterized but their reactivity and synthetic utility remains unknown.11,13 Herein, we report the preparation of stable solid triorganoindium compounds stabilized by coordination with 4-(dimethylamino)pyridine (DMAP) and their reactivity in palladium-catalyzed cross-coupling reactions.
Bearing in mind the stabilization of Group 13 derivatives by nitrogen ligands, our research started with the preparation of stable solid triphenylindium complexes by the addition of different pyridines L1–L5 to Ph3In in THF solution and removal of the solvent. In general, this procedure afforded a solid material that could be manipulated in air and whose reactivity was tested in a Pd-catalyzed cross-coupling reaction with 4-bromoacetophenone (1a, Table 1). Initial studies using a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio of Ph3In and DMAP as ligands gave the coupling product (2a) in moderate yield (Table 1, entries 2–6). Interestingly, on using the 1
2 ratio of Ph3In and DMAP as ligands gave the coupling product (2a) in moderate yield (Table 1, entries 2–6). Interestingly, on using the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio complex (Ph3In/DMAP) 2a was obtained in 73% yield with 40 mol% of the complex and PdCl2(PPh3)2 (5 mol%) as the catalyst (Table 1, entry 7), while the use of a 50 mol% of the complex led to an excellent 91% yield of 2a (Table 1, entry 8). The use of lower amounts (<50 mol%) of Ph3In(DMAP) afforded lower yields, probably due to some decomposition of the complex during the process. The stoichiometry of the complex was studied by NMR spectroscopy and a 1
1 ratio complex (Ph3In/DMAP) 2a was obtained in 73% yield with 40 mol% of the complex and PdCl2(PPh3)2 (5 mol%) as the catalyst (Table 1, entry 7), while the use of a 50 mol% of the complex led to an excellent 91% yield of 2a (Table 1, entry 8). The use of lower amounts (<50 mol%) of Ph3In(DMAP) afforded lower yields, probably due to some decomposition of the complex during the process. The stoichiometry of the complex was studied by NMR spectroscopy and a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of Ph3In/DMAP was found, whereas the use of larger amounts of DMAP gave rise to an equilibrium between ligated and non-ligated DMAP. Additionally, the use of a substoichiometric amount of DMAP (ratio Ph3In/DMAP 1
1 ratio of Ph3In/DMAP was found, whereas the use of larger amounts of DMAP gave rise to an equilibrium between ligated and non-ligated DMAP. Additionally, the use of a substoichiometric amount of DMAP (ratio Ph3In/DMAP 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8) resulted in the appearance of benzene due to the decomposition of non-ligated organometallic complexes (Fig. 1).
0.8) resulted in the appearance of benzene due to the decomposition of non-ligated organometallic complexes (Fig. 1).
| Entry | L | mol% Lb | mol% Ph3Inb | Yieldc (%) |
|---|---|---|---|---|
| a For experimental details, see the ESI. b Mol% of L and Ph3In with respect to 1a. c Isolated yields of the reaction product after column chromatography. | ||||
| 1 | — | — | 40 | — |
| 2 | L1 | 80 | 40 | 23 |
| 3 | L2 | 80 | 40 | 15 |
| 4 | L3 | 80 | 40 | 5 |
| 5 | L4 | 80 | 40 | 55 |
| 6 | L5 | 80 | 40 | 11 |
| 7 | L4 | 40 | 40 | 73 |
| 8 | L4 | 50 | 50 | 91 |
|
||||
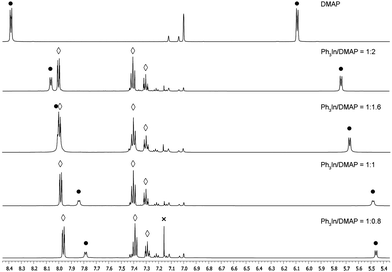 | ||
| Fig. 1 Ph3In(DMAP) stoichiometry experiments. • DMAP. ◊ Ph3In. × Benzene (resulting from Ph3In decomposition). | ||
Accordingly, the addition of DMAP (1 equiv.) to a THF solution of Ph3In at room temperature for one hour followed by evaporation of the solvent in vacuo gave a white solid with the tentative formula Ph3In(DMAP)·3LiCl (3, Scheme 1). This compound was found to be bench-stable, and was handled in air and used as a solid reagent. In order to characterize this solid, the LiCl was removed by filtration after boiling in dry toluene. This process afforded a compound that was crystallized from toluene/hexane (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). X-ray diffraction studies on the pure crystals (3′) showed a trigonal pyramidal structure in which the ideal plane formed by the phenyl groups and the indium atom is distorted by the ligand (DMAP) to give an N–In–C dihedral angle of around 100° (Scheme 1). The In–N distance was 2.27 Å and the In–C distance was 2.17 Å. Interestingly, the cross-coupling reaction with 4-bromoacetophenone carried out using the purified crystals (3′) afforded the coupling product in an excellent yield (90%) on using only 34 mol% of the solid organometallic reagent and showing the efficient transference of all three organic groups attached to the indium to the electrophile.
1). X-ray diffraction studies on the pure crystals (3′) showed a trigonal pyramidal structure in which the ideal plane formed by the phenyl groups and the indium atom is distorted by the ligand (DMAP) to give an N–In–C dihedral angle of around 100° (Scheme 1). The In–N distance was 2.27 Å and the In–C distance was 2.17 Å. Interestingly, the cross-coupling reaction with 4-bromoacetophenone carried out using the purified crystals (3′) afforded the coupling product in an excellent yield (90%) on using only 34 mol% of the solid organometallic reagent and showing the efficient transference of all three organic groups attached to the indium to the electrophile.
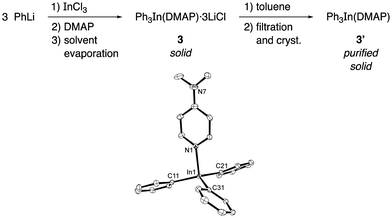 | ||
| Scheme 1 Preparation of Ph3In(DMAP) (3′) and ORTEP drawing (showing 40% probability displacement ellipsoids) of the crystal structure of Ph3In(DMAP). The hydrogen atoms are omitted for clarity. CCDC 1579360.† | ||
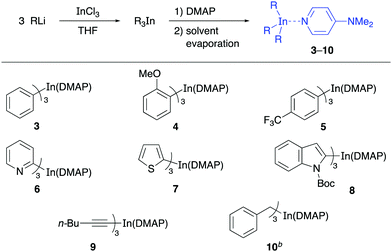
Having successfully prepared the complex Ph3In(DMAP), the protocol was applied to other triorganoindium species and the reactivity of the resulting complexes was studied in palladium-catalyzed cross-coupling reactions. With this purpose, a series of complexes R3In(DMAP)·3LiCl abbreviated as R3In(DMAP) for clarity were prepared (Table 2) including aromatic and heteroaromatic indium reagents (3–8), a trialkynylindium complex (9) and the tribenzylindium complex (10).
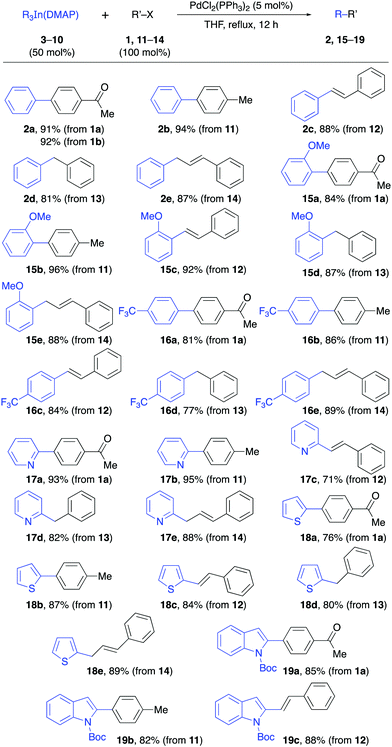
The reactivity of the solid triorganoindium reagents was tested in palladium-catalyzed cross-coupling reactions using common electrophiles under the established conditions reported for R3In prepared in situ in THF solution. The reaction of the Ph3In(DMAP) complex (3) with aryl triflate 1b or aryl bromide 11 proceeded efficiently (92–94%, Table 3). The coupling with other electrophiles such as β-bromostyrene and benzyl bromide also gave the corresponding products 2c–d in good yields (81–88%). Additionally, the Pd-catalyzed allylic substitution14 with cinnamyl bromide gave regioselectively the α-substitution product 2e in excellent yield (87%).
| a 1a: 4-bromoacetophenone; 1b: 4-acetylphenyl trifluoromethanesulfonate; 11: 4-bromotoluene; 12: β-bromostyrene; 13: benzyl bromide; 14: cinnamyl bromide. |
|---|
|
To our delight, other solid derivatives of triarylindium reagents, such as the tri(2-methoxyphenyl)indium(DMAP) (4) and tris(4-trifluoromethylphenyl)indium(DMAP) (3) also reacted efficiently with the electrophiles 1a and 11–14 to give the corresponding cross-coupling products in good to excellent yields (81–96%, Table 3). In all cases the reaction proceeded under mild reaction conditions and in short reaction times using 50 mol% of the solid indium reagents, thus showing the feasibility of using these compounds in cross-coupling reactions. The presence of DMAP as a ligand does not interfere with the reactivity of the organoindium compound since the yields are comparable to those obtained in reactions in which the R3In was generated in situ in solution.
Then, we proceeded to study the reactivity of the solid heteroaryl indium compounds 6–8. As can be seen from the results in Table 3, coupling reactions between these compounds and electrophiles 1a and 11–14 also proceeded in good yields (71–95%, Table 3) under the same experimental conditions as before. Interestingly, the reactions involving aryl halides and also a vinyl halide, benzyl bromide and an allylic bromide as electrophiles, showed the versatility of the solid indium reagents. Furthermore, the efficient coupling achieved using pyridylindium complex 6 showed that the nitrogen lone pair does not interfere in the complexation process with DMAP and the reactivity remains similar to that of the tripyridylindium in solution.
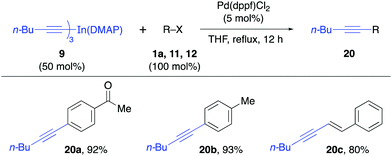
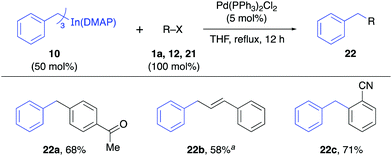
The reactivity of the solid alkynylindium complex 9 was also studied with aryl and alkenyl halides 1a, 11 and 12 under standard reaction conditions. These coupling reactions also proceeded efficiently to give excellent yields (80–93%, Table 4). Finally, the reactivity of the tribenzylindium(DMAP) complex (10) was tested. In this case, it was found that the coupling reactions with aryl bromide 1a, β-bromostyrene (12) and 2-bromobenzonitrile (21) gave satisfactory yields (58–71%, Table 5). In this case, 10 was prepared from benzylmagnesium bromide.
| a 1H NMR yield. |
|---|
|
The synthetic utility of the novel solid triorganoindium species was demonstrated not only in terms of their reactivity but also due to their integrity against decomposition over time. In this respect, Ph3In(DMAP)·3LiCl (3) was stored on the laboratory bench at room temperature under Ar and the stability of the complex evaluated by means of palladium-catalyzed cross-coupling reactions over time. The results are presented in Table 6 and it can be seen that one month after the preparation of the complex, the solid reagent remained highly reactive (78% yield of 2a, Table 6, entry 6). Interestingly, the use of purified Ph3In(DMAP) (3′, without LiCl) gave compound 2a in 95% yield after 45 days. The other organoindium complexes prepared in this study generally show high stabilities under Ar at room temperature. The stability ranges from weeks for 4 and 7, days in the case of 5, 9 and 10, and >6 h for the nitrogen heterocyclic derivatives 6 and 8 (tested by studying a Pd-catalyzed cross-coupling reaction, Table S1, ESI†).
In general, the use of organoindium species is limited to ethers as solvents, and to their preparation from the corresponding organolithium or organomagnesium reagents. For this reason, we studied the reactivity of the solid-stable R3In complexes in non-ethereal solvents. The palladium-catalyzed coupling reaction of Ph3In(DMAP) with 1a in various solvents, such as toluene, chloroalkanes or DMF, generally afforded good yields of the coupling product 2a (45–93%, Table S3, entries 2–6, ESI†).
In conclusion, a variety of bench-stable solid triorganoindium reagents have been prepared by complexation with DMAP in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry. The stability of the R3In complexes ranges from days to weeks depending on the nature of the R groups. These reagents reacted with organic electrophiles under palladium catalysis to afford the coupling products in good yields. Interestingly, only 50 mol% of the solid organometallic reagent is necessary to complete the reactions. Other solvents can be used in the coupling reactions and this demonstrates the versatility and utility of these solid reagents. Further studies on the isolation of new complexes and their applications in synthesis are underway and will be published in due course.
1 stoichiometry. The stability of the R3In complexes ranges from days to weeks depending on the nature of the R groups. These reagents reacted with organic electrophiles under palladium catalysis to afford the coupling products in good yields. Interestingly, only 50 mol% of the solid organometallic reagent is necessary to complete the reactions. Other solvents can be used in the coupling reactions and this demonstrates the versatility and utility of these solid reagents. Further studies on the isolation of new complexes and their applications in synthesis are underway and will be published in due course.
We are grateful to the Spanish Ministerio de Economía y Competitividad (CTQ2015-68369-P), Xunta de Galicia (GRC2014/042) and EDRF funds for financial support. We also thank Dr Antonio L. Llamas-Díaz (RIAIDT, Universidade de Santiago de Compostela, Spain) for the X-ray structure analysis.
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) Z.-L. Shen, S.-Y. Wang, Y.-K. Chok, Y.-H. Xu and T.-P. Loh, Chem. Rev., 2013, 113, 271 CrossRef CAS PubMed; (b) K. Zhao, L. Shen, Z.-L. Shen and T.-P. Loh, Chem. Soc. Rev., 2017, 46, 586 RSC.
- (a) I. Pérez, J. Pérez Sestelo and L. A. Sarandeses, Org. Lett., 1999, 1, 1267 CrossRef; (b) I. Pérez, J. Pérez Sestelo and L. A. Sarandeses, J. Am. Chem. Soc., 2001, 123, 4155 CrossRef; (c) R. Riveiros, D. Rodríguez, J. Pérez Sestelo and L. A. Sarandeses, Org. Lett., 2006, 8, 1403 CrossRef CAS PubMed; (d) Á. Mosquera, M. A. Pena, J. Pérez Sestelo and L. A. Sarandeses, Eur. J. Org. Chem., 2013, 2555 CrossRef; (e) M. Mato, C. Pérez-Caaveiro, L. A. Sarandeses and J. Pérez Sestelo, Adv. Synth. Catal., 2017, 359, 1388 CrossRef CAS , and references therein.
- (a) K. Takami, H. Yorimitsu, H. Shinokubo, S. Matsubara and K. Oshima, Org. Lett., 2001, 3, 1997 CrossRef CAS PubMed; (b) P. H. Lee, S.-Y. Sung and K. Lee, Org. Lett., 2001, 3, 3201 CrossRef CAS PubMed; (c) K. Lee, D. Seomoon and P. H. Lee, Angew. Chem., Int. Ed., 2002, 41, 3901 CrossRef CAS; (d) U. Lehmann, S. Awasthi and T. Minehan, Org. Lett., 2003, 5, 2405 CrossRef CAS PubMed; (e) Y.-H. Chen, M. Sun and P. Knochel, Angew. Chem., Int. Ed., 2009, 48, 2236 CrossRef CAS PubMed; (f) Z.-L. Shen, K. K. K. Goh, H.-L. Cheong, C. H. A. Wong, Y.-C. Lai, Y.-S. Yang and T.-P. Loh, J. Am. Chem. Soc., 2010, 132, 15852 CrossRef CAS PubMed; (g) D. Lee, T. Ryu, Y. Park and P. H. Lee, Org. Lett., 2014, 16, 1144 CrossRef CAS PubMed; (h) S. Kim, C.-E. Kim, B. Seo and P. H. Lee, Org. Lett., 2014, 16, 5552 CrossRef CAS PubMed; (i) S. Thapa, S. K. Gurung, D. A. Dickie and R. Giri, Angew. Chem., Int. Ed., 2014, 53, 11620 CrossRef CAS PubMed; (j) Y. Park, J. Min, D. Eom and P. H. Lee, Org. Lett., 2015, 17, 3934 CrossRef CAS PubMed.
- J. Burgess, Chem. Soc. Rev., 1996, 25, 85 RSC.
- (a) J. L. W. Pohlmann, F. E. Brinckman, G. Tesi and R. E. Donadio, Z. Naturforsch., B: J. Chem. Sci., 1965, 20, 5 Search PubMed; (b) H. C. Clark and A. L. Pickard, J. Organomet. Chem., 1967, 8, 427 CrossRef CAS; (c) E. Font-Sanchis, Á. Sastre-Santos and F. Fernández-Lázaro, Dalton Trans., 2009, 2470 RSC; (d) Z.-L. Shen, K. K. K. Goh, Y.-S. Yang, Y.-C. Lai, C. H. A. Wong, H.-L. Cheong and T.-P. Loh, Angew. Chem., Int. Ed., 2011, 50, 511 CrossRef CAS PubMed; (e) L. Adak and N. Yoshikai, J. Org. Chem., 2011, 76, 7563 CrossRef CAS PubMed; (f) S. Bernhardt, Z.-L. Shen and P. Knochel, Chem. – Eur. J., 2013, 19, 828 CrossRef CAS PubMed.
- M. Yasuda, M. Haga and A. Baba, Organometallics, 2009, 28, 1998 CrossRef CAS.
- (a) G. A. Molander and N. Ellis, Acc. Chem. Res., 2007, 40, 275 CrossRef CAS PubMed; (b) G. A. Molander, J. Org. Chem., 2015, 80, 7837 CrossRef CAS PubMed.
- J. Li, A. S. Grillo and M. D. Burke, Acc. Chem. Res., 2015, 48, 2297 CrossRef CAS PubMed.
- D. Martinez-Solorio, B. Melillo, L. Sanchez, Y. Liang, E. Lam, K. N. Houk and A. B. Smith III, J. Am. Chem. Soc., 2016, 138, 1836 CrossRef CAS PubMed.
- Selected references: (a) S. Bernhardt, G. Manolikakes, T. Kunz and P. Knochel, Angew. Chem., Int. Ed., 2011, 50, 9205 CrossRef CAS PubMed; (b) J. R. Colombe, S. Bernhardt, C. Stathakis, S. L. Buchwald and P. Knochel, Org. Lett., 2013, 15, 5754 CrossRef CAS PubMed; (c) M. Ellwart and P. Knochel, Angew. Chem., Int. Ed., 2015, 54, 10662 CrossRef CAS PubMed; (d) Y.-H. Chen, C.-P. Tüllmann, M. Ellwart and P. Knochel, Angew. Chem., Int. Ed., 2017, 56, 9236–9239 CrossRef CAS PubMed.
- Representative references: (a) H. Schumann, U. Hartmann, W. Wassermann, A. Dietrich, F. H. Görlitz, L. Pohl and M. Hostalek, Chem. Ber., 1990, 123, 2093 CrossRef CAS; (b) H. Schumann, J. Kaufmann, B. C. Wassermann, F. Girgsdies, N. Jaber and J. Blum, Z. Anorg. Allg. Chem., 2002, 628, 971 CrossRef CAS; (c) H. Schumann, F. Girgsdies, B. Heymer, J. Kaufmann, C. Marschall and W. Wassermann, Z. Anorg. Allg. Chem., 2007, 633, 2268 CrossRef CAS.
- (a) M. Yasuda, M. Haga and A. Baba, Eur. J. Org. Chem., 2009, 5513 CrossRef CAS; (b) M. Yasuda, M. Haga, Y. Nagaoka and A. Baba, Eur. J. Org. Chem., 2010, 5359 CrossRef CAS; (c) K. Kiyokawa, M. Yasuda and A. Baba, Organometallics, 2011, 30, 2039 CrossRef CAS.
- (a) D. C. Bradley, H. Dawes, D. M. Frigo, M. B. Hursthouse and B. Hussain, J. Organomet. Chem., 1987, 325, 55 CrossRef CAS; (b) K. M. Coward, A. C. Jones, A. Steiner, J. F. Bickley, L. M. Smith and M. E. Pemble, J. Chem. Soc., Dalton Trans., 2001, 41 RSC; (c) F. Thomas, T. Bauer, S. Schulz and M. Nieger, Z. Anorg. Allg. Chem., 2003, 629, 2018 CrossRef CAS; (d) X. Tian, R. Fröhlich, T. Pape and N. W. Mitzel, Organometallics, 2005, 24, 5294 CrossRef CAS.
- (a) L. Baker and T. Minehan, J. Org. Chem., 2004, 69, 3957 CrossRef CAS PubMed; (b) D. Rodríguez, J. Pérez Sestelo and L. A. Sarandeses, J. Org. Chem., 2004, 69, 8136 CrossRef PubMed; (c) R. Riveiros, R. Tato, J. Pérez Sestelo and L. A. Sarandeses, Eur. J. Org. Chem., 2012, 3018 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Tables S1 and S2, experimental procedures, compound characterization data, and copies of NMR spectra. CCDC 1579360. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7cc09344f |
| This journal is © The Royal Society of Chemistry 2018 |