Consumption of plant food supplements in the Netherlands
Suzanne M. F.
Jeurissen
 *,
Elly J. M.
Buurma-Rethans
,
Marja H.
Beukers
,
Martine
Jansen-van der Vliet
,
Caroline T. M.
van Rossum
and
R. Corinne
Sprong
*,
Elly J. M.
Buurma-Rethans
,
Marja H.
Beukers
,
Martine
Jansen-van der Vliet
,
Caroline T. M.
van Rossum
and
R. Corinne
Sprong
National Institute for Public Health and the Environment (RIVM), P.O. Box 1, 3720 BA, Bilthoven, The Netherlands. E-mail: suzanne.jeurissen@rivm.nl; Tel: +31 30 274 4353
First published on 22nd November 2017
Abstract
The use of food supplements containing herbs or other botanical ingredients (plant food supplements, PFS) is on the rise. In some cases, PFS can contain compounds that are toxic and may pose a health risk. To assess the potential health risks, information on the consumption of PFS is required, however, this was lacking for the Netherlands. In the current study, the consumption of PFS was investigated for several subgroups in the Dutch population, including children. Data from the Dutch National Food Consumption Surveys were used to get a first impression on the consumption of PFS. To obtain more detailed information, a specific PFS consumption survey was performed using online questionnaires. First, a screening survey was performed among a representative sample of 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 adults and children of the Dutch population, followed by a main survey among 739 selected PFS users in eight different age and gender subgroups. The prevalence of PFS users in the Dutch population was approximately 10% for men, 17% for women and 13% for children. A wide variety of PFS was used, with around 600 different PFS reported, containing 345 different botanicals. The most frequently used botanicals were echinacea (Echinacea purpurea), ginkgo (Ginkgo biloba), cranberry (Vaccinium macrocarpon), ginseng (Panax ginseng) and algae (such as species belonging to the genus Spirulina or Chlorella). Because PFS are widely used in the Dutch population, it is important to evaluate the potential risks associated with PFS consumption in the Netherlands, including potential herb-drug interactions. The data collected in this study are of great value to assess these risks.
100 adults and children of the Dutch population, followed by a main survey among 739 selected PFS users in eight different age and gender subgroups. The prevalence of PFS users in the Dutch population was approximately 10% for men, 17% for women and 13% for children. A wide variety of PFS was used, with around 600 different PFS reported, containing 345 different botanicals. The most frequently used botanicals were echinacea (Echinacea purpurea), ginkgo (Ginkgo biloba), cranberry (Vaccinium macrocarpon), ginseng (Panax ginseng) and algae (such as species belonging to the genus Spirulina or Chlorella). Because PFS are widely used in the Dutch population, it is important to evaluate the potential risks associated with PFS consumption in the Netherlands, including potential herb-drug interactions. The data collected in this study are of great value to assess these risks.
Introduction
The use of food supplements containing herbs or other botanical ingredients (plant food supplements, PFS) is on the rise.1 PFS have a ‘natural’ image and are often used because of their (supposed) health benefits. Food supplements are marketed in dose forms resembling medicinal products. According to the General Food Law (Regulation (EC) No. 178/20022), any food, including PFS, placed on the market has to be safe. However, in contrast to medicinal products, no pre-market safety assessment and authorization is needed when PFS, as well as other food supplements, are brought on the market in Europe.3 The legislation on botanicals in food supplements is not harmonized in Europe, and therefore PFS are subject to national legislation in EU Member States. Major differences exist among the rules for PFS. Several countries have lists with botanicals that are allowed (positive list) and/or prohibited (negative lists) for use in PFS and some have specific requirements with respect to maximum levels and labelling.4 The BELFRIT project was an initiative to harmonize these lists of botanicals in Europe, starting with the lists of Belgium, France and Italy.5,6 In the Netherlands, the Herbal Preparations Decree in the Dutch Commodities Act contains a list of botanicals and botanical ingredients that are not allowed in PFS and other herbal preparations. In addition, the Herbal Preparations Decree prohibits to place on the market any herbal preparation that contains herbal substances in amounts that are detrimental to health.7 In most EU member states, a notification procedure has to be completed before a PFS can be placed on the market. The requirements for notification vary from submission of the product label only to submission of a safety dossier.8 In the Netherlands, as well as in a few other EU member states, no notification system is set up for food supplements, including PFS, and as a consequence, there is no complete overview of PFS on the Dutch market.In spite of the common perception among consumers that ‘natural equals safe’, some PFS may contain constituents that are toxic or even genotoxic and/or carcinogenic, and can cause (serious) adverse health effects (e.g.ref. 9–11). To assess the potential health risks posed by PFS, information on the consumption of PFS is required. Recently, the results of the PlantLIBRA consumer survey, conducted among adult PFS users in six European countries (Finland, Germany, Italy, Romania, Spain and the United Kingdom) were published.12 Overall, 18.8% of the screened respondents had used at least one PFS in the preceding year (range 9.6–22.7% in the different countries). Across countries, 491 different botanicals were identified in the PFS consumed, with Ginkgo biloba (ginkgo), Oenothera biennis (evening primrose) and Cynara scolymus (artichoke) being most frequently reported. In this study, clear differences among countries in terms of botanicals used by consumers as PFS were observed. This may be due to the fact that current legislation is not harmonized at a European level, with differences between botanicals permitted in PFS among countries, as described above. In addition, some products may be on the market as (traditional) herbal medicinal products in some countries and as PFS in other countries.12 Also cultural differences may play a role.
Currently, detailed information on the consumption of PFS in the Netherlands is lacking. The aim of the current study was to investigate the consumption of PFS in various age and gender subgroups of the population, including children, in the Netherlands. Consumption data collected in the Dutch National Food Consumption Surveys were analysed to get a first impression on the percentage of PFS users in the Dutch population and to estimate the sample size needed for a specific PFS consumption survey. Thereafter, a specific PFS consumption survey was conducted among 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 adults and children in the Netherlands to obtain detailed information on the consumption of PFS in the Netherlands. The results can be used to assess the potential health risks posed by PFS and to improve the monitoring of PFS consumption in the future.
100 adults and children in the Netherlands to obtain detailed information on the consumption of PFS in the Netherlands. The results can be used to assess the potential health risks posed by PFS and to improve the monitoring of PFS consumption in the future.
Materials and methods
Definition of plant food supplements in this study
PFS are subject to the European Union (EU) Directive on Food Supplements (2002/46/EC)3 and, in the Netherlands, to the Herbal Preparations Decree in the Dutch Commodities Act.7 The EU Directive defines food supplements (including PFS) as “…foodstuffs the purpose of which is to supplement the normal diet and which are concentrated sources of nutrients or other substances with a nutritional or physiological effect, alone or in combination, marketed in dose forms, namely forms such as capsules, pastilles, tablets, pills and other similar forms, sachets of powder, ampoules of liquids, drop dispensing bottles and other similar forms of liquids and powders designed to be taken in measured small quantities”.According to the Herbal Preparations Decree in the Dutch Commodities Act, a herbal preparation is a herbal substance (a substance consisting of plant material), whether or not processed, which is intended to be used by humans, including herbal extracts. Culinary herb and spices, and cosmetics, food flavourings and medicines containing herbal substances do not fall within this definition.
The definition used for plant food supplement (PFS) in this study is a food supplement as defined by EU Directive 2002/46/EC that contains (a) substance(s) consisting of plant material (also including material from e.g. algae, fungi or lichens), including extracts thereof. This definition includes food supplements that also contain vitamins, minerals or other substances besides herbal substances. Herbal products that are not food supplements, such as (traditional) herbal medicinal products, homeopathic products and herbal teas, were excluded.
PFS consumption data from Dutch National Food Consumption Surveys (DNFCSs)
The target population of the DNFCS 2010–2012 consisted of community-dwelling men and women aged 70 years and older living in the Netherlands. Institutionalized people were excluded, as well as tube-fed or parenterally fed people, people with a high-intensity care package and people who were terminally ill. As for DNFCS 2007–2010, only people with sufficient knowledge of the Dutch language were included. The survey population was drawn from the population registered within 15 municipalities in the Netherlands, distributed to geographic region and address density. Food consumption data were collected among 739 participants from October 2010 to February 2012.14
In both surveys, food consumption data were collected by two non-consecutive 24 h dietary recalls per person (separated by 2 to 6 weeks). In the 24 h recalls not only foods and drinks, but also food supplements were registered. For each supplement the brand, type of supplement and dose was registered. The surveys covered all days of the weeks and all four seasons. An additional questionnaire was used to cover, amongst others, the frequency of use of food supplements. For each specific food supplement, the frequency of consumption during winter and the rest of the year over the preceding twelve months could be filled in.13,14 In DNFCS 2007–2010 the food frequency questionnaire provided a list of generic vitamin and mineral supplements and PFS could be listed as ‘other supplements’. ‘Other supplements’ was defined as supplements other than those containing only vitamins, minerals and/or fatty acids, and it is assumed by the authors that many of the remaining ‘other supplements’ are PFS. In DNFCS 2010–2012, the list of generic supplements also included garlic, ginseng and ginkgo supplements. Other PDF could be listed as ‘other supplements’.
PFS consumption survey
The specific survey on PFS consumption was conducted in two phases in 2014. First, a screening survey was performed to estimate the percentage of users of PFS in eight different age and gender subgroups of the Dutch population (children aged 1–8 and 9–18 years, and women and men aged 19–50 years, 51–69 years and 70 years and older) and to select PFS users for the main survey. For children, no large differences in PFS consumption between boys and girls were expected, and therefore the subgroups for children were only based on age. The screening survey was followed by the main survey, which was conducted with a PFS user selection of the screened population, to obtain detailed information on PFS consumption characteristics in the different subgroups. The field research was conducted by the market research agency GfK among members of their panel between 1 October and 1 December 2014. The activities of GfK comply with national and EU data protection legislation. The authors received anonymised data from GfK.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 729 members of the online panel aged 18 years and older were asked if they had used PFS during the preceding 12 months. Parents of children aged 1 to 18 years were asked to fill in the screening survey for their children as well. A detailed explanation of the definition of PFS was given (the questionnaire – in Dutch – can be obtained upon request). Participants could also indicate if they were not sure whether the products used were PFS. In that case, they were requested to provide details (name, brand, dose form) on the products used.
729 members of the online panel aged 18 years and older were asked if they had used PFS during the preceding 12 months. Parents of children aged 1 to 18 years were asked to fill in the screening survey for their children as well. A detailed explanation of the definition of PFS was given (the questionnaire – in Dutch – can be obtained upon request). Participants could also indicate if they were not sure whether the products used were PFS. In that case, they were requested to provide details (name, brand, dose form) on the products used.
Screening survey. The data were analysed using SAS version 9.2 (SAS Institute Inc., Cary, NC, USA). For participants that indicated that they were not sure whether the products used were PFS, the authors determined, based on the provided details (name, brand, dose form) of the products used, whether these respondents should be classified as PFS user or not. Respondents for which this was not possible based on the (lack of) information provided were excluded from further analyses and from sample selection for the main survey. The results of the screening were weighted for the background characteristics age, gender, education, size of household, geographical region, internet use, social class and degree of urbanization to obtain results that are optimally representative of the Dutch population (using the Golden Standard from 2013 of the Dutch Center for Information Based Decision Making & Marketing Research (MOA)).
Main survey. The data were analysed using SAS version 9.2 (SAS Institute Inc., Cary, NC, USA). Based on the name, brand, dose form, herbal ingredients and the uploaded pictures, the correctness of the herbal ingredients of all reported PFS was checked by the authors using label information and/or internet, and adjusted if needed. Botanicals described as ‘fruit extract’ or ‘vegetable extract’ were registered as ‘unknown fruit and/or vegetable extract’ because it was not possible to obtain information on the origin. Different species of mushrooms, algae and seaweed were registered as, respectively, ‘mushrooms’, ‘algae’ and ‘kelp/seaweed’, because detailed information on origin was not available for a large part of the supplements. Also, each PFS was classified as a single ingredient or a multi ingredient food supplement. PFS for which the herbal ingredients could not be verified were not included in the analyses. Reported PFS that did not meet the definition of a PFS and respondents that were judged not to have used at least one supplement that met the definition of PFS were excluded from the analyses.
Results
Use of PFS based on the Dutch National Food Consumption Surveys (DNFCSs)
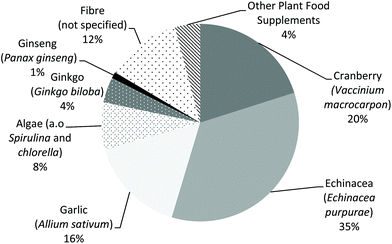
Based on the food frequency questionnaires of the DNFCSs, the percentage of respondents that reported the use of ‘other supplements’ in the preceding year ranged from 2.0 to 13.9% (Table 1). The lowest percentages were reported in children (2.8% for boys and 2.0% for girls) and the percentage increased with age. In the 24 h dietary recalls in both DNFCSs, consumption of PFS containing echinacea (Echinacea purpurea) was most frequently reported, followed by cranberry (Vaccinium macrocarpon), garlic (Allium sativum) and fibre (plant source could not be specified) (Fig. 1).| Gender and age (years) | N | % Users of ‘other supplements’ | |
|---|---|---|---|
| a Other supplements are food supplements other than those containing only vitamins, minerals and/or fatty acids. It is assumed by the authors that many of these are plant food supplements. | |||
| Boys | 7–18 | 856 | 2.8 |
| Girls | 7–18 | 857 | 2.0 |
| Men | 19–69 | 1055 | 4.6 |
| Women | 19–69 | 1051 | 10.5 |
| Men | 70+ | 373 | 8.9 |
| Women | 70+ | 366 | 13.9 |
Response in PFS consumption survey
For the PFS screening survey, 81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 729 adults were invited and 55
729 adults were invited and 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 266 (68%) responded. These adults also filled in the screening survey for in total 21
266 (68%) responded. These adults also filled in the screening survey for in total 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 242 children in the age of 1–18 years.
242 children in the age of 1–18 years.
Six children were excluded since details on their age and gender were missing. The total number of completed questionnaires was 76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 502 (Table 2). In 6.4% of the completed questionnaires, the respondents indicated that they were not sure whether the products that they or their children had used were indeed PFS. By using the information provided by the respondents on the name, brand and dose form of these products, the authors were able to conclude on the use of PFS for most of these respondents. Of the products that appeared not to be PFS, about 70–80% were food supplements with (a) vitamin(s) and/or mineral(s) and about 7–8% were medicines. The remaining products were normal foods and medical devices. For 1402 questionnaires (1.8% of the completed questionnaires) it was not clear for the authors if the products used were PFS. These were excluded for further analyses and the subjects were excluded in the sample for the main survey. After these corrections, the total number of completed questionnaires of the screening phase was 75
502 (Table 2). In 6.4% of the completed questionnaires, the respondents indicated that they were not sure whether the products that they or their children had used were indeed PFS. By using the information provided by the respondents on the name, brand and dose form of these products, the authors were able to conclude on the use of PFS for most of these respondents. Of the products that appeared not to be PFS, about 70–80% were food supplements with (a) vitamin(s) and/or mineral(s) and about 7–8% were medicines. The remaining products were normal foods and medical devices. For 1402 questionnaires (1.8% of the completed questionnaires) it was not clear for the authors if the products used were PFS. These were excluded for further analyses and the subjects were excluded in the sample for the main survey. After these corrections, the total number of completed questionnaires of the screening phase was 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100.
100.
| Children | Men | Women | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1–8 years | 9–18 years | 19–50 years | 51–69 years | 70+ years | 19–50 years | 51–69 years | 70+ years | ||
| a Corrected by excluding the respondents who indicated not to be sure if the products they used were PFS and for which a confirmation regarding the PFS status could not be obtained by the investigators. b Excluding non-respondents (n = 316), respondents that indicated that they did not use PFS during the preceding 12 months (n = 609) and respondents that did not complete the questionnaire (n = 372). c Corrected by excluding the respondents for which none of the reported products met the definition of PFS. | |||||||||
| Screening survey | |||||||||
| Respondents | 8261 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 975 975 |
8612 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 217 217 |
3779 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 796 796 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 991 991 |
1871 | 76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 502 502 |
| Respondents after correctiona | 8026 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 807 807 |
8399 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 013 013 |
3716 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 798 798 |
1841 | 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
| Main survey | |||||||||
| Sample of PFS consumers for main survey | 500 | 400 | 250 | 260 | 250 | 200 | 200 | 199 | 2259 |
| Completed questionnairesb | 159 | 112 | 123 | 119 | 121 | 109 | 114 | 105 | 962 |
| Completed questionnaires after correctionc | 75 | 76 | 91 | 102 | 104 | 98 | 96 | 97 | 739 |
For the main survey, the target was 100 subjects for each age and gender group. The number of completed questionnaires per group varied from 105 to 159 subjects. After exclusion of the subjects for whom none of the reported PFS met the definition of PFS, 75–104 completed questionnaires were left in the different age and gender groups. The reported PFS that did not meet the definition mentioned above were for instance food supplements containing only vitamins, minerals and/or other ingredients (other than botanicals), normal foods, medicines and/or medical devices. In total, 739 completed questionnaires were included in the analyses (Table 2).
Use of PFS and user characteristics based on PFS consumption survey
Based on the screening survey, the percentage of self-reported PFS users ranged from 9 to 18% in the different age and gender groups, with the lowest percentage of PFS users in men aged 51–69 years (9%) and men aged 70+ years (9%), and the highest percentage of PFS users in women aged 19–50 years (18%) and women aged 51–69 years (17%) (Table 3). Table 4 shows the sociodemographic characteristics of self-reported users and non-users of PFS. PFS users tended to have more often a low educational level but a high social class (social class is based on a combination of the profession of the respondent and the educational level of the main costs earner of the household). Also, PFS users were more often heavy internet users (≥14 h per week) compared to non-users.| Gender and age (years) | N | % Users of PFS | |
|---|---|---|---|
| a Number of subjects with correctly filled in screening questionnaire. | |||
| Children | 1–8 | 8026 | 14 |
| Children | 9–18 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 807 807 |
11 |
| Men | 19–50 | 8399 | 11 |
| Women | 19–50 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
18 |
| Men | 51–69 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 013 013 |
9 |
| Women | 51–69 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 798 798 |
17 |
| Men | 70+ | 3716 | 9 |
| Women | 70+ | 1841 | 15 |
| Children 1–18 years | Men 19 years and older | Women 19 years and older | ||||
|---|---|---|---|---|---|---|
| Users | Non-users | Users | Non-users | Users | Non-users | |
| Characteristics | (N = 2440) | (N = 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 393) 393) |
(N = 2399) | (N = 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 729) 729) |
(N = 5422) | (N = 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 717) 717) |
| a Percentages are rounded up. Percentages that differ for ≥5% between users and non-users of the same age group are printed in bold. b Low = primary school, lower vocational, low or intermediate general education; moderate = intermediate vocational education and higher general education; high = higher vocational education and university. For children, the highest educational level of the parents is reported. c Based on a combination of the profession of the respondent and the educational level of the main costs earner of the household according to the Golden Standard of the Dutch Center for Information Based Decision Making & Marketing Research (MOA). High = A; moderate = B1, B2 and C; low = D. d Light = 0–4 h per week, moderate = 5–13 h per week, heavy = ≥14 h per week. e For 4 respondents, no details on region are available. f The Western region was separately sampled for 3 big cities (Amsterdam, Rotterdam and The Hague) including 6 satellite towns and the remainder of the region. g Urbanisation: High = >1500 addresses per km2, moderate = 1000–1500 addresses per km2, low = <1000 addresses per km2. | ||||||
| Highest educational levelb | ||||||
| High | 10% | 9% | 10% | 16% | 10% | 16% |
| Moderate | 45% | 46% | 40% | 42% | 43% | 46% |
| Low | 44% | 44% | 50% | 40% | 46% | 36% |
| Unknown | 2% | 1% | 1% | 2% | 1% | 2% |
| Size of household | ||||||
| 1 | 0% | 0% | 20% | 20% | 25% | 22% |
| 2 and 3 | 21% | 18% | 53% | 53% | 51% | 53% |
| 4 | 45% | 47% | 18% | 19% | 16% | 17% |
| 5 and more | 34% | 35% | 8% | 8% | 8% | 8% |
| Social classc | ||||||
| High | 23% | 22% | 27% | 20% | 24% | 19% |
| Moderate | 53% | 56% | 52% | 51% | 47% | 45% |
| Low | 24% | 21% | 21% | 29% | 28% | 36% |
| Internet used | ||||||
| Light | 36% | 42% | 31% | 38% | 41% | 45% |
| Moderate | 34% | 32% | 32% | 32% | 31% | 30% |
| Heavy | 30% | 26% | 37% | 30% | 28% | 24% |
| Regione | ||||||
| Westf | 40% | 40% | 46% | 45% | 47% | 45% |
| North | 10% | 12% | 10% | 10% | 10% | 10% |
| East | 27% | 24% | 21% | 20% | 22% | 21% |
| South | 23% | 24% | 23% | 25% | 21% | 24% |
| Urbanisationg | ||||||
| High | 44% | 44% | 55% | 51% | 52% | 51% |
| Moderate | 22% | 21% | 16% | 19% | 19% | 19% |
| Low | 35% | 34% | 29% | 29% | 29% | 30% |
Characteristics of PFS use based on PFS consumption survey
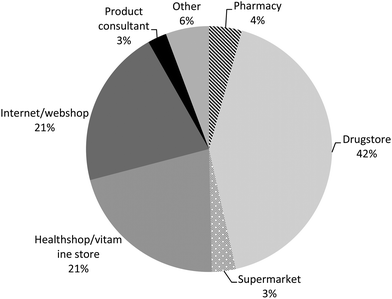
Most PFS users reported the use of one type of PFS during the preceding 12 months (children 87%, men 64% and women 58%), whereas about 10% of the children and one quarter of the adult PFS users indicated to have used two types of PFS (Table 5). The maximum number of type of PFS reported to be used was seven PFS by one woman. More than one out of three reported the additional use of other food supplements during the preceding 12 months. For most PFS, the users indicated that they were used on a daily basis (910 out of 1114 PFS, 82%), and for 354 of them (39%), the users indicated that they have used them in this frequency during the entire preceding 12 months. The PFS-users described their health mainly as ‘very well’ and ‘well’ (81% of the children, 69% of the men and 60% of the women), whereas only 4% of the children, 6% of the men and 9% of the women described their perceived health as ‘bad’ and only 1% of the women as ‘very bad’. Improvement of the immune system/defence system was the main reason for the use of PFS in children (31%), men (17%) and women (19%). Improvement of the energy level was the second most important reason for men and the third most important reason for children (with flu/cold as second reason) and women (with improvement of the urinary tract as second reason). The concomitant use of medicinal products was reported by about 20% of the children and 58% of the adults. Homeopathic medicines were used by about 20% of the children, 15% of the men and 25% of the women. About 20% of the children were treated by a complementary or alternative health care professional (for instance a chiropractor or acupuncturist) and about 40% of the men and 46% of the women (Table 5). Most PFS were purchased in a drugstore (42%), followed by internet/web shop (21%) and health shop/vitamin store (21%). Only 4% of the reported PFS were bought in a pharmacy (Fig. 2). Most products were used on own initiative (37%), followed by recommendations from friends/relatives (16%; Fig. 3).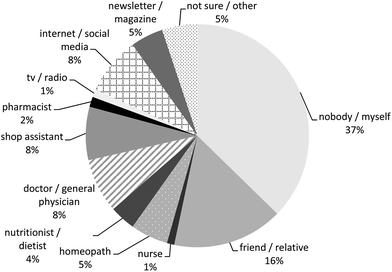 | ||
| Fig. 3 Who recommended the use of the plant food supplements (n = 1230; respondents could fill in more than one answer per plant food supplement)? | ||
| Children (N = 151) | Men (N = 297) | Women (N = 291) | |
|---|---|---|---|
| Number of PFS used | |||
| 1 | 87% | 64% | 58% |
| 2 | 11% | 25% | 27% |
| 3 | 2% | 5% | 9% |
| 4 or more | 0% | 5% | 6% |
| Top 5 reasons of use | |||
| 1 | Immune system (31%) | Immune system (17%) | Immune system (19%) |
| 2 | Flu/cold (12%) | Energy (11%) | Urinary tract (8%) |
| 3 | Energy (9%) | Heart/blood circulation (7%) | Energy (8%) |
| 4 | Relaxing (6%) | General health (6%) | Digestive function (7%) |
| 5 | General health (6%) | Digestive function (6%) | Other (7%) |
| Use of | |||
| Other food supplements | 34% | 36% | 38% |
| Medicines | 21% | 58% | 58% |
| Homeopathy | 20% | 15% | 25% |
| Complementary/alternative healthcare | 19% | 39% | 46% |
| Perceived health | |||
| Very well | 30% | 12% | 7% |
| Well | 51% | 57% | 52% |
| Neutral | 15% | 25% | 32% |
| Bad | 4% | 6% | 9% |
| Very bad | 0% | 0% | 1% |
| Total number of different products | Approximately 600 |
| Total number of botanicals | 363 |
| Total number of single ingredient food supplements used | 525 |
| Total number of multi ingredient food supplements used | 589 |
| Maximum number of botanicals per product | 54 |
| Number of brands | Approximately 250 |
| Dose form of PFS used | |
| Capsules (softgels, pearls, hard capsules) | 330 (30%) |
| Pills/tablets/lozenges | 590 (53%) |
| Liquid (extract, syrups, drops) | 116 (10%) |
| Sachets/powder | 38 (3%) |
| Ampoules | 25 (2%) |
| Others | 13 (1%) |
| Unknown | 2 (0%) |
These PFS contained altogether 345 different botanicals. It should be noted that the actual number of consumed botanicals is even larger, because some botanicals were grouped (unknown fruit and/or vegetable extract, mushrooms, algae and kelp/seaweed). Table 7 lists the botanicals that were present in at least 5 of the 1114 PFS. The most used botanicals (in >100 PFS) were echinacea (Echinacea purpurea), ginkgo (Ginkgo biloba), cranberry (Vaccinium macrocarpon), ginseng (Panax ginseng) and algae (such as species belonging to the genus Spirulina or Chlorella), followed (in >50 PFS) by citrus bioflavonoids (no detailed information on the origin available), grape (stone) (Vitis vinifera), valerian (Valeriana officinalis), rose hip (Rosa canina), garlic (Allium sativum), green tea (Camellia sinensis) and acerola (Malpighia spp.).
| Botanical (N) | Botanical (N) | Botanical (N) | Botanical (N) | Botanical (N) |
|---|---|---|---|---|
| Echinacea (175) | Black currant (25) | Siberian ginseng (14) | Cat's whisker (9) | Caraway (6) |
| Echinacea purpurea (L.) Moench | Ribes nigrum L. | Eleutherococcus senticosus (Rupr. & Maxim.) Maxim. | Orthosiphon stamineus Benth. | Carum carvi L. |
| Ginkgo (111) | St John's wort (24) | Blackberry (14) | Psyllium (9) | Clove (6) |
| Ginkgo biloba L. | Hypericum perforatum L. | Rubus fruticosus | Plantago spp. | Syzygium aromaticum (L.) Merr. & L. M. Perry |
| Cranberry (106) | Beetroot (24) | Chamomile (14) | Sage (9) | Maca (Peruvian Ginseng) (6) |
| Vaccinium macrocarpon Aiton | Beta vulgaris L. | Matricaria chamomilla L. | Salvia officinalis L. | Lepidium meyenii Malp. |
| Ginseng (105) | Saw palmetto (berry) (23) | Pollen (14) | Horsetail (8) | Monk's pepper (6) |
| Panax ginseng C.A. Mey. | Serenoa repens (W. Bartram) Small | Equisetum arvense L. | Vitex agnus-castus L. | |
| Algae (102) | Fennel (22) | Rosemary (14) | Birch (8) | Pau d'arco (6) |
| e.g. Spirulina and Chlorella | Foeniculum vulgare Mill. | Rosmarinus officinalis L. | Betula spp. | Tabebuia spp. |
| Citrus bioflavonoids (80) | Blueberry (22) | Devil's claw (13) | Mango (8) | Buckthorn (6) |
| Vaccinium myrtillus L. | Harpagophytum procumbens (Burch.) DC. ex Meisn. | Mangifera indica L. | Frangula spp. | |
| Grape and Grape stone (70) | Lemon balm (22) | Hibiscus (13) | Peach (8) | Star of Bethlehem (6) |
| Vitis vinifera L. | Melissa officinalis L. | Hibiscus spp. | Prunus persica (L.) Batsch | Ornithogalum umbellatum L. |
| Valerian (67) | Milk thistle (22) | Couch grass (13) | Propolis (8) | Brewer's yeast (5) |
| Valeriana officinalis L. | Silybum marianum (L.) Gaertn. | Agropyron repens (L.) Gould ssp. repens | Saccharomyces cerevisiae | |
| Rose hip (65) | Oat (21) | Olive (11) | Rhubarb (8) | Clematis (5) |
| Rosa canina L. | Avena sativa L. | Olea europea L. | Rheum palmatum L. | Clematis vitalba L. |
| Garlic (58) | Wheat (21) | Lemon (11) | Celery (8) | Pea (5) |
| Allium sativum L. | Triticum spp. | Citrus × limon (L.) Burm. f. | Apium graveolens L. | Pisum sativum L. |
| Green tea (57) | Evening primrose (20) | Mushroom (11) | Echinacea (8) | Fenugreek (5) |
| Camellia sinensis (L.) Kuntze | Oenothera biennis L. | Echinacea angustifolia DC. | Trigonella foenum-graecum L. | |
| Apricot (7) | ||||
| Prunus armeniaca L. | ||||
| Acerola (54) | Apple (20) | Papaya (11) | Pineapple (7) | Raspberry (5) |
| Malpighia spp. | Malus domestica Borkh. | Carica papaya L. | Ananas comosus (L.) Merr. | Rubus idaeus L. |
| Kelp/Sea weed (43) | Orange (20) | English plantain (11) | Astralagus (7) | Rockrose (5) |
| Citrus sinensis (L.) Osbeck | Plantago lanceolata L. | Astragalus spp. | Helianthemum nummularium (L.) Mill. | |
| Nettle (41) | Watercress (20) | Thyme (11) | Skullcap (7) | Juniper (5) |
| Urtica dioica L. | Nasturtium officinale W. T. Aiton | Thymus vulgaris L. | Scutellaria lateriflora L. | Juniperus communis L. |
| Turmeric (38) | Cat's claw (19) | Guarana (10) | Cauliflower (7) | Plum (5) |
| Curcuma longa L. | Uncaria tomentosa (Willd.) DC, | Paullinia cupana Kunth | Brassica oleracea L. var. botrytis L. | Prunus spp. |
| Alfalfa (31) | Hawthorn (19) | Angelica root (10) | Kale (7) | Cabbage (5) |
| Medicago sativa L. | Crataegus spp. | Angelica spp. | Brassica oleracea L. | Brassica spp. |
| Goldenrod (31) | Licorice (19) | Dong quai (10) | Boswellia (7) | Unknown vegetables or fruits extract |
| Solidago virgaurea L. | Glycyrrhiza glabra L. | Angelica sinensis (Oliv.) Diels | Boswellia spp. | |
| Spinach (31) | Elder (18) | Pomegranate (10) | Centaury (7) | Oregano (5) |
| Spinacia oleracea L. | Sambucus spp. | Punica granatum L. | Centaurium spp. | Origanum spp. |
| Carrot (31) | Tomato (17) | Mistletoe (10) | Garcinia cambogia (7) | Popular (5) |
| Daucus carota L. | Solanum lycopersicum L. | Viscum album L. | Garcinia cambogia Desr. | Populus spp. |
| Aloe (30) | Broccoli (16) | Pear (10) | Cinnamon (7) | Red yeast rice (5) |
| Aloe vera (L.) Burm. f. | Brassica oleracea L. | Pyrus spp. | Cinnamomum spp. | Monascus purpureus |
| Passion flower (30) | Pumpkin seed (16) | California poppy (10) | Senna (7) | Himalayan balsam (5) |
| Passiflora incarnata L. | Cucurbita pepo L. | Eschscholzia californica Cham. | Senna alexandrina Mill. | Impatiens glandulifera Arn. |
| Bearberry (30) | Linseed (15) | Peppermint (9) | Asparagus (6) | Rice (5) |
| Arctostaphylos uva-ursi (L.) Spreng. | Linum usitatissimum L. | Mentha piperita L. | Asparagus officinalis L. | Oryza sativa L. |
| Pepper (28) | Black cohosh (15) | (Wild) strawberry (9) | Cascara (6) | Schisandra (5) |
| Piper nigrum L. | Actaea racemosa L. | Fragaria spp. | Frangula purshiana (DC.) A. Gray | Schisandra chinensis (Turcz.) Baill. |
| Parsley (28) | Cherry (15) | Acai (9) | Cayenne pepper (6) | |
| Petroselinum crispum (Mill.) Fuss | Prunus spp. | Euterpe oleracea Mart. | Capsicum annuum L. | |
| Ginger (26) | Dandelion (15) | Barley (9) | Gotu kola (6) | |
| Zingiber officinale Roscoe | Taraxacum officinale F. H. Wigg. | Hordeum vulgare L. | Centella asiatica (L.) Urb. | |
| Soy/soy isoflavones (25) | Artichoke (14) | Hop (9) | Grapefruit (6) | |
| Glycine max (L.) Merr. | Cynara scolymus L. | Humulus lupulus L. | Citrus paradisi Macfad. |
Echinacea (Echinacea purpurea) belonged to the four most frequently used botanicals in all eight age and gender groups. Cranberry (Vaccinium macrocarpon) and algae (such as species belonging to the genus Spirulina or Chlorella) were in the top 10 of the most used botanicals in all groups, except for young children (aged 1 to 8 years) (Table 8). Ginkgo (Ginkgo biloba) and ginseng (Panax ginseng) both belonged to the three most frequently used botanicals in men and women aged 51–69 years and 70 years and more. In most cases, these two botanicals were used in multivitamins marketed for people aged 50+, 60+ or 65+. For children aged 1 to 18 years, roughly 60% of the PFS used were PFS containing multiple ingredients, mainly multivitamin/mineral supplements with one or more botanical ingredients. About 20% of the PFS used were single PFS with echinacea (Echinacea purpurea), and the remaining 20% of the PFS used were single PFS containing other botanicals.
| Children 1–8 years | Children 9–18 years | Men 19–50 years | Women 19–50 years | Men 51–69 years | Women 51–69 years | Men 70+ years | Women 70+ years | |
|---|---|---|---|---|---|---|---|---|
| a The number of PFS used containing the specific botanical is indicated between brackets. | ||||||||
| 1 | Echinacea | Echinacea | Algae (19) | Echinacea | Ginkgo | Ginkgo | Ginkgo | Echinacea |
| Echinacea purpurea (27) | Echinacea purpurea (22) | Echinacea purpurea (28) | Ginkgo biloba (19) | Ginkgo biloba (18) | Ginkgo biloba (30) | Echinacea purpurea (31) | ||
| 2 | Rose hip | Rose hip | Echinacea | Cranberry | Ginseng | Ginseng | Ginseng | Ginkgo |
| Rosa canina (17) | Rosa canina (12) | Echinacea purpurea (16) | Vaccinium macrocarpon (26) | Panax Ginseng (17) | Panax Ginseng (18) | Panax Ginseng (29) | Ginkgo biloba (28) | |
| 3 | Citrus bioflavonoids (16) | Valerian | Cranberry | Algae (20) | Algae (16) | Valerian | Echinacea | Ginseng |
| Valeriana officinalis (11) | Vaccinium macrocarpon (11) | Valeriana officinalis (14) | Echinacea purpurea (25) | Panax ginseng (25) | ||||
| 4 | Chamomile | Citrus bioflavonoids (11) | Valerian | Acerola | Echinacea | Echinacea | Cranberry | Cranberry |
| Matricaria chamomilla (8) | Valeriana officinalis (11) | Malpighia spp. (14) | Echinacea purpurea (12) | Echinacea purpurea (14) | Vaccinium macrocarpon (18) | Vaccinium macrocarpon (22) | ||
| 5 | Carrot | Algae (10) | Garlic | Citrus bioflavonoids (14) | Cranberry | Algae (11) | Grape and Grape stone | Grape and Grape stone |
| Daucus carota L. (8) | Allium sativum (10) | Vaccinium macrocarpon (11) | Vitis vinífera (17) | Vitis vinífera (21) | ||||
| 6 | Grape and Grape stone | Garlic | Ginseng | Rose hip | (Green) tea | Citrus bioflavonoids (11) | (Green) tea | (Green) tea |
| Vitis vinífera (7) | Allium sativum (6) | Panax ginseng (9) | Rosa canina (14) | Camellia sinensis (11) | Camellia sinensis (14) | Camellia sinensis (17) | ||
| 7 | Acerola | Lemon balm | Citrus bioflavonoids (9) | Alfalfa | Citrus bioflavonoids (10) | Cranberry | Saw palmetto (berry) | Turmeric |
| Malpighia spp. (6) | Melissa officinalis (6) | Medicago sativa (13) | Vaccinium macrocarpon (10) | Serenoa repens (14) | Curcuma longa (12) | |||
| 8 | Nettle | Cranberry | Kelp/Sea weed (8) | Goldenrod | Valerian | Turmeric | Algae (12) | Garlic |
| Urtica dioica (6) | Vaccinium macrocarpon (5) | Solidago virgaurea (10) | Valeriana officinalis (9) | Curcuma longa (10) | Allium sativum (11) | |||
| 9 | Spinach | Passion flower | Acerola | Parsley | Grape and Grape stone | Blueberry | Garlic | Algae (10) |
| Spinacia oleracea (6) | Passiflora incarnata (5) | Malpighia spp. (7) | Petroselinum crispum (10) | Vitis vinífera (7) | Vaccinium myrtillus (8) | Allium sativum (10) | ||
| 10 | Ginkgo | Evening primrose | Passion flower | Kelp/Sea weed (9) | Garlic | Ginger | Nettle | Bearberry |
| Ginkgo biloba (5) | Oenothera biennis (5) | Passiflora incarnata (7) | Allium sativum (7) | Zingiber officinale (7) | Urtica dioica (8) | Arctostaphylos uva-ursi (9) | ||
Discussion and conclusion
The current paper describes the consumption of PFS in various age and gender subgroups in the Netherlands. First, data collected on PFS consumption in recent DNFCSs were analysed. Thereafter, a specific PFS consumption survey among 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 respondents (screening survey) and 739 users of PFS (main survey) was conducted. Based on the screening survey, the prevalence of PFS users in the Dutch population was approximately 10% for men, 17% for women and 13% for children. The PFS used are very diverse, with approximately 600 different PFS of about 250 brands, containing a wide range of botanicals (>345 species). The most frequently used botanicals were echinacea (Echinacea purpurea), ginkgo (Ginkgo biloba), cranberry (Vaccinium macrocarpon), ginseng (Panax ginseng) and algae.
100 respondents (screening survey) and 739 users of PFS (main survey) was conducted. Based on the screening survey, the prevalence of PFS users in the Dutch population was approximately 10% for men, 17% for women and 13% for children. The PFS used are very diverse, with approximately 600 different PFS of about 250 brands, containing a wide range of botanicals (>345 species). The most frequently used botanicals were echinacea (Echinacea purpurea), ginkgo (Ginkgo biloba), cranberry (Vaccinium macrocarpon), ginseng (Panax ginseng) and algae.
The prevalences of PFS users based on the PFS consumption survey were higher, especially for children, than those estimated based on the results obtained based on the FFQs in the DNFCSs (range 2–14% among the different age-gender groups).13,14 This difference may partly be explained by a rise in the use of PFS over the last years. Another explanation may be a difference in classification of PFS between the studies due to different study design and purpose. The PFS consumption survey showed that more than half of the PFS used by children were supplements containing vitamins and/or minerals which also contained one or more botanicals. The aim of the questions on food supplement use in the DNFCSs was mainly to obtain information on the intake of vitamins, minerals and fish fatty acids. In the DNFCSs, these types of food supplements have therefore been registered as (multi)vitamin and/or mineral supplements, without annotation of a botanical ingredient. This may have led to underestimation of PFS use, especially by children. On the other hand, it was assumed that all “other supplements” reported in the FFQs of the DNFCSs were PFS, which has probably led to a slight overestimation. Because of the rise in PFS use in the last years the ongoing DNFSCs include more detailed questions on PFS use. The results of the current study will be used to further improve the questions on PFS use in the DNFSCs. Despite these limitations, it can be concluded that the prevalence of PFS use in the Netherlands is within the range previously reported for adults in six other European countries (9.6–22.7%, overall prevalence 18.8%12).
The results of the PFS consumption showed that it was difficult for respondents to judge whether the consumed products were PFS. In the screening survey, about 6% of the respondents answered that they were not sure about the products they had used. In addition, about 27% of the self-reported PFS users of the screening study that were selected for the main survey inconsistently answered on the first question of the main survey that they did not use a PFS in the preceding 12 months. This unexpected contradictory result indicates that the topic of the survey was difficult for participants. Also, many presumed PFS products (26%) reported by respondents in the main survey were actually not PFS. The products that were incorrectly classified as PFS were mainly food supplements with vitamins, minerals and other ingredients (other than botanicals). In several cases, the participants have classified these food supplements as PFS due to the flavourings (for instance banana, orange or anise extract) of these food supplements. Also traditional herbal medicinal products (THMPs), regular herbal medicinal products and homeopathic products were sometimes reported as PFS by respondents. Several respondents from the main study had to be excluded from further analysis, because none of the products reported were PFS. This was the reason that the target of 100 for each age and gender subgroup was not reached for all groups (range: 75–104 correctly completed questionnaires per subgroup). Due to the difficulties the respondents had with the correct classification of the products they used, the differences observed between characteristics of PFS users and non-users should be interpreted with care.
From the botanicals that were mostly used (in >50 PFS, Table 6), ginkgo (Ginkgo biloba), ginseng (Panax ginseng), valerian (Valeriana officinalis) and echinacea (Echinacea purpurea) were also among the ten most frequently used botanicals in the PlantLibra study,12 whereas the other mostly used botanicals were quite different. Evening primrose (Oenothera biennis) and artichoke (Cynara scolymus) are for instance listed in the top 3 of the PlantLibra study, but ranked respectively as number 52 and 37 in the current study. This is in line with the finding in the PlantLibra study that the top list of botanicals contained in PFS for each single country complied little with the overall ranking of the results of the six countries together.
To our knowledge, this is the first study that provides information on the PFS use by children in Europe. The results show that the prevalence of PFS use increases with age, and that the types of PFS used differ largely between children and adults. Most of the children (87%) that participated in the main study had used one PFS in the preceding 12 months. PFS containing echinacea (Echinacea purpurea) were mostly used, followed by PFS containing rose hip (Rosa canina) and citrus bioflavonoids. The main reason for use of the PFS as indicated by the participants was to improve the defence system/immune system (31%) or because of flu or cold (12%). This is also reflected in the type of PFS used, because children used mainly multivitamin/mineral supplements with one or more botanical ingredients, followed by single PFS with Echinacea (Echinacea purpurea).
The frequently used herbs echinacea (Echinacea purpurea), ginkgo (Ginkgo biloba), ginseng (Panax ginseng), grape (stone) (Vitis vinifera) and valerian (Valeriana officinalis) are ingredients of registered regular and traditional herbal medicinal products and/or homeopathic products in the Netherlands,15 and tablets containing cranberry (Vaccinium macrocarpon) are on the market as PFS and as medical devices. Although the difference between these type of products is apparently not always clear for consumers, the legal requirements related to the safety, quality and effectiveness of these products differ.4 Consumers may therefore not be aware of these different legal requirements and thereby possible differences in product composition.
About 20% of the children and 60% of the adults from the main survey indicated the concomitant use of medicinal products on a regular basis in the preceding year. This could pose a risk on adverse herb–drug interactions. Interaction between St. John's wort (Hypericum perforatum) and drugs metabolized by CYP3A4 is a well-known example.16 Also, there are indications that the botanicals ginkgo (Ginkgo biloba), garlic (Allium sativum) and ginseng (Panax ginseng) may interact with anticoagulants.17,18 In a follow up study, the available data can be further analysed to see if potentially harmful combinations are reported.
In conclusion, the current study provided detailed insight in the use of PFS in the Dutch population, including children. The study showed that the national food consumption surveys can be used to monitor the prevalence of PFS use and the consumption of frequently used PFS, but that for detailed information on the wide range of PFS used, a specific PFS consumption study is needed. PFS are widely used by the Dutch population, and it appeared to be difficult for consumers to make a distinction between PFS and other products containing botanicals on the market having other legal requirements with respect to safety, quality and efficacy. This emphasises the need to evaluate the potential risks associated with PFS consumption in the Netherlands, including potential herb–drug interactions. The data collected in this study are of great value to assess these risks.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to thank Jolanda van Oirschot and Marcel Temminghoff from GfK and Alicia Garcia-Alvarez (Fundación para la Investigación Nutricional, Barcelona Science Park, University of Barcelona, Barcelona, Spain) for their valuable contributions to this study. The comments and suggestions of Janneke Verkaik-Kloosterman (RIVM), Birgitte Tiesjema (RIVM), Jacqueline Castenmiller (Netherlands Food and Consumer Product Safety Authority, NVWA) and Anneke Sellis (Dutch Ministry of Health, Welfare and Sport, VWS) on the draft version of the manuscript are acknowledged. Financial support from NVWA and VWS is acknowledged.References
- L. Vargas-Murga, A. Garcia-Alvarez, B. Roman-Vinas, J. Ngo, L. Ribas-Barba and S. J. van den Berg, et al., Plant food supplement (PFS) market structure in EC Member States, methods and techniques for the assessment of individual PFS intake, Food Funct., 2011, 2, 731–739 CAS.
- Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety, Off J Eur Communities, 2002, February 1, vol. L 31, pp. 1–24 Search PubMed.
- Directive 2002/46/EC of the European Parliament and of the Council of 10 June 2002 on the approximation of the laws of the Member States relating to food supplements, Off J Eur Communities, 2002, July 12, vol. L 183, pp. 51–57 Search PubMed.
- V. Silano, P. Coppens, A. Larranaga-Guetaria, P. Minghetti and R. Roth-Ehrang, Regulations applicable to plant food supplements and related products in the European Union, Food Funct., 2011, 2, 710–719 CAS.
- G. Cousyn, S. Dalfrà, B. Scarpa, J. Geelen, R. Anton and M. Serafini, et al., Project BELFRIT, Eur. Food Feed Law Rev., 2013, 8(3), 187–196 Search PubMed.
- B. Klaus and M. Gherardini, Italy: The Italian Ministry of Health Adopts a Decree that Introduces a New, Common Positive List for the Use of Botanicals in Food Supplements, Eur. Food Feed Law Rev., 2014, 9(3), 196–198 Search PubMed.
- Dutch Ministry of Health, Welfare and Sport. Herbal Preparations Decree in the Dutch Commodities Act, Warenwetbesluit Kruidenpreparaten, In Dutch, 2001. Available via: http://wetten.overheid.nl/BWBR0012174/2014-12-13 (accessed August 5, 2016) Search PubMed.
- European Advisory Services (EAS), 28 March 2007. The use of substances with nutritional or physiological effect other than vitamins and minerals in food supplements. Available via: http://ec.europa.eu/food/safety/docs/labelling_nutrition-supplements-2007_a540169_study_other_substances_en.pdf.
- M. J. Martena, J. C. van der Wielen, L. F. van de Laak, E. J. Konings, H. N. de Groot and I. M. Rietjens, Enforcement of the ban on aristolochic acids in Chinese traditional herbal preparations on the Dutch market, Anal. Bioanal. Chem., 2007, 389, 263–275 CrossRef CAS PubMed.
- S. J. van den Berg, L. Serra-Majem, P. Coppens and I. M. Rietjens, Safety assessment of plant food supplements (PFS), Food Funct., 2011, 2, 760–768 CAS.
- C. Di Lorenzo, A. Ceschi, H. Kupferschmidt, S. Lude, E. De Souza Nascimento and A. Dos Santos, et al., Adverse effects of plant food supplements and botanical preparations: a systematic review with critical evaluation of causality, Br. J. Clin. Pharmacol., 2015, 79, 578–592 CrossRef PubMed.
- A. Garcia-Alvarez, B. Egan, S. de Klein, L. Dima, F. M. Maggi and M. Isoniemi, et al., Usage of plant food supplements across six European countries: findings from the PlantLIBRA consumer survey, PLoS One, 2014, 9, e92265 Search PubMed.
- C. T. M. Van Rossum, H. P. Fransen, J. Verkaik-Kloorsterman, E. J. M. Buurma-Rethans and M. C. Ocké, Dutch National Food Consumption Survey 2007–2010. Diet of children and adults aged 7 to 69 years, RIVM report 350050006/2011, 2011. Available via: http://www.rivm.nl.
- M. C. Ocké, E. J. M. Buurma-Rethans, E. J. De Boer, C. Wilson-Van den Hooven, Z. Eternad-Ghameshlou and J. J. M. M. Drijvers, et al., Dutch National Food Consumption Survey-Older adults 2010–2012. Diet of community-dwelling older adults, RIVM report 050413001/2013. Available via: http://www.rivm.nl.
- Medicines Evaluation Board - College ter Beoordeling van Geneesmiddelen. Medicines data bank [Geneesmiddeleninformatiebank; in Dutch]. Available from: http://www.cbg-meb.nl/geneesmiddeleninformatiebank (accessed August 5, 2016).
- A. A. Sprouse and R. B. van Breemen, Pharmacokinetic interactions between drugs and botanical dietary supplements, Drug Metab. Dispos., 2016, 44, 162–171 CrossRef CAS PubMed.
- E. F. Oga, S. Sekine, Y. Shitara and T. Horie, Pharmacokinetic herb-drug interactions: insight into mechanisms and consequencees, Eur. J. Drug Metab. Pharmacokinet., 2016, 41, 93–108 CrossRef CAS PubMed.
- X. W. Chen, K. B. Sneed, S. Y. Pan, C. Cao, J. R. Kanwar and H. Chew, et al., Herb-drug interactions and mechanistic and clinical considerations, Curr. Drug Metab., 2012, 13, 640–651 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2018 |