Far-red fluorescent probes for canonical and non-canonical nucleic acid structures: current progress and future implications
Y. V.
Suseela
,
Nagarjun
Narayanaswamy
,
Sumon
Pratihar
and
Thimmaiah
Govindaraju
 *
*
Bioorganic Chemistry Laboratory, New Chemistry Unit, Jawaharlal Nehru Centre for Advanced Scientific Research, Jakkur P.O., Bengaluru 560064, Karnataka, India. E-mail: tgraju@jncasr.ac.in
First published on 21st December 2017
Abstract
The structural diversity and functional relevance of nucleic acids (NAs), mainly deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), are indispensable for almost all living organisms, with minute aberrations in their structure and function becoming causative factors in numerous human diseases. The standard structures of NAs, termed canonical structures, are supported by Watson–Crick hydrogen bonding. Under special physiological conditions, NAs adopt distinct spatial organisations, giving rise to non-canonical conformations supported by hydrogen bonding other than the Watson–Crick type; such non-canonical structures have a definite function in controlling gene expression and are considered as novel diagnostic and therapeutic targets. Development of molecular probes for these canonical and non-canonical DNA/RNA structures has been an active field of research. Among the numerous probes studied, probes with turn-on fluorescence in the far-red (600–750 nm) region are highly sought-after due to minimal autofluorescence and cellular damage. Far-red fluorescent probes are vital for real-time imaging of NAs in live cells as they provide good resolution and minimal perturbation of the cell under investigation. In this review, we present recent advances in the area of far-red fluorescent probes of DNA/RNA and non-canonical G-quadruplex structures. For the sake of continuity and completeness, we provide a brief overview of visible fluorescent probes. Utmost importance is given to design criteria, characteristic properties and biological applications, including in cellulo imaging, apart from critical discussion on limitations of the far-red fluorescent probes. Finally, we offer current and future prospects in targeting canonical and non-canonical NAs specific to cellular organelles, through sequence- and conformation-specific far-red fluorescent probes. We also cover their implications in chemical and molecular biology, with particular focus on decoding various disease mechanisms involving NAs.
Introduction
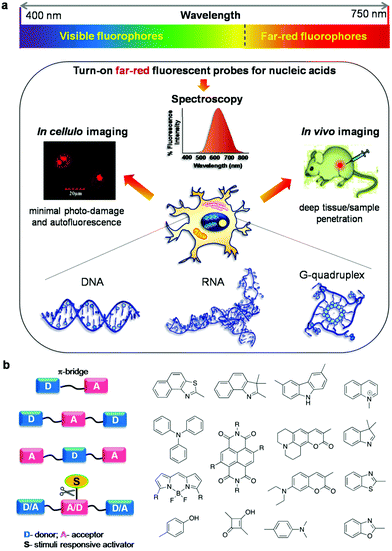
DNA and RNA are central to all biological processes as they store and transfer genetic information through replication, transcription and translation.1,2 DNA is the most robust and extensively studied form of NAs and typically adopts a right-handed, double-helix structure supported by adenine (A)–thymine (T) and guanine (G)–cytosine (C) base pairing. Like DNA, RNA contains A, G, and C, but thymine is replaced by uracil (U); it differs from DNA in both structural and functional characteristics. Unlike DNA that forms an ordered and regular structure, RNA can adopt diverse structural forms including three-dimensional structures that exhibit catalytic activity. Also, in addition to genetic information storage, RNA participates in genetic information transfer and coding for proteins. DNA is polymorphic in nature, and apart from the well-known canonical right-handed duplex conformation, it can fold into various non-canonical structures through Hoogsteen and Wobble base pairing, depending on the sequence and external environmental conditions.3 These non-canonical structures include hairpin loops, internal loops, homoduplexes [like the A-motif and poly-(GA)n duplex] and higher-ordered structures such as triplexes, G-quadruplexes, junctions, and three-way and four-way branches.4–8 Recent studies indicate that these non-canonical NA structures are required to perform diverse functions in regulating and catalysing various biological processes.9 Therefore, the structure, function and biological significance of canonical and non-canonical NAs make them attractive targets while designing treatments for several gene-related diseases such as cancer, and parasitic and viral infections among others.10–12 Over the years, various analytical tools such as UV-vis absorption spectroscopy, circular dichroism (CD) spectroscopy, gel-electrophoresis, DNA-foot printing assay, viscosity measurements, isothermal calorimetry (ITC), mass spectrometry analysis, X-ray crystallography and nuclear magnetic resonance (NMR) spectroscopy have been employed for studying both canonical and non-canonical DNA structures and their interactions with small molecules.13–15 However, most of these spectroscopic tools work in vitro only and are unsuitable under in vivo conditions. To investigate the structural and functional aspects of NAs inside the cell, it is necessary to design fluorescent probes with turn-on emission upon interacting with the target NA structure.16 In this regard, fluorescence spectroscopy has become a powerful tool in cell biology and has been playing a vital role in the modern era of active research, encompassing bioimaging and diagnostic applications.17,18 Undeniably, day-to-day research activities in molecular, cell and chemical biology and allied areas would be unimaginable without the study of DNA and RNA using fluorescence-based techniques. Fluorescence techniques provide the additional advantage of real-time monitoring of structural reorganisation and biological functions of biomacromolecules in living cells, with high temporal and spatial resolution.19,20 A variety of small molecular fluorescent probes have been designed and developed to target a broad range of biomolecules including constituents of the cell surface, cell membrane, proteins, NAs and enzyme substrates.21–24 In comparison to other biomolecules, relatively very few fluorescent probes are available for NAs and these are limited by their selectivity and sensitivity towards the structure and conformational changes of NAs. An ideal fluorescent probe for the imaging of cellular components including NAs, proteins and other biomolecules must have the following properties: (i) structure- and sequence-specificity, (ii) high binding affinity, (iii) cell permeability, (iv) good water solubility, (v) turn-on fluorescence behaviour, (vi) high quantum yield, (vii) high photostability and (viii) non-toxicity upon binding.Fluorescent probes ranging from small molecular probes,20–23 to fluorescent proteins,25 inorganic nanoparticles and nanocrystals26 have been employed for targeting various biological components with many advantages such as high fluorescence quantum yield and high photostability. In particular, fluorescent labelling-based probes for targeting NAs have become indispensable tools in diverse fields ranging from cell biology, molecular biology and chemical biology to clinical diagnosis and drug discovery.18 However, their inherent toxicity and tricky synthetic procedures limit their potential use in biological applications. In this regard, small molecular probes offer advantages to fine-tune the chemical and photophysical properties by functionalising with electron donating and accepting groups and enhancing their suitability for a broad range of applications.27,28 The fluorescence properties of small molecular probes can be fine-tuned by suitably choosing the electron donor (D) and acceptor (A) moieties. The appropriate combination of D and A units by a conjugated π-electron chain (D–π–A) has the potential to provide a novel approach to modulate the absorption and emission wavelengths by means of modifying the structure and number of conjugated double bonds between donor and acceptor moieties. The architecture of donor–acceptor interplay has brought in different combinations like D–A–D, A–D–A and simple D–π–A systems, which is evident in almost all the designs presented throughout this review (Fig. 1).
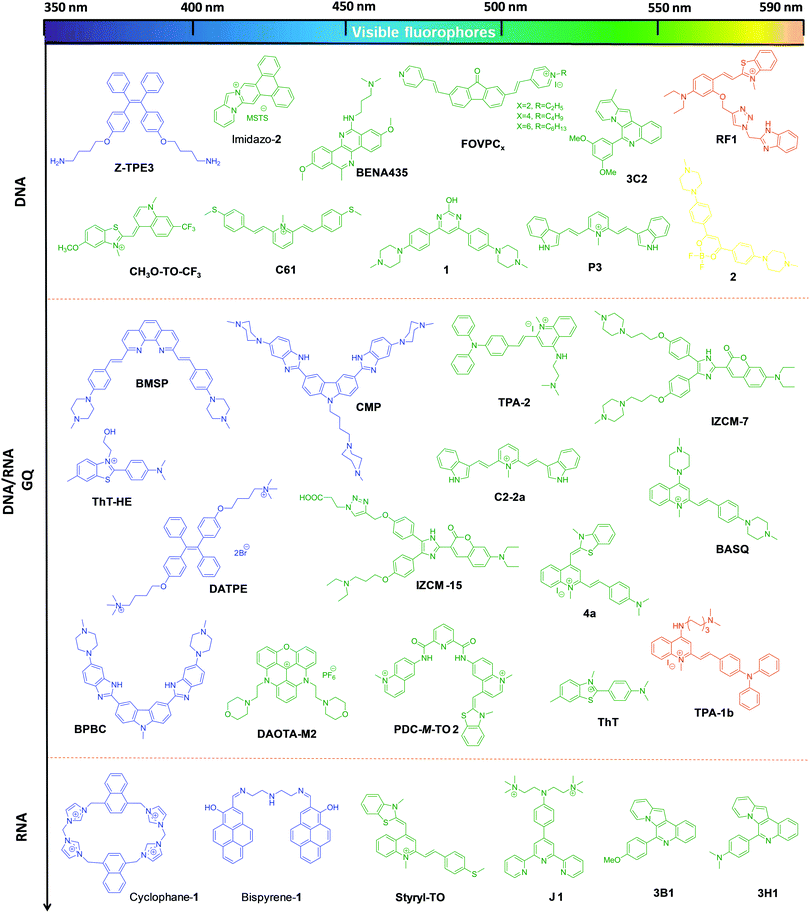
The A–D–A system can be rendered with stimuli responsiveness by adding chemical or enzymatic trigger appendages, which would be activated in the presence of external stimuli conditions. Probes with stimuli-responsive properties and their detailed study have been discussed separately in the miscellaneous section. The restriction of molecular rotation around the π-conjugated methine bridge between D and A impacts the push–pull effects, and this process can be specific to a conformation of NAs, giving rise to different spectroscopy responses. A systematic and timely survey of fluorescent probes of various canonical and non-canonical NA structures is necessary to foster future advancements in this active research area which includes in vitro, in cellulo and in vivo imaging of biomolecules and organelles. Among the small molecular fluorescent probes, Hoechst (bis-benzimidazole probes), 4′,6-diamidino-2-phenylindole (DAPI), ethidium bromide (EtBr),29,30 propidium iodide (PI),31,32 SYBR green I,33,34 PicoGreen35,36 and thiazole orange (TO)37,38 are the commonly used ones for staining DNA in gel-electrophoresis, the cell nucleus, real-time PCR analysis, flow cytometry and many other biological applications.39,40 Numerous small molecular fluorescent probes with emission in the lower wavelength region of the visible spectrum have been reported for the detection of non-canonical G-quadruplex structures and therapeutic applications targeting these DNA polymorphic structures (Fig. 2).41–46 However, these fluorescent probes suffer from common problems like non-specificity, poor aqueous solubility, cell impermeability, absorption and emission in the lower wavelength region of the UV-Vis spectrum, cellular toxicity, low fluorescence quantum yield, and insufficient chemical and photostability. In addition, auto-fluorescence from cellular biomolecules results in high background signal, leading to high noise-to-signal ratios in the lower wavelength of the visible (blue and green) region. In contrast, small molecular probes with absorption in the longer wavelength of the visible region and emission in the red or near infrared (NIR) regions are preferred for in vivo or in situ penetration.20 To the best of our knowledge, there are no in-depth reviews that have covered far-red (red and near infrared i.e., 600–750 nm region of the electromagnetic spectrum) fluorescent probes for NAs, especially for DNA, RNA and their non-canonical structures. It is, hence, essential to provide researchers with one-stop cumulative information on such highly sought-after probes, with thorough discussion and clear outlook for future developments in the area. In this review article, we cover research publications from the past decade (2006 to present), reporting mainly far-red fluorescence turn-on probes for canonical (DNA/RNA) and non-canonical (mainly G-quadruplex) structures of NAs and their potential applications including in vitro detection and quantification, in cellulo imaging, chromosomal staining, parasite detection in human erythrocytes, flow cytometry analysis, FRET study, in vivo imaging and diagnostic applications (Fig. 1). Keeping in mind the tremendous progress made over the decades in developing visible fluorescent probes capable of providing optimal image resolution, we provide a brief survey of fluorescent probes of canonical and non-canonical NAs with emission in the visible region of 400–600 nm, for the reader's benefit.47–75 However, we direct the reader's attention to primary literature as well as some of the review articles for detailed information on these visible fluorescence (400–600 nm) probes.47–76 The molecular structures and characteristic properties, including excitation–emission wavelengths, quantum yield, binding constant, NA conformation selectivity and in cellulo localisation of selected and relevant probes, and their primary literature are shown in Fig. 3 and Tables 1 and 2, respectively. Notably, research activity in the area of development of far-red fluorescent probes has picked up only in the last few years. In this review article, we restrict our coverage and critical discussion to far-red fluorescent probes by highlighting their molecular design concepts and purpose, achievements, chemical and photostability, quantum yields, limitations of the probes and methods, and possible suggestions to improve the selectivity, specificity, and sensitivity based on organic molecular platforms. Furthermore, we highlight the scope of future research, entirely in the hope of achieving highly selective recognition and imaging of specific canonical and non-canonical NA structures and possibly targeting longer sequences than the fewer nucleobases of canonical and non-canonical DNA and RNA structures.
| Probe | λ exc (nm) | λ em (nm) | Stokes shift (nm) | Quantum yield (ΦF) bound/unbound | Binding constant | Mode of binding | In vitro conformation specificity | In cellulo imaging (localization) |
|---|---|---|---|---|---|---|---|---|
| Z-TPE3 47 | 330 | 480 | 150 | — | — | Electrostatic | DNA | — |
| 1 48 | 416 | 503 | 87 | 0.12/0.001 | — | Groove | AT-rich DNA | Live HEK293 (nucleus) |
| 2 48 | 502 | 558 | 56 | 0.14/0.002 | — | Intercalation | AT-rich DNA | Live HEK293 (nucleus) |
| C61 49 | 425 | 540 | 115 | 0.0675 | — | — | DNA | Live and fixed A549 and HeLa cells (nucleus) |
| FOVPCx 50 | 400 | 540 | 140 | ∼0.4–0.6 | — | Intercalation | DNA | 3T3 cells (nuclei) |
| P3 51 | 447 | 537 | 90 | 0.26 | — | Minor groove | AT-rich | Fixed HeLa |
| Imidazo-252 | 470 | 536 | 66 | 0.32 | ∼4.5 × 105 M−1 | Intercalation | AT-rich | Live HeLa cells (nuclei) |
| CH3O-TO-CF3 53 | 526 | 560 | 34 | 0.04 | — | — | DNA | HeLa cells |
| BENA435 54 | 435 | 426, 484 | ∼49 | 0.138 | — | Intercalation | AT-rich DNA and RNA | Live and fixed mouse fibroblasts (nuclei) |
| RF1 55 | 490 | 585 | 95 | 0.5 | K a = 9.5 × 105 M−1 | Minor groove | AT-rich DNA | Fixed HeLa cells (nuclei) |
| 3C2 56 | 447, 467 | 526 | 79, 59 | 0.112 | — | — | DNA | Live HeLa, MCF 7, NIH-3T3 cells |
| Cyclophane-157 | 350 | 449 | 99 | — | 4.9 × 106 M−1 | End stacking, electrostatic interaction | RNA | Fixed HeLa cells C. Elegans (both nuclei and cytoplasm) |
| Bispyrene-158 | 348 | 470 | 122 | — | — | — | RNA | Live HeLa cells |
| Styryl-TO 59 | 476 | 535 | 59 | 0.056/0.0016 | 12.32 × 105 M−1 | — | RNA | Fixed PC3 cells |
| J1 60 | 800 (two photon) 405 (single photon) | 550 | 145 (single photon) | 0.19 (unbound) | 1.8 × 104 M−1 | Groove | RNA | Live HepG2, HeLa, MCF7 and MRC5 cells (nucleoli) |
| 3H1 56 | 437 | 544 | 107 | 0.43 | — | — | RNA | Live HeLa, MCF 7, NIH-3T3 cells |
| 3B1 56 | 441, 457 | 507 | ∼66 | 0.128 | — | — | RNA | Live HeLa, MCF 7, NIH-3T3 cells |
| Probe | λ exc (nm) | λ em (nm) | Stokes shift (nm) | Quantum yield (ΦF) bound/unbound | Binding constant | Mode of binding | In vitro conformation/sequence specificity of GQ | In cellulo imaging (localization) |
|---|---|---|---|---|---|---|---|---|
| BPBC 61 | 408 | 462 | 54 | 0.249 | 0.5–5 × 106 M−1 | End stacking | Parallel | — |
| BMSP 62 | 338 | 512 | 174 | 0.203 | 0.13 μM | End stacking | Antiparallel | Live MCF-7 cells (cytoplasm) |
| ThT-HE 63 | 415 | 485 | 70 | 0.53 | 13–23 μM | End stacking | Parallel (27MYC) | — |
| CMP 64 | 330 | 466 | 136 | — | K D = 0.13–0.34 μM | End stacking | — | Fixed HeLa cells (nucleus) |
| DATPE 65 | 330 | ∼475 | 145 | 0.069–0.334 | K a = 3–7 × 106 M−1 | Intercalation | Multimeric GQ | — |
| TPA-2 66 | 470 | 595 | 125 | — | ∼9.08 × 106 M−1 | — | No selectivity | Fixed HepG2 cells (nucleoli) |
| BASQ 67 | 456 | 587 | 131 | — | 0.1–3.36 × 106 M−1 | Partial stacking | — | — |
| DAOTA-M2 68 | 436 | 581 | 145 | 3.3–4.9/0.032 | log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ka = 5.7–6.1 Ka = 5.7–6.1 |
— | Live osteosarcoma cell line U2OS | |
| PDC-M-TO 2 69 | 500 | 549 | 49 | 0.31 | — | — | ckit2 and cmyc genes | — |
| 4a 70 | 475 | 630 | 155 | — | 9.65 × 105 M−1 | End stacking | — | Fixed PC3 cells (nucleus) |
| ThT 71,72 | 425 | 490 | 65 | 0.25 | ∼2.85 × 105 M−1 | End stacking and groove | DNA/RNA GQ | — |
| IZCM-7 73 | 450 | 525 | 75 | 0.518/0.012 | 3.9 × 106 M−1 | End stacking | Parallel (Pu22) | — |
| IZCM-15 74 | 450 | 525 | 75 | 0.44 | — | End stacking with groove interactions | Parallel (c-kit2) | — |
| TPA-1b 75 | 470 | 595 | 125 | — | 3.59 × 107 M−1 | End stacking | HRAS gene | Live Hep-G2 cells (nucleoli) |
| C2-2a 76 | 486 | 550 | 64 | 0.24 | 5.02 × 105 M−1 | — | Antiparallel (telo21) | Live PC3 cells |
Canonical structures
DNA
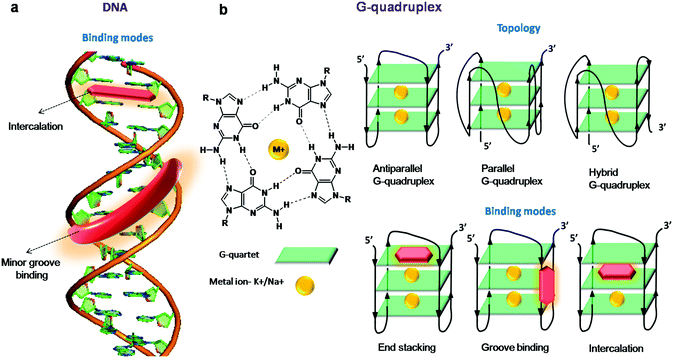
The typical edge-to-edge hydrogen bonding between nucleobases keeps the complementary strands organised into a right-handed double-helical structure termed B-DNA, the most common form of DNA under physiological conditions. The canonical B-DNA or RNA adopt the double helix conformation wherein two antiparallel strands are held together by standard (canonical) Watson–Crick hydrogen bonding between A and T (A![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) T) and G and C (G
T) and G and C (G![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C) base pairs, with the exception of A
C) base pairs, with the exception of A![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) U for RNA. Remarkably, NAs (DNA and RNA) are central to gene expression and in vitro studies, and direct in vivo visualisations are highly useful in unravelling the complex information related to their structure and function. Therefore, novel far-red turn-on fluorescent probes and methods for the rapid and reliable non-invasive detection of NAs are invaluable for understanding genetic mutations and disorders, with far-reaching implications for developing effective diagnostics and therapeutics.
U for RNA. Remarkably, NAs (DNA and RNA) are central to gene expression and in vitro studies, and direct in vivo visualisations are highly useful in unravelling the complex information related to their structure and function. Therefore, novel far-red turn-on fluorescent probes and methods for the rapid and reliable non-invasive detection of NAs are invaluable for understanding genetic mutations and disorders, with far-reaching implications for developing effective diagnostics and therapeutics.
Fluorescence spectroscopy and imaging are widely used to study DNA under in vitro conditions and stain the nuclei of live or fixed cells, respectively; they are also used to probe the structural reorganisation of the chromosomes in the cell nucleus.24 Small molecular fluorescent probes interact with duplex DNA mainly through intercalation and minor groove binding modes (Fig. 2a). These fluorescent probes are stabilised through various non-covalent interactions, including aromatic, van der Waals and hydrogen bonding interactions between nucleobases and electrostatic interactions with the negatively charged phosphate backbone. The molecular mechanisms underlying NA-induced fluorescence enhancement of the turn-on fluorescence dyes originate either from restriction of intramolecular rotation (viz., cyanine, benzimidazole, carbazole derivatives and AIE: aggregation-induced emission probes) or from protection against water-mediated nonradiative deactivation provided by the NA (viz., NA staining agents, porphyrins and metal complexes).
In recent years, cyanine-based far-red probes have been gaining the attention of the scientific community due to their deep sample penetration, minimal interference from auto-fluorescence from the cellular milieu leading to improved signal-to-noise ratios, non-fluorescence in the unbound state and exhibition of turn-on or enhanced fluorescence upon binding to the target, high quantum yields in the bound state and good photostability, all of which enable imaging and quantification of NAs in live cells and organisms.
Xiaojun Peng and co-workers reported a cyanine probe 1-ethyl-4-{3-[3-(4-diethylamino)-butyl-2-benzothiazolinylidene] propenyl}quinolinium iodide (DEAB-TO-3) that acts as a structural analogue of the fluorescence DNA binding probe TO-3 with excitation (626 nm) and emission (649 nm) in a longer wavelength region of the visible spectrum (Fig. 4a).77 In DEAB-TO-3, methyl groups on quinoline and benzothiazole were replaced with ethyl and N,N-diethyl-amino butyl groups, respectively, compared to the parent TO-3 dye. Probe DEAB-TO-3 alone was weakly fluorescent in a buffer solution with meager fluorescence quantum yield (ΦF = ∼0.0037). Upon binding ct-DNA, the quantum yield increased by 97.3-fold (ΦDNAF = 0.36). Interestingly, the probe showed ∼80.3-fold (at 647 nm) and 18.9-fold (at 661 nm) fluorescence enhancement in the presence of A:T and G:C rich DNA, respectively (Fig. 4a). The observed fluorescence enhancement of DEAB-TO-3, in the presence of DNA, mainly originates from the restriction of intramolecular rotation of unsymmetrical cyanine dyes. Furthermore, low fluorescence of the probe in the presence of G:C rich DNA may be presumed to be due to photo-induced electron transfer (PET) from the probe to guanine residues. In the presence of ct-DNA, CD spectra showed induced positive and negative CD-signals at 616 and 580 nm respectively, which suggests binding of the probe in the minor groove of DNA. Also, live cell fluorescence imaging of HeLa cells using DEAB-TO-3 (2 μM) showed selective staining of the cell nucleus over the cytoplasm (Fig. 4b). In their subsequent work, Xiaojun Peng and co-workers designed a bright red fluorescence cyanine TO3-CN probe with good photostability and a Stokes shift of 68 nm for imaging NAs (both DNA and RNA) in a non-selective manner (Fig. 4c).78 They introduced an electron withdrawing cyano (–CN) group in the TO3 probe to improve its photostability by suppressing the reactivity with singlet oxygen. It was presumed that introducing an electron withdrawing group into TO3 would not influence the binding selectivity of the probe to DNA. However, the new probe TO3-CN (λex = ∼543 nm and λem = 611 nm) showed a blue shift in the absorption and emission wavelength maxima compared to parent probe TO3 (λex = ∼626 nm and λem = 647 nm), whereas the emission remained in the red region. TO3-CN showed a large fluorescence enhancement in the presence of NAs (ct-DNA and RNA) with a significant increase in quantum yield (ΦF(bound) = ∼0.70; ΦF(unbound) = ∼0.017), which is higher than that of the TO3 probe (Fig. 4c). The photostability of TO3-CN was tested by continuously irradiating with UV-light for 3 h, along with TO3 as a control. After 3 h, probe TO3-CN retained ∼90% of its optical density, whereas TO3 lost ∼80% under similar conditions (Fig. 4d). Confocal fluorescence imaging of MCF7 cells using TO3-CN (2 μM) showed clear nucleolar staining with weak nuclear and cytoplasmic staining (Fig. 4e). However, TO3-CN showed ∼70% fluorescence in cells upon continuous radiation for 5 min while TO3 showed merely ∼30% fluorescence intensity. Furthermore, DNA and RNA digestion studies in MCF7 cells showed binding of TO3-CN to both DNA and RNA. The cell viability assay showed low-toxicity at 5 μM of TO3-CN with ∼80% viable cells. Nevertheless, these studies suggest that the introduction of electron withdrawing groups such as –CN group in the cyanine backbone significantly increases their photostability, whereas DNA/RNA selectivity of this class of probes needs to be addressed along with the determination of the binding constant and other characteristic parameters. Our research group is particularly interested in the design and development of red-NIR fluorescent probes for various biomolecules and amyloid aggregates.79–82 Recently, we reported hemicyanine-based, cell-permeable red fluorescent probes for the selective detection of DNA containing AT-base pairs, which are useful for multiple biological applications including nuclear staining, cell-cycle analysis and malarial parasite (Plasmodium falciparum) detection in infected human erythrocytes, among others.83 We designed three hemicyanine-based probes, among which thiazole-coumarin (TC) with excitation at 528 nm and weak emission in the longer wavelengths of the visible region (641 nm) showed better turn-on fluorescence (Fig. 5). These hemicyanine probes are a new class of donor–π bridge–acceptor (D–π–A) molecules, wherein the intramolecular charge transfer (ICT) process results in a large Stokes shift. Generally, these probes are non-fluorescent or weakly fluorescent in the unbound form due to intramolecular twisting; upon binding with NAs, the intramolecular rotation freezes and molecular rigidity and planarity are attained, leading to significant fluorescence enhancement. Interestingly, TC is almost non-fluorescent in a buffer solution with meager fluorescence quantum yields, which is a necessary attribute for turn-on fluorescence DNA binding probes. Among three probes, TC showed promising fluorescence enhancement in the presence of A20:T20 compared to G10:C10, single-stranded DNA (ssDNA) and proteins (Fig. 4a). In the presence of A10:T10, the absorption spectrum of TC showed a significant bathochromic shift (Δλmax = 14 nm) with hypochromicity. In addition, TC displayed fluorescence enhancement of ∼16-fold, ∼6-fold and ∼2-fold with a variable number of consecutive A:T base pairs, i.e., 20, 4 (two sets) and 2 (two sets), respectively. Unbound probe TC showed low fluorescence quantum yield (ΦF = 0.03) while exhibiting a high fluorescence quantum yield of ΦF = 0.36 in the presence of A20:T20. These results suggest a significant increase in red fluorescence intensity of TC with an increasing number of A:T base pairs. Furthermore, TC was employed for staining of DNA containing A:T base pairs in gel electrophoresis studies. Probe TC showed promisingly higher fluorescence intensity in the presence of A:T-rich DNA over G:C-rich DNA in the following order: A20:T20 > (D1)mix > (D2)mix > G10:C10, where (D1)mix (5′-CGATAAGCGCTTATCG-3′) and (D2)mix (5′-CGGTACCGCGGTACCG-3′) are self-complementary mixed sequence DNA. On the other hand, EtBr showed relatively low fluorescence intensity in the presence of A:T-rich DNAs compared to G:C-rich DNAs. Surprisingly, competitive binding studies revealed fluorescence resonance energy transfer (FRET) between Hoechst 33258 (donor) and probe TC (acceptor). Density functional theory (DFT) calculations showed that TC preferably binds the DNA in an intercalation mode and charge transfer transition from the excited TC moiety to guanine results in fluorescence quenching in the presence of G:C base pairs. Selective fluorescence enhancement in the presence of DNA motivated us to study the cellular uptake properties of probe TC. Confocal fluorescence images of TC in HeLa and HEK 293 cells (Fig. 5b) showed selective staining of the cell nucleus with maximum co-localisation with Hoechst 33258. Moreover, staining of metaphase chromosomes of HEK 293 cells showed dense intensity in the centromeric region, which is known to be rich in A:T base pairs. Furthermore, we demonstrated live fluorescence imaging of Plasmodium falciparum as the probe selectively stained the red blood cells containing parasites compared to control blood cells at lower concentrations of the probe (Fig. 5c). This result is especially impressive as the Plasmodium falciparum genome contains ∼80% A:T base pairs and even low concentrations of the probe were sufficient to selectively stain inside the anucleated red blood cells. This probe, therefore, has implications for developing novel diagnostic tools for malaria. The fluorescence-activated cell sorting (FACS) analysis using probe TC in HEK 293 cells showed a similar or marginally improved staining pattern of the cell-cycle phase compared to that achieved with PI (Fig. 5d). Remarkably, FACS analysis using TC does not require RNAse treatment and can be performed on live cells, unlike PI, which is highly toxic and impermeable to live cells and can only be used for the analysis of dead cells. Overall, these results on red fluorescent probe TC demonstrated its selective recognition of DNA containing A:T base pairs, preferential staining of the cell nucleus and malaria parasites in human blood cells and its potential use in diagnostic kits. Furthermore, TC can be employed as a promising biomarker for DNA quantification through FACS analysis, FRET analysis within DNA sequences and possibly PCR applications, all of which make it a multifunctional DNA probe.
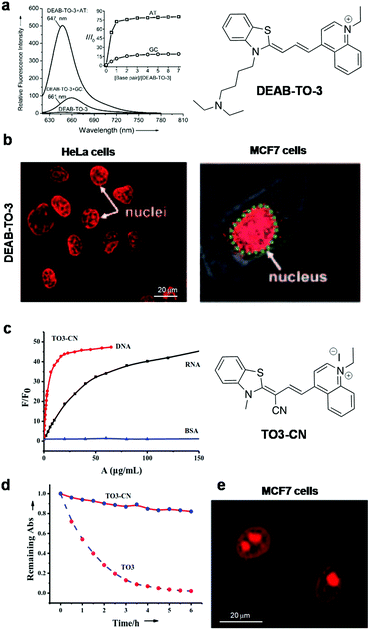 | ||
| Fig. 4 DEAB-TO-3 and TO3-CN. (a) Fluorescence spectra of DEAB-TO-3 (1 μM) in the presence of poly(dA–dT)2 and poly(dG–dC)2. Inset: The corresponding concentration-dependent fluorescence response of DEAB-TO-3. (b) Fluorescence images of DEAB-TO-3 treated live HeLa cells and MCF-7 cells at 5 μM and 2 μM concentration, respectively. Reproduced from ref. 77 with permission from Wiley-VCH, copyright 2011, (c) fluorescence response of TO3-CN (1 μM) to DNA, RNA and bovine serum albumin (BSA), (d) photostability response of probes TO3-CN, and (e) confocal fluorescence images of TO3-CN (2 μM) in live MCF-7 cells. Reproduced from ref. 78 with permission from Royal Society of Chemistry, copyright 2014. | ||
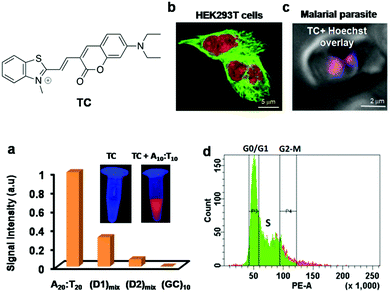 | ||
| Fig. 5 (a) Fluorescence response of TC (10 μM) in the presence of various AT and GC-rich double stranded DNAs. Inset: Photographs of solutions of TC and TC + A10:T10 under UV light (365 nm). (b) Fluorescence imaging of fixed HEK 293T cells incubated with anti α-tubulin marker (green) and TC (red), and (c) live fluorescence imaging of the AT-rich genome of parasitized red blood cells using TC (1 μM). Reproduced from ref. 83 with permission from Nature Publishing Group, copyright 2014. | ||
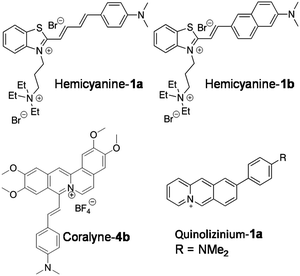
Recently, Jian-Feng Ge and co-workers reported quaternary ammonium group-bearing hemicyanine probes for the detection of NAs (Fig. 6).84 The probes hemicyanine-1a and hemicyanine-1b contain a D–π–A architecture with a positively charged quaternary ammonium group, which assists the probes’ binding to negatively charged NAs with high affinity. Absorption spectra in the presence of DNA and RNA showed a large red shift in the absorption maxima of hemicyanine-1a (80 nm and 29 nm with DNA and RNA, respectively) and hemicyanine-1b (61 nm and 42 nm with DNA and RNA, respectively). Emission spectra of hemicyanine-1a showed ∼33-fold and ∼16-fold fluorescence enhancement at 706 nm upon addition of increasing concentrations of DNA and RNA, respectively, with only slight increase in the quantum yields (ΦF = 0.125 and ΦF = 0.116, respectively) compared to unbound hemicyanine-1a (ΦF = 0.008). Similarly, probe hemicyanine-1b showed ∼33-fold and ∼39-fold fluorescence enhancement at 712 nm in the presence of DNA and RNA, respectively, along with minor increment in the quantum yields (ΦF = 0.058 and ΦF = 0.049, respectively) compared to unbound hemicyanine-1b (ΦF = 0.003) (Fig. 6). The major drawback of probes hemicyanine-1a and 1b is the lack of selectivity as they are incapable of distinguishing between DNA and RNA. Confocal fluorescence imaging of probes hemicyanine-1a and 1b (2 μM) in fixed HeLa cells showed staining of nucleoli and the cytoplasm, although DNAse and RNAse experiments indicated that the probes were not responsive for DNA binding inside cells; hence, the authors have claimed RNA imaging alone in fixed cells. Hemicyanine-1a and 1b are attractive NIR fluorescence markers for NAs in fixed cells, though selective reporting of DNA and RNA, quantum yields and live cell imaging need to be addressed and improved through appropriate structural modifications.
Various heterocyclic alkaloids (berberine, coralyne, palamatine etc.) known to target different NAs have been known to stabilise GQ DNA and show anti-telomerase and anti-leukemic activity.85,86 Notwithstanding such positive attributes, their major drawback is fluorescence quenching upon interaction with NAs. Ihmels and his group succeeded in transforming coralyne into a potential turn-on fluorescent probe (Coralyne-4b) by extending the π-system of the core molecule by conjugating with styryl substituent (Fig. 6).87 Coralyne-4b, upon excitation at 400 nm, showed an emission band at 480 nm (green emission). Interestingly, the presence of either ct-DNA or quadruplex DNA (22AG) resulted in fluorescence quenching at 480 nm and the appearance of a new red-shifted band at 695 nm (red emission). The ct-DNA showed 125-fold fluorescence increase compared to GQ DNA (53-fold) with binding constants 2.3 × 105 and 0.33 × 105 M−1, respectively. However, the probe failed to differentiate the duplex and GQ conformations of NAs. Nevertheless, fluorometric detection of live cells using Coralyne-4b has been reported in NIH 3T3 mouse fibroblasts.
Bortolozzi et al. reported a multicolour fluorescent probe for various biomacromolecules with different emission profiles. The multicolour probe 9-(4-dimethylaminophenyl) benzo[b]quinolizinium (quinolizinium-1a) has been found to be sensitive to environmental conditions such as polarity and viscosity, making it useful for detecting NAs and other biomolecules (Fig. 6).88 Protonation of the molecule and viscosity of the solution were found to enhance the emission intensity. Probe Quinolizinium-1a exhibited an intense red-shifted emission band at 699 nm upon excitation at 470 nm in a glycerol–water mixture, suggesting intramolecular charge transfer (ICT) through the restriction of intramolecular rotation. As a consequence, a characteristic change in the fluorescence behaviour of Quinolizinium-1a was observed in the presence of duplex and quadruplex DNA. In the presence of ct-DNA, the probe showed 38-fold enhancement in emission intensity with an emission maximum at 728 nm. On the other hand, the probe exhibited a 96-fold increase in emission intensity with an emission peak at 697 nm in the presence of quadruplex-forming oligonucleotide 5′-A(GGGTTA)3GGG-3′. The binding parameters of the probe with duplex DNA and quadruplex DNA were determined from photometric titrations using a scatchard plot. The binding constants for duplex ct-DNA and quadruplex DNA were found to be Kb = 1.3 × 104 M−1 and Kb = 8.3 × 104 M−1, respectively. The probe exhibited change in fluorescence in the presence of bovine serum albumin with emission maxima at 609 nm. Thus, the fluorescence properties (emission intensity and maxima) of the probe are highly sensitive to the environment, making it suitable for multicolour imaging of various biomolecules and subcellular organelles. Incubation of HeLa cells with the probe (2.5 μM) for 1 h revealed cellular uptake and red emission in the nucleolar region, corresponding to binding with DNA. The red staining was observed in the cytoplasm due to RNA binding, besides showing blue emission for lysosomes and green emission for proteins. This probe was claimed to be a potential probe for multicolour-based imaging of various biomolecules in different compartments of the cell with higher resolution. However, the selectivity among various NA structures and distinct binding modes need to be assessed accurately for quantitative imaging and analysis. Multicolour fluorescence analysis is of high utility, and developing efficient probes in this area would be of great importance for imaging and diagnostic applications.
A new class of quinone cyanine (QCy7) dyes is fast becoming a versatile molecular platform to develop NIR fluorescent probes. QCy7 dyes are typically one-donor-two-acceptor-based molecular systems (D2A) composed of a donor phenolic-moiety conjugated with positively charged N-alkylated heterocyclic acceptors such as quinolines, pyridines, iodolines and benzothiazoles.80,81,89–94 The deprotonation of the phenolic-moiety generates a phenolate ion, which donates electrons to the positively charged nitrogen atom of one of the acceptors (N-alkylated heterocyclic group). This process triggers internal charge transfer (ICT) between the two positively charged acceptors, making the molecule a highly delocalised π-electron system similar to the Cy7 backbone. Recently, we designed a bent-shaped quinone cyanine-dithiazole (QCy-DT) NIR fluorescent probe for selective detection and imaging of DNA containing A:T base pairs through turn-on response (Fig. 7a).95 At physiological pH, QCy-DT mostly exists in the phenolate form, which results in the ICT process to form a delocalised π-electron system; it showed very basal level NIR fluorescence (λmax= ∼680 nm) upon excitation at 530 nm owing to delocalisation of π-electrons from the negatively charged phenolic oxygen to the positively charged p-substituted benzothiazolium vinyl group (Fig. 7a). Thus, being weakly fluorescent or non-fluorescent in the unbound state and exhibiting a large Stokes shift (Δλmax = ∼150 nm) makes QCy-DT a suitable dye to study DNA binding properties. The fluorescence response of QCy-DT was examined in the presence of DNA duplexes rich in A:T base pairs such as A20:T20, self-complementary d(ATAT)5, Drew-AT – a self-complementary 14 base pair sequence with a 6 AT-base pair core (5′-GCGCAAATTTGCGC-3′) and G:C-rich DNA duplex G20:C20, self-complementary (D1)mix and ct-DNA. A fluorescence study showed ∼250, ∼200, ∼55, ∼50, ∼40, ∼8 and 2-fold fluorescence enhancement at 650 nm in the presence of A20:T20, Drew-AT, (D1)mix, d(ATAT)5, ct-DNA, G20:C20, and ssDNA, respectively. Furthermore, CD-spectra of QCy-DT showed induced positive CD signals at 575 and 366 nm and a negative CD signal at 476 nm in the presence of A20:T20, confirming the minor groove binding ability of QCy-DT. Surprisingly, we observed a 4-fold difference in the fluorescence enhancement of QCy-DT in the presence of A20:T20, and self-complementary d(ATAT)5 with alternative AT-base pairs, indicating the sequence-selective recognition of a particular combination of A:T base pairs in DNA. The sequence-specific recognition ability of QCy-DT was evaluated by recording fluorescence spectra in the presence of self-complementary DNA duplexes (12-base pairs sequences) containing variable (A/T)4 base pairs within 5′-GCGC-Bn-GCGC-3′ (where B indicates nucleobases with n = 4 AT rich central cores such as 5′-ATAT-3′ (DM1), 5′-ATTA-3′ (DM2), 5′-AAAA-3′ (DM3) and 5′-AATT-3′ (DM4) in the central groove region) (Fig. 7b). QCy-DT showed maximum and minimum fluorescence enhancement in the presence of 5′-AATT-3′ (DM4) and 5′-ATAT-3′ (DM1) sequences, respectively (Fig. 7b). Furthermore, local variations around 5′-AATT-3′, i.e., 5′-X![[A with combining low line]](https://www.rsc.org/images/entities/char_0041_0332.gif)
![[A with combining low line]](https://www.rsc.org/images/entities/char_0041_0332.gif)
![[T with combining low line]](https://www.rsc.org/images/entities/char_0054_0332.gif)
![[T with combining low line]](https://www.rsc.org/images/entities/char_0054_0332.gif) Y-3′ (X = A/T/G/C and Y is the complementary base of X) sequences were performed. Remarkably, QCy-DT showed large fluorescence enhancement for DNA with a 5′-A
Y-3′ (X = A/T/G/C and Y is the complementary base of X) sequences were performed. Remarkably, QCy-DT showed large fluorescence enhancement for DNA with a 5′-A![[A with combining low line]](https://www.rsc.org/images/entities/char_0041_0332.gif)
![[A with combining low line]](https://www.rsc.org/images/entities/char_0041_0332.gif)
![[T with combining low line]](https://www.rsc.org/images/entities/char_0054_0332.gif)
![[T with combining low line]](https://www.rsc.org/images/entities/char_0054_0332.gif) T-3′ core sequence, which was ∼4-fold higher than that of DNA containing a 5′-AATT-3′ core sequence. Thus, QCy-DT is the first NIR fluorescence turn-on probe to recognise the minor groove of a DNA duplex containing A:T base pairs in a sequence-specific manner (Fig. 7b). Confocal fluorescence imaging of QCy-DT in fixed HeLa and MCF-7 cells showed selective staining of the cell nucleus at a submicromolar concentration (0.5 μM), confirming the cell permeability and DNA staining by the probe (Fig. 7d). The cell viability assay (MTT assay) showed that QCy-DT is non-toxic up to ∼8 μM with >76% cells remaining viable after a 24 h time period of incubation, which enables live cell imaging applications. Furthermore, QCy-DT was found to inhibit the trophozoite stage of P. falciparum parasites with an IC50 value of ∼4 μM similar to netropsin (Fig. 7c). Live fluorescence imaging of Plasmodium falciparum within the red blood cells showed that probe QCy-DT specifically stains the parasite nuclei, but not the cytoplasmic part of red blood cells (Fig. 7e). Overall, ease of synthesis, non-fluorescence in the unbound state and turn-on NIR fluorescence upon binding to DNA, large Stokes shift, cell permeability, low-toxicity, live cell imaging and Plasmodium parasite staining makes probe QCy-DT a superior and multifunctional NIR fluorescent probe for a wide range of biological applications. It is noteworthy to mention here that the selective staining of Plasmodium falciparum in red blood cells and inhibition of parasites indicate QCy-DT is an extraordinary probe and opens up newer avenues towards the development of effective diagnostic and therapeutic tools for malaria through a structure–activity relationship study. Furthermore, the molecular structure of QCy-DT with phenolic functionality offers numerous ways to develop stimuli-responsive probes to detect various biomolecules and study bimolecular interactions.
T-3′ core sequence, which was ∼4-fold higher than that of DNA containing a 5′-AATT-3′ core sequence. Thus, QCy-DT is the first NIR fluorescence turn-on probe to recognise the minor groove of a DNA duplex containing A:T base pairs in a sequence-specific manner (Fig. 7b). Confocal fluorescence imaging of QCy-DT in fixed HeLa and MCF-7 cells showed selective staining of the cell nucleus at a submicromolar concentration (0.5 μM), confirming the cell permeability and DNA staining by the probe (Fig. 7d). The cell viability assay (MTT assay) showed that QCy-DT is non-toxic up to ∼8 μM with >76% cells remaining viable after a 24 h time period of incubation, which enables live cell imaging applications. Furthermore, QCy-DT was found to inhibit the trophozoite stage of P. falciparum parasites with an IC50 value of ∼4 μM similar to netropsin (Fig. 7c). Live fluorescence imaging of Plasmodium falciparum within the red blood cells showed that probe QCy-DT specifically stains the parasite nuclei, but not the cytoplasmic part of red blood cells (Fig. 7e). Overall, ease of synthesis, non-fluorescence in the unbound state and turn-on NIR fluorescence upon binding to DNA, large Stokes shift, cell permeability, low-toxicity, live cell imaging and Plasmodium parasite staining makes probe QCy-DT a superior and multifunctional NIR fluorescent probe for a wide range of biological applications. It is noteworthy to mention here that the selective staining of Plasmodium falciparum in red blood cells and inhibition of parasites indicate QCy-DT is an extraordinary probe and opens up newer avenues towards the development of effective diagnostic and therapeutic tools for malaria through a structure–activity relationship study. Furthermore, the molecular structure of QCy-DT with phenolic functionality offers numerous ways to develop stimuli-responsive probes to detect various biomolecules and study bimolecular interactions.
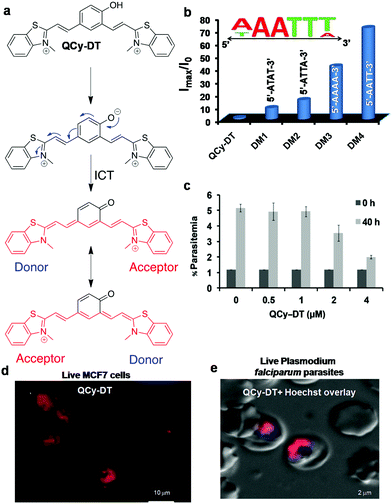 | ||
| Fig. 7 QCy-DT: DNA minor groove binding NIR fluorescent probe. (a) Activation of QCy-DT upon deprotonation of phenolic group to transform the probe into a NIR emitting D2A (one donor two acceptor) system. (b) Fluorescence response of probe QCy-DT (2 μM) against the variable (A/T)4 sequence. Inset: Sequence selectivity of QCy-DT with maximum fluorescence response observed for 5′-AAATTT-3′ up to six base pairs. (c) IC50 determination of QCy-DT for the inhibition of the early trophozoite stage of the malarial parasite. (d) Confocal fluorescence images of probe QCy-DT (0.5 μM) in live MCF7 cells, and (e) live fluorescence imaging of Plasmodium falciparam in blood cells using QCy-DT (0.5 μM). Reproduced from ref. 95 with permission from Oxford University Press, copyright 2015. | ||
Yin et al. developed a primary amine-containing, water-soluble, multifunctional squarine-based indocyanine dye (D1) and studied its interactions with DNA (Fig. 8).96 The cationic D1 dye showed an absorption maximum at 638 nm, and the corresponding emission band around 647 nm with a narrow Stokes shift. Dye D1 retained its fluorescence after 7-day air-exposure, suggesting excellent photostability. Initially, the authors assumed that cationic D1 would interact with negatively charged NAs or other biomolecules and organelles such as phospholipids of the cell membrane and eventually, the dye was used for staining the cellular membrane and nucleus (Fig. 8a). To prove this assumption, the authors studied the interaction of D1 with DNA and phospholipids using isothermal titration calorimetry (ITC). ITC measurements showed that interaction between D1 and DNA was entropically favourable with an association constant (Ka) of 1.9 × 105 M−1. The fluorescence staining of Drosophila and mouse cells with D1 dye showed the maximum fluorescence in the cell membrane compared to other cellular organelles. Furthermore, studies with the membrane-localised marker CD8-GFP in Drosophila salivary glands showed good co-localisation of D1 dye with the membrane marker CD8-GFP (Fig. 8a). On the other hand, confocal images of D1-treated fixed tissues showed selective staining of the cell nucleus with promising co-localisation of DAPI. These studies suggested that the dye D1 selectively stains the cell membrane in live tissues and the cell nucleus in fixed cells or tissues. The squarine-based molecular platform can be further modified chemically to achieve poised hydrophilicity and hydrophobicity to make it a selective staining probe for DNA in live cells.
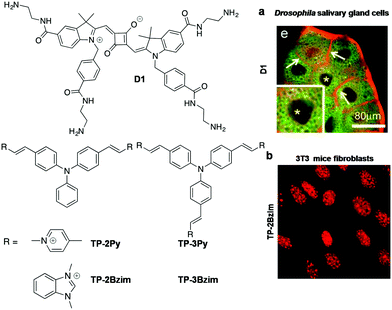 | ||
| Fig. 8 Squarine and triphenyl methane family probes. (a) Confocal images of cultured Drosophila salivary gland cells stained with D1 (red). * indicates a nucleus. Reproduced from ref. 96 with permission from Wiley-VCH, copyright 2013. (b) Fluorescence imaging of AT-rich centromeres in mice fibroblasts (3T3) using TP-2Bzim (1 μM). Reproduced from ref. 97 with permission from American Chemical Society, copyright 2013. | ||
Teulade-Fichou and co-workers reported di-branched and tri-branched triphenylamine N-alkylated benzimidazolium-based two-photon fluorescent probes for A:T-rich DNA (Fig. 8).97 These two-photon fluorescent probes 2,2′-((1E,1′E)-((phenylazanediyl)bis(4,1-phenylene))-bis(ethene-2,1-iyl))bis (1,3-dimethyl-1H-benzo[d]imidazol-3-ium) Iodide (TP-2Bzim), and 2,2′,2′′-((1E,1′E,1′′E)-(nitrilotris(benzene-4,1-diyl))tris-(ethene-2,1-diyl))tris(1,3-dimethyl-1H-benzo[d]imidazol-3-ium) Iodide (TP-3Bzim) were employed for studying their DNA binding properties. The photophysical properties of TP-2Bzim, TP-3Bzim, TP-2Py and TP-3Py were studied in glycerol and buffer solution in the absence and presence of ssDNA and DNA. TP-2Bzim showed a blue shift (∼52–58 nm) in the absorption and fluorescence spectra, with promising fluorescence enhancement (∼140-fold) at 600 nm upon addition of increasing concentration of Drew-AT. However, TP-3Bzim and TP-Py showed moderate fluorescence improvement in the presence of Drew-AT DNA. These results suggest that the V-shaped TP-2Bzim probe attains maximum molecular rigidity and planarity with high π-electron delocalisation upon binding DNA, leading to massive fluorescence enhancement. In addition, CD and molecular modelling studies have supported higher propensity of the V-shaped TP-2Bzim probe to interact with the DNA minor groove. Furthermore, two photon absorption spectra of TP-Bzim and TP-Py probes showed maximum peak intensity at 840 nm with cross-section values (δ) ranging from 250 to 1080 GM. Confocal fluorescence imaging of TP-2Bzim (0.1 and 1 μM) showed nuclear staining of HT29 cells with an excellent signal-to-noise ratio. The fluorescence imaging of TP-2Bzim in the 3T3 cell lines showed accumulation of high dye density foci in the centromeric A:T-rich regions (Fig. 8b). The photostability of TP-2Bzim was studied in comparison with commercial red DNA staining agents in MCF7 cells. Overall, the triphenylamine di-branched and tri-branched probes are a new class of two-photon fluorescent probes for in cellulo imaging and can be useful in many biological applications. The characteristic properties of far-red probes of canonical structures are summarised in Table 3.
| Probe | λ exc (nm) | λ em (nm) | Stokes shift (nm) | Quantum yield (ΦF) | Binding constant | Mode of binding | In vitro conformation specificity | In cellulo imaging |
|---|---|---|---|---|---|---|---|---|
| DEAB-TO-3 77 | 626 | 649 | 23 | 0.36 | — | Grooves | AT rich DNA | Live HeLa cells |
| TO3-CN 78 | 543 | 604 | 68 (unbound); 56 (DNA), 49 (RNA) | 0.73 (DNA) 0.72(RNA) | — | Minor groove | DNA and RNA | Live MCF7 cells |
| TC 83 | 521 | 610 | 89 | 0.36 | — | Intercalation | AT-rich DNA | Live cells, parasites red blood cell |
| Hemicyanine-1a84 | 633 | 706-DNA, 707-RNA | 69-DNA, 121-RNA | 0.125 (DNA), 0.116 (RNA) | — | — | DNA and RNA | Fixed HeLa cells |
| Hemicyanine-1b84 | 633 | 712, 714 | 137, 158 |
0.058 (DNA),
0.049 (RNA) |
— | — | DNA and RNA | Fixed cells |
| Coralyne-4b87 | 400/500 | 480, 695 | 125-DNA, 53-GQ | — |
2.3 × 105 M−1 (DNA);
0.33 × 105 M−1 (GQ) |
DNA, GQ | Live NIH 3T3 mouse fibroblasts | |
| Quinozolium-1a88 | 490 |
728 (DNA)
697 (GQ) |
238-DNA, 207-GQ | — |
1.3 × 104 M−1 (DNA);
8.3 × 104 M−1 (GQ) |
— | DNA, GQ | Live HeLa cells |
| QCy-DT 95 | 530 | 650 | 86 | 0.32 | 2.9 × 106 M−1 | Minor groove | AT-rich DNA (sequence specific) | Live (MCF7) and fixed (HeLa) cells |
| D1 96 | 638 | 647 | 9 | 0.3 | 1.9 × 105 M−1 | Minor groove | DNA | Fixed tissues |
| TP-2Bzim 97 | 430/476 | 640/585 | 109 | 0.54 | 107 M−1 | Minor groove | Drew AT DNA | Live 3T3 cells |
| CP 114 | 598 | 658 | 60 | 0.22 (in DCM) | — | — | RNA | Live HeLa, A549, L929 cells |
| CP3 115 | 584 | 638 | 54 | 0.555 (in DCM) | — | — | RNA and lysosome | Live HeLa, A549, L929 cells |
| CP6 115 | 595 | 655 | 60 | 0.338 (in DCM) | — | — | RNA and lysosome | Live HeLa, A549, L929 cells |
| F22 116 | 548 | 620 | 72 | 0.0075 | — | — | RNA | Live Hela, A549, 3T3-L1 |
| Hsd + CB7 119 | 458 | 682 | 224 | 0.016 | — | — | RNA | Fixed cell (Hsd alone), live cell (Hsd + CB7) |
| FLETH 120 | 490 | 620 | — | — | — | Intercalation | RNA | Live MCF7 cells |
RNA
Over the last decade, there has been increasing evidence that RNA is responsible for varied functions in live cells, including essential catalytic roles and regulation/silencing of gene expression. Unlike DNA, designing RNA-specific fluorescent probes is a challenging task due to their flexible secondary and tertiary structural conformations. RNA in the nucleus is concentrated mostly in the nucleolus, which is the key site for ribosomal RNA (rRNA) transcription, processing and assembly.98,99 Furthermore, RNA is also found in the cytoplasm during transport of small (tRNAs and microRNAs) and large RNA (mRNAs and ribosomal RNAs) molecules from the nucleus through nuclear pore complexes for gene expression. In recent times, dedicated efforts have been directed at visualising cellular RNA and understanding its role in various physiological processes inside the cell, using several techniques established for RNA detection in living cells; these include fluorescence-labelled RNA microinjection,100,101 fluorescence in situ hybridisation (FISH),102–106 molecular beacons,107 green fluorescence protein (GFP)-tagged RNA binding proteins108,109 and small molecular probes.40 Small molecular fluorescent probes are, in fact, complementary tools to antibodies, which are preferred for live cell imaging owing to good membrane permeability, unlike proteins and antibodies that require membrane disruption by fixing of cells. Moreover, it is hard to study the nucleolar dynamics in prefixed cells and, therefore, it is extremely beneficial to have RNA-selective fluorescent probes to monitor the RNA content and distribution in comparison with DNA organisation inside the nucleus.110 However, RNA-staining small molecular probes are rare due to difficulty in designing probes with differential selectivity for RNA over DNA and poor nuclear membrane permeability. SYTO RNAselect, a green fluorescent dye, is the only commercial probe available for RNA imaging in live cells; remarkably, its molecular structure has not been reported yet.111Despite the difficulty, considerable efforts have been directed at developing RNA imaging probes, of which very few far-red fluorescent probes are reported.112,113 Wang and co-workers designed a probe for the imaging of nucleolar RNA in live cells using a crescent-shaped probe (CP) (Fig. 9).114CP in Tris-HCl.EDTA buffer solution showed an emission band around 658 nm and Stokes shift of 60 nm upon excitation corresponding to the absorption maxima (598 nm). The significant Stokes shift of the emission band into the red region arises from intramolecular charge transfer (ICT) and attainment of molecular planarity in bound CP. In vitro studies have shown fluorescence enhancement in CP upon selective binding with RNA compared to DNA. The probe exhibited good cellular and nuclear permeability in living cells (both normal [L929] and cancer [A549] cells). Interestingly, CP in the nucleolus was found to emit 30 times more intensely compared to that in the nucleus (Fig. 9a). DNase digestion of cells showed retention of fluorescence intensity, whereas RNase digestion led to complete blackout of the fluorescence signals of CP in the nucleolus, corroborating the fact that fluorescence enhancement of CP is due to its selective binding to RNA, not DNA. Cell viability studies on HeLa cell lines revealed much cellular tolerance at the imaging concentration of 5 μM. CP was reported to exhibit superior photostability compared to the commercially available dye SYTO RNASelect. CP (5 μM) was successfully used to image the dynamics of nucleolar RNA in real time during various phases of mitosis in HeLa cells without affecting the cell division process. Further, CP was employed to visualise the changes in nucleolar RNA during cisplatin-induced apoptosis. Subsequently, the same group expanded this family of dyes (CP3 and CP6) by primarily altering the amino-functionality on the two constituent dye moieties.115 All the CP-family dyes in buffer solution exhibited absorption maxima in the 584–600 nm region and the corresponding emission maxima in the range of 638–658 nm. The quantum yields for CP3 and CP6 were found to be ΦF = 0.555 and 0.338 (in dichloromethane), respectively, whereas in buffer ΦF = 0.041 (CP3) and ΦF = 0.015 (CP6). The structural variability on molecular structures of this class of probes allowed the detection of various cellular organelles like mitochondria, lysosomes and the nucleolus. In live cell imaging, CP3 and CP6 probes distinctly stained the nucleolus of the cells along with the lysosomes in the cytoplasm. Probes CP3 and CP6 reproduced a similar staining pattern in A549 cancer cells as well as in normal L929 cells, as shown in Fig. 9b and c, respectively. Selective staining of RNA in nucleoli by CP3 and CP6 was demonstrated by DNase and RNase digest experiments performed using fixed permeabilised HeLa cells using SYTO RNASelect as a control marker for cellular RNA. Both CP3 and CP6 probes proved to possess better photostability compared to SYTO RNASelect or MTG, making them potential candidates for use in studying the dynamic changes in nucleolar RNA in living cells. The selectivity of CP probes, however, needs significant improvement, and a combinatorial library with structural diversity and stringent screening would lead to modulation of various requisite characteristics to make them superior RNA-selective probes. Furthermore, the low quantum yield of the CP class of dyes also calls for further fine-tuning of the molecular structure of the probe. Finally, advanced experimental and theoretical studies have to be performed to understand the origin of RNA selectivity, which, in turn, help in designing probes with superior selectivity and sensitivity. Chang and co-workers screened a library of styryl derivatives using in vitro RNA assay and live cell imaging methods, and narrowed it down to a potential probe F22 fluorescing in the red region (λem = 620 nm) (Fig. 10a).116,117F22 exhibited efficient fluorescence at a low working concentration of 1 μM in HeLa cells and was found to possess high photostability, low phototoxicity and good cell tolerability (Fig. 10a). The utility of the probe was assessed by the fluorescence staining of cells that showed distinct nucleolar staining and faint staining of the cytoplasm. F22 is one of the rare red-emitting probes for RNA with good selectivity and photostability in comparison to the commercial probe SYTO RNASelect, especially for staining the RNA-rich nucleolus in live cells. Although treatment of cells with DNase or RNase led to an overall reduction in the fluorescence intensity, DNase treatment retained the bright nucleolar stain owing to relative selectivity for RNA. The quantum yield of F22 (ΦRNAF(unbound) = 0.0007) increased 10-fold upon binding to RNA, (ΦRNAF = 0.0075), which is still very low and contributes to high noise to signal ratio. F22 bears an orthogonal relationship with the DNA-selective dyes Hoechst and DAPI and was used in combination for the simultaneous detection of DNA and RNA. This styryl dye molecular platform overcomes challenges such as very low quantum yield, to achieve relatively high selectivity for RNA. In another interesting approach, Peng et al. judiciously transformed a DNA binding probe into an RNA-selective probe through structural modifications. DNA binding probe 2-[2-[4-(dimethylamino)phenyl]ethenyl]-3-methylbenzo thiazolium iodide (Hs)118 was structurally modified by introducing positive charge and steric bulk, to obtain probe 2,4-bis[(E)-N-methyl-2-benzothiazoliumvinyl]-1-dimethylaminobenzenediiodide (Hsd) (Fig. 10b).119 The relatively bulkier Hsd (compared to Hs) becomes too large for the narrow minor grooves of DNA but fits the RNA structure, although the actual mode of binding is yet to be established. Hsd (10 μM) showed emission maxima at 682 nm with a shoulder peak at 580 nm upon excitation at 458 nm. The NIR emission might arise from extended π-conjugation in the long cyanine backbone of the probe. A 65-fold enhancement in the fluorescence intensity with a blue shift of 8 nm was observed in the presence of 30 equiv. RNA (ΦF = 0.016) compared to 24-fold fluorescence enhancement and 12 nm red shift observed with DNA. The probe stained the nucleolar RNA in fixed HeLa cells distinctly and also the cytoplasmic ribosomal RNA. Generally, RNA probes suffer from poor cell penetrating ability, which seriously limits their living cell imaging applications. To overcome this serious limitation, the authors came up with the idea of a supramolecular host–guest complex of a macromolecular cage curcubit[7]uril (CB7) and Hsd, and the complex was found to cross the cell membrane in live HeLa cells without introducing any intrinsic cytotoxicity (Fig. 10b). The probe Hsd, in the presence of CB7, showed 24-fold enhancement in fluorescence with 24 nm blue shift. Herein, CB7 acts as a host and the guest molecule Hsd is captured into the host cavity through the benzothiazolium moiety through hydrophobic and ion-pair interaction. However, this probe suffers from poor quantum yields and still lacks differential selectivity between DNA and RNA. Nevertheless, the enhancement in fluorescence intensity upon binding with NAs (DNA/RNA) was attributed to the restricted rotation of the benzothiazolium vinyl group due to interaction with the phosphate backbone and the water excluded-hydrophobic environment offered by higher ordered structures. The larger hydrophobic pocket of RNA secondary structures accommodated Hsd better than the DNA duplex or single-stranded DNA, resulting in relatively higher fluorescence enhancement in the presence of the former. Designing a FRET-based probe (FLEth) was attempted by Turro and co-workers by covalently linking the intercalating dye EtBr (Eth) to the fluorescein dye (FL) as an RNA intercalating probe using visible light excitation (Fig. 10).120 The probe FLEth, upon excitation under visible light (488 nm) in the absence of RNA, resulted in an emission band of fluorescein at 520 nm, whereas in the presence of RNA, strong emission at 620 nm was observed due to energy transfer from fluorescein to intercalating EtBr, with a 9-fold increase in fluorescence intensity compared with the existing fluorogenic intercalator PI (Fig. 10c). The probe showed an energy transfer efficiency of ≈77% upon binding to RNA, indicating that the majority of the photons absorbed by fluorescein were efficiently transferred to the intercalated fluorophore. Nevertheless, in the presence of DNA, minimal change in the fluorescence of the probe was observed, signifying its selectivity towards RNA. However, further studies to understand these findings in detail are not available. Cellular uptake of FLEth was evaluated in mammalian breast cancer cell lines, and dual colour emission of the probe in the green and red channels showed maximum intensity in the nucleolus, the dense region of RNA, and cytoplasm. FLEth bound to RNA has been reported to exhibit longer fluorescence lifetime, an attribute that warrants its potential use in time-resolved experiments.
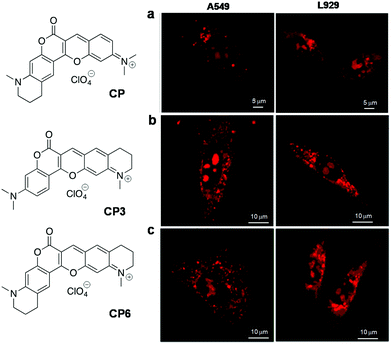 | ||
| Fig. 9 Crescent-shaped probes (CP). Fluorescence imaging of live A549 and L929 cells using (a) CP (5 μM), (b) CP3(7 μM), and (c) CP6 (7 μM). Reproduced from ref. 114 and 115 with permission from Elsevier, copyright 2015 and American Chemical Society, copyright 2015 respectively. | ||
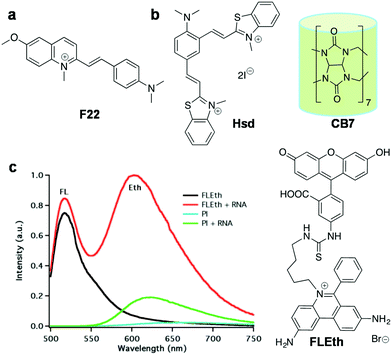 | ||
| Fig. 10 RNA probes. (a) F22, (b) Hsd and macromolecular cage curcubit[7]uril (CB7), and (c) fluorescence response of the FLEth probe and propidium iodide (PI) to yeast RNA. Reproduced from ref. 120 with permission from American Chemical Society, copyright 2008. | ||
Non-canonical structures
Single-stranded regions of the genome containing a repeating sequence of nucleobases form alternative secondary structures through non-canonical base pairs (such as Wobble and Hoogsteen base pairs). These secondary structures act as central blockades for various proteins processing the single-stranded regions formed during replication, transcription, repair and homologous recombination. The most well-studied and highly characterised secondary structure in the past two decades is the G-quadruplex (GQ), which is known to be formed by guanine-rich sequences. GQs are non-canonical secondary structures of DNA/RNA assembled by the stacking of two or more G-tetrads with either intra- or intermolecular association. In the G-tetrad, the four guanines are hydrogen-bonded to each other and stabilised by Hoogsteen hydrogen bonding (Fig. 2b). In the 1980s, studies revealed that G-rich repeat sequences in the human telomere were capable of forming GQ structures in vitro, and these DNA structures were shown to inhibit the telomerase activity, an enzyme which is regularly overexpressed in cancer cells, immortalising them.121,122 GQs display a broad range of structural diversity based on the sequence, orientation of strands and loop length and type (e.g., diagonal loops, lateral loops and double chain reversal loops), presence of alkali metal ions and molecular crowding conditions.123–125 GQs may be categorised as parallel, anti-parallel or mixed based on the strand direction (5′ → 3′) and conformation of guanine glycosidic torsion angles (Fig. 2). In a parallel GQ, all the four strands run in the same direction (5′ → 3′) with guanines in either all anti- or syn-conformation. In the case of anti-parallel folding, alternate/opposite strands run in opposite directions (one in 5′ → 3′ and the other 3′ → 5′) and have both syn- and anti-guanines. In hybrid structures, a mixed combination of both the strand orientations is found. In addition, each GQ consists of four grooves that are different from a duplex and can be narrow, medium and wider based on syn- and anti-guanines. The binding modes of the GQ structure include intercalation and groove binding, as in the duplex structure; in addition, the end-stacking mode is commonly encountered in these structures (Fig. 2b).The GQ structure has attracted the exceptional attention of researchers due to its in vitro and in vivo biological applications. Recent literature has underscored its biological relevance by the distribution of GQ sequences in the human genome (370![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 sequences)126–129 and their existence in the telomeric regions and gene promoters (more than 40% of human genome promoters consist of quadruplex-forming sequences). Putative GQ sequences have been identified in the human genome, predominantly in telomeres, ribosomal DNA (rDNA), promoter regions, untranslated regions (UTRs) of mRNA, and first exons and first introns of many genes. The existence and distribution of GQs evoked their implication in various biological functions like transcription regulation, chromosomal stability and, in particular, telomerase activity due to the formation of a GQ at the end of telomeres.130 The formation of GQs in the promoter regions of several human genes (insulin,131 VEGF,132 retinoblastoma133) and oncogenes (c-myc,134–138 c-kit,139,140 bcl-2,141–144 k-ras,145 and RET oncogenes146) highlights its potential role in the growth of cancer cells. In addition to the nuclear genome, recent reports have suggested the presence of nearly 200 putative GQ-forming sequences in mitochondrial DNA, about which very little is known and much is yet to be explored.147,148 It has already been reported that both DNA and RNA GQs in living cells are associated with various cellular processes and human diseases like cancer and diabetes, which makes GQs an unambiguously significant diagnostic and therapeutic target.11,149–153 Recently, the presence of both DNA and RNA GQ structures in human cells was visualised by using an engineered structure-specific antibody.149,150 However, the cellular impermeability of highly expensive antibodies limits their use for detecting RNA/DNA GQs in living cells. Therefore, developing GQ-selective small molecules paves the way to probe their structure, localisation, distribution and function, and various quadruplex structure-associated diseases. In particular, fluorescent probes with high affinity and selectivity are important tools for characterising the conformational dynamics, folding and localisation in live cells as well as whole organisms. Moreover, structure-specific fluorescent probes of GQs are useful in developing potential therapeutic agents. There have been a number of reviews highlighting ligands stabilising the GQ structure and fluorescent probes for various GQ structures.44–46 In this review, we restrict ourselves to recent far-red fluorescent probes, which are selective and capable of discriminating GQs from other NAs and ensuring their in cellulo imaging in fixed and live cells.
000 sequences)126–129 and their existence in the telomeric regions and gene promoters (more than 40% of human genome promoters consist of quadruplex-forming sequences). Putative GQ sequences have been identified in the human genome, predominantly in telomeres, ribosomal DNA (rDNA), promoter regions, untranslated regions (UTRs) of mRNA, and first exons and first introns of many genes. The existence and distribution of GQs evoked their implication in various biological functions like transcription regulation, chromosomal stability and, in particular, telomerase activity due to the formation of a GQ at the end of telomeres.130 The formation of GQs in the promoter regions of several human genes (insulin,131 VEGF,132 retinoblastoma133) and oncogenes (c-myc,134–138 c-kit,139,140 bcl-2,141–144 k-ras,145 and RET oncogenes146) highlights its potential role in the growth of cancer cells. In addition to the nuclear genome, recent reports have suggested the presence of nearly 200 putative GQ-forming sequences in mitochondrial DNA, about which very little is known and much is yet to be explored.147,148 It has already been reported that both DNA and RNA GQs in living cells are associated with various cellular processes and human diseases like cancer and diabetes, which makes GQs an unambiguously significant diagnostic and therapeutic target.11,149–153 Recently, the presence of both DNA and RNA GQ structures in human cells was visualised by using an engineered structure-specific antibody.149,150 However, the cellular impermeability of highly expensive antibodies limits their use for detecting RNA/DNA GQs in living cells. Therefore, developing GQ-selective small molecules paves the way to probe their structure, localisation, distribution and function, and various quadruplex structure-associated diseases. In particular, fluorescent probes with high affinity and selectivity are important tools for characterising the conformational dynamics, folding and localisation in live cells as well as whole organisms. Moreover, structure-specific fluorescent probes of GQs are useful in developing potential therapeutic agents. There have been a number of reviews highlighting ligands stabilising the GQ structure and fluorescent probes for various GQ structures.44–46 In this review, we restrict ourselves to recent far-red fluorescent probes, which are selective and capable of discriminating GQs from other NAs and ensuring their in cellulo imaging in fixed and live cells.
DNA GQs
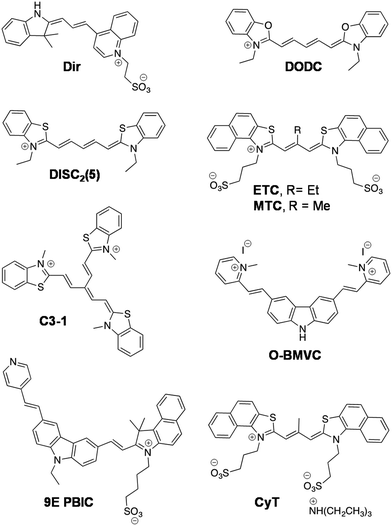
Cyanine (polymethine) dyes are among the earliest synthetic dyes used as fluorescent probes for NAs owing to their narrow absorption bands, high extinction coefficients (200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1) and enhanced fluorescence intensity upon efficient DNA binding. The turn-on fluorescence response of this class of probes, upon binding, is attributed to the restriction in the rotation of the conjugated methine-bridge, thus reducing non-radiative decay. Thiazole orange (TO), a cyanine dye, mostly emits in the green region and binds to all forms of NAs. Several research groups have undertaken the challenging task of chemical modification of TO in order to enhance its binding affinity and selectivity and push its emission wavelength into the far-red region. Renjun Pei and co-workers recently modified a non-specific TO dye by extending the polymethine chain, conjugating a bulky dimethylindole heterocycle in place of benzothiazole and substituting an anionic propylsulphonate on the quinoline ring (Fig. 11).154 This probe Dir was found to be a specific red-emitting probe for parallel c-myc quadruplexes, reporting 36-fold fluorescence enhancement at 651 nm. While a 16-fold increase for hybrid type quadruplex HT22 was observed, a 7-fold increase was seen in the case of Hras; negligible fluorescence was observed for DNA and ssDNA. The binding constant was determined to be in the order of 107 for Dir and quadruplex complexation. The fluorescence quantum yields of Dir in buffer (0.7%) enhanced to 49% in the presence of c-myc parallel quadruplex DNA, confirming the relative specificity of the probe for quadruplex DNA, which is remarkable compared to the parent dye TO that binds to almost all forms of NAs. However, the binding mode of probe Dir has not been investigated and substantially lacks cellular imaging. Nevertheless, the probe was further modified to a C3-symmetric trimer (Dir-Trimer) which showed selective turn-on fluorescence for the parallel c-myc GQ similar to Dir and was utilised for visualisation of GQs in cells.155 Later, Wong et al. reported another modified TO probe by fusing it with benzofuroquinolinium (1) (Fig. 12), which showed high selectivity and fluorescence enhancement for telo21 and c-myc quadruplexes through end-on stacking interactions over DNA (Fig. 12a and b).156 The probe benzofuroquinolium-1 showed relatively good quantum yields in the presence of a GQ (ΦF = 0.25 and 0.18 for the telomeric and c-myc GQ, respectively) compared to the probe alone (ΦF = 0.0012). The probe was shown to detect both DNA and RNA quadruplexes in fixed MCF7 cell lines (Fig. 12c). However, the fluorescence signal partially resulting from duplex DNA binding cannot be ruled out and warrants further investigation. The binding characteristics such as mode of interaction and binding affinity need to be determined, to encourage chemical modification of the probe and make it more specific to a particular conformation of quadruplex, apart from achieving complete selectivity over DNA, RNA and ssDNA.
000 M−1 cm−1) and enhanced fluorescence intensity upon efficient DNA binding. The turn-on fluorescence response of this class of probes, upon binding, is attributed to the restriction in the rotation of the conjugated methine-bridge, thus reducing non-radiative decay. Thiazole orange (TO), a cyanine dye, mostly emits in the green region and binds to all forms of NAs. Several research groups have undertaken the challenging task of chemical modification of TO in order to enhance its binding affinity and selectivity and push its emission wavelength into the far-red region. Renjun Pei and co-workers recently modified a non-specific TO dye by extending the polymethine chain, conjugating a bulky dimethylindole heterocycle in place of benzothiazole and substituting an anionic propylsulphonate on the quinoline ring (Fig. 11).154 This probe Dir was found to be a specific red-emitting probe for parallel c-myc quadruplexes, reporting 36-fold fluorescence enhancement at 651 nm. While a 16-fold increase for hybrid type quadruplex HT22 was observed, a 7-fold increase was seen in the case of Hras; negligible fluorescence was observed for DNA and ssDNA. The binding constant was determined to be in the order of 107 for Dir and quadruplex complexation. The fluorescence quantum yields of Dir in buffer (0.7%) enhanced to 49% in the presence of c-myc parallel quadruplex DNA, confirming the relative specificity of the probe for quadruplex DNA, which is remarkable compared to the parent dye TO that binds to almost all forms of NAs. However, the binding mode of probe Dir has not been investigated and substantially lacks cellular imaging. Nevertheless, the probe was further modified to a C3-symmetric trimer (Dir-Trimer) which showed selective turn-on fluorescence for the parallel c-myc GQ similar to Dir and was utilised for visualisation of GQs in cells.155 Later, Wong et al. reported another modified TO probe by fusing it with benzofuroquinolinium (1) (Fig. 12), which showed high selectivity and fluorescence enhancement for telo21 and c-myc quadruplexes through end-on stacking interactions over DNA (Fig. 12a and b).156 The probe benzofuroquinolium-1 showed relatively good quantum yields in the presence of a GQ (ΦF = 0.25 and 0.18 for the telomeric and c-myc GQ, respectively) compared to the probe alone (ΦF = 0.0012). The probe was shown to detect both DNA and RNA quadruplexes in fixed MCF7 cell lines (Fig. 12c). However, the fluorescence signal partially resulting from duplex DNA binding cannot be ruled out and warrants further investigation. The binding characteristics such as mode of interaction and binding affinity need to be determined, to encourage chemical modification of the probe and make it more specific to a particular conformation of quadruplex, apart from achieving complete selectivity over DNA, RNA and ssDNA.
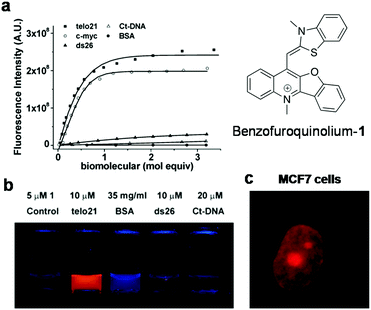 | ||
| Fig. 12 (a) Fluorescence spectra of probe benzofuroquinolium-1 (5 μM) in the presence of different DNA GQs (telo21 and c-myc), DNA (ds26 and ct-DNA) and BSA, (b) photograph of solutions of benzofuroquinolium-1 in the presence of canonical and non-canonical structures, which selectively show bright fluorescence for non-canonical Tel21 (a DNA GQ) under UV light (λex = 302 nm), and (c) fluorescence microscopy image of fixed MCF7 cells treated with benzofuroquinolium-1 (2 μM). Reproduced from ref. 156 with permission from Royal Society of Chemistry, copyright 2011. | ||
The dimeric form of the symmetric 3,3′-diethyloxadicarbocyanine (DODC) dye is a known DNA minor groove binder, which was subsequently reported to bind various dimeric hairpin quadruplex structures (Fig. 11).157DODC displayed an induced exciton CD signal that might have resulted from π-stacking in grooves and with a Kd in the micromolar range.158 The fluorescence intensity of DODC increased by 18% in the presence of tetramolecular parallel TG4T quadruplex, whereas no significant change was observed with duplex DNA, DNA hairpins and ssDNA. Recently, DODC was even used as a reporter for DNA quadruplexes in a ‘mix and measure’ fluorescence screening assay.159 Tang and co-workers have demonstrated in vitro recognition of DNA quadruplexes by the supramolecular self-assembly of a cyanine derivative.160 The symmetrical cyanine dye 3,3′-di(3-sulphopropyl)-4,5,4′,5′-dibenzo-9-ethyl-thiacarbocyaninetriethylammonium salt (ETC) recognised mixed hybrid DNA quadruplexes of human telomeres through its supramolecular self-assembly involving transformation between the J-aggregates and monomer at low ionic strength (Fig. 11). Furthermore, the significant changes in the absorbance and strong fluorescence enhancement (70 fold, λmax = 600 nm) of the ETC monomer, upon addition of various quadruplex structures (parallel/hybrid GQs), allowed their specific detection in gel electrophoresis (PAGE) experiments. The authors have devoted significant efforts to investigating the interactions of the probe with topologies of different GQs and shown specific interactions of ETC through the end-stacking mode and with loops of parallel/hybrid GQs. However, under physiological conditions and under high ionic strength (140 mM K+, 10 mM Na+ and 2 mM Mg2+), the transformation of ETC from J-aggregates to monomer formation was hindered due to strong intermolecular forces. Therefore, ETC was subjected to further chemical modification to derive 3,3′-di(3-sulphopropyl)-4,5,4′,5′-dibenzo-9-methyl-thiacarbocyanine triethyl ammonium salt (MTC), by replacing ethyl (9-position substituent) with a methyl group, which decreased the ability of the probe to form aggregates and enabled the detection of GQs in high ionic strength (Fig. 11).161 In the presence of parallel/hybrid GQs, the absorption band at 660 nm corresponding to the H-aggregate of the probe disappeared and the monomer absorption band appeared (at 580 nm) accompanied by 1000-fold fluorescence enhancement (λem = 600 nm), which was 200-times compared to the fluorescence intensity recorded for duplex and ssDNA. Further, the H-aggregates of MTC were shown to recognise the self-assembled parallel/hybrid GQs on Au-film. However, the shortcoming of this work is that the binding characteristics and utility of ETC and MTC for cell-based imaging or assays have not been reported or investigated.
Klaus Weisz and co-workers reported another symmetrical dye, the dicarbocyanine dye 3,3′-diethylthiadicarbocyanine (DiSC2(5)) accompanied by detailed characterisation of its binding to the c-myc quadruplex employing a combination of several spectroscopic techniques (Fig. 11).162 The NMR spectroscopy data revealed that two dye molecules tightly bind to the c-myc quadruplex through end-stacking, although with stronger interactions at the 5′-terminal G-tetrad. Interestingly, FRET was observed in the UV-spectral region upon complexation between the dye and the c-myc quadruplex. The c-myc DNA has broad absorbance spectra with maxima at λ = 280 and 250 nm. However, maximum fluorescence enhancement of the probe was observed in the presence of c-myc upon excitation of the sample at 254 nm compared to direct excitation (λex = 600 nm), which is attributed to the energy transfer process from DNA to the probe. The association constant (Ka) determined based on fluorescence quenching of guanine upon binding of DiSC2(5) to the c-myc GQ was found to be 4.8 × 105 M−1. On the other hand, negligible energy transfer was observed upon the addition of thrombin binding aptamer (TBA), GC-rich duplex DNA or ssDNA to the probe, which is indicative of specificity of DiSC2(5) for c-myc parallel quadruplex. The significant energy transfer between the c-myc quadruplex and DiSC2(5) allowed the visual detection of their [DiSC2(5):c-myc] complexes after UV-irradiation using sensitised dye fluorescence in the longer wavelength region >640 nm. Nevertheless, the key disadvantage of this probe is UV excitation and inadequate information available on binding parameters. Furthermore, the binding and discrimination abilities of the probe with various potential quadruplex-forming oncogenes have not been studied. In addition, it is essential to determine the quantum yields of the probe bound to different NA structures to assess its practical utility, as the optical detection highly depends on the quantum yield.
Inspired by the C3-symmetric dyes as quadruplex binders,163 Ihmels et al. developed a triangle-shaped [2.2.2]heptamethinecyanine dye C3-1 for the fluorescence detection of DNA GQs (Fig. 11).164 Probe C3-1 showed a significantly red-shifted absorption band at 677 nm accompanied by hyperchromicity, upon the addition of DNA quadruplex, as a consequence of dissociation of the cyanine aggregates and binding of the monomer to the quadruplex structure.165,166 The cyanine C3-1, which is weakly fluorescent in aqueous solution (ΦF < 0.005), showed a broad emission band upon the addition of a DNA GQ with a maximum at 715 nm, a shoulder at 660 nm (λex = 560 or 580 nm) and a 106-fold increase in fluorescence. However, C3-1 exhibited a similar emission band in the presence of a DNA duplex, albeit with a relatively low (57-fold) increase in intensity. The analysis of fluorescence data yielded a binding constant of 8.2 × 105 M−1 and binding stoichiometry of n = 2.4. The relative selectivity of C3-1 towards DNA GQs compared to DNA duplexes was further confirmed by the high thermal stability of DNA GQs complexed with C3-1. 1H NMR and CD studies revealed some information on the binding mode similar to TO, which is known to bind GQs through the end-on stacking mode. However, C3-1 is incapable of differentiating diverse GQ conformations, and the actual mode of binding, quantum yield upon binding to GQs and cellular imaging studies are not available.
Carbazole derivatives are another important class of probes used for the fluorescence detection of GQs. Chang and co-workers reported the synthesis of a novel carbazole derivative featuring two vinylpyridinium branches at the 3 and 6 positions of carbazole and studied, in detail, their binding to GQs (Fig. 11). The probe 3,6-bis(1-methyl-4-vinylpyridinium) carbazole diiodide (BMVC) was shown to significantly stabilise the human telomeric form of the quadruplex shown by the melting temperature studies (ΔTm = 13 °C). However, the BMVC derivative showed emission below 600 nm (λem = 575 nm), which was subsequently modified by XueningFei et al., to obtain a novel compound 3-(2-(4-vinylpyridine))-6-(2-(1-(4-sulphobutyl))-3,3-dimethyl-2-vinylbenz[e]indole)-9-ethyl-carbazole (9EPBIC) with a longer emission wavelength (λem = 600 nm and λex = 520 nm).167 The probe 9EPBIC (Fig. 11) showed 100-fold increase in fluorescence in the presence of parallel GQs (especially with c-myc) and 10–30-fold in the presence of other GQs, showing negligible fluorescence for the DNA duplex and ssDNA. Overall, the probe exhibited good selectivity for the c-myc 2345 GQ, with apparent binding constants on the order of 105. Molecular docking studies show that the mode of binding is through end-on stacking with a parallel c-myc quadruplex. The cellular imaging studies and quantum yield data are not available for this probe. In another attempt at modifying BMVC to push the emission into the longer wavelength region, TO was conjugated with a carbazole moeity. The resultant probe TO-CZ showed turn-on fluorescence (λem = 613 nm and λex = 467 nm) in the presence of GQs with moderate quantum yield (ΦF = 0.155), as shown in Fig. 13.168 The ability of TO-CZ to sense GQs in cellulo was assessed in SiHa cells and the probe was found to stain both DNA and RNA GQs. Zhi-Shu Huang et al. developed a colorimetric and red-emitting fluorescence dual probe ISCH-1 for the detection of GQs. ISCH-1 was obtained by incorporating a coumarin–hemicyanine fluorophore within an isaindigotone framework, which emerged as a donor–π–acceptor (D–π–A) hemicyanine-like dye with an enhanced push–pull effect due to electron-withdrawing fluorine atoms on the quinazolone moiety (Fig. 13).169 The absorption maxima of ISCH-1 at 530 nm decreased with a large red-shifted band observed at 600 nm upon the addition of GQs and resulted in a vivid colour change from pink to blue under ambient light, signifying its naked eye detection. The weakly fluorescent ISCH-1 (λem = 640 nm and λex = 570 nm; ΦF = 0.0008) showed decent fluorescence enhancement at 653 nm with a quantum yield (ΦF) of 0.186 in the presence of GQ compared to other NA structures. The probe was further investigated for intracellular fluorescence staining in live and fixed HeLa cells, which showed localisation of the probe in nucleoli (Fig. 13a). This is an indication of potential sensing of rDNA regions (abundant in GQ forming sequences) and was further validated by a chromatin immunoprecipitation (CHIP) assay. Yu-Jing Lu et al. developed a D–A–D architecture-based Indole-3c for targeting GQ DNA and it was prepared by conjugating a 1-methylquinolinium scaffold with a plain indolyl moiety (Fig. 13).170 Indole-3c showed 150–210 fold enhancement in fluorescence at 617 nm (λex = 531 nm) for GQ DNA (telo21, htg22, ckit2, pu22) compared to <50-fold enhancement for duplex DNA, ssDNA and RNA. These results demonstrated that Indole-3c possesses reasonably good selectivity to discriminate DNA GQs from other NA structures. However, it fails to discriminate among different topologies and sequences of DNA GQs. The probe showed reasonable quantum yields in the presence of a DNA GQ (ΦF = 0.13–0.26), though a detailed binding mode analysis is not available. Indole-3c was shown to enter the nucleus and stain nucleoli in live PC3 cells.
 | ||
| Fig. 13 Molecular structures of cyanine-based fluorescent probes and (a) confocal fluorescence microscopy image of fixed HeLa cells treated with ISCH-1 (5 μM). Reproduced from ref. 169 with permission from Royal Society of Chemistry, copyright 2014. | ||
The triphenylmethane (TPM) class of dyes include malachite green, methyl green and crystal violet, which have been known for more than a century.171,172 These TPM dyes are non-emissive in most organic solvents due to their twisted intramolecular state resulting from mutual rotation of phenyl rings. Xiang Zhou et al. reported a cyanovinyl-pyridiniumtriphenylamine (CPT) derivative for the selective recognition of antiparallel DNA GQs (Fig. 14).172 In the CPT class of molecules, the electron-rich triphenylamine core acts as the electron donor while the N-methylated heterocycles play a key role as the electron-acceptor and also assist in the DNA binding of the probe. The rotation of the methane bridge impacts the push–pull effects between the donor and acceptor moieties; this rotation is restricted upon specific DNA binding and gives rise to an enhanced optical response. Remarkably, CPT2 (Fig. 14) exhibited 190-fold increase in fluorescence emission at 620 nm (λex = 521 nm) upon binding to an antiparallel GQ (22AG). The probe has been reported to have the potential to induce a topological change and specificity towards antiparallel quadruplex formation, as shown by the CD data. Molecular dynamics simulation studies have shown binding of the probe in grooves through hydrogen bonding interactions. The fluorescence enhancement of the probe in the presence of GQs with antiparallel (22AG) and mixed hybrid (G3T3, H-RAS, and c-kit) conformations was reported to be higher than that for other parallel GQs, duplexes and ssDNA. Interestingly, the probe, when bound, eventually converted the quadruplexes with mixed type conformation (G3T3, H-RAS, and c-kit) into antiparallel conformation, which was responsible for substantial fluorescence enhancement. Overall, CPT2 has the potential to be used as a fluorescence turn-on probe for antiparallel GQ structures. This probe can be of further interest if detailed studies on quantum yields, binding constants and cellular imaging can be made available. Recently, Wang et al. have reported a group of modified triphenylamine-based dyes (TPA-1, TPA-2a and TPA-2b) (Fig. 14).173TPA-1 and TPA-2a were designed by linking indoliumhemicyanine and N-methylquinoline units, respectively, to the triphenylamine core via a methine chain, whereas TPA-2b possesses a functional amine side chain compared to TPA-2a (Fig. 14). Among the three dyes, TPA-2b showed high affinity for the c-kit1 (parallel) GQ by exhibiting maximum hypochromism (41.3%) in the absorption maxima at 450 nm and an apparent red shift of 45 nm. In comparison, TPA-2a, in the presence of c-kit1, showed 20 nm red shift and 31.2% hypochromism; only 11.9% hypochromism was observed for TPA-1. The binding constants (Kb) of TPA-1, TPA-2a and TPA-2b to c-kit1 were found to be 0.12 × 106, 5.47 × 106 and 14.2 × 107 M−1, respectively. The red shift (45 nm) in the absorption spectrum of TPA-2b gave rise to a vivid colour change from pale yellow to pink under daylight, which is useful for the naked-eye detection of c-kit1. TPA-2b, upon the addition of the c-kit1 GQ, showed an increase in emission peak at 627 nm (λex = 462 nm) and displayed a detection limit of ∼0.16 μM. The quantum yields of these probes in the bound and unbound states are not reported. The fluorescence measurement of TPA-2b showed its good selectivity for DNA GQs over dsDNA or ssDNA and was in sound agreement with the absorption measurements. Similar to CPT2, probe TPA-2b induced the parallel GQ conformation in c-kit1 in the absence of monovalent cations, as validated by the CD measurements and fluorescence turn-on response (up to 32-fold) in the presence of GQs. In spite of the notable fluorescence enhancement of TPM dyes in the presence of GQs, the inadequate characterisation of binding modes and poor quantum yields limit their potential applications.
Squaraine dyes are a new class of cyanine dyes with a four-membered ring core linked to electron-rich moieties.174 These dyes show sharp and intense optical features in the therapeutic window (650–850 nm) and also exhibit good photostability.175–177 However, very few reports are available for sensing NA using the squaraine family of dyes. In 2014, Xiang Zhou and co-workers reported the squarylium dye-based probe (4z)-4-[(3-methyl-6-iodo-1,3-benzothiazol-3-ium-2-yl)methylidene]-2-[(Z)-(3-methyl-6-iodo-1,3-benzothiazol-2(3H)-ylidene)methyl]-3-xocyclobut-1-en-1-olate (TSQ1) for the sensitive and naked eye detection of GQs (Fig. 14).178TSQ1 is a nonlinear optical probe consisting of two electron-donating endo-groups (D) and a central electron-withdrawing unit (A) forming a D–A–D bridge. The authors monitored the fluorescence emission of TSQ1 at 668 nm (λex = 620 nm) in the presence of parallel (c-src), various anti-parallel (22AG, G3T3, G4TTA and h-Telo) and mixed type (c-kit and H-RAS) GQ-forming gene sequences with NaCl in the buffer medium. The probe showed fluorescence enhancement for parallel (c-src) GQs and a negligible response for DNA and ssDNA, which is visible to the naked eye under UV light irradiation. However, the unconvincing selectivity of the probe for different topologies of GQs and poor in cellulo imaging demands structure refinement. Around the same time, Dihua Shangguan and co-workers reported another squaraine dye CSTS (Fig. 14) with a sulphonate backbone similar to TSQ1, which has emission in the NIR region and has been found to be selective for parallel GQs.179CSTS has the tendency to form H-aggregates (absorption maxima at 613 nm with a shoulder near 650–700 nm) in Tris–HCl buffer, and the V-shaped conformation of the probe is believed to contribute to the specificity observed for all the parallel GQ forming sequences. CSTS exhibited spectral changes in the presence of parallel GQs (EAD, ckit, pu22), indicating dissociation of H-aggregates into the monomeric form. This transformation of molecular aggregates to monomers gave rise to strong fluorescence enhancement (λex = 680 nm and λem = 710 nm), which was almost quenched in the aggregated form. The quantum yield of CSTS (ΦF = 0.01 in unbound form) was shown to increase significantly in the presence of a parallel GQ (ΦF = 0.42 in bound form). The dissociation constants for CSTS in the presence of parallel GQs were found to be in the micromolar range. The 1H NMR titration, molecular docking studies and G4/hemin peroxidase activity inhibition suggested an end-stacking mode of binding of CSTS to parallel GQs. CSTS can be a useful probe for discriminating parallel GQs from other DNAs. The negatively charged sulphonic groups of CSTS affected cellular uptake that, in turn, limited its in cellulo studies and applications. Subsequently, they functionalised the core structure of squaraine by employing click chemistry to improve its solubility, affinity for different parallel GQs and cellular permeability compared to CSTS.180 The modified squaraine derivative CSTS-4 with tertiary amine groups facilitated its higher affinity for parallel GQs (ckit2) with significant fluorescence increase at 710 nm (λex = 670 nm) (Fig. 14). CSTS-4 showed a higher apparent binding constant of Ka = 2.27 × 107 M−1 and good quantum yield (ΦF = 0.358). The cellular imaging in the MCF7 cells showed localisation of CSTS-4 in lysosomes. The two tertiary amine groups of the probe are also believed to enhance its cellular uptake and imaging. Recently, a dicyanovinyl-substituted amphiphilic squaraine derivative, SQgl, was reported as an NIR fluorescent probe (λex = 660 nm & λem = 744 nm) for discriminating parallel GQs (Fig. 14).181 The amphiphilic squaraine SQgl strongly self-assembled in water, forming a non-fluorescent aggregate. However, parallel GQs among various NAs could displace squaraine from its aggregate, forming a sandwich-like π-complex in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 stoichiometric ratio, which is of a fluorescent supramolecular architecture with a binding constant of 105 M−1 and ΦF = 0.358. The observed selectivity and sensitivity would be promising for in cellulo imaging and in vivo applications, which are yet to be reported.
2 stoichiometric ratio, which is of a fluorescent supramolecular architecture with a binding constant of 105 M−1 and ΦF = 0.358. The observed selectivity and sensitivity would be promising for in cellulo imaging and in vivo applications, which are yet to be reported.
Mauro Freccero et al. reported a naphthalenediimide (NDI)-based fluorescence dyad for the application of GQ detection (Fig. 14).182,183 The optoelectronic properties of NDIs can be effectively tuned by aromatic core substitution184–188 to prune their absorption and emission in the red region of the spectroscopic window. Therefore, NDIs are promising candidates for fluorescence imaging and photodyanamic therapy, and it is particularly appealing to develop NDI-based probes for GQ.189 The authors designed a dyad (cNDI-1) by covalently conjugating electron-rich coumarin (D) to electron-deficient core-disubstituted NDI (A) via a flexible alkyl (CH2)7 spacer (Fig. 14).183 The mutual fluorescence quenching of NDI and coumarin moieties rendered cNDI-1 non-fluorescent in the unbound state. The long spacer allowed strong interaction between aromatic cores and the quenching of NDI emission caused by intramolecular electron transfer from coumarin (D) to NDI (A), as investigated by laser flash photolysis (LFP). The unbound cNDI-1 dyad had undetectable emission (ΦF < 0.001, λex = 638 nm) unlike the cNDI (without coumarin moiety), which had a quantum yield ΦF = 0.19. The cNDI-1 exhibited a single wavelength emission at 498 nm in solution and far-red emission at 666 nm in the presence of GQ. The cNDI-1 was observed to bind to GQ through its NDI moiety, which keeps NDI and coumarin apart and inhibits the quenching by electron transfer associated with D–A interaction, restoring the intrinsic fluorescence of both the fluorophores. The dyad performed better as a turn-on probe for GQ upon excitation at 452 nm corresponding to the coumarin moiety, and emission was observed in the far-red region (λem = 666 nm). The maximum fluorescence emission was observed for parallel GQs (ckit2), which also exhibited maximum apparent binding constant (K11 = 6.9 × 106 M−1) followed by antiparallel GQs (HRAS). Negligible enhancement was observed for RNA GQs, DNA duplex and ssDNA, indicating the discriminating ability of the probe. Although the probe cNDI-1 has some remarkable properties, its selectivity towards GQ topology is not impressive and in cellulo and in vivo applications are likely to be difficult due to low quantum yield. The same group recently reported core-extended NDIs (c-exNDIs) for detecting nuclear DNA GQs with emission in the red region (Fig. 15).190c-exNDIs were also shown to display anti-HIV-1 activity due to their ability to bind viral GQs with high affinity.191 The aggregation of c-exNDI in water caused fluorescence quenching, though GQ binding triggered the transformation of the aggregate to monomeric form, which resulted in turn-on fluorescence. Aggregates of c-exNDI in water showed two broad absorption bands at 555 and 605 nm while the monomeric form had two emission bands at 617 and 670 nm. Among all the GQs studied (anti-parallel form: HRAS, hTel22 in Na+ and TBA; hybrid form: hTel22 in K+; parallel form: c-kit1, c-kit2 and c-myc and controls: ssDNA and dsDNA), hTel22 (hybrid GQ) showed maximum fluorescence enhancement of c-exNDI (λex = 600 nm) at λem = 617 nm (Fig. 15a). c-exNDI binds to human telomeric hTel22 with a binding constant of K1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 = 5.34 × 106 M−1 and quantum efficiency (ΦF) of 0.24 (ΦF in unbound form is 0.08). The interaction of c-exNDI with hTel22GQ was further confirmed by CD studies, which showed an induced CD (ICD) signal corresponding to the probe absorption wavelength and inhibition of Taq polymerase progression in a concentration-dependent manner. c-exNDI was investigated for cellular entry and found to have preferential localisation in the nuclei of 293T cells, which was further confirmed by co-localisation with 1H6 GQ-specific antibody (Fig. 15b). The interaction of the probe with nucleolar GQs was studied by the comparative nucleolin (NCL, GQ-binding protein) displacement assay, which induced relocalisation of NCL to the nucleoplasm outside the nucleoli. c-exNDI is one among few small molecules to achieve in situ detection of DNA GQs within the cell nuclei. Nevertheless, selectivity of the probe can be further improved to achieve better sequence selectivity. The characteristic properties of far-red probes of non-canonical GQ structures are summarised in Table 4.
1 = 5.34 × 106 M−1 and quantum efficiency (ΦF) of 0.24 (ΦF in unbound form is 0.08). The interaction of c-exNDI with hTel22GQ was further confirmed by CD studies, which showed an induced CD (ICD) signal corresponding to the probe absorption wavelength and inhibition of Taq polymerase progression in a concentration-dependent manner. c-exNDI was investigated for cellular entry and found to have preferential localisation in the nuclei of 293T cells, which was further confirmed by co-localisation with 1H6 GQ-specific antibody (Fig. 15b). The interaction of the probe with nucleolar GQs was studied by the comparative nucleolin (NCL, GQ-binding protein) displacement assay, which induced relocalisation of NCL to the nucleoplasm outside the nucleoli. c-exNDI is one among few small molecules to achieve in situ detection of DNA GQs within the cell nuclei. Nevertheless, selectivity of the probe can be further improved to achieve better sequence selectivity. The characteristic properties of far-red probes of non-canonical GQ structures are summarised in Table 4.
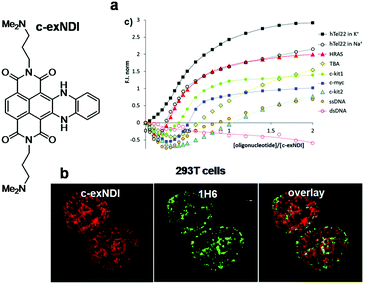 | ||
| Fig. 15 NDI-based DNA GQ probe (c-exNDI). (a) Fluorescence enhancement plotted as a function of the [oligonucleotide]/[c-exNDI] ratio for the titrations of c-exNDI with various NAs, and (b) cells stained with c-exNDI(red signal), with anti-G4 antibody 1H6 (green signal) and overlay of both c-exNDI (red) and G4 (green) on the right. Reproduced from ref. 190 with permission from Royal Society of Chemistry, copyright 2017. | ||
| Probe | λ exc (nm) | λ em (nm) | Stokes shift (nm) | Quantum yield (ΦF) bound/unbound | Binding constant | Mode of binding | In vitro conformation/sequence specificity of DNA/RNA GQ | In cellulo imaging (localization) |
|---|---|---|---|---|---|---|---|---|
| Dir 154 | 600 | 651 | 51 | 0.49/0.007 | ∼107 M−1 | — | Parallel/cmyc DNA | — |
| Benzofuroquinolium-1156 | 527 | 573 | 46 | 0.25/0.0012 | — | End stacking | Parallel and antiparallel | Fixed MCF7 cells |
| ETC 160 | 584 | 600 | 16 | — | — | End stacking | Hybrid/parallel | — |
| MTC 161 | 530 | 600 | 70 | 0.86/0.011 | — | |||
| DiSc2(5)162 | 600 & 254 | 680 | 80 | — | K a = 4.8 × 105 M−1 | End stacking | Parallel | — |
| C3-1 164 | 560 or 580 | 715 with shoulder at 660 | 135 | — | 8.2 × 105 M−1 | End stacking | — | |
| 9E-PBIC 167 | 526 | 600 | 74 | 0.94 (in DCM)/0.014 | K app = 1.14 × 105 M−1 | End stacking | Parallel/cmy | — |
| TO-CZ 168 | 467 | 613 | 146 | 0.1554 | — | End stacking | No selectivity | Fixed SiHa cells (nucleoli) |
| ISCH-1 169 | 570 | 653 | 83 | 0.186/0.0008 | — | — | — | Live and fixed HeLa cells (nucleolus) |
| Indole-3c170 | 531 | 617 | 86 | 0.13–0.26 | 2.5–7 × 105 M−1 | — | — | Live PC3 cells (nucleoli) |
| CPT2 172 | 521 | 620 | 99 | — | — | Grooves | Antiparallel | — |
| TPA-2b 173 | 462 | 627 | 165 | — | ∼107 M−1 | — | Parallel | — |
| TSQ1 178 | 610 | 668 | 58 | — | — | — | No selectivity | Fixed A549 cells |
| CSTS 179 | 680 | 710 | 30 | 0.423/0.01 | K d ∼ μM | End stacking | Parallel | — |
| CSTS-4 180 | 670 | 698–725 | ∼30 | 0.35–0.37 | K a = 2.27 × 107 M−1 | End stacking | Parallel (ckit2) | Live MCF7 cells (lysosomes) |
| SQgl 181 | 660 | 744 | 84 | 0.61 | ∼105 M−1 | End-stacking | Parallel | — |
| cNDI-1 183 | 452 | 666 | 214 | 0.19/<10−3 | K 11(app) = 6.9 × 106 M−1 | End stacking | Minimal selectivity (ckit1 parallel) | — |
| c-exNDI 190 | 600 | 613 | 13 | 0.24/0.08 | 5.34 × 106 M−1 | — | Hybrid | Fixed 293T cells |
| GQR 192 | 360 | 597 | 237 | 0.28/0.014 | K d = 25.18 μM | Grooves | Parallel | — |
| DFHBFSI 194 | 513 | 606 | 93 | 0.39/0.0052 | K d = 1.27 μM | End stacking | Parallel | Live CCRF-CEM cells |
| TMPyP4 197,198 | 424 | 657 and 722 | 233, 298 | — |
K
d = 50–300 nM (duplex);
K d = 50–300 nM (GQ) |
End stacking and Intercalation | Non-specific (duplex, GQ, triplex) | — |
| NMM 196,201 | 399 | 614 | 215 | — |
K
d = 200 μM (duplex);
K d = 2 μM (GQ) |
End stacking | Parallel GQ | — |
| CyT 216 | 532 | 595 | 63 | 0.95 | ∼105 M−1 | — | RNA GQ | A549 (live & fixed) cells |
| TOHdPP 219 | 467 | 732 | 265 | — | 2.86–1.12 × 106 M−1 | 5′-Tetrad stacking | Parallel RNA GQ (TERRA rUAG4) | — |
As comprehended from the examples discussed above, the selectivity of fluorescent probes for specific quadruplex topology is a challenge and targeting a particular sequence with different topologies would be an even more daunting task. Young-Tae Chang and co-workers reported a BODIPY-based dye specific to the parallel quadruplex topology.192 These authors have screened as many as 5000 compounds using the diversity-oriented fluorescence library approach (DOFLA) to uncover primary hits for quadruplex recognition.193 The hit compound BODIPY-based fluorescence sensor (E)-3-(5,5-difluoro-7-(4-methoxystyryl)-9-methyl-5H-4λ4,5λ4-dipyrrolo[1,2-c:2′,1'-f][1,3,2]diazaborinin-3-yl)-N-isopropyl-N-(isopropylcarbamoyl)propanamide (GQR) showed unprecedented selectivity to a parallel GQ (93del, with four medium grooves and exposed ends), over different parallel and anti-parallel GQs (Fig. 16a). GQR has weak fluorescence (582 nm) in buffer due to aggregation-induced quenching; the addition of a GQ resulted in the disaggregation of GQR molecules, leading to the recovery of inherent fluorescence emission accompanied by a bathochromic shift of 12 nm (λem = 597 nm; λex = 360 nm). The probe interestingly showed pronounced fluorescence enhancement in the presence of parallel GQ 93del (ΦF(unbound) = 0.014; ΦF(bound) = 0.28) compared to other parallel GQs (J19, T95, T95) with a dissociation constant of Kd = 25.18 ± 0.02 μM (Fig. 14b). In contrast, another set of parallel stranded GQs (ckit1 and pu24T) showed negligible emission, as they possess additional snapback motifs covering the G-tetrad core, thereby inhibiting the ligand binding. This selectivity of the probe indicates its potential to detect specific ones among various parallel GQ conformations. 1H NMR and molecular docking studies have suggested an association of GQR with the groove region of 93del through carboxamide hydrogen bonding interactions. Nevertheless, there is ample scope for further improvement in performance of the probe through dedicated structure–activity modulation to improve the quantum yield and explore its applications for cellular imaging of GQs. Despite numerous reports on the design of far-red emission probes, the inadequate information on binding constants, low quantum yields, lack of detailed and thorough investigation in the presence of different quadruplex-forming sequences related to oncogenes, unsatisfactory binding mode characterisation, and in cellulo or in vivo toxicity discourage the use of some of these probes for in cellulo or in vivo imaging and analysis of GQs. The selectivity and discriminatory potential of probes towards different topologies of GQs is another very important aspect that needs to be addressed in order to allow detailed structural and functional studies with superior spatial resolution of this relatively unexplored world of GQs under in cellulo and in vivo conditions. Nevertheless, characteristic features of various far-red probes discussed above in terms of their molecular structure, structural evolution and optical properties towards achieving sequence- and conformation-specific recognition of DNA GQs would certainly guide the future developments in this area to design superior far-red probes for GQs with all the necessary attributes.
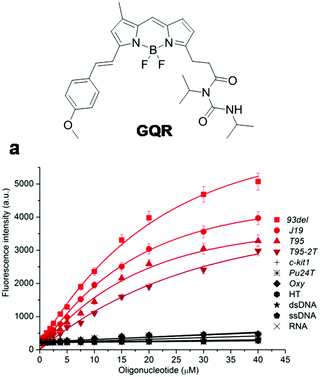 | ||
| Fig. 16 BODIPY-based DNA GQ probe GQR. Fluorescence response of GQR (10 μM) in the presence of various GQs, DNA and RNA. Reproduced from ref. 192 with permission from Nature Publishing Group, copyright 2014. | ||
Recently, Zhou Nie and co-workers reported a DNA-based mimic of red fluorescent protein (RFP), wherein the RFP-chromophore analogue was stabilised by confining it in a GQ environment.194 The RFP chromophore analogues were synthesised such that the fluorescence emission spanned from red to far-red region (583–668 nm). The GQ tetrad formed by Hoogsteen hydrogen bonding among the four guanines forms the ideal docking site for RFP chromophore-analogues and ensures interaction through end staking, which restricts the intramolecular rotation and prevents twisted intramolecular charge transfer (TICT) induced radiationless relaxation in the confined environment. Thus, the photophysical property of the RFP chromophore-analogues was tuned to shift their emission to the red and near infrared region in the presence of GQs. Furthermore, two new RFP chromophore-analogues with fluorine substituents viz., DFHBSI and DFHBNI were developed to ensure that the phenolic hydroxyl group remains in the phenolate form at neutral pH, which is necessary to achieve red emission. Upon complexation with a GQ sequence (NG16), these probes emit at 590 nm and 612 nm, respectively. Further structural modifications led to a set of new chromophores with emission ranging from 583–668 nm in the presence of NG16. From the panel of RFP chromophore analogues, (4Z)-(3,5-difluoro-4-hydroxybenzylidene)-2-((E)-2,5-difluorostyryl)-1-methyl-1H-imidazol-5 (4H)-one (DFHBFSI) was found to be the best DNA RFP mimic in terms of quantum yield (0.39) in the bound state with a GQ (Fig. 17). The probe exhibits enhancement in fluorescence emission at 606 nm (λex = 513 nm) in the presence of NG16 with an appreciable Stokes shift of 90 nm. DFHBFSI exhibited good photostability compared to conventional RFP mCherry and high binding affinity (Kd = 1.27 μM) towards GQs with end stacking interactions. The probe showed high selectivity for parallel GQ DNA over other GQ conformations (TBA, Hum21), duplex DNA (ds26) and ssDNA (A21), unlike thiazole orange (TO), crystal violet (CV) and N-methyl mesoporphyrin IX (NMM) which show poor discriminating abilities for parallel GQs (Fig. 17a). Furthermore, the DFHBFSI–GQ complex was used to selectively illuminate the protein biomarker on the cancer cell surface. A reporter strand bearing two repeat NG16 was hybridised to DFHBFSI with a recognition strand bearing an aptamer sgc8, which targets protein tyrosine kinase 7 (PTK7, a transmembrane receptor protein over expressed in many cancers). This study was performed in CCRF-CEM cells (T-cell acute lymphoblastic leukaemia cell line) wherein specific and significant illumination of the cellular periphery upon two-photon excitation corroborated with the high expression levels of PTK7 in the cell membrane (Fig. 17b). Taking advantage of the two-photon property of the DFHBFSI-GQ complex, imaging of tissue sections from CCRF-CEM cell xenograft mice was performed. The spatial distribution of PTK7 was monitored in real tissue samples and the images were collected at different depths to construct a three-dimensional box (3D) as shown in Fig. 17c. However, further studies to visualise GQs under different in cellulo and in vivo conditions are necessary to evaluate the potential applications of the probe.
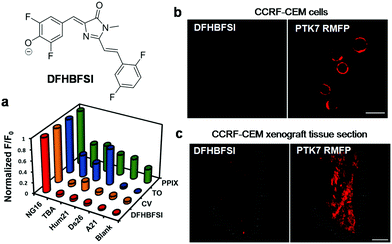 | ||
| Fig. 17 RFP chromophore analogue DFHBFSI. (a) Fluorescence responses of GQ binding dyes (3 μM) towards different DNA conformations (15 μM). (b) Two-photon image of CCRF-CEM cells (excitation: 750 nm) treated with PTK7RMFP. Scale bar: 20 μm. (c) 3D reconstruction of two-photon images of a PTK7RMFP treated tissue section from a CCRF-CEM cell xenograft. Scale bar: 100 μm. Reproduced from ref. 194 with permission from Oxford University Press, copyright 2017. | ||
Porphyrins and pthalocyanines are π-conjugated macrocycles that show intense absorption and fluorescence bands in the visible region.195 Porphyrins with different substituents on the periphery, such as negatively charged (carboxylic acid groups for N-methyl mesoporphyrin IX, NMM) and positively charged groups (quaternary pyridinium at meso position) or metalated derivatives, exhibit complementary π-electron character with the G-quartet and are shown to target GQs via π–π stacking interactions (Fig. 18 and Table 4).196–200 However, they are non-selective and mostly studied for their therapeutic properties rather than as fluorescent probes. Among them 5,10,15,20-tetra(N-methyl-4-pyridyl)porphyrin (TMPyP4) has been extensively studied as a GQ ligand, which also binds to duplex, triplex, single-stranded and bulk genomic DNA with similar affinities (Kd ≅ 200 nM) (Table 4).198 Therefore, TMPyP4 is a non-selective ligand and causes cytotoxicity to normal cell lines. In contrast, anionic porphyrin N-methyl mesoporphyrin (NMM) consisting of a mixture of four regioisomers presented good selectivity for parallel GQs,196,198 albeit with low affinity (Kd ≅ 2–10 μM) and the change in fluorescence of NMM was used to detect GQ DNA in vitro.201 Nevertheless, some of the peripherally modified (pyridinium- and ammonium-containing porphyrazine) and metalated (Cu, Ni and Zn) derivatives of porphyrin and phthalocyanines exhibited relatively improved selectivity for GQ DNA197,202,203 Phthalocyanines consisting of four isoindole groups linked through nitrogen atoms display a larger π-planar surface compared to porphyrins. As a consequence, phthalocyanines are expected to attain high affinity interaction with GQ structures through π–π interactions; yet phthalocyanine-based fluorescent probes for GQs are seldom studied.195 For the first time, Nathan W. Luedtke and co-workers reported zinc phthalocyanine substituted with guanidinium groups (tetrakis(diisopropylguanidinio) zinc phthalocyanine, Zn-DIGP) and studied GQ (H-Telo and c-myc) binding and cellular uptake properties (Table 5).204 Interestingly, Zn-DIGP was found to exhibit 200-fold turn-on fluorescence (λex = 620 nm, λem = 705 nm) in the presence of GQ DNA. Zn-DIGP showed specificity to the c-myc GQ with at least 100- and 5000-fold higher affinity compared to tRNA and ct-DNA, respectively. The GQ specificity was confirmed by fluorescence quenching of 5’-fluorescein-labelled c-myc DNA upon titration of Zn-DIGP in the presence of 1000-fold excess ct-DNA, wherein the probe showed high apparent affinity (Kd ≤ 2 nM) for the c-myc GQ. On the other hand, Zn-TMPyP4 showed 10-fold loss in apparent binding affinity, which is consistent with its unrestrained and non-specific binding to various NA structures (Table 5). The utility of Zn-DIGP (3 μM) was demonstrated for in cellulo imaging of living and fixed cells including HeLa, MCF7, B16F10, SH-SY5Y, E. coli BL-21 and SK-Mel-28. Furthermore, the probe caused rapid threefold knock-down of c-myc mRNA at a relatively small dose of 1 μM. Therefore, Zn-DIGP can be used as a theranostic probe to evaluate the relationship between GQ folding and transcriptional control.
Metal complexes are promising candidates for NA detection as they offer several advantages like absorption in the visible region, large Stokes shifts (>150 nm), and chemical and photostability. The DNA light-switch effect (non-photoluminescence in the unbound state and intense photoluminescence upon binding to DNA) has been observed with the [Ru(bpy)2(dppz)]2+ (bpy = 2,20-bipyridine, dppz =dipyrido[3,2-a:2′,3’-c]phenazine) complex upon metal-to-ligand charge transfer (MLCT) excitation.205 A number of polypyridyl coordination complexes of d6 octahedral metal ions have been developed as highly sensitive and structure-specific DNA probes.206–209 Jim A. Thomas and co-workers reported two dinuclear Ru(II) complexes [(Ru-(phen)2)2(tpphz)]4+3 and [(Ru-(bpy)2)2(tpphz)]4+4 (phen = 1,10-phenanthroline, tpphz = tetrapyridophenazine) for NA binding and in cellulo staining applications (Table 5).210,211 These complexes are non-emissive in water but show immense luminescence when bound to DNA (light-switch effect) due to shielding of the phenazine nitrogen atoms of the tpphz ligand from water and resultant emission from the MLCT state. Interaction of these complexes with duplex (ct-DNA) and GQ DNA [5′-AGGG-(TTAGGG)3, G3] was studied. Complexes 3 and 4 displayed >60-fold luminescence enhancement in the presence of ct-DNA with λem = 658 nm and 647 nm, respectively. Surprisingly, complexes 3 and 4 displayed 2.5-fold blue-shifted (∼32 nm) enhancement of luminescence (λem(3)= 631 nm and λem(4) = 605 nm) in the presence of GQ DNA. The emission lifetimes of the complexes bound to GQs (τ(3) = 129 ns and τ(4) = 123 ns) were notably longer compared to that of the duplex (τ(3) = 84 ns and τ(4) = 92 ns). The cellular uptake and viability of these complexes were examined in MCF7 cells. Complex 3 (500 μM) displayed intense luminescence (λem = 630–670 nm and λex = 458 nm) localised in the nucleus of live cells without affecting their viability. Moreover, in vitro studies showed that 3 was capable of distinguishing duplex and GQ DNA structures with distinct emission maxima (λem(GQ) = 631 nm; λem(duplex) = 650 nm). In cellulo lambda stacking experiments revealed maxima at 680 and 630 nm corresponding to duplex and GQ conformations, respectively for a single excitation energy (λex = 458 nm). Therefore, cellular localisation of the probe, with respect to duplex and GQ structures, was monitored at the red (670–700 nm) and yellow (630–640 nm) channels; the ability of 3 to induce or stabilise the GQ structure was visualised by formaldehyde fixation. Furthermore, complex 3 showed a large increase in emission around 630–640 nm in the L5178Y-R cell line at the concentrations (>200 μM) required for cellular permeability and reasonable nuclear staining. While the cellular uptake of these metal complexes remains a barrier for developing such DNA binding probes for live cells and in vivo applications, a detailed toxicity study involving DNA and off-target interactions needs to be pursued with the utmost care.
RNA GQs
The modes of binding and fluorescence properties of molecular probes that recognise RNA are significantly dependent on their secondary structure. RNA GQs play a crucial role in a number of cellular processes such as pre-mRNA slicing and polyadenylation, mRNA targeting and translation.12,212 As a number of putative RNA GQ motifs have been identified within the human transcriptome,213–215 there arises a critical need for the development of specific and reliable detection methods for these structures. Tang and co-workers recently reported a cyanine-based dye CyT as a promising sensor for the selective detection of RNA GQs among other RNA secondary structures (Fig. 11).216 So far, CyT is the only red emission-based molecular probe reported for the detection of RNA GQs in live cells of human origin. The sensing capability of the probe was studied in the presence of various forms of RNA, including single-stranded, double-stranded, hairpin structure, and three- and four-way junctions, in addition to the quadruplex structure. CyT exhibited negligible fluorescence in the unbound state and fluorescence enhancement (λem = 595 nm and λex = 532 nm) of over 1000-fold was observed in the presence of an RNA GQ compared to a mere 25-fold increase with non-GQ RNAs. Selective recognition of RNA GQs results from the disassembly of CyT J-aggregates and binding of the monomer to RNA GQs, which result in restricting the molecular rotation and prevention of non-radiating transition. In agreement with the binding preference, CyT displayed a relatively high binding constant (order of 105 M−1) for RNA GQs compared to non-GQ RNA structures (order of 104 M−1). Remarkably, the probe exhibited high quantum yields upon binding to two equivalents of RNA GQs (ΦF = 0.95 for Tel22, 0.92 for VEGF, 0.79 for TRF2, 0.74 for BCL-2, and 0.58 for NRAS oligonucleotides). The selective staining of non-canonical RNA GQs in the presence of various non-GQ RNA forms (like ssRNA, duplex RNA, short hairpin RNA and RNA junction structures) was demonstrated in a polyacrylamide gel electrophoresis experiment. The probe was successfully implemented to detect RNA GQs in fixed and live cells of human origin. Fluorescence microscopy study revealed that incubation of live A549 cells with CyT (5 μM) for 24 h illuminated the cytoplasm in red, reiterating the potential of the probe for the detection of endogenous RNA GQs in real time. However, there remains a dearth of conformation-specific fluorescent probes, specially far-red probes for efficient detection and imaging of different RNA GQs under in cellulo and in vivo conditions.Human telomeric repeat-containing RNA (TERRA) plays an important role, along with its DNA counterpart, in the process of telomere shortening and elongation.217 TERRA of various lengths solely adopts a parallel GQ structure (TERRA GQ) under in vitro and in vivo conditions and the major challenge is to selectively distinguish it from DNA GQs that possess polymorphic structures including parallel (3 + 1) hybrid, basket and chair topologies.218 Yong Shao and co-workers identified 5,10,15,20-tetrakis(3,5-dihydroxyphenyl) porphyrin (TOHdPP) from the pool of porphyrin derivatives as a selective fluorescent probe for the TERRA GQ (Fig. 18 and Table 4).219TOHdPP interacts with the TERRA GQ tetrad through π-stacking and triggers efficient electron communication between the tetraphenyl substituents and porphyrin macrocycle, as required by the hyperporphyrin effect. This was further validated by the observed enhanced and red-shifted fluorescence emission band at 732 nm. TOHdPP showed 60-fold brighter fluorescence intensity (λex = 467 nm) with an RNA GQ (rUAG4), compared to its DNA counterpart (TAG4). It is interesting to note that TERRA parallel GQ structures selectively convert TOHdPP to the hyperporphyrin state, and similar transformation is unfavourable for varieties of DNA GQs with parallel topology. The hydrogen bonding interactions of hydroxyl groups of TOHdPP with the phosphate backbone and/or the ribose/U residues have been suggested as being responsible for the observed exclusive RNA GQ selectivity. These results are interesting attributes, making TOHdPP a promising probe for RNA GQs, especially TERRA.
In contrast to known classical turn-on probes, David Monochaud and co-workers devised an alternative strategy, referred to as “twice-as-smart” GQ ligands.220–222 These ligands act as smart probes with high affinity and selectivity for GQs, and exhibit fluorescence turn-on properties based on fluorogenic electron distribution. This unprecedented design strategy that employs the red-edge effect (REE: a characteristic property defined as the dependence of the wavelength of the emission maximum on the excitation wavelength λmaxem = f[λex]) has immensely widened the scope of the probe's emission wavelength despite the lack of emission in the red region, exclusively. The probe operates through a quadruplex-promoted conformational switch that assembles its four guanines into an intramolecular G-quartet (smart ligand). The first smart ligand PyroTASQ for DNA/RNA GQs exhibited turn-on fluorescence through a quadruplex-promoted conformational switch and was exploited to light up the quadruplex in polyacrylamide gel electrophoresis. However, it was only partially successful in in cellulo imaging due to its propensity for aggregation.220 To overcome this limitation of aggregation, naphtho-TASQ (N-TASQ) was designed and studied exclusively for cellular imaging. N-TASQ exists in its open conformation when free in solution, and the fluorescence of its naphthalene template is quenched by the four surrounding guanines via intramolecular-photoinduced electron transfer. However, upon interaction with GQ, the intramolecular G-quartet formation leads to the redistribution of the guanine electrons onto the four guanines and relieves the template from its electronic restraint, restoring the probe fluorescence (Fig. 19a). Although N-TASQ absorbs below 320 nm, hampering its application in confocal imaging (commonly equipped with lasers adjusted at 408, 488 and 555 nm), its multiphoton absorption (>600 nm) property has allowed unbiased detection of RNA GQs in live cells (no fixation or forced permeabilisation is necessary) by two-photon microscopy.221 The fluorescence response of the probe is associated with better accessibility of the external G-quartet created by the loop-free environment of any quadruplex, thus offering selectivity for particular GQs over other NA conformations. However, given the authors' judicious hypothesis for N-TASQ with the unique spectroscopic property of REE, compatibility of N-TASQ for confocal imaging was discovered.222 Hence, they could explore the options of convenient visualisation of RNA GQs in live cells using all the standard emission filters of a confocal microscope (DAPI: 495 nm; FITC: 495–590 nm; Alexa: 585 nm and above), observing better quantum gain through the FITC filter (Fig. 19b). This unparalleled strategy elicited small molecule-based fluorescent probes for GQ imaging in live cells, contrary to antibody-based approaches that are limited to fixed and permeabilised cells.
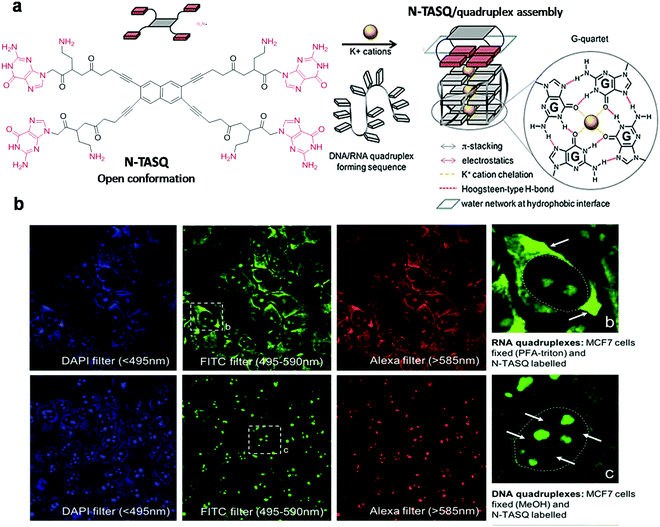 | ||
| Fig. 19 N-TASQ as a ‘twice-as-smart’ GQ probe. (a) Chemical structure of N-TASQ and schematic representation of the quadruplex forming sequence in its unfolded and folded states. The bioinspired interaction between N-TASQ and the quadruplex takes place through quartet–quartet recognition. (b) Images of fixed MCF7 cells (PFA-Triton) post-labelled with N-TASQ (100 μM), and (c) images of fixed MCF7 cells (MeOH) post-labelled with N-TASQ (100 μM). Reproduced from ref. 221 with permission from Nature Publishing Group, copyright 2016. | ||
Miscellaneous
In the past few years, stimuli-responsive fluorescent probes have gained more attention, owing to on-demand or analyte-specific activation to target, and space-time specificity.223–225 These probes provide an additional opportunity to detect the generation, accumulation and transportation of specific analyte flux across subcellular organelles such as the cell nucleus, cell membrane and mitochondria. In particular, fluorescence combination probes with signalling assisted by the additional recognition event (DNA or protein binding), which are provided with built-in correction for false positives, are highly beneficial for probing specific biological activity in a cellular environment and have the potential to be used in diagnostic and therapeutic applications.226–230 These probes also help in avoiding unwanted background fluorescence from the other biomolecules and cellular compartments, apart from increased sensitivity and selectivity towards the stimuli (pH, ROS or chemical substrates) or analyte (biomolecules) of interest. The majority of DNA binding probes are almost non-fluorescent in the unbound state and exhibit a turn-on fluorescence response in the presence of DNA. Such a turn-on fluorescence response, specifically in the presence of DNA, inspired us to design a stimuli-responsive fluorescent probe in combination with DNA, particularly making use of our turn-on NIR fluorescent probe QCy-DT (Fig. 20a).95 In probe QCy-DT, a free phenolic-OH group is available for functionalisation with a large variety of chemically or enzymatically cleavable groups to develop promising stimuli-responsive probes for various biological applications. We developed a phenylboronic acid-functionalised quinone-cyanine probe QCy-BA, in combination with DNA (i.e., QCy-BA⊂DNA), which serves as a superior NIR fluorescence combination probe for cellular hydrogen peroxide (H2O2) over other reactive oxygen species (ROS) (Fig. 20a).231 In the presence of H2O2, probe QCy-BA converts to DNA minor groove binding QCy-DT, which shows turn-on NIR-fluorescence in the presence of exogenous or endogenous (cellular) DNA. In the absence of exogenous or cellular DNA, the probe QCy-BA does not show any fluorescence response in the NIR-region, and this helps in achieving a double check (built-in-correction) of the observed signal output. The selective turn-on NIR-fluorescence response of the combination sensor QCy-BA⊂DNA, in the presence of H2O2, encouraged us to study its cellular uptake properties and detection of the exogenous and endogenous levels of H2O2 in normal and primary cells. Furthermore, a cell viability assay in HeLa cells showed that more than 80% of cells were viable at 25 μM concentration. These results revealed that probe QCy-BA is cell-permeable and non-toxic at the working concentration (5 μM). To evaluate the detection of endogenously elevated levels of H2O2, live HeLa cells were treated with epidermal growth factor (EGF, 50 ng mL−1). EGF binds to the epidermal growth factor receptor (EGFR), which activates the NOx/PI3K signalling pathways and stimulates the production of H2O2 endogenously. In the presence of QCy-BA (5 μM), FACS analysis showed an increase in fluorescence on the PerCPchannel, and the presence of H2O2 scavenger N-acetyl-L-cysteine (NAC) resulted in decreased fluorescence in the PerCPchannel (Fig. 20c). This confirms that probe QCy-BA is capable of detecting endogenous levels of H2O2 in live cells. Furthermore, probe QCy-BA (5 μM) detected endogenously elevated concentrations of H2O2 by DNA damaging agents such as BrdU and doxorubicin in HeLa as well as primary MRC5 cells (human primary lung fibroblasts) (Fig. 20b). Furthermore, the glucose oxidase assay confirmed the use of the combination probe QCy-BA⊂DNA for probing H2O2 generated in situ by the oxidation of glucose to gluconic acid. In the presence of catalase enzyme which converts H2O2 to water and oxygen, QCy-BA⊂Drew-AT showed reduced fluorescence emission at 650 nm, and hence, the combination probe is a promising tool for monitoring in situ turnover of H2O2. Interestingly, QCy-BA showed 10-fold higher fluorescence in cells compared to the commercially available ROS probe 2′,7′-dichlorofluorescein diacetate (DCFDA), which generally exhibits 3 to 4-fold fluorescence response. The confocal fluorescence images of QCy-BA (5 μM) in the cancer cell-line HeLa and primary MRC5 cells showed promising red fluorescence enhancement in the cell nucleus in the presence of H2O2 (100 μM), whereas the cells incubated with QCy-BA alone did not show any fluorescence in the red channel (Fig. 20d). This suggests that probe QCy-BA is a superior probe for the detection of exogenous and endogenously elevated levels of H2O2 in normal and primary cells. These versatile properties make our probe QCy-BA a potential NIR-fluorescent probe for the detection of H2O2 flux in the cell and open new avenues to ascertain a relationship between the oxidative stress in cancer and ageing-related diseases.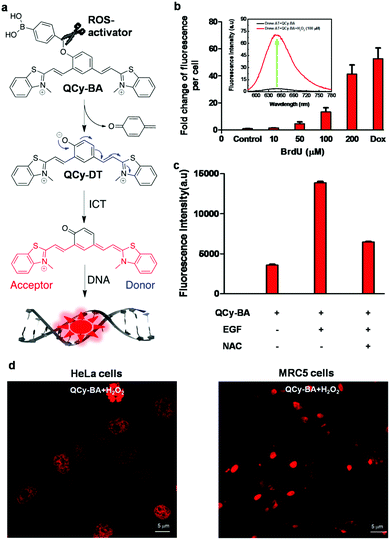 | ||
| Fig. 20 (a) Molecular structure of stimuli responsive NIR fluorescent probe QCy-BA and its transformation to DNA minor groove binder and NIR fluorescent probe QCy-DT in the presence of a H2O2 stimulus, and (b) detection of H2O2 generated in response to BrdU (0 to 200 μM) or doxorubicin (0.1 μM) for 48 h, in live HeLa cells using QCy-BA (5 μM). Inset: Fluorescence response of probe QCy-BA (5 μM) to externally added H2O2 (100 μM) in the presence of exogenous DNA. (c) Fluorescence response of probe QCy-BA (5 μM) in HeLa cells upon incubation with epidermal growth factor (EGF) (50 ng mL−1) and N-acetyl-L-cysteine (8 mM) in the PerCP channel, and (d) confocal fluorescence images of QCy-BA (5 μM) in cancer HeLa and primary MRC5 cells in the absence and presence of H2O2. Reproduced from ref. 231 with permission from Royal Society of Chemistry, copyright 2016. | ||
Therefore, this stimuli-responsive fluorescence combination probe (QCy-BA) with signalling assisted by an additional recognition event (DNA binding) is highly beneficial for probing specific biological molecules, events or activities in a cellular environment and has the potential to be used in numerous diagnostic and therapeutic applications. Thus, our fluorescence combination probe provides an additional advantage of detecting the generation, accumulation and transportation of H2O2 in cells. Also, unwanted background fluorescence from the other parts of cellular organisms is avoided and this improves the selectivity towards the stimuli of interest. To the best of our knowledge, combination probe QCy-BA⊂DNA is the first of its kind used for the detection of normal and elevated levels of H2O2 in live cells and in primary cells with NIR-fluorescence. We believe that the design concept of this combination probe will inspire the future development range of effective far-red fluorescent probes for the detection, quantification and detailed analysis of several biomolecules for in cellulo and in vivo imaging, diagnostic and theranostic applications.
Simultaneous detection of different RNA targets can provide a bigger picture and deeper insight into their subcellular dynamics. However, imaging endogenous RNA accurately in live cells in real time is not an easy task. Some of the powerful methods for imaging specific mRNAs enlist the use of fluorescent proteins, labelled antisense probes, aptamers and recently reported bioorthogonal conjugation that enable real-time mRNA visualisation.232–234 However, it requires careful optimisation of absorption and emission profiles to avoid cross-excitation among different probes and simultaneous detection of various targets. There have been reports describing the techniques involved in imaging RNA,107,232 and herein we chose to describe a few labelled antisense probes and the bioorthogonal conjugation strategy with far-red fluorescence labels to achieve high signal-to-background ratio for RNA imaging.233,235,236 Seitz and co-workers exploited, for the first time, single dye forced intercalation (FIT) probes, emitting in the red region (≥590 nm), to address this challenge (Fig. 21a).235 This technique involved hybridisation of a DNA FIT-probe containing quinoline blue (QB) with a complementary RNA target, resulting in the intercalation of the chromophore between the nucleotide base pairs. Restricted rotation around the methine bridge leads to increased lifetime of the excited state, leading to fluorescence enhancement. The red emission (λem = 610 nm and λex = 560 nm, Fig. 21b) and enhanced fluorescence (190-fold) of QB upon hybridisation with complementary RNA enabled its use for multiplexing, in combination with the orthogonal BO (cyan emission) and TO (green emission) probes, without any cross-excitation. The use of locked NA (LNA) monomer on the backbone, adjacent to the dye nucleotide, enhanced its quantum yield (ΦF(DNA) = 0.25; ΦF (LNA) = 0.46) as compared to when the DNA backbone was present. The TO- and QB-FIT probes were used for dual colour imaging of polyadenylated and oskar mRNA in oocytes of Drosphila melanogaster by means of wash-free fluorescent in situ hybridisation (FISH) and super resolution microscopy (STED). In a similar study, Yavin et al. reported red emitting quinoline blue (BisQ) as an FIT probe attached to peptide NA (PNA) to detect single-point mutation of mRNA in malignant cell lines using KRAS as the model gene (Fig. 21c).236 One of the probes PNA1 ((D-Lys)4-CCTCGA[BisQ]TACCGCATCC-NH2) had the dye located opposite to Uracil/Thymine, and the other probe PNA2 ((D-Lys)4-CCTCGACT[BisQ]TACCGCATCC-NH2) had the dye positioned opposite to Guanine. Both probes were designed to be complementary to mRNA of the Panc1 cancer cells with a single mismatch adjacent to the dye. The KRAS oncogene has frequent single-base mutations associated with tumorigenesis. To detect mRNA from KRAS in living cells, the probes PNA1 and PNA2 were incubated with three cancer cell lines (Panc1, BxPC-3 and HT-29); among these, only Panc1 contained the mutated form of mRNA (G to A point mutation) in the KRAS gene. A red fluorescence signal was observed solely for the hybridisation of mRNA of Panc1 with PNA1, while the other two cell lines produced a negligible response. However, in spite of the promising fluorescence response of PNA2 with DNA and RNA in test tubes, it gave a poor signal with live cells. The unique property of PNA1 for selective imaging of the live cells with a point mutation in KRAS makes it a promising approach for the detection of cancer and other disease conditions.
Bioorthogonal conjugation is a robust strategy to accomplish the requisite sensitivity and selectivity for NA detection and imaging in live cells.237 For instance, antisense probes are designed such that the reactive groups are brought into proximity upon hybridisation with the RNA template for fluorogenic reaction, which produces a detectable fluorescence signal. Neal K. Devaraj and co-workers used tetrazine ligation-based bioorthogonal conjugation for the detection of mRNA in live cells (Fig. 22).233 NIR emitting quinone cyanine dye functionalised with a phenyl vinyl ether group was used as a dienophile for tetrazine ligation reaction, which cages the phenoxide moiety, and fluorescence of the dye was quenched through internal charge transfer (ICT) (Fig. 22a).231 In particular, the RNA binding sequences with a vinyl ether (rBP-VE-Cy) and tetrazine group (rBP-Tz) were subjected to tetrazine ligation and turn-on fluorescence in the presence of in vitro transcribed mRNA transcript sfGFP-3′BT (Fig. 22b), which encodes for fluorescence protein GFP bearing two probe RNA recognition sequences within the 3′-UTR region. Utility of RNA template-assisted tetrazine ligation reaction and in situ generation of the probe was tested in CHO cells transfected with sfGFP-3′BT and appropriately functionalised oligonucleotides rBP-VE-Cy (25 nM) and rBP-Tz (25 nM). As expected, significant fluorescence emission was observed with predominant localisation of the in situ generated probe in the cytoplasm (Fig. 22c). A similar bioorthogonal conjugation strategy that employs a red fluorescence trimethine cyanine probe and sequence-specific short PNAs has been attempted for the detection of parallel GQ DNA.237 In general, the development of sensitive and specific far-red fluorogenic template-assisted and bioorthogonal reaction-derived probes offers effective methods to detect and monitor RNA/DNA in cells and tissues, provided limitations of in cellulo and in vivo probe delivery are addressed.
 | ||
| Fig. 22 (a) Schematic of fluorogenic bioorthogonal probe design using a tetrazine-vinyl ether uncaging reaction, (b) cartoon depicting fluorescence turn on of the probe in the presence of the target sfGFP mRNA transcript, and (c) fluorescence response in CHO cells treated with 25 nM NIR fluorogenic antisense probes (pink) after being transfected with sfGFP-3′BT plasmid (green). Reproduced from ref. 233 with permission from American Chemical Society. copyright 2016. | ||
Summary and future perspectives
The selected examples of far-red fluorescent probes discussed in this review article illustrate the incredible growth of fluorescence turn-on probes for canonical and non-canonical nucleic acid (NA) structures in recent years. In view of rapid development in the area of ultrasensitive imaging techniques, it is essential to note that brightness, lifetime, ability to avoid auto-fluorescence from the surrounding environment and photostability of the far-red fluorescent probe would enhance the limit of spatial and temporal resolution, a sought-after feature for single molecule imaging-based techniques and applications. Therefore, it is imperative to appreciate the bright future of far-red fluorescent probes owing to their design, bioimaging properties, and diagnostic and theranostic applications. The structure and function of canonical and non-canonical DNA and RNA directly or indirectly play a fundamental role in almost all the biological processes. In this context, structure or conformation-selective far-red fluorescent probes become indispensable molecular tools to understand the dynamics, localisation, transport and expression of canonical and non-canonical DNA and RNA in real time. In particular, various non-canonical NA structures (hairpin loops, internal loops, junctions, triplexes, G-quadruplexes and higher-ordered structures, homoduplexes [like A-motif and poly-(GA)n duplex], three-way and four-way branches) need special attention in order to understand the structure–function relationship and biological implications. This is indeed a largely unexplored area waiting to be conquered by designing very selective, sensitive and biologically benign probes.In particular, designing highly target-specific fluorescent probes can create a revolution in addressing the drawbacks of immuno fluorescence technology as it has concerns of imaging only in fixed cells due to impermeability of the antibodies used.
The intracellular dynamics of mRNA synthesis and localisation can be analysed at higher temporal resolution using small molecule-based selective fluorescent probes. In addition to bioimaging and structure–function studies, these molecular probes can be utilised in conventional diagnostic and theranostic studies under several situations, through physical encapsulation or chemical conjugation. In addition, NIR probes find application in photodynamic therapy for selective destruction of cancer cells in tumour tissues.238 Moreover, far-red turn-on probes of NAs, which are specific to cellular organelles with high resolution, would substantially contribute to the advancements in the field of life sciences and medicine.19 The selective targeting of NAs (DNA/RNA/G-quadruplexes and other non-canonical structures), in particular organelles like the nucleus and mitochondria, using highly conformation-specific and stimuli-responsive turn-on probes is an area that still needs to be explored. Contribution in this direction aids in understanding the interdependence of organelles and their cellular dynamics. For instance, mitochondria are the only organelle within the cell with extra-chromosomal DNA and are under the control of both the nuclear and mitochondrial genome. Currently, mitochondria are a favourite topic of research, with recent discoveries of mitochondrial DNA (mtDNA) mutations and reactive oxygen/nitrogen species (ROS/RNS) leading to mitochondrial dysfunction that contributes massively to disease conditions such as cancer, hypertension,239 age-related disorders and neurodegenerative diseases including Alzheimer's, Parkinson and amyotrophic lateral sclerosis (ALS).240–242 Thus, developing selective far-red fluorescent probes for the detection of DNA mutations and damage specific to mitochondria243 might provide greater insights into the role of mtDNA under various disease conditions. This enhances our knowledge on disease pathogenesis and aids the development of effective diagnostic and theranostic probes (Fig. 23).
 | ||
| Fig. 23 Current progress, applications, future challenges and prospects of far-red fluorescent probes of canonical and non-canonical NA structures. | ||
It is essential to design conformation-specific probes to understand the intervention of quadruplex structural dynamics in a cellular process. Research findings over the last decade and ongoing research efforts reveal the ubiquitous presence of DNA and RNA G-quadruplexes and their possible involvement in the modulation of innumerable biological processes.130 Therefore, employing far-red fluorescent probes is an ideal strategy for studying structural dynamics of DNA and RNA G-quadruplexes, for their structure–function relation and role in various cellular processes. For example, a recent survey shows that the DNA sequence of the insulin-linked polymorphic region (ILPR) contains G-rich repeats (5′-ACAG4TGTG4-3′), which form non-canonical G-quadruplex structures and can easily be targeted to bridge the gap of the disease mechanism and therapeutic approach.244,245 However, many reported probes for DNA G-quadruplex and RNA G-quadruplex detection are not selective or conformation-specific, and only a few of them have been used for cellular visualisation. These limitations need to be considered while designing new molecular probes. One must be cautious when a new probe is developed for DNA or RNA G-quadruplexes and greater care and attention must be devoted to determining whether the signal is coming solely from the specific DNA/RNA G-quadruplex or from other non-canonical and higher order DNA or RNA conformations or their complexes with biomolecules such as proteins. Such careful studies and observations not only help in designing conformation-specific turn-on probes but may also lead to the serendipitous discovery of selective probes for other non-canonical NA structures or their interactions with proteins, an area untouched so far. Developing probes for non-canonical DNA/RNA structures (not considered for developing probes so far) such as triplexes, i-motifs, homoduplexes (A-motif and poly-(GA)n duplex), hairpin loops, internal loops, junctions, three-way and four-way branches and higher-ordered structures is a challenging task and needs to be addressed with greater prominence.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank Prof. C. N. R. Rao FRS for constant support and encouragement, JNCASR, the Department of Science and Technology (DST), New Delhi, India (Science and Engineering Research Board grant: SB/S1/OC-47/2013 and SwarnaJayanti Fellowship grant DST/SJF/CSA-02/2015-2016), and Sheikh Saqr Laboratory (SSL), ICMS-JNCASR, Alexander von Humboldt Foundation, Germany for financial support and CSIR and UGC for research fellowships to N. N. and S. P.Notes and references
- G. M. Blackburn, Nucleic acids in chemistry and biology, Royal Society of Chemistry, 2006 Search PubMed.
- B. Alberts, Molecular Biology of the Cell, Garland, 2008 Search PubMed.
- J. Choi and T. Majima, Chem. Soc. Rev., 2011, 40, 5893–5909 RSC.
- A. Rich and S. Zhang, Nat. Rev. Genet., 2003, 4, 566–572 CrossRef CAS PubMed.
- D. R. Gallie, Gene, 1998, 216, 1–11 CrossRef CAS PubMed.
- G. Yagil, Crit. Rev. Biochem. Mol. Biol., 1991, 26, 475–559 CrossRef CAS PubMed.
- E. H. Blackburn, Cell, 2001, 106, 661–673 CrossRef CAS PubMed.
- G. Aubert and P. M. Lansdorp, Physiol. Rev., 2008, 88, 557–579 CrossRef CAS PubMed.
- G. Wang and K. M. Vasquez, DNA Repair, 2014, 19, 143–151 CrossRef CAS PubMed.
- L. H. Hurley, Nat. Rev. Cancer, 2002, 2, 188–200 CrossRef CAS PubMed.
- S. Balasubramanian, L. H. Hurley and S. Neidle, Nat. Rev. Drug Discovery, 2011, 10, 261–275 CrossRef CAS PubMed.
- A. Cammas and S. Millevoi, Nucleic Acids Res., 2017, 45, 1584–1595 Search PubMed.
- N. C. Garbett, P. A. Ragazzon and J. B. Chaires, Nat. Protoc., 2007, 2, 3166–3172 CrossRef CAS PubMed.
- N. J. Buurma and I. Haq, Methods, 2007, 42, 162–172 CrossRef CAS PubMed.
- J. Kypr, I. Kejnovská, D. Renčiuk and M. Vorlíčková, Nucleic Acids Res., 2009, 37, 1713–1725 CrossRef CAS PubMed.
- H. A. Crissman and G. T. Hirons, Methods Cell Biol., 1994, 41, 195–209 CrossRef CAS PubMed.
- R. P. Haugland, The handbook: a guide to fluorescent probes and labeling technologies, Molecular probes, 2005 Search PubMed.
- L. Yuan, W. Lin, K. Zheng, L. He and W. Huang, Chem. Soc. Rev., 2013, 42, 622–661 RSC.
- M. Fernández-Suárez and A. Y. Ting, Nat. Rev. Mol. Cell Biol., 2008, 9, 929–943 CrossRef PubMed.
- J. Zhang, R. E. Campbell, A. Y. Ting and R. Y. Tsien, Nat. Rev. Mol. Cell Biol., 2002, 3, 906–918 CrossRef CAS PubMed.
- R. W. Sinkeldam, N. J. Greco and Y. Tor, Chem. Rev., 2010, 110, 2579–2619 CrossRef CAS PubMed.
- L. M. Wysocki and L. D. Lavis, Curr. Opin. Chem. Biol., 2011, 15, 752–759 CrossRef CAS PubMed.
- L. D. Lavis and R. T. Raines, ACS Chem. Biol., 2008, 3, 142–155 CrossRef CAS PubMed.
- D. Zink and T. Cremer, Curr. Biol., 1998, 8, R321–R324 CrossRef CAS PubMed.
- B. N. Giepmans, S. R. Adams, M. H. Ellisman and R. Y. Tsien, Science, 2006, 312, 217–224 CrossRef CAS PubMed.
- E. Cassette, M. Helle, L. Bezdetnaya, F. Marchal, B. Dubertret and T. Pons, Adv. Drug Delivery Rev., 2013, 65, 719–731 CrossRef CAS PubMed.
- J. Chan, S. C. Dodani and C. J. Chang, Nat. Chem., 2012, 4, 973–984 CrossRef CAS PubMed.
- N. Johnsson and K. Johnsson, ACS Chem. Biol., 2007, 2, 31–38 CrossRef CAS PubMed.
- M. Waring, J. Mol. Biol., 1965, 13, 269–282 CrossRef CAS PubMed.
- C.-C. Tsai, S. C. Jain and H. M. Sobell, Proc. Natl. Acad. Sci. U. S. A., 1975, 72, 628–632 CrossRef CAS.
- D. J. Arndt-Jovin and T. M. Jovin, Methods Cell Biol., 1989, 30, 417–448 CrossRef CAS PubMed.
- C. Riccardi and I. Nicoletti, Nat. Protoc., 2006, 1, 1458–1461 CrossRef CAS PubMed.
- H. Zipper, H. Brunner, J. Bernhagen and F. Vitzthum, Nucleic Acids Res., 2004, 32, e103 CrossRef PubMed.
- S. Giglio, P. T. Monis and C. P. Saint, Nucleic Acids Res., 2003, 31, e136 CrossRef PubMed.
- A. Dragan, J. Casas-Finet, E. Bishop, R. Strouse, M. Schenerman and C. Geddes, Biophys. J., 2010, 99, 3010–3019 CrossRef CAS PubMed.
- V. L. Singer, L. J. Jones, S. T. Yue and R. P. Haugland, Anal. Biochem., 1997, 249, 228–238 CrossRef CAS PubMed.
- J. Nygren, N. Svanvik and M. Kubista, Biopolymers, 1998, 46, 39–51 CrossRef CAS PubMed.
- H. S. Rye, S. Yue, D. E. Wemmer, M. A. Quesada, R. P. Haugland, R. A. Mathies and A. N. Glazer, Nucleic Acids Res., 1992, 20, 2803–2812 CrossRef CAS PubMed.
- Q. Li, Y. Kim, J. Namm, A. Kulkarni, G. R. Rosania, Y.-H. Ahn and Y.-T. Chang, Chem. Biol., 2006, 13, 615–623 CrossRef CAS PubMed.
- J. W. Lee, M. Jung, G. R. Rosania and Y.-T. Chang, Chem. Commun., 2003, 1852–1853 RSC.
- S. Neidle, Nat. Prod. Rep., 2001, 18, 291–309 RSC.
- P. B. Dervan, Bioorg. Med. Chem., 2001, 9, 2215–2235 CrossRef CAS PubMed.
- A. Boutorine, D. Novopashina, O. Krasheninina, K. Nozeret and A. Venyaminova, Molecules, 2013, 18, 15357–15397 CrossRef CAS PubMed.
- A. C. Bhasikuttan and J. Mohanty, Chem. Commun., 2015, 51, 7581–7597 RSC.
- B. R. Vummidi, J. Alzeer and N. W. Luedtke, ChemBioChem, 2013, 14, 540–558 CrossRef CAS PubMed.
- D. Monchaud and M.-P. Teulade-Fichou, Org. Biomol. chem., 2008, 6, 627–636 CAS.
- L. Xu, Z. Zhu, D. Wei, X. Zhou, J. Qin and C. Yang, ACS Appl. Mater. Interfaces, 2014, 6, 18344–18351 CAS.
- J. N. Wilson, J. Wigenius, D. R. G. Pitter, Y. Qiu, M. Abrahamsson and F. Westerlund, J. Phys. Chem. B, 2013, 117, 12000–12006 CrossRef CAS PubMed.
- S. Feng, Y. Kyung Kim, S. Yang and Y.-T. Chang, Chem. Commun., 2010, 46, 436–438 RSC.
- S. Chi, L. Li and Y. Wu, J. Phys. Chem. C, 2016, 120, 13706–13715 CAS.
- P. Gaur, A. Kumar, S. Bhattacharyya and S. Ghosh, J. Mater. Chem. B, 2016, 4, 4895–4900 RSC.
- P. Bosch, V. García, B. S. Bilen, D. Sucunza, A. Domingo, F. Mendicuti and J. J. Vaquero, Dyes Pigm., 2017, 138, 135–146 CrossRef CAS.
- E. E. Rastede, M. Tanha, D. Yaron, S. C. Watkins, A. S. Waggoner and B. A. Armitage, Photochem. Photobiol. Sci., 2015, 14, 1703–1712 CAS.
- A. Erve, Y. Saoudi, S. Thirot, C. Guetta-Landras, J.-C. Florent, C.-H. Nguyen, D. S. Grierson and A. V. Popov, Nucleic Acids Res., 2006, 34, e43 CrossRef PubMed.
- P. Gaur, A. Kumar, R. Dalal, S. Bhattacharyya and S. Ghosh, Chem. Commun., 2017, 53, 2571–2574 RSC.
- S. Kwon, D. I. Kwon, Y. Jung, J. H. Kim, Y. Lee, B. Lim, I. Kim and J. Lee, Sens. Actuators, B, 2017, 252, 340–352 CrossRef CAS.
- B. Shirinfar, N. Ahmed, Y. S. Park, G.-S. Cho, I. S. Youn, J.-K. Han, H. G. Nam and K. S. Kim, J. Am. Chem. Soc., 2013, 135, 90–93 CrossRef CAS PubMed.
- Y. Liu, E. J. Jun, G. Kim, A.-R. Lee, J.-H. Lee and J. Yoon, Chem. Commun., 2014, 50, 2505–2507 RSC.
- Y.-J. Lu, Q. Deng, D.-P. Hu, Z.-Y. Wang, B.-H. Huang, Z.-Y. Du, Y.-X. Fang, W.-L. Wong, K. Zhang and C.-F. Chow, Chem. Commun., 2015, 51, 15241–15244 RSC.
- W. Du, H. Wang, Y. Zhu, X. Tian, M. Zhang, Q. Zhang, S. C. De Souza, A. Wang, H. Zhou, Z. Zhang, J. Wu and Y. Tian, ACS Appl. Mater. Interfaces, 2017, 9, 31424–31432 CAS.
- B. Jin, X. Zhang, W. Zheng, X. Liu, C. Qi, F. Wang and D. Shangguan, Anal. Chem., 2014, 86, 943–952 CrossRef CAS PubMed.
- S. Wu, L. Wang, N. Zhang, Y. Liu, W. Zheng, A. Chang, F. Wang, S. Li and D. Shangguan, Chem. – Eur. J., 2016, 22, 6037–6047 CrossRef CAS PubMed.
- Y. Kataoka, H. Fujita, Y. Kasahara, T. Yoshihara, S. Tobita and M. Kuwahara, Anal. Chem., 2014, 86, 12078–12084 CrossRef CAS PubMed.
- B. Maji, K. Kumar, M. Kaulage, K. Muniyappa and S. Bhattacharya, J. Med. Chem., 2014, 57, 6973–6988 CrossRef CAS PubMed.
- Q. Zhang, Y.-C. Liu, D.-M. Kong and D.-S. Guo, Chem. – Eur. J., 2015, 21, 13253–13260 CrossRef CAS PubMed.
- M.-Q. Wang, S. Liu, C.-P. Tang, A. Raza, S. Li, L.-X. Gao, J. Sun and S.-P. Guo, Dyes Pigm., 2017, 136, 78–84 CrossRef CAS.
- M.-Q. Wang, Y. Wu, Z.-Y. Wang, Q.-Y. Chen, F.-Y. Xiao, Y.-C. Jiang and A. Sang, Dyes Pigm., 2017, 145, 1–6 CrossRef CAS.
- A. Shivalingam, M. A. Izquierdo, A. Le Marois, A. Vyšniauskas, K. Suhling, M. K. Kuimova and R. Vilar, Nat. Commun., 2015, 6, 8178 CrossRef CAS PubMed.
- P. Yang, A. De Cian, M.-P. Teulade-Fichou, J.-L. Mergny and D. Monchaud, Angew. Chem., 2009, 121, 2222–2225 CrossRef.
- Y.-J. Lu, Q. Deng, J.-Q. Hou, D.-P. Hu, Z.-Y. Wang, K. Zhang, L. G. Luyt, W.-L. Wong and C.-F. Chow, ACS Chem. Biol., 2016, 11, 1019–1029 CrossRef CAS PubMed.
- J. Mohanty, N. Barooah, V. Dhamodharan, S. Harikrishna, P. I. Pradeepkumar and A. C. Bhasikuttan, J. Am. Chem. Soc., 2013, 135, 367–376 CrossRef CAS PubMed.
- S. Xu, Q. Li, J. Xiang, Q. Yang, H. Sun, A. Guan, L. Wang, Y. Liu, L. Yu, Y. Shi, H. Chen and Y. Tang, Sci. Rep., 2016, 6, 24793 CrossRef CAS PubMed.
- M.-H. Hu, S.-B. Chen, R.-J. Guo, T.-M. Ou, Z.-S. Huang and J.-H. Tan, Analyst, 2015, 140, 4616–4625 RSC.
- M.-H. Hu, X. Chen, S.-B. Chen, T.-M. Ou, M. Yao, L.-Q. Gu, Z.-S. Huang and J.-H. Tan, Sci. Rep., 2015, 5, 17202 CrossRef CAS PubMed.
- M.-Q. Wang, Z.-Y. Wang, Y.-F. Yang, G.-Y. Ren, X.-N. Liu, S. Li, J.-W. Wei and L. Zhang, Tetrahedron Lett., 2017, 58, 3296–3300 CrossRef CAS.
- Y.-J. Lu, D.-P. Hu, K. Zhang, W.-L. Wong and C.-F. Chow, Biosens. Bioelectron., 2016, 81, 373–381 CrossRef CAS PubMed.
- X. Peng, T. Wu, J. Fan, J. Wang, S. Zhang, F. Song and S. Sun, Angew. Chem., Int. Ed., 2011, 50, 4180–4183 CrossRef CAS PubMed.
- S. Zhang, J. Fan, Z. Li, N. Hao, J. Cao, T. Wu, J. Wang and X. Peng, J. Mater. Chem. B, 2014, 2, 2688–2693 RSC.
- D. Maity, A. Raj, D. Karthigeyan, T. K. Kundu and T. Govindaraju, RSC Adv., 2013, 3, 16788–16794 RSC.
- D. Maity and T. Govindaraju, Org. Biomol. Chem., 2013, 11, 2098–2104 CAS.
- D. Maity, A. Raj, P. K. Samanta, D. Karthigeyan, T. K. Kundu, S. K. Pati and T. Govindaraju, RSC Adv., 2014, 4, 11147–11151 RSC.
- K. Rajasekhar, N. Narayanaswamy, N. A. Murugan, G. Kuang, H. Ågren and T. Govindaraju, Sci. Rep., 2016, 6, 23668 CrossRef CAS PubMed.
- N. Narayanaswamy, M. Kumar, S. Das, R. Sharma, P. K. Samanta, S. K. Pati, S. K. Dhar, T. K. Kundu and T. Govindaraju, Sci. Rep., 2014, 4, 6476 CrossRef CAS PubMed.
- X.-L. Wang, R. Sun, J.-T. Miao, X.-S. Li and J.-F. Ge, Sens. Actuators, B, 2016, 236, 627–634 CrossRef CAS.
- Y.-X. Xiong, Z.-S. Huang and J.-H. Tan, Eur. J. Med. Chem., 2015, 97, 538–551 CrossRef CAS PubMed.
- B. S. Patro, B. Maity and S. Chattopadhyay, Antioxid. Redox Signaling, 2010, 12, 945–960 CrossRef CAS PubMed.
- P. M. Pithan, D. Decker, S. I. Druzhinin, H. Ihmels, H. Schonherr and Y. Voß, RSC Adv., 2017, 7, 10660–10667 RSC.
- R. Bortolozzi, H. Ihmels, L. Thomas, M. Tian and G. Viola, Chem. – Eur. J., 2013, 19, 8736–8741 CrossRef CAS PubMed.
- N. Karton-Lifshin, E. Segal, L. Omer, M. Portnoy, R. Satchi-Fainaro and D. Shabat, J. Am. Chem. Soc., 2011, 133, 10960–10965 CrossRef CAS PubMed.
- N. Karton-Lifshin, L. Albertazzi, M. Bendikov, P. S. Baran and D. Shabat, J. Am. Chem. Soc., 2012, 134, 20412–20420 CrossRef CAS PubMed.
- E. Kisin-Finfer and D. Shabat, Bioorg. Med. Chem., 2013, 21, 3602–3608 CrossRef CAS PubMed.
- O. Redy-Keisar, E. Kisin-Finfer, S. Ferber, R. Satchi-Fainaro and D. Shabat, Nat. Protoc., 2014, 9, 27–36 CrossRef CAS PubMed.
- S. Gnaim and D. Shabat, Acc. Chem. Res., 2014, 47, 2970–2984 CrossRef CAS PubMed.
- D. Maity, A. Raj, D. Karthigeyan, T. K. Kundu and T. Govindaraju, Supramol. Chem., 2015, 27, 589–594 CrossRef CAS.
- N. Narayanaswamy, S. Das, P. K. Samanta, K. Banu, G. P. Sharma, N. Mondal, S. K. Dhar, S. K. Pati and T. Govindaraju, Nucleic Acids Res., 2015, 43, 8651–8663 CrossRef CAS PubMed.
- J. Li, K. Guo, J. Shen, W. Yang and M. Yin, Small, 2014, 10, 1351–1360 CrossRef CAS PubMed.
- B. Dumat, G. Bordeau, E. Faurel-Paul, F. Mahuteau-Betzer, N. Saettel, G. Metge, C. L. Fiorini-Debuisschert, F. Charra and M.-P. Teulade-Fichou, J. Am. Chem. Soc., 2013, 135, 12697–12706 CrossRef CAS PubMed.
- M. O. Olson, K. Hingorani and A. Szebeni, Int. Rev. Cytol., 2002, 219, 199–266 CrossRef CAS PubMed.
- Y. W. Lam, L. Trinkle-Mulcahy and A. I. Lamond, J. Cell Sci., 2005, 118, 1335–1337 CrossRef CAS PubMed.
- M. M. Mhlanga, D. Y. Vargas, C. W. Fung, F. R. Kramer and S. Tyagi, Nucleic Acids Res., 2005, 33, 1902–1912 CrossRef CAS PubMed.
- G. S. Wilkie and I. Davis, Cell, 2001, 105, 209–219 CrossRef CAS PubMed.
- A. M. Femino, F. S. Fay, K. Fogarty and R. H. Singer, Science, 1998, 280, 585–590 CrossRef CAS PubMed.
- R. W. Dirks, C. Molenaar and H. J. Tanke, Methods, 2003, 29, 51–57 CrossRef CAS PubMed.
- J. Guo, J. Ju and N. J. Turro, Anal. Bioanal. Chem., 2012, 402, 3115–3125 CrossRef CAS PubMed.
- E. V. Volpi and J. M. Bridger, BioTechniques, 2008, 45, 385–386 CrossRef CAS PubMed.
- B. Simon, M. Sandhu and K. L. Myhr, J. Neurosci. Res., 2010, 88, 55–63 CrossRef CAS PubMed.
- G. Bao, W. J. Rhee and A. Tsourkas, Annu. Rev. Biomed. Eng., 2009, 11, 25–47 CrossRef CAS PubMed.
- J. S. Andersen, Y. W. Lam, A. K. Leung, S.-E. Ong, C. E. Lyon, A. I. Lamond and M. Mann, Nature, 2005, 433, 77–83 CrossRef CAS PubMed.
- E. Bertrand, P. Chartrand, M. Schaefer, S. M. Shenoy, R. H. Singer and R. M. Long, Mol. Cell, 1998, 2, 437–445 CrossRef CAS PubMed.
- D. Hernandez-Verdun, P. Roussel and J. Gébrane-Younès, J. Cell Sci., 2002, 115, 2265–2270 CAS.
- R. P. Haugland, Assays for cell viability, proliferation and function. In The Handbook, A Guide to Fluorescent Probes and Labeling Technologies, 10th edn, 2005 Search PubMed.
- X. Liu, Y. Sun, Y. Zhang, F. Miao, G. Wang, H. Zhao, X. Yu, H. Liu and W.-Y. Wong, Org. Biomol. Chem., 2011, 9, 3615–3618 CAS.
- G. Song, Y. Sun, Y. Liu, X. Wang, M. Chen, F. Miao, W. Zhang, X. Yu and J. Jin, Biomaterials, 2014, 35, 2103–2112 CrossRef CAS PubMed.
- B. Zhou, W. Liu, H. Zhang, J. Wu, S. Liu, H. Xu and P. Wang, Biosens. Bioelectron., 2015, 68, 189–196 CrossRef CAS PubMed.
- W. Liu, B. Zhou, G. Niu, J. Ge, J. Wu, H. Zhang, H. Xu and P. Wang, ACS Appl. Mater. Interfaces, 2015, 7, 7421–7427 CAS.
- Q. Li, Y. Kim, J. Namm, A. Kulkarni, G. R. Rosania, Y.-H. Ahn and Y.-T. Chang, Chem. Biol., 2006, 13, 615–623 CrossRef CAS PubMed.
- Q. Li and Y.-T. Chang, Nat. Protoc., 2006, 1, 2922–2932 CrossRef CAS PubMed.
- C.-Q. Zhu, S.-J. Zhuo, H. Zheng, J.-L. Chen, D.-H. Li, S.-H. Li and J.-G. Xu, Analyst, 2004, 129, 254–258 RSC.
- Z. Li, S. Sun, Z. Yang, S. Zhang, H. Zhang, M. Hu, J. Cao, J. Wang, F. Liu and F. Song, Biomaterials, 2013, 34, 6473–6481 CrossRef CAS PubMed.
- N. Stevens, N. O’connor, H. Vishwasrao, D. Samaroo, E. R. Kandel, D. L. Akins, C. M. Drain and N. J. Turro, J. Am. Chem. Soc., 2008, 130, 7182–7183 CrossRef CAS PubMed.
- A. M. Zahler, J. R. Williamson, T. R. Cech and D. M. Prescott, Nature, 1991, 350, 718–720 CrossRef CAS PubMed.
- C. W. Greider and E. H. Blackburn, Cell, 1985, 43, 405–413 CrossRef CAS PubMed.
- O. Doluca, J. M. Withers and V. V. Filichev, Chem. Rev., 2013, 113, 3044–3083 CrossRef CAS PubMed.
- S. Neidle and S. Balasubramanian, Quadruplex nucleic acids, Royal Society of Chemistry, 2006 Search PubMed.
- J. Bidzinska, G. Cimino-Reale, N. Zaffaroni and M. Folini, Molecules, 2013, 18, 12368–12395 CrossRef CAS PubMed.
- A. K. Todd, M. Johnston and S. Neidle, Nucleic Acids Res., 2005, 33, 2901–2907 CrossRef CAS PubMed.
- J. L. Huppert and S. Balasubramanian, Nucleic Acids Res., 2005, 33, 2908–2916 CrossRef CAS PubMed.
- A. Bourdoncle, A. Estevez Torres, C. Gosse, L. Lacroix, P. Vekhoff, T. Le Saux, L. Jullien and J.-L. Mergny, J. Am. Chem. Soc., 2006, 128, 11094–11105 CrossRef CAS PubMed.
- P. S. Shirude, B. Okumus, L. Ying, T. Ha and S. Balasubramanian, J. Am. Chem. Soc., 2007, 129, 7484–7485 CrossRef CAS PubMed.
- D. Rhodes and H. J. Lipps, Nucleic Acids Res., 2015, gkv862 Search PubMed.
- A. Lew, W. J. Rutter and G. C. Kennedy, Proc. Natl. Acad. Sci. U. S. A., 2000, 97, 12508–12512 CrossRef CAS PubMed.
- D. Sun, K. Guo, J. J. Rusche and L. H. Hurley, Nucleic Acids Res., 2005, 33, 6070–6080 CrossRef CAS PubMed.
- A. I. Murchie and D. M. Lilley, Nucleic Acids Res., 1992, 20, 49–53 CrossRef CAS PubMed.
- T. Simonsson, M. Kubista and P. Pecinka, Nucleic Acids Res., 1998, 26, 1167–1172 CrossRef CAS PubMed.
- A. Ambrus, D. Chen, J. Dai, R. A. Jones and D. Yang, Biochemistry, 2005, 44, 2048–2058 CrossRef CAS PubMed.
- A. Rangan, O. Y. Fedoroff and L. H. Hurley, J. Biol. Chem., 2001, 276, 4640–4646 CrossRef CAS PubMed.
- A. Siddiqui-Jain, C. L. Grand, D. J. Bearss and L. H. Hurley, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 11593–11598 CrossRef CAS PubMed.
- V. Dhamodharan, S. Harikrishna, A. C. Bhasikuttan and P. Pradeepkumar, ACS Chem. Biol., 2014, 10, 821–833 CrossRef PubMed.
- S. Rankin, A. P. Reszka, J. Huppert, M. Zloh, G. N. Parkinson, A. K. Todd, S. Ladame, S. Balasubramanian and S. Neidle, J. Am. Chem. Soc., 2005, 127, 10584–10589 CrossRef CAS PubMed.
- H. Fernando, A. P. Reszka, J. Huppert, S. Ladame, S. Rankin, A. R. Venkitaraman, S. Neidle and S. Balasubramanian, Biochemistry, 2006, 45, 7854–7860 CrossRef CAS PubMed.
- J. Dai, T. S. Dexheimer, D. Chen, M. Carver, A. Ambrus, R. A. Jones and D. Yang, J. Am. Chem. Soc., 2006, 128, 1096–1098 CrossRef CAS PubMed.
- T. S. Dexheimer, D. Sun and L. H. Hurley, J. Am. Chem. Soc., 2006, 128, 5404–5415 CrossRef CAS PubMed.
- J. Dai, D. Chen, R. A. Jones, L. H. Hurley and D. Yang, Nucleic Acids Res., 2006, 34, 5133–5144 CrossRef CAS PubMed.
- P. Sengupta, S. Chattopadhyay and S. Chatterjee, Drug Discovery Today, 2017, 22, 1165–1186 CrossRef CAS PubMed.
- S. Cogoi and L. E. Xodo, Nucleic Acids Res., 2006, 34, 2536–2549 CrossRef CAS PubMed.
- K. Guo, A. Pourpak, K. Beetz-Rogers, V. Gokhale, D. Sun and L. H. Hurley, J. Am. Chem. Soc., 2007, 129, 10220–10228 CrossRef CAS PubMed.
- D. W. Dong, F. Pereira, S. P. Barrett, J. E. Kolesar, K. Cao, J. Damas, L. A. Yatsunyk, F. B. Johnson and B. A. Kaufman, BMC genomics, 2014, 15, 677 CrossRef PubMed.
- S. K. Bharti, J. A. Sommers, J. Zhou, D. L. Kaplan, J. N. Spelbrink, J.-L. Mergny and R. M. Brosh, J. Biol. Chem., 2014, 289, 29975–29993 CrossRef CAS PubMed.
- G. Biffi, M. Di Antonio, D. Tannahill and S. Balasubramanian, Nat. Chem., 2014, 6, 75–80 CrossRef CAS PubMed.
- G. Biffi, D. Tannahill, J. McCafferty and S. Balasubramanian, Nat. Chem., 2013, 5, 182–186 CrossRef CAS PubMed.
- G. W. Collie and G. N. Parkinson, Chem. Soc. Rev., 2011, 40, 5867–5892 RSC.
- Y. Chen and D. Yang, Curr. Protoc. Nucleic Acid Chem., 2012, 17.15.11–17.15.17 Search PubMed.
- S. Cosconati, A. Rizzo, R. Trotta, B. Pagano, S. Iachettini, S. De Tito, I. Lauri, I. Fotticchia, M. Giustiniano and L. Marinelli, J. Med. Chem., 2012, 55, 9785–9792 CrossRef CAS PubMed.
- X. Chen, J. Wang, G. Jiang, G. Zu, M. Liu, L. Zhou and R. Pei, RSC Adv., 2016, 6, 70117–70123 RSC.
- G. Jiang, X. Chen, L. Xu, Y. Cao, S. Hong, M. Liu, W. Cao and R. Pei, ChemistrySelect, 2017, 2, 2783–2788 CrossRef CAS.
- Y.-J. Lu, S.-C. Yan, F.-Y. Chan, L. Zou, W.-H. Chung, W.-L. Wong, B. Qiu, N. Sun, P.-H. Chan and Z.-S. Huang, Chem. Commun., 2011, 47, 4971–4973 RSC.
- Q. Chen, I. D. Kuntz and R. H. Shafer, Proc. Natl. Acad. Sci. U. S. A., 1996, 93, 2635–2639 CrossRef CAS.
- E. W. White, F. Tanious, M. A. Ismail, A. P. Reszka, S. Neidle, D. W. Boykin and W. D. Wilson, Biophys. Chem., 2007, 126, 140–153 CrossRef CAS PubMed.
- S. Paramasivan and P. H. Bolton, Nucleic Acids Res., 2008, 36, e106 CrossRef PubMed.
- Q. Yang, J. Xiang, S. Yang, Q. Li, Q. Zhou, A. Guan, X. Zhang, H. Zhang, Y. Tang and G. Xu, Nucleic Acids Res., 2010, 38, 1022–1033 CrossRef CAS PubMed.
- Q. Yang, J.-F. Xiang, S. Yang, Q. Li, Q. Zhou, A. Guan, L. Li, Y. Zhang, X. Zhang and H. Zhang, Anal. Chem., 2010, 82, 9135–9137 CrossRef CAS PubMed.
- B. Karg, A. Funke, A. Ficht, A. Sievers-Engler, M. Lämmerhofer and K. Weisz, Chem. – Eur. J., 2015, 21, 13802–13811 CrossRef CAS PubMed.
- H. Bertrand, A. Granzhan, D. Monchaud, N. Saettel, R. Guillot, S. Clifford, A. Guédin, J. L. Mergny and M. P. Teulade-Fichou, Chem. – Eur. J., 2011, 17, 4529–4539 CrossRef CAS PubMed.
- H. Ihmels and L. Thomas, Org. Biomol. Chem., 2013, 11, 480–487 CAS.
- H. J. Karlsson, M. Eriksson, E. Perzon, B. Åkerman, P. Lincoln and G. Westman, Nucleic Acids Res., 2003, 31, 6227–6234 CrossRef CAS PubMed.
- T. Mahmood, A. Paul and S. Ladame, J. Org. Chem., 2009, 75, 204–207 CrossRef PubMed.
- D. Lin, X. Fei, Y. Gu, C. Wang, Y. Tang, R. Li and J. Zhou, Analyst, 2015, 140, 5772–5780 RSC.
- M.-H. Hu, R.-J. Guo, S.-B. Chen, Z.-S. Huang and J.-H. Tan, Dyes Pigm., 2017, 137, 191–199 CrossRef CAS.
- J.-W. Yan, S.-B. Chen, H.-Y. Liu, W.-J. Ye, T.-M. Ou, J.-H. Tan, D. Li, L.-Q. Gu and Z.-S. Huang, Chem. Commun., 2014, 50, 6927–6930 RSC.
- Y.-J. Lu, X.-L. Guo, M.-H. Xu, W.-W. Chen, W.-L. Wong, K. Zhang and C.-F. Chow, Dyes Pigm., 2017, 143, 331–341 CrossRef CAS.
- J.-H. Guo, L.-N. Zhu, D.-M. Kong and H.-X. Shen, Talanta, 2009, 80, 607–613 CrossRef CAS PubMed.
- H. Lai, Y. Xiao, S. Yan, F. Tian, C. Zhong, Y. Liu, X. Weng and X. Zhou, Analyst, 2014, 139, 1834–1838 RSC.
- M.-Q. Wang, W.-X. Zhu, Z.-Z. Song, S. Li and Y.-Z. Zhang, Bioorg. Med. Chem. Lett., 2015, 25, 5672–5676 CrossRef CAS PubMed.
- D. dos Santos Pisoni, C. L. Petzhold, M. P. de Abreu, F. S. Rodembusch and L. F. Campo, C. R. Chim., 2012, 15, 454–462 CrossRef.
- U. Mayerhöffer, M. Gsänger, M. Stolte, B. Fimmel and F. Würthner, Chem. – Eur. J., 2013, 19, 218–232 CrossRef PubMed.
- U. Mayerhöffer, B. Fimmel and F. Würthner, Angew. Chem., Int. Ed., 2012, 51, 164–167 CrossRef PubMed.
- B. Wang, J. Fan, S. Sun, L. Wang, B. Song and X. Peng, Dyes Pigm., 2010, 85, 43–50 CrossRef CAS.
- Y. Chen, S. Yan, L. Yuan, Y. Zhou, Y. Song, H. Xiao, X. Weng and X. Zhou, Org. Chem. Front., 2014, 1, 267–270 RSC.
- B. Jin, X. Zhang, W. Zheng, X. Liu, J. Zhou, N. Zhang, F. Wang and D. Shangguan, Anal. Chem., 2014, 86, 7063–7070 CrossRef CAS PubMed.
- X. Zhang, Y. Wei, T. Bing, X. Liu, N. Zhang, J. Wang, J. He, B. Jin and D. Shangguan, Sci. Rep., 2017, 7, 4766 CrossRef PubMed.
- V. Grande, F. Doria, M. Freccero and F. Würthner, Angew. Chem., Int. Ed., 2017, 56, 7520–7524 CrossRef CAS PubMed.
- F. Doria, A. Oppi, F. Manoli, S. Botti, N. Kandoth, V. Grande, I. Manet and M. Freccero, Chem. Commun., 2015, 51, 9105–9108 RSC.
- M. Zuffo, F. Doria, V. Spalluto, S. Ladame and M. Freccero, Chem. – Eur. J., 2015, 21, 17596–17600 CrossRef CAS PubMed.
- F. Doria, C. M. Gallati and M. Freccero, Org. Biomol. Chem., 2013, 11, 7838–7842 CAS.
- C. Röger and F. Würthner, J. Org. Chem., 2007, 72, 8070–8075 CrossRef PubMed.
- N. Sakai, J. Mareda, E. Vauthey and S. Matile, Chem. Commun., 2010, 46, 4225–4237 RSC.
- F. Würthner, S. Ahmed, C. Thalacker and T. Debaerdemaeker, Chem. – Eur. J., 2002, 8, 4742–4750 CrossRef.
- Y. V. Suseela, S. Das, S. K. Pati and T. Govindaraju, ChemBioChem, 2016, 17, 2162–2171 CrossRef CAS PubMed.
- F. Doria, I. Manet, V. Grande, S. Monti and M. Freccero, J. Org. Chem., 2013, 78, 8065–8073 CrossRef CAS PubMed.
- F. Doria, M. Nadai, M. Zuffo, R. Perrone, M. Freccero and S. N. Richter, Chem. Commun., 2017, 53, 2268–2271 RSC.
- R. Perrone, F. Doria, E. Butovskaya, I. Frasson, S. Botti, M. Scalabrin, S. Lago, V. Grande, M. Nadai, M. Freccero and S. N. Richter, J. Med. Chem., 2015, 58, 9639–9652 CrossRef CAS PubMed.
- L. Zhang, J. C. Er, K. K. Ghosh, W. J. Chung, J. Yoo, W. Xu, W. Zhao, A. T. Phan and Y.-T. Chang, Sci. Rep., 2014, 4, 3776 CrossRef PubMed.
- M. Vendrell, D. Zhai, J. C. Er and Y.-T. Chang, Chem. Rev., 2012, 112, 4391–4420 CrossRef CAS PubMed.
- G. Feng, C. Luo, H. Yi, L. Yuan, B. Lin, X. Luo, X. Hu, H. Wang, C. Lei, Z. Nie and S. Yao, Nucleic Acids Res., 2017, 45, 10380–10392 CrossRef PubMed.
- H. Yaku, T. Fujimoto, T. Murashima, D. Miyoshi and N. Sugimoto, Chem. Commun., 2012, 48, 6203–6216 RSC.
- J. M. Nicoludis, S. T. Miller, P. D. Jeffrey, S. P. Barrett, P. R. Rablen, T. J. Lawton and L. A. Yatsunyk, J. Am. Chem. Soc., 2012, 134, 20446–20456 CrossRef CAS PubMed.
- E. Boschi, S. Davis, S. Taylor, A. Butterworth, L. A. Chirayath, V. Purohit, L. K. Siegel, J. Buenaventura, A. H. Sheriff, R. Jin, R. Sheardy, L. A. Yatsunyk and M. Azam, J. Phys. Chem. B, 2016, 120, 12807–12819 CrossRef CAS PubMed.
- J. Ren and J. B. Chaires, Biochemistry, 1999, 38, 16067–16075 CrossRef CAS PubMed.
- H. Yaku, T. Murashima, D. Miyoshi and N. Sugimoto, Chem. Commun., 2010, 46, 5740–5742 RSC.
- D. P. Gonçalves, R. Rodriguez, S. Balasubramanian and J. K. Sanders, Chem. Commun., 2006, 4685–4687 RSC.
- H. Arthanari, S. Basu, T. L. Kawano and P. H. Bolton, Nucleic Acids Res., 1998, 26, 3724–3728 CrossRef CAS PubMed.
- L. Ren, A. Zhang, J. Huang, P. Wang, X. Weng, L. Zhang, F. Liang, Z. Tan and X. Zhou, ChemBioChem, 2007, 8, 775–780 CrossRef CAS PubMed.
- S. C. Karunakaran, P. S. S. Babu, B. Madhuri, B. Marydasan, A. K. Paul, A. S. Nair, K. S. Rao, A. Srinivasan, T. K. Chandrashekar, C. M. Rao, R. Pillai and D. Ramaiah, ACS Chem. Biol., 2013, 8, 127–132 CrossRef CAS PubMed.
- J. Alzeer, B. R. Vummidi, P. J. C. Roth and N. W. Luedtke, Angew. Chem., Int. Ed., 2009, 48, 9362–9365 CrossRef CAS PubMed.
- A. E. Friedman, J. C. Chambron, J. P. Sauvage, N. J. Turro and J. K. Barton, J. Am. Chem. Soc., 1990, 112, 4960–4962 CrossRef CAS.
- X. Li, A. K. Gorle, T. D. Ainsworth, K. Heimann, C. E. Woodward, J. Grant Collins and F. R. Keene, Dalton Trans., 2015, 44, 3594–3603 RSC.
- H. Huang, P. Zhang, B. Yu, Y. Chen, J. Wang, L. Ji and H. Chao, J. Med. Chem., 2014, 57, 8971–8983 CrossRef CAS PubMed.
- M. Matson, F. R. Svensson, B. Nordén and P. Lincoln, J. Phys. Chem. B, 2011, 115, 1706–1711 CrossRef CAS PubMed.
- M. R. Gill and J. A. Thomas, Chem. Soc. Rev., 2012, 41, 3179–3192 RSC.
- C. Rajput, R. Rutkaite, L. Swanson, I. Haq and J. A. Thomas, Chem. – Eur. J., 2006, 12, 4611–4619 CrossRef CAS PubMed.
- M. R. Gill, J. Garcia-Lara, S. J. Foster, C. Smythe, G. Battaglia and J. A. Thomas, Nat. Chem., 2009, 1, 662–667 CrossRef CAS PubMed.
- S. Millevoi, H. Moine and S. Vagner, Wiley Interdiscip. Rev.: RNA, 2012, 3, 495–507 CrossRef CAS PubMed.
- J. L. Huppert, A. Bugaut, S. Kumari and S. Balasubramanian, Nucleic Acids Res., 2008, 36, 6260–6268 CrossRef CAS PubMed.
- J.-D. Beaudoin and J.-P. Perreault, Nucleic Acids Res., 2010, 38, 7022–7036 CrossRef CAS PubMed.
- O. Kikin, Z. Zappala, L. D’Antonio and P. S. Bagga, Nucleic Acids Res., 2008, 36, D141–D148 CrossRef CAS PubMed.
- S. Xu, Q. Li, J. Xiang, Q. Yang, H. Sun, A. Guan, L. Wang, Y. Liu, L. Yu and Y. Shi, Nucleic Acids Res., 2015, gkv1040 CrossRef PubMed.
- P. Agarwala, S. Pandey and S. Maiti, Org. Biomol. Chem., 2015, 13, 5570–5585 CAS.
- L. Liu, Y. Shao, J. Peng, C. Huang, H. Liu and L. Zhang, Anal. Chem., 2014, 86, 1622–1631 CrossRef CAS PubMed.
- Y. Wang, Y. Hu, T. Wu, H. Liu, L. Zhang, X. Zhou and Y. Shao, Analyst, 2015, 140, 5169–5175 RSC.
- A. Laguerre, L. Stefan, M. Larrouy, D. Genest, J. Novotna, M. Pirrotta and D. Monchaud, J. Am. Chem. Soc., 2014, 136, 12406–12414 CrossRef CAS PubMed.
- A. Laguerre, K. Hukezalie, P. Winckler, F. Katranji, G. Chanteloup, M. Pirrotta, J.-M. Perrier-Cornet, J. M. Y. Wong and D. Monchaud, J. Am. Chem. Soc., 2015, 137, 8521–8525 CrossRef CAS PubMed.
- A. Laguerre, J. M. Wong and D. Monchaud, Sci. Rep., 2016, 6, 32141 CrossRef CAS PubMed.
- J. Rautio, H. Kumpulainen, T. Heimbach, R. Oliyai, D. Oh, T. Järvinen and J. Savolainen, Nat. Rev. Drug Discovery, 2008, 7, 255–270 CrossRef CAS PubMed.
- H.-M. Lee, D. R. Larson and D. S. Lawrence, ACS Chem. Biol., 2009, 4, 409–427 CrossRef CAS PubMed.
- A. Deiters, ChemBioChem, 2010, 11, 47–53 CrossRef CAS PubMed.
- M. I. Sánchez, J. Martínez-Costas, F. Gonzalez, M. A. Bermudez, M. E. Vazquez and J. L. Mascareñas, ACS Chem. Biol., 2012, 7, 1276–1280 CrossRef PubMed.
- M. I. Sánchez, C. Penas, M. E. Vázquez and J. L. Mascareñas, Chem. Sci., 2014, 5, 1901–1907 RSC.
- Q. Hu, M. Gao, G. Feng and B. Liu, Angew. Chem., Int. Ed., 2014, 53, 14225–14229 CrossRef CAS PubMed.
- R. Kumar, J. Han, H.-J. Lim, W. X. Ren, J.-Y. Lim, J.-H. Kim and J. S. Kim, J. Am. Chem. Soc., 2014, 136, 17836–17843 CrossRef CAS PubMed.
- E.-J. Kim, S. Bhuniya, H. Lee, H. M. Kim, C. Cheong, S. Maiti, K. S. Hong and J. S. Kim, J. Am. Chem. Soc., 2014, 136, 13888–13894 CrossRef CAS PubMed.
- N. Narayanaswamy, S. Narra, R. R. Nair, D. K. Saini, P. Kondaiah and T. Govindaraju, Chem. Sci., 2016, 7, 2832–2841 RSC.
- F. Hövelmann and O. Seitz, Acc. Chem. Res., 2016, 49, 714–723 CrossRef PubMed.
- H. Wu, S. C. Alexander, S. Jin and N. K. Devaraj, J. Am. Chem. Soc., 2016, 138, 11429–11432 CrossRef CAS PubMed.
- B. A. Armitage, Curr. Opin. Chem. Biol., 2011, 15, 806–812 CrossRef CAS PubMed.
- F. Hövelmann, I. Gaspar, J. Chamiolo, M. Kasper, J. Steffen, A. Ephrussi and O. Seitz, Chem. Sci., 2016, 7, 128–135 RSC.
- N. Kolevzon, D. Hashoul, S. Naik, A. Rubinstein and E. Yavin, Chem. Commun., 2016, 52, 2405–2407 RSC.
- K. Meguellati, G. Koripelly and S. Ladame, Angew. Chem., 2010, 122, 2798–2802 CrossRef.
- D. T. Jayaram, S. Ramos-Romero, B. H. Shankar, C. Garrido, N. Rubio, L. Sanchez-Cid, S. B. Gómez, J. Blanco and D. Ramaiah, ACS Chem. Biol., 2016, 11, 104–112 CrossRef CAS PubMed.
- F. H. Wilson, A. Hariri, A. Farhi, H. Zhao, K. F. Petersen, H. R. Toka, C. Nelson-Williams, K. M. Raja, M. Kashgarian and G. I. Shulman, Science, 2004, 306, 1190–1194 CrossRef CAS PubMed.
- V. K. Mootha, C. M. Lindgren, K.-F. Eriksson, A. Subramanian, S. Sihag, J. Lehar, P. Puigserver, E. Carlsson, M. Ridderstråle and E. Laurila, Nat. Genet., 2003, 34, 267–273 CrossRef CAS PubMed.
- K. F. Petersen, D. Befroy, S. Dufour, J. Dziura, C. Ariyan, D. L. Rothman, L. DiPietro, G. W. Cline and G. I. Shulman, Science, 2003, 300, 1140–1142 CrossRef CAS PubMed.
- A. K. Reeve, K. J. Krishnan and D. Turnbull, Ann. N. Y. Acad. Sci., 2008, 1147, 21–29 CrossRef CAS PubMed.
- T. Ozawa, Y. Natori, M. Sato and Y. Umezawa, Nat. Methods, 2007, 4, 413–419 CAS.
- M. C. Hammond-Kosack, B. Dobrinski, R. Lurz, K. Docherty and M. W. Kilpatrick, Nucleic Acids Res., 1992, 20, 231–236 CrossRef CAS PubMed.
- G. C. Kennedy, M. S. German and W. J. Rutter, Nat. Genet., 1995, 9, 293–298 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2018 |