Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Independent validation and regulatory agency approval for high rate algal ponds to treat wastewater from rural communities†
Howard J.
Fallowfield
 *a,
Paul
Young
*a,
Paul
Young
 a,
Michael J.
Taylor
a,
Neil
Buchanan‡
a,
Nancy
Cromar
a,
Alex
Keegan
b and
Paul
Monis
b
a,
Michael J.
Taylor
a,
Neil
Buchanan‡
a,
Nancy
Cromar
a,
Alex
Keegan
b and
Paul
Monis
b
aHealth & the Environment Group, College of Science and Engineering, Flinders University, Adelaide, South Australia. E-mail: howard.fallowfield@flinders.edu.au
bAustralian Water Quality Centre, PO Box 1751, Adelaide, 5001, South Australia
First published on 8th November 2017
Abstract
Despite the many recognised benefits, the application of high rate algal ponds (HRAP) to manage wastewater treatment in small communities has been limited. To be incorporated into the South Australian Community Wastewater Management Scheme (CWMS), new wastewater treatment systems are required to undergo validation and obtain regulatory approval from the South Australian Department of Health, Wastewater Management Group. A HRAP system at Kingston on Murray, South Australia, underwent validation to be incorporated into the CWMS. The process was consistent with the Australian National Guidelines which requires the demonstration of the log10 reduction values (LRV) for indicator organisms achieved by the wastewater treatment system. These were required to be measured twice weekly, over a 10 week period in below average solar radiation and temperature conditions, by an independent National Association of Testing Authorities accredited laboratory. The Australian Water Quality Centre was commissioned to assess the removal of Escherichia coli, F-RNA bacteriophage and aerobic spore-forming bacteria. Flinders University of South Australia concurrently monitored the removal of the same organisms and other standard wastewater parameters. While ASFB were shown to be unsuitable indicators of protozoa in natural pond systems, the system effectively removed E. coli and F-RNA bacteriophage with the treated effluent meeting the limits set by the guidelines for effluent reuse for non-food crop irrigation: a 5th percentile LRV of >1.0 for F-RNA bacteriophage and a median E. coli concentration of <4.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10E. coli MPN 100 mL−1. Based on these results two configurations of HRAP systems were approved to be incorporated into the CWMS.
log10E. coli MPN 100 mL−1. Based on these results two configurations of HRAP systems were approved to be incorporated into the CWMS.
Water impactHRAPs occupy less surface area and have lower capital costs than other pond systems. Communities lacking centralised sewage systems are often in water-scarce regions – shorter HRAP retention times and consequently reduced evaporation increases effluent volume for reuse. The validation of these systems by a regulatory agency legitimises them as alternatives to other pond systems, facilitating more wide-scale application of HRAPs. |
Introduction
In rural South Australian communities, treatment of wastewater is managed by Community Wastewater Management Schemes (CWMS) with the assistance of the Local Government Association of South Australia (LGA SA). As of 2016, 172 CWMS were operating in 45 district councils, treating wastewater from approximately 180![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 individuals or approximately 15% of the South Australian population. Ninety of these were waste stabilisation pond (WSP)-based systems, reflecting a preference for these systems. Drivers for this preference include the limited expertise available to manage, operate and maintain electro-mechanical wastewater treatment plants in these communities; and increasing awareness in rural communities of issues associated with energy supply, cost of operation and associated greenhouse gas emissions.
000 individuals or approximately 15% of the South Australian population. Ninety of these were waste stabilisation pond (WSP)-based systems, reflecting a preference for these systems. Drivers for this preference include the limited expertise available to manage, operate and maintain electro-mechanical wastewater treatment plants in these communities; and increasing awareness in rural communities of issues associated with energy supply, cost of operation and associated greenhouse gas emissions.
In CWMS, the first stage of treatment is performed in on-site septic tanks where the bulk solid portion of the waste is settled out and undergoes anaerobic digestion. The treated liquid phase is then reticulated to a centralised WSP system for further treatment before disposal or beneficial reuse. The recommended WSP system configuration comprises five cells, each with a recommended depth of 1.2 m. The first WSP is a facultative pond, required to have a theoretical hydraulic retention time (THRT) of 36 d, while the remaining four are maturation ponds, operated in series, each having a THRT of 7.5 d. This equates to a recommended total THRT of 66 d for CWMS WSP systems.
In 2009, the Health and Environment Group at Flinders University of South Australia (FUSA) commissioned the construction of a high rate algal pond (HRAP) system for research on the treatment of wastewater at the Kingston on Murray CWMS. The initial aims of the project were: to compare the treatment performance of a CWMS WSP system with the HRAP at Kingston on Murray; determine the optimum operating conditions to maximise HRAP performance, and to provide criteria for HRAP design and operation in South Australia. This research showed that, in comparison to a CWMS WSP operated at Lyndoch, South Australia, the HRAP at Kingston on Murray achieved Escherichia coli die-off rates and 5-day biochemical oxygen demand (BOD5) removal rates 4 to 6 times higher and ammonia removal rates 8 to 17 times higher with at least 50% less evaporative losses.1,2 This reduction in treatment time reduces area requirement and consequently construction costs, while the reduced evaporative loss means more water is available for beneficial reuse in water-scare regions, such as, rural Australia.3
After establishing the many benefits HRAPs provide over WSPs, approval for HRAPs to be included as an alternative treatment option to WSPs in the CWMS design guidelines was sought from the South Australian Department of Health, Wastewater Management Group (DoHWMG). The validation process required for approval is consistent with the Australian National Guidelines for Water Recycling: Managing Health and Environmental Risks (Phase 1),4 which employ the concept of disability-adjusted life years (DALYs) with the tolerable risk accepted as 10−6 DALYs per capita per year, equivalent to an annual risk of diarrhoeal illness of 1 per 1000 people. The public health risk associated with exposure to waterborne pathogens in treated wastewaters intended for disposal or reuse are managed by health-based performance targets derived from the guidelines to ensure the tolerable risk is not exceeded. The initial concentration of the organisms in the wastewater, data relating to their passage through components of the wastewater treatment train, the frequency of exposure and likely ingestion volume associated with the reuse water are considered in the derivation of the target log10 reduction values (LRV) of indicators for bacterial, viral and protozoan pathogens. The treated wastewater from CWMS is most commonly used to irrigate non-food crops, typically woodlots. The target LRVs for this reuse application for enteric organisms are 5.0 for viruses, 4.0 for bacteria and 3.5 for protozoa, with an additional treated wastewater quality objective of a median concentration of <4.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10E. coli 100 mL−1 (NRMMC, 2006). A minimum 5th percentile of 1.0
log10E. coli 100 mL−1 (NRMMC, 2006). A minimum 5th percentile of 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10 reduction of viruses is required following treatment since on-site controls can contribute further to exposure reduction. E. coli and F-RNA bacteriophage were used as indicators for pathogenic bacteria and viruses as recommended by the guidelines.4 Following consultation with DoHWMG, aerobic spore-forming bacteria (ASFB) were chosen as indicators for pathogenic protozoa.
log10 reduction of viruses is required following treatment since on-site controls can contribute further to exposure reduction. E. coli and F-RNA bacteriophage were used as indicators for pathogenic bacteria and viruses as recommended by the guidelines.4 Following consultation with DoHWMG, aerobic spore-forming bacteria (ASFB) were chosen as indicators for pathogenic protozoa.
The validation took place between 1 August and 10 October 2013. It was required to be carried out in below average solar radiation and temperature conditions with twenty inlet and twenty outlet samples taken over 10 weeks, with the 5th percentiles of the LRVs used as the performance values for the validation. This sampling strategy is employed to reflect the worst-case scenario when determining system performance. It was also a requirement for validation that sample collection and microbiological analysis be conducted by a National Association of Testing Authorities (NATA) accredited laboratory. Consequently, the Australian Water Quality Centre (AWQC), South Australian Water Corporation was engaged by the LGA SA to undertake this analysis. This involved the manual collection of inlet and outlet samples over the ten week period, followed by laboratory analysis of the samples within 24 hours of collection. Concurrently during the validation, FUSA employed an auto-sampler to collect composite treated wastewater samples, which were stored at 1 °C before retrieval and microbiological analysis similar to that conducted by the AWQC. The required validation of wastewater treatment systems in rural and remote communities is logistically difficult and expensive. Uniquely, this validation enabled comparison and evaluation of two different sampling strategies, daily ‘grab’ sampling versus composite daily sampling and refrigerated storage.
To the authors' knowledge, this is the first validation and approval of a HRAP system by a regulatory agency in Australia or elsewhere. This paper details the methodology and results of the validation process for the HRAP at Kingston on Murray for inclusion in the CWMS design guidelines by DoHWMG in 2016.
Material and methods
Wastewater treatment plant site
The HRAP system was operated by FUSA. It consisted of two HRAPs operated in series at the Kingston on Murray wastewater treatment site (34.242816° S, 140.330197° E). The HRAPs were both single loop, HDPE sheet lined raceways, each 30 m long with a single channel width of 2.5 m. Within the HRAPs, wastewater was circulated at a mean surface velocity of 0.2 m s−1 by an 8 blade, stainless steel paddlewheel. Over the course of the validation, both HRAPs were operated at a depth of 0.30 m, with a surface area of 200 m2 and a THRT of 5 days.The first HRAP in the series (HRAP1) received septic tank-treated domestic wastewater produced by the South Australian rural town Kingston on Murray. The town had a population of approximately 300 permanent residents, with the usual variety of commercial activities associated with a small rural Australian town, as well as a school and a seasonal backpacker hostel. Wastewater depth within HRAP1 was controlled by a calibrated ultrasonic depth sensor (U-Gage, Banner Engineering Corp., Minneapolis) activating a submersed pump which transferred the wastewater from HRAP1 into the second HRAP in the series (HRAP2). The treated effluent from HRAP2 was pumped, again under ultrasonic depth control, to the storage pond before discharge via an irrigation system.
Wastewater inflow into HRAP1 was monitored via Mag-Flow meters (ABB Ltd, Zurich, Switzerland) installed on both the HRAP inlet and outlet pipes. Over that period the average daily inflow was 12.13 m3 d−1, with a minimum of 6.8 m3 d−1 and a maximum of 18.9 m3 d−1. The observed variation in the daily flows was due to the fluctuations in the population of the township and was not subject to a regular, predictable pattern. The mean daily flow was 12 m3, consistent with the long-term average. The daily inflow comes from a central pumping station in the township, which is activated and deactivated by float switches. The height between the activating and deactivating float switches was set so that each pumping consisted of approximately 2000 L delivered over 20 minutes (100 L min−1). Theoretically, the pump was set to activate 6 times per day. In practice, the pump was activated in clusters, typically 2 pump activations in the morning, another in the early afternoon, 2 more activations in the evening and a final activation just after midnight.
Sampling strategy
Sampling was carried out between 1 August and 10 October 2013 during below average solar radiation and temperature conditions. Grab samples from both HRAPs and the inlet were collected on Monday and Thursday of each week at approximately 7 am and shipped immediately on ice by road freight to Adelaide for analysis. Samples were processed within 5–8 hours of collection by the AWQC laboratories. FUSA collected samples over the same period as the AWQC. Effluent samples from the two HRAPs were collected twice daily, at 3 am and 3 pm, by a refrigerated (1 °C) auto-sampler, (Avalanche® Sampler, Teledyne ISCO Lincoln, NE). The two samples collected each day formed a daily composite sample (1 L). The results for these samples were considered an average over the day. The median sample storage time in the auto-sampler at 1 °C was 12.5 d (range 8–14 d). To obtain a fresh sample of wastewater entering the pond, during every visit to retrieve the samples taken by the auto-sampler, a single wastewater grab sample (1 L) was taken from the inlet when the septic tank effluent was pumped from the transfer station into the pond. After the samples had been retrieved they were transported, while being refrigerated at 1 °C in the dark, and analysed within 24 h.Microbiological analysis
FUSA quantified E. coli for each sample using a single Colilert Quanti-Tray® (IDEXX Laboratories, Inc. Westbrook, ME) according to the manufacturer's instructions. The values were reported as E. coli Most Probable Number (MPN) 100 mL−1.
F-RNA bacteriophage quantification was carried out at FUSA using a double layer agar plaque assay method.7,8 Duplicate 5 mL aliquots were used for each HRAP sample. 1 mL of each inlet sample was diluted in 9 mL of tryptone water (Oxoid Ltd), which was divided into 5 mL aliquots both of which were enumerated.
To enumerate ASFB, FUSA used the filtration and pasteurisation method described in Young, Buchanan,9 which was adapted from Rice, Fox.10
Wastewater analysis
Daily minimum air temperature (°C) and maximum air temperature (°C) over the validation period were collected from the weather station at Renmark Aerodrome, SA (34.20° S, 140.68° E) (Bureau of Meteorology). This weather station was ∼34 km away from the HRAP system.
The data from the inlet and HRAP samples collected on the same day were used for the calculation of the LRV for the respective day. The calculation of LRVs for HRAP1 using FUSA data required a different approach, as this data set did not have an inlet sample collected on the same day of each composite HRAP sample collected by auto-sampler. To calculate LRVs for each HRAP1 sample, the inlet sample that was collected on the date closest to the sample was used.
Statistical analyses
Statistical analysis and graphical preparation were carried out using Analyse-it for Microsoft Excel (version 2.20; Analyse-it Software, Ltd, http://www.analyse-it.com/, 2009); R statistical software13 with the additional packages rcmdr14 and ggplot2 (ref. 15); and IBM SPSS Statistics 23.16Microbiological results from each laboratory were statistically compared where the sampling regimes aligned. All data sets were tested for normality using Shapiro–Wilk test for normality (ESI† S1). Data sets found to be normally distributed were analysed using independent-samples t-test for equality of means while those found to violate normality were compared using independent-samples Mann–Whitney U test. Significance was tested to the 0.05 level for all statistical comparisons.
Results and discussion
Prevailing weather conditions during the validation period
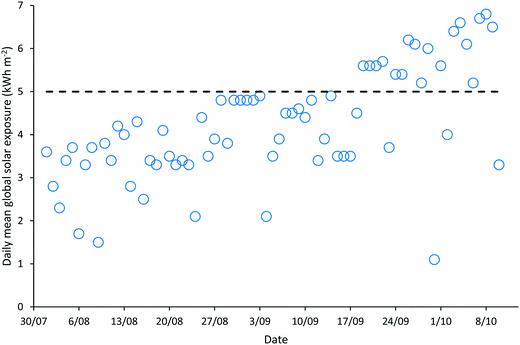
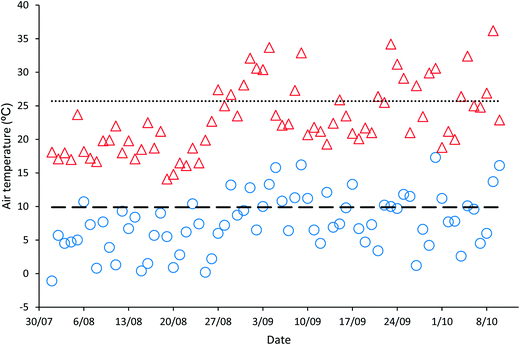
‘Natural’ wastewater treatment systems, which are largely dependent upon prevailing weather conditions for their effectiveness, are required to be validated when solar irradiance and temperature, the main contributors to pathogen inactivation and algal growth in HRAPs, are low.17–22 The validation of the HRAP at Kingston on Murray was conducted over 10 weeks in the winter and spring of 2013. During the 10 weeks of validation, the daily mean global solar exposure, 4.23 ± 1.29 kW h m−2, was 15.4% less than the 2013 daily annual mean of 5.00 kW h m−2, although it increased towards the end of the validation period (Fig. 1). The mean daily minimum air temperature during the validation was 7.61 ± 4.19 °C, 23.13% lower than the annual mean minimum air temperature, 9.9 °C, recorded for 2013 (Fig. 2). Similarly, the mean daily maximum air temperature, 23.11 ± 5.22 °C, during the validation was 10.08% less than the annual mean daily maximum air temperature of 25.7 °C.Wastewater characteristics during the validation period
The BOD5, suspended solids and turbidity of the wastewater within HRAPs 1 and 2 during the validation period was typical of that associated with HRAPs treating domestic wastewater. The mean inlet BOD5 concentration to HRAP1 from the Kingston on Murray septic tanks was 180.83 ± 72.55 mg BOD5 L−1 (Table 1). Following treatment in HRAP1, the mean BOD5 concentration was reduced by 90.6% to 16.95 ± 14.06 mg BOD5 L−1, which was then the inlet concentration to HRAP2. The mean BOD5 removal from the inlet wastewater following treatment in HRAP1 was consistent with that reported for longitudinal studies on the same pond, 91.76% and 93.4%,1,23 and similar to removal rates reported for other HRAPs treating domestic wastewater.3 Following treatment in HRAP2, the mean BOD5 concentration at the outlet of HRAP2 was 23.85 ± 10.92 mg BOD5 L−1, higher than the HRAP2 inlet water supplied from HRAP1 and independent-samples Mann–Whitney U test showed that this difference was statistically significant (p = 0.007; n = 40). The most likely reason for the increased BOD5 concentration in HRAP2 is that the ageing biomass in the pond was degrading and releasing extracellular material into suspension, increasing the organic matter concentration. The median filtered BOD5 concentration over the 10 week period for HRAPs 1 and 2 compared favourably with the acceptable annual median guideline value of 20 mg BOD5 L−1.4| 5-day biochemical oxygen demand (mg BOD5 L−1) | Suspended solids (mg L−1) | Turbidity (NTU) | Chlorophyll a (mg L−1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Inlet | HRAP1 | HRAP2 | Inlet | HRAP1 | HRAP2 | Inlet | HRAP1 | HRAP2 | HRAP1 | HRAP2 | |
| Mean | 180.83 | 16.95 | 23.85 | 56.67 | 141.65 | 119.58 | 83.67 | 185.28 | 161.54 | 1.99 | 1.56 |
| Standard deviation | 72.55 | 14.06 | 10.92 | 14.17 | 59.80 | 42.94 | 22.37 | 60.47 | 53.08 | 1.25 | 0.86 |
| Median | 205.5 | 11 | 19.5 | 61 | 134 | 125 | 94 | 165 | 163 | 1.35 | 1.47 |
| n | 6 | 20 | 20 | 6 | 69 | 69 | 3 | 69 | 69 | 69 | 69 |
The suspended solids concentrations in the HRAPs were slightly less than those reported for HRAPs treating domestic wastewater.24–26 The mean suspended solids (mg L−1) concentration of the inlet wastewater to HRAP1 was 56.67 ± 14.17 mg L−1, and biomass production in HRAP1 increased this three-fold to 141.65 ± 59.80 mg L−1 (Table 1). The suspended solids decreased slightly in HRAP2 to 119.58 ± 42.94 mg L−1, providing supporting evidence that the ageing biomass was degrading.
The mean chlorophyll a concentrations of the HRAP wastewaters, a surrogate measure of algal biomass, were similar in the HRAPs: 1.99 ± 1.25 mg L−1 in HRAP1 and 1.56 ± 0.86 mg L−1 in HRAP2 (Table 1). The lower chlorophyll a concentration in HRAP2 adds additional supporting evidence that the ageing biomass was degrading. These chlorophyll a concentrations were comparable to those reported for other HRAPs treating domestic wastewater.24–26
The mean turbidity of the wastewater in HRAP1, 185.28 ± 60.47 NTU, and HRAP2, 161.54 ± 53.08 NTU, was double that of the original inlet wastewater from septic tanks: 83.67 ± 22.37 NTU (Table 1). This increased turbidity from the inlet to the HRAPs was most likely caused by the algal biomass growing in the ponds.
Microbiological validation of HRAP performance
| E. coli log10 reduction values | F-RNA bacteriophage log10 reduction values | Aerobic spore-forming bacteria log10 reduction values | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HRAP1 | HRAP2 | In series | HRAP1 | HRAP2 | In series | HRAP1 | HRAP2 | In series | |
| Mean | 1.81 | 1.49 | 3.30 | 1.17 | 1.16 | 2.32 | 0.18 | −0.24 | −0.05 |
| Standard deviation | 0.46 | 1.21 | 1.28 | 0.38 | 0.73 | 0.74 | 0.47 | 0.29 | 0.37 |
| Median | 1.76 | 0.93 | 2.90 | 1.30 | 0.88 | 2.08 | 0.04 | −0.15 | −0.20 |
| 5th percentile | 1.24 | 0.37 | 1.82 | 0.62 | 0.35 | 1.61 | −0.30 | −0.52 | −0.40 |
| n | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 |
| E. coli log10 reduction values | F-RNA bacteriophage log10 reduction values | Aerobic spore-forming bacteria log10 reduction values | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HRAP1 | HRAP2 | In series | HRAP1 | HRAP2 | In series | HRAP1 | HRAP2 | In series | |
| Mean | 2.00 | 0.88 | 2.89 | 2.25 | 0.63 | 2.87 | 0.07 | 0.24 | 0.31 |
| Standard deviation | 0.58 | 0.52 | 0.75 | 0.64 | 0.72 | 0.89 | 0.31 | 0.24 | 0.35 |
| Median | 1.91 | 0.86 | 2.61 | 2.15 | 0.42 | 2.83 | 0.02 | 0.24 | 0.32 |
| 5th percentile | 1.22 | 0.13 | 2.00 | 1.37 | −0.23 | 1.50 | −0.34 | −0.01 | −0.27 |
| n | 42 | 42 | 42 | 67 | 67 | 68 | 57 | 57 | 57 |
The mean E. coli LRVs for HRAP1 determined for both AWQC and FUSA were similar to those reported for other HRAPs.27,28 Notably, the mean E. coli LRVs for HRAP1 operated at a 5 d THRT were similar to the 2.02 ± 0.65 LRV reported for the facultative WSP operated at a 27.5 d THRT at the CWMS at Lyndoch, South Australia.1
The median concentration of E. coli in the effluent following treatment in the HRAPs with a combined THRT of 10 d was measured at 3.13 log10E. coli MPN 100 mL−1 by AWQC and 3.30 log10E. coli MPN 100 mL−1 by FUSA (Table 6).
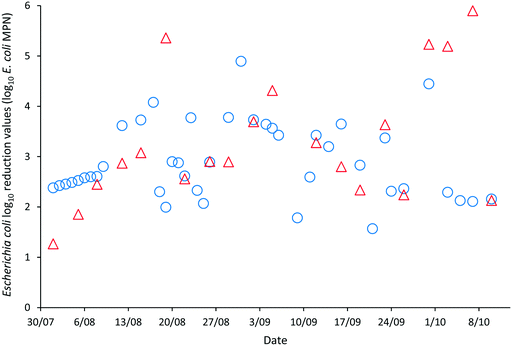
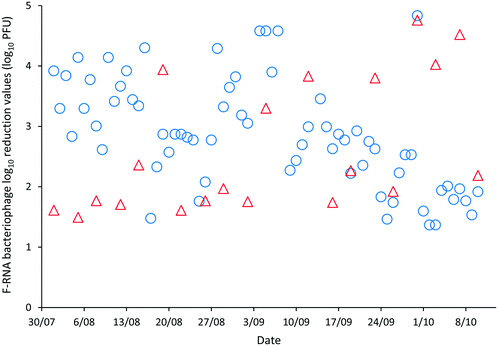
The LRVs for F-RNA bacteriophage ranged between 1.61–4.76 as determined by AWQC and 1.13–5.04 as determined by FUSA (Fig. 4). The F-RNA bacteriophage mean LRVs followed a similar pattern to those determined for E. coli with the AWQC derived values for the mean LRV higher for HRAP1 and lower for HRAP2 than those obtained by FUSA. The mean LRVs for F-RNA bacteriophage for the HRAPs operated in series measured by AWQC over the validation period was 2.32 ± 0.74 (Table 2) compared with 2.87 ± 0.89 determined by FUSA (Table 3). The 5th percentile LRVs for F-RNA bacteriophage were 1.61 and 1.50 as determined by AWQC and FUSA respectively.
AWQC and FUSA data both showed that the HRAPs consistently inactivated F-RNA bacteriophage over the validation period. There are no data available in the literature for F-RNA bacteriophage inactivation by other HRAPs. Davies-Colley, Craggs28 reported approximately a 1 LRV for somatic phage by a HRAP treating domestic wastewater during summer. An F-RNA bacteriophage LRV of 1.3 has been reported for facultative WSPs with THRT of 18 d (ref. 29) which compares with the mean 1.17 and 2.25 LRVs determined by AWQC and FUSA for HRAP1 with a 5 d THRT. The mean F-RNA bacteriophage LRVs for HRAP2 were less than the annual mean LRV of 1.72 reported for a pilot maturation WSP;30 however, the LRV for the WSP reduced to 0.42 when considering only the winter data, less than the LRVs reported for the HRAP.30
Overall, the HRAPs showed inactivation of E. coli and F-RNA bacteriophage equivalent to those reported for WSPs. However, the inactivation rates were achieved using considerably shorter THRTs than those commonly employed for WSPs. The shorter THRTs reduce both the area requirement and the cost of construction for HRAPs compared to WSPs typically employed in CWMS in rural South Australia.
ASFB were shown to be unsuitable indicators for protozoa in open systems as analysis by both laboratories frequently showed higher concentrations of ASFB in the HRAP's treated effluent than was entering in the influent from septic tanks. Young, Buchanan9 proposed the likely causes of increased ASFB in the HRAP effluent were ASFB being transported into the HRAPs by wind-blown soil and/or by propagation of influent spores in the HRAPs triggered by increases in temperature. It was concluded that ASFB were an unsuitable indicator for Cryptosporidium spp. and other protozoa in natural pond systems and E. coli should be used as an indicator in HRAPs.23
The influence of environmental parameters on the LRVs achieved in HRAP1 is explored in more detail in Inactivation of indicator organisms in wastewater treated by a high rate algal pond system.9 This publication details a longitudinal study on HRAP1 disinfection carried out by FUSA between July 2013 to May 2014, of which some of the data presented here is a component. Data presented in both publications includes E. coli, F-RNA bacteriophage, ASFB, BOD5, chlorophyll a concentrations in the inlet and HRAP1 as well the LRVs achieved by HRAP1 for all indicator organisms.
AWQC's independent validation data for the HRAP system showed the treated effluent met the limits set by the NRMMC4 guidelines for effluent reuse for non-food crop irrigation with a winter 5th percentile LRV of >1.0 for F-RNA bacteriophage and a median E. coli concentration of <4.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10E. coli MPN 100 mL−1. Based on these disinfection results, in 2016, DoHWMG approved a HRAP based system comprising of a single HRAP receiving septic tank effluent operated at depths between 0.3–0.5 m at a 10 d THRT to be an alternative to installing the standard 5 cell 1.2 m deep WSP system with a 66 d THRT when new systems are required. Additionally, based on these results and those in Buchanan,1 the DoHWMG approved a second configuration of a HRAP based system, one which would replace existing facultative WSPs in need of upgrade with a single HRAP operated at a depth between 0.3–0.5 m at a 5 d THRT, while retaining the traditional in series, 4 cell (30 d THRT) maturation WSPs. The removal of helminths was not considered in the validation since they are not endemic in most parts of Australia. In areas where helminths infections are prevalent, a minimum 25 d total treatment time is required based NRMMC.4 As such, the configuration approved by DoHWMG to ensure helminth die-off was a 10 d THRT in a HRAP with an additional 15 d THRT in a storage lagoon before discharge or reuse. These design guidelines were published in Design Guideline for a High Rate Algal Pond (HRAP) – as an Element in Wastewater Treatment Trains.
log10E. coli MPN 100 mL−1. Based on these disinfection results, in 2016, DoHWMG approved a HRAP based system comprising of a single HRAP receiving septic tank effluent operated at depths between 0.3–0.5 m at a 10 d THRT to be an alternative to installing the standard 5 cell 1.2 m deep WSP system with a 66 d THRT when new systems are required. Additionally, based on these results and those in Buchanan,1 the DoHWMG approved a second configuration of a HRAP based system, one which would replace existing facultative WSPs in need of upgrade with a single HRAP operated at a depth between 0.3–0.5 m at a 5 d THRT, while retaining the traditional in series, 4 cell (30 d THRT) maturation WSPs. The removal of helminths was not considered in the validation since they are not endemic in most parts of Australia. In areas where helminths infections are prevalent, a minimum 25 d total treatment time is required based NRMMC.4 As such, the configuration approved by DoHWMG to ensure helminth die-off was a 10 d THRT in a HRAP with an additional 15 d THRT in a storage lagoon before discharge or reuse. These design guidelines were published in Design Guideline for a High Rate Algal Pond (HRAP) – as an Element in Wastewater Treatment Trains.
Comparison between grab and refrigerated auto-sampler sampling methods
This study also enabled comparison between two methods of sampling and subsequent analysis. The mean E. coli inlet concentrations measured by the two laboratories were similar with the AWQC reporting a value of 6.19 ± 0.31 log10E. coli MPN 100 mL−1 and FUSA reporting a mean of 6.16 ± 0.39 log10E. coli MPN 100 mL−1 (Table 4). The same value, 5.05 ± 0.50 log10 PFU 100 mL−1, for mean inlet F-RNA bacteriophage concentration was obtained by both AWQC and FUSA, although the median values differed (Table 4). Independent-samples t-test for equality of means was performed between the results obtained by each laboratory for both organisms found that for both E. coli (p = 0.97; n = 12) and FRNA bacteriophage (p = 0.65; n = 12) there was no statistically significant difference between the results. As both laboratories employed grab sampling for the inlet, the results of the analysis suggest that the analytical methods used by both laboratories for enumeration of these organisms in wastewater were equivalent.| Escherichia coli concentration (log10E. coli MPN 100 mL−1) | F-RNA bacteriophage concentration (log10 PFU 100 mL−1) | |||
|---|---|---|---|---|
| AWQC | FUSA | AWQC | FUSA | |
| Mean | 6.19 | 6.16 | 5.05 | 5.05 |
| Standard deviation | 0.31 | 0.39 | 0.50 | 0.50 |
| Median | 6.11 | 6.07 | 4.95 | 4.81 |
| n | 20 | 6 | 20 | 6 |
Independent-samples t-test for equality of means analysis also showed there was no statistically significant difference between the mean HRAP2 concentrations of E. coli as determined by AWQC using grab sampling and FUSA using composite sampling (Table 6). The AWQC mean concentration value for E. coli was 2.89 ± 1.19 log10E. coli MPN 100 mL−1 and the FUSA mean was 3.17 ± 0.72 log10E. coli MPN 100 mL−1 (p = 0.51; n = 40). Independent-samples Mann–Whitney U test indicated there was no statistically significant difference between the mean F-RNA bacteriophage concentration in HRAP2 determined by the each of the laboratories (Table 6). The F-RNA mean concentration for HRAP2 determined by AWQC was 2.43 ± 1.06 log10 PFU 100 mL−1 and the mean concentration determined by FUSA was 2.11 ± 0.92 log10 PFU 100 mL−1 (p = 0.19; n = 40). Considering the result of the statistical analysis for the inlet samples, the result of the statistical analysis of the HRAP2 samples, which only differed in methodology by FUSA collecting samples by refrigerated auto-sampler, suggests that the different sampling strategies employed did not produce results for the enumeration of either organisms which were statistically significantly different.
Contrasting with the previous results, an independent-samples Mann–Whitney U test indicated there was a statistically significant difference between the results obtained by each laboratory for mean concentration F-RNA bacteriophage in HRAP1 determined by AWQC using grab sampling and FUSA using refrigerated, composite sampling (Table 5). The mean F-RNA bacteriophage concentration determined by AWQC was 3.88 ± 0.50 log10 PFU 100 mL−1, and the mean determined by FUSA was 2.74 ± 0.63 log10 PFU 100 mL−1 (p < 0.001; n = 40). It is unclear why the result from this statistical analysis differs from the previous results given that all sampling was carried out at the same time, the same sampling strategies were employed, and the same enumeration methods were used for both HRAP1 and HRAP2. Without understanding the cause for this difference, it is difficult to construe the significance, if any, of this result. Independent-samples t-test for equality of means analysis suggested there was no statistically significant difference between the E. coli concentrations measured by both laboratories in HRAP2. The E. coli mean concentration determined by AWQC was 4.38 ± 0.41 log10E. coli MPN 100 mL−1 and the FUSA mean was 4.05 ± 0.54 log10E. coli MPN 100 mL−1 (p = 0.07; n = 40). This result provides additional support that the different sampling strategies employed by each laboratory did not affect the microbiological analysis.
| Escherichia coli concentration (log10E. coli MPN 100 mL−1) | F-RNA bacteriophage concentration (log10 PFU 100 mL−1) | |||
|---|---|---|---|---|
| AWQC | FUSA | AWQC | FUSA | |
| Mean | 4.38 | 4.05 | 3.88 | 2.74 |
| Standard deviation | 0.41 | 0.54 | 0.5 | 0.63 |
| Median | 4.25 | 3.95 | 3.91 | 2.78 |
| n | 20 | 42 | 20 | 67 |
There have been few studies on the dark die-off of E. coli in wastewater stored for the length of time utilised during this validation. Mayer, Vierheilig31 measured dark die-off of E. coli in wastewater stored in a refrigerated auto-sampler at 5 °C. They reported a dark die-off of approximately 0.8 log10E. coli MPN 100 mL−1 over 11 d: similar to the mean time, 11.83 d, the samples were left in the auto-sampler before collection during the validation.31 This result is supported by Buchanan 2014 who measured the dark die-off of E. coli in wastewater stored at 2.5 °C in the laboratory to be approximately 0.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log10E. coli MPN 100 mL−1 at 11 d. The significance of these results to what was happening to the organisms in the refrigerated auto-samplers during the validation is unclear particularly when considering the lower storage temperature used in the validation, 1 °C, and the values for the dark die-off of E. coli being similar to the standard deviation of the mean concentrations of E. coli measured in both HRAPs by each laboratory (Tables 5 and 6).
log10E. coli MPN 100 mL−1 at 11 d. The significance of these results to what was happening to the organisms in the refrigerated auto-samplers during the validation is unclear particularly when considering the lower storage temperature used in the validation, 1 °C, and the values for the dark die-off of E. coli being similar to the standard deviation of the mean concentrations of E. coli measured in both HRAPs by each laboratory (Tables 5 and 6).
| Escherichia coli concentration (log10E. coli MPN 100 mL−1) | F-RNA bacteriophage concentration (log10 PFU 100 mL−1) | |||
|---|---|---|---|---|
| AWQC | FUSA | AWQC | FUSA | |
| Mean | 2.89 | 3.17 | 2.43 | 2.11 |
| Standard deviation | 1.19 | 0.72 | 1.06 | 0.92 |
| Median | 3.13 | 3.30 | 2.75 | 2.04 |
| n | 20 | 42 | 20 | 68 |
As the regulator validates new wastewater treatment systems based on final LRVs, the most important result from the statistical analyses was that there was no statistically significant difference between the final LRVs determined by each laboratory for E. coli using independent-samples t-test for equality of means (p = 0.37; n = 40) and F-RNA bacteriophage using independent-samples Mann–Whitney U test (p = 0.20; n = 40).
Validation of wastewater treatment systems in rural and remote communities is a challenging and expensive process. The Kingston on Murray HRAP system was a 500 km round trip from Adelaide, the location of both analytical laboratories. Personnel were required on-site to conduct manual ‘grab’ sampling twice per week over a 10 week period and to arrange transport on ice to AWQC to enable analysis to be conducted within 24 h of sampling. The use of refrigerated (1 °C) auto-samplers to collect and store the samples before retrieval was an alternate approach which may significantly reduce both the cost and logistical complexity associated with the validation of treatment plants in remote locations. Furthermore, application of refrigerated auto-samplers enables samples to be taken more frequently, resulting in a larger dataset for the validation. Further research is required to elucidate the behaviour of organisms stored in dark refrigerated auto-samplers for extended periods, but considering the results of this study, the employment of refrigerated, portable auto-samplers should be considered an economical option for validation of rural wastewater treatment systems.
Conclusions
To the authors' knowledge, this is the first time the independent validation of a HRAP has been accepted by a regulatory agency. The results from the validation provide robust evidence that HRAPs are an effective alternate treatment option to other conventional natural pond systems, such as WSPs. The results also demonstrated the HRAP treated effluent met the Australian reuse guideline requirements for irrigation of non-food crops. Consequently, HRAPs were approved to be incorporated into the South Australian CWMS as an alternative option to the conventional WSP systems currently used. The comparison between the AWQC and FUSA methodology suggests that refrigerated auto-samplers may present a simpler and cheaper method for monitoring remote wastewater treatment systems.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are grateful to the Local Government Association of South Australia for providing financial assistance to both the validation program and the operation of the HRAPs, Loxton Waikerie District Council for support and access to the Kingston on Murray wastewater treatment facility, Raj Indela, Neil Lian and Amy Hawley, Flinders University for technical support and Richard Gayler, Gayler Professional Services, for project management services. Dr. David Cunliffe, Tony Farror and Michelle Wittholz Department of Health and Ageing, South Australia for guidance on the application of the national recycling guidelines.References
- A. N. Buchanan, Comparing the performance of a High Rate Algal Pond with a Waste Stabilisation Pond in rural South Australia, Flinders University, 2014 Search PubMed.
- N. Buchanan, N. Cromar, N. Bolton and H. Fallowfield, Comparison of a High Rate Algal Pond with a ‘standard'secondary facultative Waste Stabilisation Pond in rural South Australia, International Water Association Small Sustainable Solutions for Water Conference; 18th – 22nd April, 2011; Venice, Italy, 2011 Search PubMed.
- P. Young, M. Taylor and H. Fallowfield, Mini-review: high rate algal ponds, flexible systems for sustainable wastewater treatment, World J. Microbiol. Biotechnol., 2017, 33(6), 117 CrossRef CAS PubMed.
- NRMMC E, (2006) Australian Guidelines for Water Recycling: Managing Health and Environmental Risks (Phase 1), ed. AHMC, Natural Resource Management Ministerial Council, Environment Protection and Heritage Council, Australian Health Ministers' Conference, Australia, 2006 Search PubMed.
- Standards Australia, AS 4276.21-2005 Method 21: Examination for coliforms and Escherichia coli—Determination of most probable number (MPN) using enzyme hydrolysable substrates, Water microbiology, Sydney, NSW, Australia, 2005 Search PubMed.
- USEPA USEPA, in UV Disinfection Guidance Manual, ed. Agency EP, Washington, D.C., 2006 Search PubMed.
- R. T. Noble, I. M. Lee and K. C. Schiff, Inactivation of indicator micro-organisms from various sources of faecal contamination in seawater and freshwater, J. Appl. Microbiol., 2004, 96(3), 464–472 CrossRef CAS PubMed.
- J. Debartolomeis and V. J. Cabelli, Evaluation of an Escherichia coli host strain for enumeration of F male-specific bacteriophages, Appl. Environ. Microbiol., 1991, 57(5), 1301–1305 CAS.
- P. Young, N. Buchanan and H. Fallowfield, Inactivation of indicator organisms in wastewater treated by a high rate algal pond system, J. Appl. Microbiol., 2016, 121(2), 577–586 CrossRef CAS PubMed.
- E. W. Rice, K. R. Fox, R. J. Miltner, D. A. Lytle and C. H. Johnson, Evaluating plant performance with endospores, J. - Am. Water Works Assoc., 1996, 88(9), 122–130 CAS.
- WTW, Operating manual: system OxiTop® OxiTop® OC100 controller; OxiTop®-C measuring heads, 2006, Available from: http://www.globalw.com/downloads/WQ/oxitop_oc_100.pdf Search PubMed.
- Standard Methods for the Examination of Water and Wastewater, ed. A. E. Greenberg, L. S. Clesceri and A. D. Eaton, American Water Works Association, American Public Health Association, Washington, D.C., 1992 Search PubMed.
- R Core Team, R: A language and environment for statistical computing, 2012 Search PubMed.
- J. Fox, Getting Started With the r commander: A basic-statistics graphical user interface to r, J. Stat. Softw., 2005, 14(9), 1–42 Search PubMed.
- H. Wickham, ggplot2: elegant graphics for data analysis, Springer, 2009 Search PubMed.
- IBM Corp, IBM SPSS Statistics for Windows, IBM Corp., Armonk, NY, 23 edn, 2015 Search PubMed.
- N. Bolton, N. Cromar, P. Hallsworth and H. Fallowfield, A review of the factors affecting sunlight inactivation of micro-organisms in waste stabilisation ponds: preliminary results for enterococci, Water Sci. Technol., 2010, 61(4), 885–890 CrossRef CAS PubMed.
- S. Benchokroun, B. Imziln and L. Hassani, Solar inactivation of mesophilic Aeromonas by exogenous photooxidation in high-rate algal pond treating waste water, J. Appl. Microbiol., 2003, 94(3), 531–538 CrossRef CAS PubMed.
- J. C. Goldman, Outdoor algal mass cultures—II. Photosynthetic yield limitations, Water Res., 1979, 13(2), 119–136 CrossRef CAS.
- J. U. Grobbelaar, The influence of light/dark cycles in mixed algal cultures on their productivity, Bioresour. Technol., 1991, 38(2–3), 189–194 CrossRef.
- H. Maynard, S. Ouki and S. Williams, Tertiary lagoons: a review of removal mecnisms and performance, Water Res., 1999, 33(1), 1–13 CrossRef CAS.
- H. Fallowfield and M. Garrett, The photosynthetic treatment of pig slurry in temperate climatic conditions: a pilot-plant study, Agric. Wastes, 1985, 12(2), 111–136 CrossRef CAS.
- P. Young, N. Buchanan and H. Fallowfield, Inactivation of indicator organisms in wastewater treated by a high rate algal pond system, J. Appl. Microbiol., 2016, 121(2), 577–586 CrossRef CAS PubMed.
- B. Picot, H. El Halouani, C. Casellas, S. Moersidik and J. Bontoux, Nutrient removal by high rate pond system in a Mediterranean climate (France), Water Sci. Technol., 1991, 23(7–9), 1535–1541 CAS.
- B. Picot, A. Bahlaoui, S. Moersidik, B. Baleux and J. Bontoux, Comparison of the purifying efficiency of high rate algal pond with stabilization pond, Water Sci. Technol., 1992, 25(12), 197–206 CAS.
- P. Chen, Q. Zhou, J. Paing, H. Le and B. Picot, Nutrient removal by the integrated use of high rate algal ponds and macrophyte systems in China, Water Sci. Technol., 2003, 48(2), 251–257 CAS.
- R. Davies-Colley, R. Craggs and J. Nagels, Disinfection in a pilot-scale advanced pond system (APS) for domestic sewage treatment in New Zealand, Water Sci. Technol., 2003, 48(2), 81–87 CAS.
- R. Davies-Colley, R. Craggs, J. Park, J. Sukias, J. Nagels and R. Stott, Virus removal in a pilot-scale advanced pond system as indicated by somatic and F-RNA bacteriophages, Water Sci. Technol., 2005, 51(12), 107–110 CAS.
- C. Campos, A. Guerrero and M. Cardenas, Removal of bacterial and viral faecal indicator organisms in a waste stabilization pond system in Choconta, Cundinamarca (Colombia), Water Sci. Technol., 2002, 45(1), 61–66 CAS.
- L. Alcalde, G. Oron, L. Gillerman, M. Salgot and Y. Manor, Removal of fecal coliforms, somatic coliphages and F-specific bacteriophages in a stabilization pond and reservoir system in arid regions, Water Sci. Technol., 2003, 3(4), 177–184 CAS.
- R. Mayer, J. Vierheilig, L. Egle, G. Reischer, E. Saracevic and R. Mach, et al., Automated sampling procedures supported by high persistence of bacterial fecal indicators and Bacteroidetes genetic microbial source tracking markers in municipal wastewater during short-term storage at 5 C, Appl. Environ. Microbiol., 2015, 81(15), 5134–5143 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ew00228a |
| ‡ Dr Neil Buchanan deceased, 2 July 2015. |
| This journal is © The Royal Society of Chemistry 2018 |