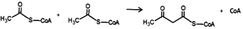Molecular actions of hypocholesterolaemic compounds from edible mushrooms
Alicia
Gil-Ramírez
,
Diego
Morales
 * and
Cristina
Soler-Rivas
* and
Cristina
Soler-Rivas
Department of Production and Characterization of Novel Foods, CIAL – Research Institute in Food Science (UAM+CSIC), C/Nicolas Cabrera 9, Campus de Cantoblanco, Universidad Autonoma de Madrid, 28049 Madrid, Spain. E-mail: diego.morales@uam.es
First published on 8th November 2017
Abstract
Cholesterol levels are strictly regulated to maintain its homeostasis; therefore, if it is not absorbed with the diet, the cholesterol biosynthetic pathway is enhanced and vice versa. Nowadays, the commonly prescribed therapeutic treatments for hypocholesterolemic patients are targeted toward the reduction of both cholesterol intestinal absorption and/or its endogenous biosynthesis. But, when hypercholesterolemia is still moderate the consumption of food products with cholesterol-lowering capacities is more desirable than using drugs. Marketed foods supplemented with hypocholesterolemic compounds are only inhibiting mechanisms for cholesterol absorption (i.e. phytosterols and cereal β-glucans). However, certain fungal extracts obtained from edible mushrooms might be able to modulate cholesterol levels by both strategies, pharmaceutical drugs and functional foods. In vitro and in vivo studies indicated that fungal sterols down-regulated genes involved in cholesterol homeostasis (such as Srebf2 and Nr1h4 (FXR)) and other specific mushroom extracts (β-glucans and other water-soluble compounds) also stimulated transcriptional profiles similar to simvastatin or ezetimibe (two hypocholesterolemic drugs). These and other observations suggested that the hypocholesterolemic effect of mushroom extracts could be due to transcriptional and post-transcriptional modulations besides other indirect effects.
1. Introduction
Although cardiovascular disease (CVD) incidence has been decreasing over the past decades due to medical advances and advice, they are still the second leading cause of premature death in the Western world after cancer.1 Already for many years, health authorities have been reinforcing efforts to inform people about what CVDs are, their main symptoms, their health consequences and the way to decrease the risk of suffering them.CVD risks are influenced by genetic factors such as specific tendencies to obesity, hypertension, etc., and gender or age; however, many risk factors are also modulated by lifestyle habits such as smoking, sedentary/involved in sports or in-/adequate diets2 and this is the reason that CVDs are considered as multifactorial diseases.
Several biomarkers are used to evaluate the real individual CVD risk, i.e. elevated homocysteine, coronary artery calcium-phosphate calcification or elevated fibrinogen blood level but, the most useful marker for CVD risk is the LDL (low-density lipoprotein)-cholesterol concentration in serum, one of the major transport cholesterol complexes. LDL-cholesterol constitutes 60–70% of total serum cholesterol and a blood concentration higher than 200 mg dL−1 is considered, nowadays, as a risky level.3,4
Due to the increasing interest of modern society for natural products as agents able to decrease the consumption of pharmaceutical drugs, new food products with high levels of hypocholesterolemic compounds are being explored. Nowadays, the cholesterol-lowering effect of compounds such as plant sterols (phytosterols, phytostanols and derivatives);5,6 dietary fibers such as β-glucans or chitosans;7 peptides such as those derived from soy proteins8 or bovine milk β-lactoglobulins9 have been studied and therefore, several functional food products supplemented with these compounds are available at the supermarkets.10–14
Some edible mushroom species are able to lower cholesterol levels in serum since they are a good source of fungal sterols (with high structural similarity to phytosterols), dietary fibers such as β-glucans and chitins (fungal polysaccharides), β-lactones, specific proteo/glucan complexes or compounds such as eritadenine.10 Recent studies pointed out that ergosterol-enriched extracts and other fungal compounds were also able to modulate cholesterol-related gene expression in animal intestine and liver.15
Therefore, in this work, the molecular events occurring during cholesterol synthesis and absorption are reviewed analyzing the influence of some hypocholesterolemic compounds obtained from edible mushrooms.
2. Cholesterol metabolism
Cholesterol, together with phospholipids, modulates membrane fluidity influencing transport through them, permeability, configuration of membrane proteins or enzyme activities. Furthermore, cholesterol is involved in many metabolic pathways since it is a precursor of a wide range of biological molecules such as bile acids (i.e. cholic acid), steroid hormones (i.e. testosterone) and lipophilic vitamins (i.e. vitamin D3).7 This sterol is synthesized mainly in the liver, besides other organs such as adrenal glands, intestine or ovaries. But, it can also be incorporated from the diet after the digestion process. Thus, well-balanced mechanisms of cholesterol synthesis, bile acid catabolism, cholesterol intake and excretion through faeces will maintain healthy and stable cholesterol values in the serum (homeostasis). Until a few years ago, the liver was considered the main control center of cholesterol homeostasis; however, more recent studies indicated the intestine as a tissue highly involved in the regulation of plasma cholesterol levels and homeostasis.13,16,172.1. Molecular events occurring during cholesterol digestion
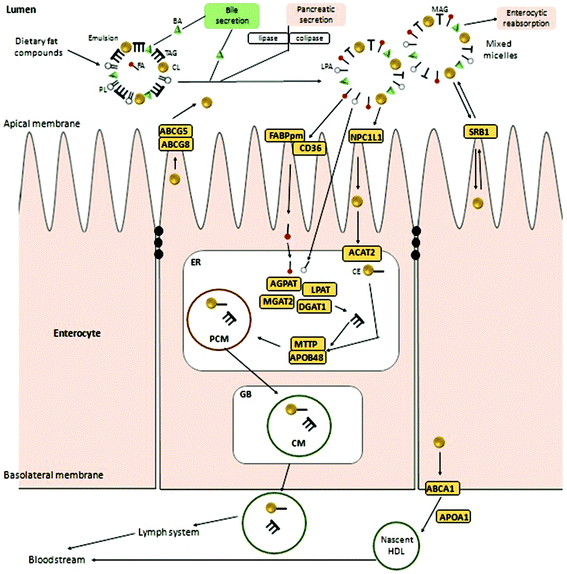
Although food digestion in humans starts in the mouth with mechanical chewing and starch degradation by salivary enzymes (mastication), fat remains undigested until it reaches the stomach. Gastric digestion is mainly oriented toward protein degradation; however, some lipid-degraded enzymes are also active at this step. Afterwards, the main fat digestion take place in the duodenal lumen where all lipid compounds from the gastric bolus (including free or esterified diet cholesterol) are mixed with the bile and pancreatic juices.Cholesterol molecules from diet, bile, intestinal secretions and desquamated cells together with the bile secreted compounds such as phospholipids (lecithin) and bile acids (salts of taurocholic and deoxycholic acids, etc.) form small emulsified droplets. Then, pancreatic lipase, phospholipase A2 and cholesterol esterase transform the emulsified particles into a series of colloidal structures including vesicles, micelles or dietary mixed micelles (DMM).10
2.2. Molecular events occurring during cholesterol absorption
Most of the micellated lipid-like compounds are incorporated into the organism through the second part of the small intestine (jejunum), except for bile acids that can be absorbed at the jejunum and ileum levels by an apical sodium-dependent bile acid transporter (ASBT).18,19 The SR-B1 scavenger receptor (encoded by the SCARB1 gene in humans), mainly located at both apical and basolateral membranes17,20 of adrenal glands, hepatocytes and enterocytes (Fig. 1), is involved in the regulation of endocrine metabolism, vitamin absorption or bile secretion. It also plays a role in cholesterol transport through membranes as a receptor of HDL (high-density lipoprotein)-cholesterol but not in the small intestine absorption context.20,21 SR-B1 transport allows a passive bi-directional cholesterol efflux depending on concentration gradients22 pointing to SR-B1 as an important modulator of reverse cholesterol transport (RCT) (described later).20 However, although SR-B1 contributes to enterocytic cholesterol absorption, recent studies demonstrated that Niemann-Pick C1-like protein (NPC1L1) is the main sterol transporter from the intestinal lumen to the enterocyte cytoplasm, being imperative for non-esterified cholesterol absorption.23NPC1L1 is involved in this transmembrane sterol efflux due to a sterol-sensing domain (SSD) and it is co-localized at the cellular and intracellular vesicular membranes. The distribution of non-esterified cholesterol determines the main location of NPC1L1 proteins. At low intracellular concentrations, NPC1L1 will be mostly exposed at the brush-border enterocyte membrane and it will be translocate inside the cell at high levels of non-esterified cholesterol.24 In humans, NPC1L1 genes are not only expressed in the enterocytes of the small intestine but also in the liver where they are expressed in large amounts. Human hepatocytic NPC1L1 protein is located at the canalicular membrane facilitating the uptake of newly secreted biliary cholesterol and therefore, showing a role similar to intestinal NPC1L1.20 The transcriptional regulation of NPC1L1 has not yet been elucidated but, it seems to be influenced by sterol regulatory element-binding protein or SREBP2 (encoded by SREBF2 gene in humans) that are sensors activating different answers depending on intracellular cholesterol concentrations. At low cholesterol levels, SCAP (integral membrane protein) goes along with SREBP2 from the endoplasmic reticulum (ER) to the Golgi body (GB) for subsequent processing and activation. In contrast, at high or enough cholesterol levels the SCAP-SREBP2 complex is retained by INSIG proteins (Insulin induced gene 1 protein located in the ER membrane) to avoid SREBP2 maturation impairing its transcription.25 Moreover, other reports indicated PPARδ (peroxisome-proliferator-activated receptor δ) as another NPC1L1 modulator since down-regulation of the cholesterol transporter has been induced by PPARδ activation in mice.11
Intracellular non-esterified cholesterol concentrations could also be modulated by ATP-binding cassette (ABC) transporters such as ABCG5/ABCG8 and ABCA1, located respectively at apical and basolateral enterocyte sides. ABCG5 and ABCG8 proteins, expressed in the liver and small intestine, are involved in the reverse cholesterol transport (RCT) of sterols, from the intracellular environment to the lumen. Independent expression of both genes is necessary for the proper function of this heterodimer.26 Over-expression of the ABCG5/8 heterodimer increases non-esterified cholesterol excretion to the lumen reducing its internal concentration and consequently inducing activation of the cholesterol synthesis rate.27 In the small intestine, ABCG5/8 gene expression seems to be regulated by an LXR-dependent member of the nuclear receptor family named RXR (retinoid X receptor)26–28 while in the liver, the heterodimer is directly modulated by LXR.30 Apparently, the latter receptor, along with PPARδ, is also a modulator of ABCA1 expression.10,26 ABCA1 is a transport protein directly involved in the excretion of exceeding non-esterified cholesterol into HDL. LXR agonist administration or high cholesterol concentrations in the cytosol stimulate a direct effect on the transcriptional modulation of these transport proteins although its specific regulatory mechanisms remain still unclear. A protein–protein interaction with another transcription factor affecting the transcription rate of the ABC proteins has been hypothesized.29
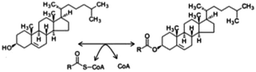
Due to confusions noticed in several publications, it is worth defining the specific role of two widely mentioned enzymes involved in the transferring of acyl groups within the cholesterol metabolism. Acetyl-Coenzyme A transferase (ACAT) and sterol O-acyltransferase (SOAT) are two enzymes belonging to the acyltransferase family; however, they do not catalyze the same reaction (Table 1). ACAT isoforms (ACAT1/ACAT2) are responsible for the reversible formation of acetoacetyl-CoA from two molecules of acetyl-CoA. ACAT1 and ACAT2 genes are respectively located in chromosome 11 (11q22.3) and chromosome 6 (6q25.3).30,31 However, SOAT isoforms (SOAT1/SOAT2) catalyze the formation of fatty acid-cholesterol esters from cholesterol and acyl-CoA molecules and are encoded by two genes located at loci 1q25.2 and 12q13.13.32,33 Then, since in some of the mentioned works the text wrongly referred to ACAT when it should indicate SOAT, personal advice (AvS mark) was given pointing attention where required.
Once non-esterified cholesterol reaches the cytoplasm it becomes a substrate of ACAT2(AvS), an integral membrane protein mainly expressed in the small intestine and liver. ACAT2(AvS) decreases cytoplasmic amounts of non-esterified cholesterol promoting its esterification and integration into the ER pre-chylomicrons modulating the cholesterol transmembrane absorption rate from the intestinal lumen.34 It also plays an important role in maintaining the dynamic equilibrium (homeostasis) between free-cholesterol and esterified-cholesterol.35 More than 50% of sterol esterification within the enterocytes is carried out by ACAT2(AvS) with a higher affinity for cholesterol esterification rather than for other non-cholesterol sterols.
The internal ER triglyceride re-assemblage is carried out by several enzymes such as lysophosphatidate acyltransferase (AGPAT), phosphatidate phosphatase (LPAP), 2-acylglycerol O-acyltransferase 2 (MGAT2) and diacylglycerol O-acyltransferase 1 (DGAT1). Then, esterified-cholesterol together with the triglycerides is packed into pre-chylomicrons by the microsomal triglyceride transfer protein (MTTP) and apolipoprotein B48 (an isoform derived from the APOB gene characteristic of enterocytes). MTTP and APOB48 proteins constitute an active heterodimeric complex linked by ionic interactions with a particular feedback assembly and secretion system i.e. the larger the APOB48 subunit is, the lower binding capacity with MTTP is noticed and less APOB48 is secreted. Therefore, the APOB48-MTTP binding process plays an important role in lipoprotein biogenesis.36–38
The combined regulatory effect of NPC1L1, ABCA1, ABCG5/8 and ACAT2(AvS) activities play a critical role in modulating the amount of esterified cholesterol that will be integrated in the pre-chylomicrons with the assistance of apolipoporotein B48 (APOB48), microsomal triglyceride transfer protein (MTTP) and diacylglycerol-O-acyltransferase (DGAT1/2).39
Once prechylomicrom structure is assembled, it is further transformed into chylomicrons in the GB and excreted by exocytosis into the lymph system through the enterocyte basolateral membrane. On the other hand, the non-esterified cholesterol remaining in the cytoplasm could bind to the APOA1 protein for further transport to the lymphatic vessels leading to nascent HDL lipoproteins. Thus, HDL as well as chylomicrons are released free into the bloodstream and transported to the liver and peripheral organs such as adrenal glands.11
2.3. Molecular events occurring during cholesterol synthesis
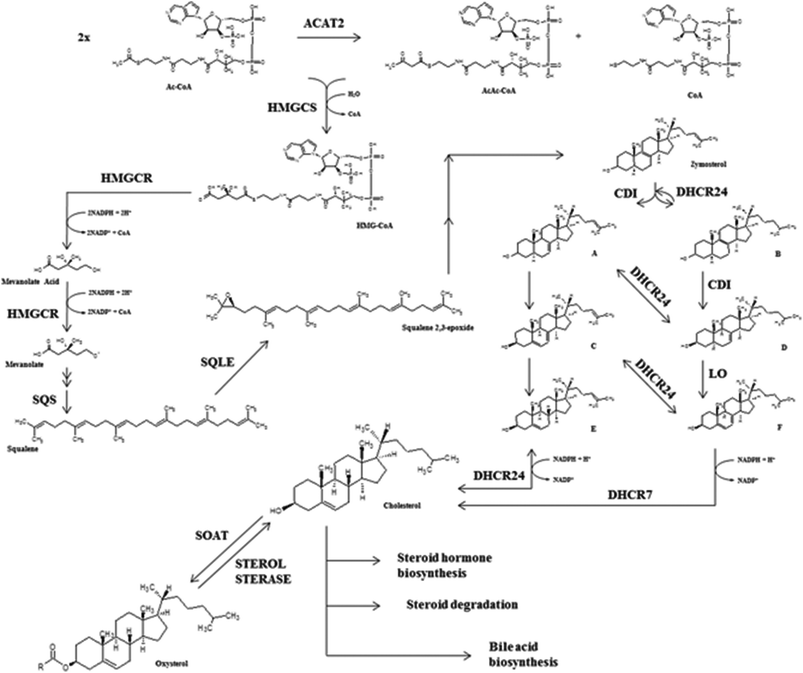
Total blood cholesterol levels are not only dependent on exogenous cholesterol absorption but also on endogenous cholesterol synthesis. Several tissues are involved in de novo cholesterol biosynthesis i.e. enterocytes, adrenal glands, ovaries or testicles but, mostly it is generated by hepatic cells. In fact, one of the liver's main roles is the production of the bile salts from cholesterol as constitutive compounds of biliary fluids needed for the digestion processes while the cholesterol synthesized in adrenal glands or intestine is used respectively as a hormone precursor and a cholesterolemia modulator.11 Cholesterol biosynthesis is carried out by a combination of mevalonate and steroid biosynthetic pathways40 (Fig. 2).3-Hydroxy-3-methylglutaryl coenzyme A reductase (HMGCR) is considered the key enzyme of cholesterol synthesis although the activities of many enzymes involved in the biosynthetic pathway, such as ACAT2, hydroxymethylglutaryl-CoA synthase (HMGCS), delta24-sterol reductase (DHCR24), farnesyl-diphosphate farnesyltransferase (FDFT1/SQS) or 7-dehydrocholesterol reductase (DHCR7), are also susceptible to modulation. Recently, Gill et al. (2011) suggested that squalene monoxygenase (SQLE) might be the second critical modulatory point despite its lower specificity within cholesterol metabolism compared to HMGCR (after in vitro experiments).41
However, other enzymes such as SQS and SQLE are also gaining attention as a potential stop-point of cholesterol biosynthesis because SQS is involved in the transformation of farnesyl pyrophosphate into squalene, being the first specific reaction at the branching point between sterol and non-sterol biosynthesis. The SQS transcriptional product and protein are modulated by cholesterol since low levels of this sterol activate the SQS promoter by sterol regulatory element binding proteins (mostly SREBP2). In contrast, SQS mRNA concentration decreases in response to cholesterol excess. SQLE is a monoxygenase (also named squalene epoxidase) that catalyzes the next step after squalene transformation by SQS, a crucial oxygenation process yielding squalene 2,3-epoxide (Fig. 2). Modulation of SQLE activity seems also cholesterol-dependent and apparently regulation is mediated by proteosome activity.41
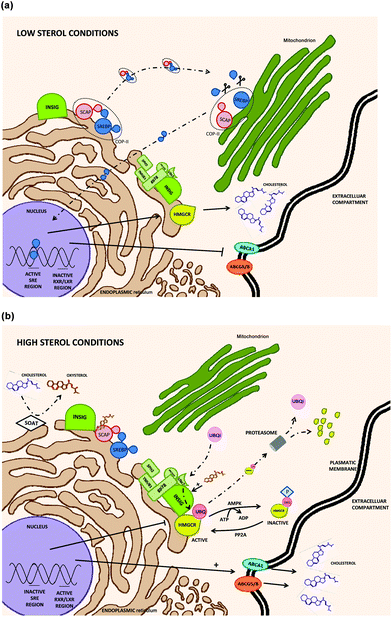
HMGCR includes seven domains inserted in the ER (endoplasmic reticulum) membrane with an active carboxyl chain located at the cytosol. HMGCR is ubiquitously expressed i.e. in immune (white blood cells), nervous, muscle, small intestine or reproductive (ovary cells) human tissues.42 HMGCR transcription and degradation depends on a sterol/non-sterol feedback regulation but is not directly controlled by the cholesterol molecule. Under cholesterol depleted conditions, the SREBP-SCAP complex is formed in the ER membrane (without any INSIG interaction); then, it is transported to GB where SREBP is activated by proteolytic events facilitating its translocation into the nucleus to activate HMGCR transcription (Fig. 3a). In contrast, under cholesterol exceeding conditions, high amount of oxysterols are synthesized by the mitochondrial sterol 27-hydroxylase (CYP27A) and several mechanisms reducing HMGCR transcriptional rates are activated.43 HMGCR regulatory mechanisms could be classified as INSIG-dependent (modulating at transcriptional and post-transcriptional levels), or INSIG-independent.
INSIG-dependent HMGCR regulation mechanisms (Fig. 3b):
- INSIG can disrupt SREBP activation by binding to SCAP when high sterol concentrations are noticed in the cytosol. Once the INSIG-SCAP complex is formed, SCAP is structurally altered impairing SREBP recognition and stopping the assembly of the SREBP-SCAP complex for its further transport from the ER (endoplasmic reticulum) to the GB (Golgi body). In consequence, SREBP is not translocated to the nucleus and HMGCR transcription is inactivated.17,44
- Under similar conditions, INSIG can also bind to the N-terminal region of HMGCR and conjugate it with ubiquitin. Ubiquitation is carried out by gp78 (membrane-bound ubiquitin E3 ligase) assisted by Ubc7 (an E2 ubiquitin conjugating enzyme) providing active ubiquitins and a few other enzymes. Then, ubiquitinated HMGCR is released into the cytosol and subsequently proteosome-degraded with the participation of p97/VCP (ATPase associated to the membrane).
However, the presence of high levels of sterols is not mandatory for the ubiquitination process but it stimulates HMGCR degradation by enhancing INSIG-HMCR bindings.44
INSIG-independent HMGCR regulation mechanisms (Fig. 3b):
- HMGCR activity could be also modulated in situations of cellular stress (low ATP levels) by AMP kinase (AMP-activated protein kinase). In this case, HMGCR is inactivated by a serine phosphorylation due to AMP kinase activity. It is a reversible reaction and HMGCR can also be activated by protein phosphatase 2A (PP2A).44,45
- Non-sterol isoprenoids might also modulate HMGCR translation by a mechanism still unclear, according to Burg et al. (2011).44
2.4. Molecular events occurring during cholesterol excretion
For several decades, classical reverse cholesterol transport (RCT) was considered the main mechanism to eliminate cholesterol; however, recent studies suggest another possible pathway called transintestinal cholesterol excretion (TICE).17RCT is a derivative branch of the hepatobiliary pathway. Lipoproteins such as HDL or LDL make available cholesterol for hepatic absorption as esterified or non-esterified molecules. Esterified cholesterol is transformed by the hepatic cholesteryl ester hydrolase (NCEH1) into the non-esterified form after hydrolysis of the ester linkage. Thus, the generated forms are directly excreted through the ABCG8/5 heterodimer protein or transformed into bile salts.46 CYP7A1 (cholesterol 7-alpha-monooxygenase) is the enzyme responsible for cholesterol transformation into primary bile salts (cholic and chenodeoxycholic acid) within the neutral bile acid pathway in the liver. Synthesized bile salts are secreted to the bile canaliculus by the bile salt export pump (BSEP) or the multidrug resistance-associated protein 2 (MRP2) and become part of bile fluids. Recent in vivo studies have suggested the involvement in the RCT of other cholesterol transporters such as NPC1L120,47 although its role in biliary cholesterol excretion has not yet been elucidated. But, it might involve adjustments in cholesterol balance to avoid excessive loss of the metabolite through the intestinal track.
TICE has been suggested as an alternative cholesterol excretion mechanism where the sterol is directly eliminated from blood through the intestinal mucosa and excreted via the feces.17 The hypothesis was drawn after unexpected results obtained by several authors that showed an unwarranted balance between cholesterol inputs and outputs in mouse models. They showed a higher amount of fecal cholesterol than the sum of dietary intake and biliary secretion48 or an unaltered cholesterol excretion rate in knockout NPC1L1 mice with a decreasing of 90% biliary excretion.49
These results questioned the complete RCT classical concept50 as well as the contribution of biliary or non-biliary cholesterol to its RCT excretion.51 Although the involvement of several membrane transports in TICE have been studied (i.e. SR-BI,52 NPC1L1,53 APOA1,54 LDLR or ABCG5/855), it is still unknown whether TICE is carried out through basolateral or apical transporters or whether HDLs are involved;56 therefore, the mechanism is not yet elucidated. There is also scientific controversy about the importance of TICE; some authors suggest that it could be only a compensatory cholesterol excretion mechanism in case of biliary cholesterol depletion but, other authors indicated it as the main mechanism in cholesterol excretion. Van der Velde et al. (2007, 2010) estimated the contribution of TICE as 70 and 30% of total cholesterol were excreted in mice and humans, respectively.48,56
2.5. Molecular events occurring during cholesterol transport
HDL, IDL (intermediate-density lipoprotein), LDL and VLDL (very low-density lipoprotein) are the connecting structures responsible for transporting cholesterol molecules through the bloodstream from one tissue to another until they are detected by cellular membrane receptors such as SR-B1 for HDL or LDLR (LDL-receptor) for both structures.The non-esterified cholesterol eliminated by ABCA1 through the basolateral membrane of enterocytes is bound to APOA1 generating nascent-HDL and the esterified cholesterol is similarly assembled with APOB48 in ER synthetized pre-chylomicrons further transformed into chylomicrons in GA and excreted by exocytosis to the intracellular space. Therefore, both structures become cholesterol transporters and they distribute it via the bloodstream to the rest of the organism.
Nascent HDL is transformed into mature HDL by the accumulation of non-esterified cholesterol molecules secreted by hepatocyte and enterocyte ABCA1 protein. Once mature HDLs are formed, some cholesterol molecules are esterified by the action of lecithin–cholesterol acyltransferase (LCAT) along with their blood transport. Esterified and non-esterified cholesterol are detected by SR-B1 located in the basolateral membrane of cells from the liver, small intestine or gland adrenals allowing the incorporation of esterified cholesterol inside the cell. Intracellular esterified cholesterol is transformed into non-esterified cholesterol (by SOAT action) and HDL is turned into LDL. The non-esterified cholesterol can be used for bile salt synthesis and their further intestinal secretion by BSEP or to be directly excreted via ABCA1 (basolateral membrane) and ABCG5/ABCG8 (apical membrane) activity. In turn, secreted non-esterified cholesterol again could be attached to nascent HDL to create mature HDL and continue with cholesterol transport.
LDL molecules are recognized by the liver, intestine and adrenal gland LDLRs as esterified cholesterol suppliers. LDL particles can be generated not only by VLDL transformation (by SR-B1 activity) but also by the addition of esterified cholesterol to APOB100 (apolipoprotein isoform characteristic of hepatocytes) leading to very low density lipoproteins (VLDL). These structures are secreted to the bloodstream by hepatocytes. VLDL lipids are used by the muscle and peripheral tissues as they pass through the bloodstream generating IDL and LDL by lipoprotein lipase (LPL) activity. Moreover, the esterified cholesterol of chylomicron structures is also recognized by LDLR providing to the hepatocyte those cholesterol molecules assembled in enterocytic ER after the digestion process.
The complex cholesterol biosynthesis and absorption engineering and the multifactorial regulation system make the control of cholesterol metabolism a difficult challenge for the scientific community, particularly because some of the involved compounds are also intermediates of other metabolic pathways i.e LXR modulates ABCG5/8 activity but also DIO1, a selenoprotein involved in thyroid hormone metabolism. However, these factors also make it a flexible system that could be modulated from different critical points.
3. Fungal molecules modulating cholesterol metabolism
Many food compounds have been described as modulators of cholesterol homeostasis interacting with specific control points and inhibiting or altering cholesterol metabolism; even some in vivo studies suggest synergistic hypocholesterolaemic activities between several inhibitors.57–59 Those compounds showed different targets depending on whether they modulate cholesterol absorption, synthesis, excretion or transport.The intake of dietary fibers (DF) from cereals etc., increases cholesterol and bile acid excretion rates lowering their bioavailability because they might act as bile acid scavengers. This mechanism impairs bile acid re-absorption stimulating the hepatic synthesis of new bile acids from cholesterol reducing its blood concentration10,60,61 although for particular β-glucans, modulation of cholesterol-related gene expressions have been also noticed.62
Plant sterols (phytosterols and phytostanols) are considered direct cholesterol competitors for their incorporation into DMMs because of their structural similarity and due to the limited capacity of DMMs to solubilize lipophilic and water-insoluble molecules.63–65 Some reports also suggested that phytosterols might modulate SOAT activities although it still remains partially unclear. Cholesterol enterocytic esterification by ACAT(AvS) was decreased by sterol competition although the enzyme showed lower esterification efficiency for plant sterol than cholesterol. Other authors suggested that plant sterols influenced MTTP and APOB48 lipoprotein in vitro models.65
Compounds such as novel amino β-lactam derivates66 or curcuminoid polyphenols were described as potential NPC1L1 inhibitiors by showing high binding affinity or acting by indirect influence on SREBP1.37 ABCG5/8 postranscriptional regulations were also described for spironolactones or polyphenols from Aronia melanocarpa.67,68
Other compounds from natural sources have been reported as ACAT(AvS) inhibitors reducing cholesterol esterification rates such as alkamides from Piper nigrum,69 shikonin derivatives from Lithospermum erythrorhizon,70 an isoprenyl flavonoid identified as grabol from licorice roots,71 ursolic acid (indirect inhibition via (PPAR)-α activation)72 or flucoxanthin from marine plants.73
3.1. Hypocholesterolemic compounds of edible mushrooms
A few specific bioactive compounds are already indicated as responsible for the hypocholesterolaemic properties of mushrooms. The most extensively investigated are fungal sterols (ergosterol and its derivatives such as ergost-22-ene-1,3-diol, ergosta-5,7-dien-3b-ol, (22E)-ergosta-1,4,6,22-tetraen-3-one, etc.) and β-glucans together with other dietary fibres.Ergosterol (ergosta-5,7,22-trien-3β-ol) is considered the major fungal sterol (from 53% up to 80% of the fungal sterols, w/w) followed by ergosterol derivatives such as ergosta-5,8,22-trien-3-ol, ergosta-7,22-dien-3-ol, ergosta-5,7-dien-3-ol and ergosta-7-en-3-ol (fungisterol).74 However, their precise concentration in the different mushroom strains was dependent on environmental conditions (developmental stage, cultivation conditions, etc.) or species. For instance, Chantharellus cibarius and Craterellus cornucopioides showed almost exclusively ergosterol, while other species showed more ergosta-5,7-dienol than fungisterol (Lyophyllum shimeji or Pleurotus ostreatus). Flammulina velutipes contained high amounts of ergosta 5,8,22-trien-3β-ol.10
Apparently, the hypocholesterolaemic activities of fungal sterols were mainly due to their structural similarity with cholesterol as noticed for plant sterols. Therefore, these compounds such as phytosterols and phytostanols were able to hinder cholesterol incorporation into DMMs. Ergosterol-enriched fractions obtained using green technologies, such as supercritical fluid extraction (SFE), were more effective than β-sitosterol in displacing cholesterol from DMMs during an in vitro digestion model.59 Moreover, fungal sterol-enriched extracts also showed HMGCR inhibitory activities in vitro75 and ergosterol was also considered as a competitive inhibitor of C24-reductase due to its double bond at C-22 in the side chain of its structure (as also noticed for stigmasterol and brasicasterol). C24-Reductase is another enzyme involved in the cholesterol biosynthetic pathway down-stream from HMGCR.76
Fungal β-glucans showed different molecular structures from cereal polysaccharides. Their branching profiles are (1→3) and (1→3)(1→6) conferring on them many biological activities such as antitumoral, antioxidant, immunomodulatory, etc. apart from hypocholesterolaemic properties.10,77,78 Some reports suggested that those bioactive properties are due to their different tridimensional configuration depending on their monomer composition, branching degree or conformation while other authors considered their water solubility (that is also partially dependent on their glycosidic linkages) a more relevant factor, considering those water-insoluble as responsible for the cholesterol lowering activities by impairing cholesterol absorption. Moreover, other publications indicated β-glucan degradation products (generated i.e. after a digestion process) as hypocholesterolemic molecules more bioactive than their larger precursors.10,79 However, until now the precise structural requirements for the observed hypocholesterolemic action of fungal β-glucans remain unclear awaiting further studies.
β-Glucans from a large number of mushroom species have been studied such as lentinan from shiitake mushrooms (Lentinula edodes), schizophyllan from Schizophyllum commune, grifolan from Grifola frondosa, and many others from Agaricus blazei, Ganoderma lucidum (a medicinal mushroom consumed as a nutraceutical or dietary supplement), and P. ostreatus as well as (1→3) β-glucans and glucuronoxylomannans from Auricularia polytricha and Tremella fuciformis.80,81 All showed different conformations, and solubilities and therefore biological properties.
Chitin, a special type of water-insoluble β-(1→4)-glucan including N-acetylglucosamine monomers, is also considered a hypocholesterolaemic polysaccharide while its derivative, named chitosan, was more studied because of its antitumor and immunomodulation activities.10,77 Several polysaccharide fractions (containing high percentages of fungal β-, α-glucans and chitooligosacharides among others) from L. edodes, P. ostreatus and A. bisporus extracted by using pressurized technologies (PSE) showed bile acid binding capacities in vitro similar to those shown by cereal fibers82 indicating that this might impair cholesterol absorption as previously suggested for plant β-glucans.
According to suggestions by Gunde-Cimerman et al. (1993, 1995), lovastatin an inhibitor of HMGCR (3-hydroxy-3-methylglutaryl-Coenzime A reductase) was naturally present in some Pleurotus spp. fruiting bodies such as P. ostreatus or P. eryngii83,84 and in other species such as A. bisporus or B. edulis. However, recent studies did not detect any statin in mushroom species showing interesting HMGCR inhibitory activity pointing to some water soluble polysaccharides and proteoglucans as responsible for the noticed in vitro inhibition.10,75
HMGCR inhibition was also mediated by an AMP kinase via phosphorylation and green and black tea polyphenols induced a direct increase of HMGCR phosphorylation possibly via AMP kinase phosphorylation. Their precise mechanism of action is still unclear but, it seems to involve the activation of regulatory factors such as PPAR.85,86
Only a few compounds are still nowadays indicated as potential inhibitors of SQS such as resveratrol from wine87 as well as zaragozic acids isolated from the liquid broth of certain ascomycetes.88
24(S),25-Epoxycholesterol was also pointed out as an inhibitor of DHCR24 activity. This enzyme catalyzes the transformation of desmosterol into cholesterol. The inhibitor did not modify DHCR24 protein levels, but increased desmosterol accumulation decreasing cholesterol levels in in vitro studies due to its structural similarity with desmosterol.89
3.2 Molecular events modulated by mushroom extracts
The presence of certain natural molecules in mushrooms does not only modify cholesterol absorption or the metabolic pathway of consumers but can also modulate the expression of some genes related to cholesterol homeostasis. Recent studies carried out on particular edible mushrooms studied their influence as an attempt to further identify the most interesting fungal compounds to treat moderate hypercholesterolemia.Mushrooms such as Pleurotus ostreatus, Grifola frondosa and Hypsizigus marmoreus were able to differently modulate the gene expression patterns of livers of mice fed with each mushroom (10–14%) for 4 weeks. Triglyceride levels in the liver and plasma decreased in the mice fed with P. ostreatus compared with those in the control group. Moreover, liver cholesterol decreased while plasma total cholesterol increased probably due to HDL values that were also increased. Cholesterol in the liver was lower in the group fed with G. grondosa than in the control group but no changes were found in the H. marmoreus-fed group. DNA microarray analysis of the livers revealed that CTP1A and FABP families were upregulated in the P. ostreatus-fed group, which were considered to promote lipid transport and β-oxidation. In the G. frondosa-fed group, not only the gene involved in signal transduction of innate immunity via TLR3 and interferon but also virus resistance genes (such as MX1, RSAD2 and OAS1) were upregulated.90
Administration of Agaricus brasiliensis (also known as Agaricus blazei) to hypercholesterolemic albino Fischer rats during 6 weeks lowered cholesterol levels in serum and induced significant changes in the expression of cholesterol-related genes. HMGCR mRNA expression was not influenced but LDLR upregulation was noticed together with the upregulation of CYP7A1, the rate-limiting enzyme for bile acid synthesis, and mRNA levels of the ABCG5/8 carriers. These increases were accompanied by a significant increase in the content of cholesterol excreted in the faeces and by a concomitant increase in NR1H3 (LXR) mRNA levels. However, in this case PPAR-α was not significantly upregulated compared with levels noticed in the hypercholesterolemic control group.91
In a few reports specific extracts, and not the whole fruiting body, were studied. For instance, ethanol (95%) extracts obtained from P. ostreatus were administered to hypercholesterolemic male Wistar rats for 14 days and a reduction of triglycerides and HDL cholesterol was noticed in the plasma. Moreover, the extract was able to upregulate the genes that were downregulated after hypercholesterolemia induction such as those related to fatty acid oxidation such as acyl CoA oxidase (ACO) and synthetase (ACS) together with carnityl palmityl transferase-1 (CPT-1) and PPAR-α. They also downregulated genes related to fatty acid biosynthesis and cholesterol metabolism (FAS fatty acid synthase, SREBF1, APOC3) to similar expression than during normocholesterolemia.92
Hericium erinaceus hot water and ethanol extracts were administered, together with a high-fat diet, to C57BL76J mice for 4 weeks. Incorporation of the extracts in the diet resulted in a significant decrease in body weight, fat and serum and hepatic trigliceride levels compared to control. The ethanol extract acted as an agonist of PPAR-α since it was able to up-regulate mRNAs usually modulated by PPAR-α (ACAT, ApoA1, LPL or SREBP1) in spite of the fact that the PPAR-α expression itself did not change.93
An extract obtained from the cauliflower mushroom (Sparassis crispa) significantly enhanced hepatic cholesterol catabolism when administered to male Sprague-Dawley rats fed a cholesterol-rich diet for 4 weeks because they were able to up-regulate CYP7A1 mRNA expression concomitant with HMGCR downregulation after. Additionally, the extract supplementation resulted in cholesterol and bile acid fecal excretion.94
3.3 Molecular events modulated by fungal sterols
Since plant and fungal sterols share structural similarities, similar modulation of cholesterol related genes might also be expected. However, in the few studies carried out using fungal sterols different effects were noticed.Ergosterol was able to regulate sterol regulatory element binding protein (SREBP) cleavage in yeast as response to cellular oxygen levels95 suggesting that it might also modulate these elements in mammalians. Studies carried out using cell cultures revealed that DMMs isolated from the in vitro digestion of ergosterol and extracts containing ergosterol mixed with cholesterol did not influence the transcriptional levels of SREBF1 differently than when only cholesterol was administered. Moreover, only a slight inhibition was noticed for SREBF2 mRNA of Caco2 cells when treated with an ergosterol-containing extract but not with ergosterol itself. However, other cholesterol related genes were also overexpressed such as LDLR (when an ergosterol-enriched extract from Agaricus bisporus was applied).15 When the lower compartment of the Caco2 cells supplemented with the sterol-containing digested extracts were added to HepG2 cells, higher modulation of genes related to lipid metabolism was noticed than those more directly related to cholesterol homeostasis such as DGAT2 upregulation.
When similar extracts were given for 4 weeks to C57BL/6JRj mice previously fed for 4 weeks with hypercholesterolemic diets, inhibition of SREBF2 and NR1H4 (gene enconding FXR) were noticed in the jejunum up to similar levels than hypocholesterolemic drugs such as ezetimibe and simvastatin.15 This downregulation was due to their ergosterol content because supplementation with purified ergosterol induced similar modulation. FXR is the farnesoid X receptor, a nuclear receptor also involved in the regulation of cholesterol homeostasis.96 FXR is involved in the activation or inhibition of bile acid synthesis and transport acting as a sensor of their concentration.97 The extract induced the downregulation of DGAT2 in the liver as observed in cell cultures but upregulation of FDFT1 might be to compensate for the reduction of the ratio TC/HDL recorded. Reduction of the triglyceride levels was also noticed in the liver, perhaps because of the observed modulation of DGAT2 gene transcription. Moreover, this extract induced the overexpression of DIO1 mRNA in the jejunum, the tissue where cholesterol absorption is higher.98 Type I iodothyronine deiodinase is a selenoprotein encoded by the DIO1 gene and plays a major role in normal thyroid function, but it also indirectly influences cholesterol homeostasis by regulating LDLR gene expression. Increased DIO1 activity in the liver correlated with up-regulation of LDLR mRNA and lowering of TC, TG and LDL levels in serum.15 However, no significant effect was noticed by Gil-Ramirez et al., 201598 on the latter gene; perhaps the supplemented concentration was sufficient to stimulate DIO1 but it did not revert on LDLR readjustments. In contrast, ergosterol administered as a single compound induced the downregulation of DIO1 mRNA; therefore the effect of the ergosterol enriched extract could be due to some other sterol or compound present in the extract.98
However, when ergosterol containing extracts were incorporated into lard and the mixture was supplemented together with the diet to induce hypercholesterolemia, insignificant effects were noticed on any SREBP, other cholesterol regulatory nuclear receptor or genes involved in cholesterol metabolism except for ABCG5 and 8 mRNAs expression in cecum that were up-regulated. Perhaps the addition of the extract as an ingredient inside a lipid matrix prevented their proper bioavailability (because it was also unable to lower cholesterol levels in serum, HDLc or LDLc) or perhaps the beneficial properties of ergosterol were not sufficient to overcome the detrimental effect of lard and the hypercholesterolemic diet when administered simultaneously.99
The transcription of some ABC transporters (ABCG5/8 etc.) was induced by the LXR factor in enterocytes (but not in hepatocytes)65 although, there is a controversy about its influence on other transporters (ABCA1). Oxysterols such as 22(R)-hydroxycholesterol, 24(S),25-epoxycholesterol or 27-hydroxycholesterol, etc., are considered as endogenous natural LXR agonists; however, plant sterol derivatives showed higher LXR agonist activity. Brasicasterols from unicellular algae and Brassica sp. (i.e. rapeseed) induced large variations in the gene expression of ABC transporters due to their ability to act as the LXR factor in mice. Moreover, sitostanol (in mice), sitosterol (in Caco2 cells) and a few 4-desmethylsterol derivatives were also able of inducing ABCA1 up-regulation using their LXR agonist activity.5,11,13,65 However, no direct effects of phytosterols or fungal sterols were noticed on the transcriptional levels of the transporters because, on the one hand, in ABCA1 and ABCG5/8-deficient mice decreasing of cholesterol intestinal absorption was also noticed after phytosterol administration indicating that ABC transporters were not their direct targets. On the other hand, no significant overexpression or repression was noticed in hypercholesterolemic C57BL/6JRj mice treated with ergosterol-containing extracts.15
Other reports indicated that phytosterol/stanols modulated HMGCR expression via ACAT(AvS) inhibition. Apparently, lower ACAT(AvS) activity led to higher free cholesterol amounts, inhibiting the cholesterol biosynthetic pathway and HMGCR expression beside others. It also reduced chylomicron assembling, and promoted the back efflux of non-esterified sterols to the lumen.11,65,100 However, their influence on SOAT seemed to be by chemical inhibition more than by molecular modulation. Direct down-regulation of HMGCR mRNA (together with inhibition of the enzyme) was only noticed in organic extracts obtained from the Reishi mushroom (Ganoderma lucidum) containing oxygenated lanosterol derivatives. They also inhibited cholesterol synthesis in T9A4 hepatocytes and reduced total cholesterol in hamsters.101
Several in vitro studies pointed out that SREBP2, NPC1L1 and SR-B1 gene expression was modulated by plant sterols such as stigmasterol and β-sitosterol toward the reduction of cholesterol absorption. Surprisingly, HepG2 cells treated with these sterols showed simultaneous down-regulation of NPC1L1 and SR-B1 when an opposite effect on these two molecules could be expected. This modulation was not noticed when fungal sterols were applied to hepatic cells15 or when administered to mice together with a hypercholesterolemic diet.99 Studies using homozygous and heterozygous knockout mice (NPC1L1−/− and NPC1L1+/−) showed a lower cholesterol absorption in homozygous animals; however, heterozygous mice showed higher HMGCR mRNA levels in gut and liver tissues than wild-type animals without changes in ABC transporter expression rates. Authors explained their results by compensatory effects: difficulties for cholesterol absorption were compensated by a stimulation of endogenous cholesterol synthesis to maintain physiological plasma levels.102,103 Moreover, when the knockout mice were treated with plant sterols or ezetimibe, the wild-type and heterozygous animals showed a reduction in cholesterol and TG levels. Absorption of campesterol and β-sitosterol was reduced in NPC1L1+/− and almost absent in NPC1L1−/− mice indicating that certain modulation of NPC1L1 took place.102,103 However, no modulation of NPC1L1 expression was noticed in C57BL/6 mice fed a fungal sterol extract together with a hypercholesterolemic diet.99
Moreover, β-sitosterol addition to Caco2 cultures induced the down-regulation of HMGCR expression although this effect was not noticed in mice. Only sitosterolemic individuals showed reduction in HMGCR activity in the ileum; so apparently only large amounts of plant sterols or long-term 2% (w/w) plant sterol administration can induce such an effect.11,104
Other reports indicated that phytosterols might modulate the expression of other closely related genes such as those encoding the hepatic cholesterelogenic farnesyl phosphate synthase (FFPS), liver CYP7A1 (cytochrome P450 family 7 subfamily A polypeptide 1) or the annexin 2-caveolin 1 (ANXA2-CAV1) protein complex.11 Therefore, fungal sterols might be involved in the modulation of CYP7A1 noticed during the administration of the complete mushroom fruiting bodies to hypercholesterolemic mice.91
3.4 Molecular events modulated by fungal polysaccharides
At present, not many studies have been carried out concerning the molecular effect of dietary fibers independently of their plant or fungal origin. However, already in 1996, reports by Cheung suggested that fungal polysaccharide extracts could modulate cholesterol related enzyme activity (HMGCR) by several mechanisms and not only via direct inhibition. Later on, several studies were carried out to investigate the molecular role of fungal polysaccharides on cholesterol metabolism.78Fukushima et al. (2000)105 examined the effects of a fiber extract obtained from Agaricus bisporus on LDLR mRNA expression after feeding rats with cholesterol-free diets during 4 weeks. Despite a higher relative liver weight in the control group (cellulose supplementation) compared with those animals fed with the mushroom fiber extract as supplement, serum total cholesterol, VLDL and LDL concentrations in the control group were significantly greater than in animals fed the A. bisporus fiber. Added to this, once LDLR mRNA hepatic levels were analyzed, results showed that mushroom LDLR up-regulation was significantly higher than that induced by cellulose fiber supplement.105 One year later, the same group reported that maitake (Grifola frondosa) and enokitake (Flammulina velutipes) fiber extracts lowered total serum cholesterol levels by two mechanisms, by scavenging cholesterol molecules inducing their fecal excretion and by enhancement of LDLR mRNA expression in rat liver.106 However, no up-regulation was significantly noticed when rats were fed shiitake (Lentinula edodes) fiber extracts.
Dietary fibre fractions from P. ostreatus, L. edodes and A. bisporus fruiting bodies obtained as described by Jeurink et al. (2008)107 including a low α-glucan amount and high concentrations of chitin derivatives and other β-glucans82 were also added to Caco2 cells to study their effect on the most interesting cholesterol-related genes. The transcriptomic profile was studied after 1 and 24 h application and results indicated that the fiber extracts obtained from P. ostreatus were able to modulate the transcription of more genes related to cholesterol metabolism than the other two mushrooms studied at longer incubation times. Up-regulation of FDFT1 and NPC1L1 was noticed together with slight modulation of a few other mRNAs; therefore, in vivo experiments were carried out using P. ostreatus fiber extract using two different experimental settings. In the first one, C57BL/6J mice were firstly administered hypercholesterolemic diets and then, supplemented with the fiber extract (as palliative treatment) and in the second experiment, mice were simultaneously fed with hypercholesterolemic diet plus fiber extract (preventive treatment). Results indicated that their molecular responses were completely dependent on the supplementation setting. In the palliative setting, administration of the fiber extract reduced hepatic triglyceride levels and it might be because of the DGAT1 downregulation also recorded. In the preventive setting, the fiber extract modulated cholesterol-related gene expression similar to simvastatin and ezetimibe in the liver (i.e. by inhibiting FDFT1, NR1H3 (LXR) and NR1H4 (FXR) mRNA expression) although no changes in plasma and liver biochemical data were recorded.108 Later on, when a similar extract was applied in a higher concentration and mixed with lard, a hypocholesterolemic effect was noticed (as reduction of TC and LDLc levels) but no relevant modulation of cholesterol-related gene transcription was noticed despite the overexpression of NPC1L1 in mice liver and jejunum.99
The molecular modulations noticed for fungal β-glucans seemed to be different from other dietary fibers obtained from plants i.e. high viscosity oat or barley β-glucan extracts demonstrated their ability to down-regulate SREBF2 gene expression in intestinal cells (NCl-H716)62 but no with in vivo testing. However, according to Hu, Wang & Xu (2008) corn bran dietary fiber up-regulated the expression of other genes such as FXR in ileal cells or PPAR in liver.109
Hepatic HMGCR up-regulation was observed after the administration of hydroxylpropylmethycellulose or inulin-oligofructose (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to animal models;110,111 however, barley β-glucan administration induced no changes in HMGCR expression.112 Jones (2008)112 noticed hepatic SREBF2 up-regulation when soluble dietary fiber (guar gum, a galactomannan) was administered to pigs. In consequence, LDLR expression was enhanced and reduced the LDLs from the bloodstream. Fungal fibers instead seemed to modulate LDLR mRNA expression directly.105,106 Hepatic ABCG5/8 gene expression was also enhanced by guar gum consumption leading a higher cholesterol efflux from the liver to the intestinal lumen.112 A similar enhancement together with ABCA1 mRNA was noticed in jejunum from mice administered P. ostreatus fibers (palliative treatment) but without significant cholesterol reduction.108
1) to animal models;110,111 however, barley β-glucan administration induced no changes in HMGCR expression.112 Jones (2008)112 noticed hepatic SREBF2 up-regulation when soluble dietary fiber (guar gum, a galactomannan) was administered to pigs. In consequence, LDLR expression was enhanced and reduced the LDLs from the bloodstream. Fungal fibers instead seemed to modulate LDLR mRNA expression directly.105,106 Hepatic ABCG5/8 gene expression was also enhanced by guar gum consumption leading a higher cholesterol efflux from the liver to the intestinal lumen.112 A similar enhancement together with ABCA1 mRNA was noticed in jejunum from mice administered P. ostreatus fibers (palliative treatment) but without significant cholesterol reduction.108
Other studies indicated that the scavenging of bile acids by plant fiber (β-glucans) activated CYP7A1 to convert cholesterol into new bile acids109,113 provoking a hepatic cholesterol decrease that up-regulated LDLR expression and reduced cholesterol blood levels. Due to these changes, the biosynthetic pathway was also activated via HMGCR up-regulation to compensate for the lack of hepatic cholesterol.113,114 However, the effect of fungal fibers on CYP7A1 is still, at the present, unknown.
Other studies were carried out using extracts containing water soluble polysaccharides from L. edodes. This fraction contained α- and β-glucans and fucomannogalactans that when digested (in vitro) were applied to Caco2 cell cultures and no significant effect was noticed on the modulation of cholesterol-related gene expression. But, when the lower compartment of the cell monolayer was applied to HepG2, modulation of some mRNAs was noticed after 24 h incubation. However, the modulatory pattern fitted more with a low cholesterol level response since enzymes such as HMGCR, FDFT1 and ACAT1 were overexpressed; perhaps the HMGCR inhibitory activity showed by this extract induced a posttranscriptional cholesterol reduction that activated the biosynthetic pathway after 24 h.115 Later on, when this extract was administered to normocholesterolemic mice for 4 weeks similar overexpression of HMGCR (in liver) and FDFT1 (in jejunum) was noticed. However, when the extract was administered to hypercholesterolemic mice the modulation profile showed similarities with those generated after the administration of ezetimibe, simvastatin or both such as for instance the down-regulation of FDFT1 (in the ileum), SREBF2 or NR1H4 (in the jejunum) or the up-regulation of ABCG8 or ACAT1 in the jejunum.
Other polysaccharides extracted from 3 different strains of Pleurotus tuber-regium, when administered to obese-diabetic rats for 8 weeks also showed a hypolipidemic effect that was associated with up-regulated liver PPAR-alpha mRNA expression and protein levels. Moreover, hyperglycemia was also attenuated by polysaccharides; the elevated serum total cholesterol, triglycerides and low-density lipoprotein (LDL) concentrations were controlled, and parallel restoration of decreased high-density lipoprotein (HDL) levels was noticed after their supplementation.116
3.5 Molecular events modulated by other fungal compounds
An adenosine analogue alkaloid (eritadenine) is another compound from L. edodes able to inhibit S-adenosylhomocysteine hydrolase, a key enzyme of hepatic phospholipid metabolism. This inhibition could be related to the lowering of cholesterol levels in serum noticed in animal studies.117,118 Eritadenine increases hepatic microsomal phosphatidylethanolamine (PE) concentration and decreases liver microsomal Δ6-desaturase activity, altering the fatty acid and molecular species profile in liver and plasma. When it was administered to rats, suppression of Δ6-desaturase activity was accompanied by a significant reduction in the abundance of mRNA for the enzyme suggesting that dietary eritadenine might suppress the activity of liver microsomal Δ6-desaturase by altering the microsomal phospholipid profile and this effect was mediated by the regulation of enzyme transcription.119 Eritadenine was also involved in the up-regulation of the CYP7A1 expression noticed in the liver of hypercholesterolemic mice fed the standard compound or 5, 10 or 20% L. edodes.120Water extracts obtained by PWE (pressurized water extractions) from normal and selenium-enriched A. bisporus were tested in vitro as HMGCR inhibitors. Selenium supplementation enhanced the inhibitory activity of statins and therefore, the latter extracts might improve their HMGCR inhibitory capacity. However, no significant differences were found; both similarly inhibited the enzyme only if the extracts were not thermally treated. When the extracts were applied to HepG2 cells to study their effect at the molecular level they also induced similar responses in most of the cholesterol-related genes. However, after 1 h application overexpression of LDLR mRNA was noticed in non Se-fortified extracts. After 24 h LDLR mRNA was downregulated together with FDFT1. Similar downregulation of the SQS mRNA was also noticed in Se-fortified extracts indicating that non-thermal water extracts from A. bisporus could inhibit transcription of this enzyme that is also a key enzyme downstream the cholesterol metabolic pathway.121 Reduced expression of GPX3 (glutathione peroxidase, a selenoprotein largely influenced by the presence of selenium in the media) was shown to increase the cell-mediated oxidation of LDL and selenium supplementation (1 ppm) also induced downregulation of APOB and HMGCR expression during hypercholesterolemia in rat models. However, no significant modulation of GPX398 and APOB121 expression was noticed either in non- or Se-fortified extracts; only a slight up-regulation of HMGCR after 1 h for non-fortified extracts and after 24 h for Se-fortified ones perhaps to compensate the inhibition of the FDFT1 transcription was noticed.121
4 Conclusion
Consumption of edible mushrooms or functional foods containing specific fungal extracts should be encouraged among people with low to moderate hypercholesterolemia (before the use of pharmaceutical drugs) to lower their cholesterol levels in serum. As noticed for plants, cereals and other food derivatives, they contain specific compounds that can modulate cholesterol homeostasis via different transcriptional and post-translational mechanisms that are nowadays not completely understood but that they could be different than those described for plants. Thus, more studies are necessary to broaden the knowledge toward the molecular effect of fungal compounds on human health since the results obtained up to now are promising, suggesting that their use as hypocholesterolemic foods might be more effective than those products actually offered in the markets.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the national R + D program from the Spanish Ministry of Science and Innovation (projects AGL2010-21537 and AGL2014-56211-R) and the regional program from the Community of Madrid, Spain (S2013/ABI-2728).References
- F. Araujo, C. Gouvinhas, F. Fontes, C. La Vecchia, A. Azevedo and N. Lunet, Trends in cardiovascular diseases and cancer mortality in 45 countries (1980-2010), Eur. J. Prev. Cardiol., 2014, 21, 1004–1017 CrossRef PubMed.
- I. Graham, D. Atar, K. Borch-Johnsen, G. Boysen, G. Burell, R. Cifkova, J. Dallongeville, G. De Backer, S. Ebrahim, B. Gjelsvik, C. Herrmann-Lingen, A. Hoes, S. Humphries, M. Knapton, J. Perk, S. G. Priori, K. Pyorala, Z. Reiner, L. Ruilope, S. Sans-Menendez, W. S. O. Reimer, P. Weissberg, D. Wood, J. Yarnell and J. L. Zamorano, European guidelines on cardiovascular disease prevention in clinical practice: Executive summary, Atherosclerosis, 2007, 194, 1–45 CrossRef CAS PubMed.
- S. M. Grundy, D. Becker, L. T. Clark, R. S. Cooper, M. A. Denke, W. J. Howard, D. B. Hunninghake, R. Illingworth, R. V. Luepker, P. McBride, J. M. McKenney, R. C. Pasternak, N. J. Stone, L. Van Horn, H. B. Brewer, J. I. Cleeman, N. D. Ernst, D. Gordon, D. Levy, B. Rifkind, J. E. Rossouw, P. Savage, S. M. Haffner, D. G. Orloff, M. A. Proschan, J. S. Schwartz, C. T. Sempos, S. T. Shero, E. Z. Murray, S. A. Keller and A. J. Jehle, Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report, Circulation, 2002, 106, 3143–3421 CrossRef.
- D. S. Goodman, S. B. Hulley, L. T. Clark, C. E. Davis, V. Fuster, J. C. LaRosa, A. Oberman, E. J. Schaefer, D. Steinberg, W. V. Brown, S. M. Grundy, D. Becker, E. Bierman, J. Sooter-Bochenek, R. Mullis, N. Stone, D. B. Hunninghake, J. M. Dunbar, H. N. Ginsberg, R. Illingworth, H. C. Sadin, G. Schonfeld, J. I. Cleeman, B. Brewer Jr., N. Ernst, W. Friedewald, J. M. Hoeg, B. Rifkind and D. Gordon, Report of the National Cholesterol Education Program Expert Panel on Detection, Evaluation and Treatment of high blood cholesterol in adults, Arch. Intern. Med., 1988, 148, 36–69 CrossRef.
- E. Kaneko, M. Matsuda, Y. Yamada, Y. Tachibana, I. Schimomura and M. Makishima, Induction of intestinal ATP-binding cassette transporters by a phytosterol derived liver X receptor agonist, J. Biol. Chem., 2003, 278, 36091–36098 CrossRef CAS PubMed.
- S. Rozner and N. Garti, The activity and absorption relationship of cholesterol and phytosterols, Colloids Surf., A, 2006, 282–283, 435–456 CrossRef.
- C. S. Brennan and L. J. Cleary, The potential use of cereal (1→3, 1→4)-β-D-glucans as functional food ingredients, J. Cereal Sci., 2005, 42, 1–13 CrossRef CAS.
- F. Zhong, X. Zhang, J. Ma and C. F. Shoemaker, Fractionation and identification of a novel hypocholesterolemic peptide derived from soy protein Alcalase hydrolysates, Food Res. Int., 2007, 40, 756–762 CrossRef CAS.
- S. Nagaoka, Y. Futamura, K. Miwa, T. Awano, K. Yamauchi, Y. Kanamaru, K. Tadashi and T. Kuwata, Identification of novel hypocholesterolemic peptides derived from bovine milk beta-lactoglobulin, Biochem. Biophys. Res. Commun., 2001, 281, 11–17 CrossRef CAS PubMed.
- A. Gil-Ramirez and C. Soler-Rivas, The use of edible mushroom extracts as bioactive ingredients to design novel functional foods with hypocholesterolemic activities. Chapter 2, in Mushrooms: Cultivation, Antioxidant Properties and Health Benefits, ed. G. Pesti. Nova Science Publishers, Inc., New York, 2014, pp. 43–73, ISBN: 978-1-63117-521-3 Search PubMed.
- L. Calpe-Berdiel, J. C. Escola-Gil and F. Blanco-Vaca, New insights into the molecular actions of plant sterols and stanols in cholesterol metabolism, Atherosclerosis, 2009, 203, 18–31 CrossRef CAS PubMed.
- Y. Park and T. P. Carr, Unsaturated fatty acids and phytosterols regulate cholesterol transporter genes in Caco-2 and HepG2 cell lines, Nutr. Res., 2013, 33, 154–161 CrossRef CAS PubMed.
- T. Sudhop, D. Lutjohann and K. von Bergmann, Sterol transporters: target of natural sterols and new lipid lowering drugs, Pharmacol. Ther., 2005, 105, 333–341 CrossRef CAS PubMed.
- P. Costet, Molecular pathways and agents for lowering LDL-cholesterol in addition to statins, Pharmacol. Ther., 2010, 126, 263–278 CrossRef CAS PubMed.
- A. Gil-Ramirez, V. Caz, R. Martin-Hernandez, F. R. Marin, C. Largo, A. Rodriguez-Casado, M. Tabernero, A. Ruiz-Rodriguez, G. Reglero and C. Soler-Rivas, Modulation of cholesterol-related gene expression by ergosterol and ergosterol-enriched extracts obtained from Agaricus bisporus, Eur. J. Nutr., 2016, 55, 1041–1057 CrossRef CAS PubMed.
- J. K. Kruit, A. K. Groen, T. J. van Berkel and F. Kuipers, Emerging roles of the intestine in control of cholesterol metabolism, World J. Gastroenterol., 2006, 12, 6429–6439 CrossRef CAS PubMed.
- M. Y. M. van der Wulp, H. J. Verkade and A. K. Groen, Regulation of cholesterol homeostasis, Mol. Cell. Endocrinol., 2013, 368, 1–16 CrossRef CAS PubMed.
- J. Y. Chiang, Bile acids: regulation of synthesis, J. Lipid Res., 2009, 50, 1955–1956 CrossRef CAS PubMed.
- J. Geyer, T. Wilke and E. Petzinger, The solute carrier family SLC10: more than a family of bile acid transporters regarding function and phylogenetic relationships, Naunyn Schmiedeberg's Arch. Pharmacol., 2006, 372, 413–431 CrossRef CAS PubMed.
- A. Dikkers and U. J. Tietge, Biliary cholesterol secretion: more than a simple ABC, World J. Gastroenterol., 2010, 16, 5936–5945 CAS.
- A. van Bennekum, M. Werder, S. T. Thuanhai, C. H. Han, P. Duong, D. L. Williams, P. Wettstein, G. Schulthess, M. C. Phillips and H. Hauser, Class B scavenger receptor-mediated intestinal absorption of dietary beta-carotene and cholesterol, Biochemistry, 2005, 44, 4517–4525 CrossRef CAS PubMed.
- S. D. Turley and J. M. Dietschy, Sterol absorption by the small intestine, Curr. Opin. Lipidol., 2003, 14, 233–240 CrossRef CAS PubMed.
- L. Jia, J. L. Betters and L. Yu, Niemann-pick C1-like 1 (NPC1L1) protein in intestinal and hepatic cholesterol transport, Annu. Rev. Physiol., 2011, 73, 253–259 CrossRef PubMed.
- L. Yu, S. Bharadwaj, J. M. Brown, Y. Ma, W. Du, M. A. Davis, P. Michaely, P. Liu, M. C. Willingham and L. L. Rudel, Cholesterol-regulated translocation of NPC1L1 to the cell surface facilitates free cholesterol uptake, J. Biol. Chem., 2006, 281, 6616–6624 CrossRef CAS PubMed.
- L. J. Sharpe, E. C. L. Cook, N. Zelcer and A. J. Brown, The UPS and downs of cholesterol homeostasis, Trends Biochem. Sci., 2014, 39, 527–535 CrossRef CAS PubMed.
- H. Kusuhara and Y. Sugiyama, ATP-binding cassette, subfamily G (ABCG family), Pflugers Arch., 2007, 453, 735–744 CrossRef CAS PubMed.
- L. Yu, J. Li-Hawkins, R. E. Hammer, K. E. Berge, J. D. Horton, J. C. Cohen and H. H. Hobbs, Overexpression of ABCG5 and ABCG8 promotes biliary cholesterol secretion and reduces fractional absoprtion of dietary cholesterol, J. Clin. Invest., 2002, 110, 671–680 CrossRef CAS PubMed.
- K. E. Berge, H. Tian, G. A. Graf, L. Yu, N. V. Grishin, J. Schultz, P. Kwiterovich, B. Shan, R. Barnes and H. H. Hobbs, Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters, Science, 2000, 290, 1771–1775 CrossRef CAS PubMed.
- J. J. Repa, K. E. Berge, C. Pomajzl, J. A. Richardson, H. H. Hobbs and D. J. Mangelsdorf, Regulation of ATP-binding cassette sterol transporters ABCG5 and ABCG8 by the liver X receptors alpha and beta, J. Biol. Chem., 2002, 277, 18793–18800 CrossRef CAS PubMed.
- Acetiyl-CoA acetyltransferase 1, the GeneCardsHuman. Weizmann Institute of Science, Rehovot, Israel, http://www.genecards.org/cgi-bin/carddisp.pl?gene=ACAT1 accessed 05.04.17.
- Acetyl-CoA acetyltransferase 2, the GeneCards Human. Weizmann Institute of Science, Rehovot, Israel. http://www.genecards.org/cgi-bin/carddisp.pl?gene=ACAT2 accessed 05.04.17.
- Sterol O-acyltransferase 1, the GeneCards Human. Weizmann Institute of Science, Rehovot, Israel. http://www.genecards.org/cgi-bin/carddisp.pl?gene=SOAT1 accessed 05.04.17.
- Sterol O-acyltransferase 2, the GeneCards Human. Weizmann Institute of Science, Rehovot, Israel. http://www.genecards.org/cgi-bin/carddisp.pl?gene=SOAT2 accessed 05.04.17.
- T. Y. Chang, B. L. Li, C. C. Y. Chang and Y. Urano, Acyl-coenzyme A: cholesterol acyltransferases, Am. J. Physiol. Endocrynol. Metab., 2009, 297, E1–E9 CrossRef CAS PubMed.
- I. Tabas, Cholesterol in health and disease, J. Clin. Invest., 2002, 110, 583–590 CrossRef CAS PubMed.
- M. M. Hussain, J. Shi and P. Dreizen, Microsomal triglyceride transfer protein and its role in apoB-lipoprotein assembly, J. Lipid Res., 2003, 44, 22–32 CrossRef CAS.
- A. Sahebkar, G. T. Chew and G. F. Watts, Recent advances in pharmacotherapy for hypertriglyceridemia, Prog. Lipid Res., 2014, 56, 47–66 CrossRef CAS PubMed.
- M. M. Hussain, A proposed model for the assembly of chylomicrons, Atherosclerosis, 2000, 148, 1–15 CrossRef CAS PubMed.
- F. Lammert and D. Q. H. Wang, New insights into the genetic regulation of intestinal cholesterol absorption, Gastroenterology, 2005, 192, 718–734 CrossRef PubMed.
- N. Plana, Peroxidacion lipidica y factores de riesgo cardiovascular, in Departamento de Medicina y Cirugia de la Facultad de Medicina de la Universidad de Rovira i Virgili, Universidad Rovira y Virgili, Reus, 1993 Search PubMed.
- S. Gill, J. Stevenson, I. Kristiana and A. J. Brown, Cholesterol-dependent degradation of squalene monooxygenase, a control point in cholesterol synthesis beyond HMG-CoA reductase, Cell Metab., 2011, 13, 260–273 CrossRef CAS PubMed.
- 3-Hydroxy-3-Methylglutaryl-CoA Recutase. The GeneCardsHuman. Weizmann Institue of Science, Rehovot, Israel. http://www.genecards.org/cgi-bin/carddisp.pl?gene=HMGCR accessed 05.04.17.
- E. Ikonen, Mechanisms for cellular cholesterol transport: Defects and human disease, Physiol. Rev., 2006, 86, 1237–1261 CrossRef CAS PubMed.
- J. S. Burg and P. J. Espenshade, Regultation of HMG-CoA reductase in mammals and yeast, Prog. Lipid Res., 2011, 50, 403–410 CrossRef CAS PubMed.
- J. P. Lee, A. Brauweiler, M. Rudolph, J. E. Hooper, H. A. Drabkin and R. M. Gemmil, The TRC8 ubiquitin ligase is sterol regulated and interacts with lipid and protein biosynthetic pathways, Mol. Cancer Res., 2010, 8, 93–106 CrossRef CAS PubMed.
- M. Norlin and K. Wikvall, Enzymes in the conversion of cholesterol into bile acids, Curr. Mol. Med., 2007, 7, 199–218 CrossRef CAS PubMed.
- R. E. Temel, W. Tang, Y. Ma, L. L. Rudel, M. C. Willingham, Y. A. Ioannou, J. P. Davies, L. M. Nilsson and L. Yu, Hepatic Niemann-Pick C1-like 1 regulates biliary cholesterol concentration and is a target of ezetimibe, J. Clin. Invest., 2007, 117, 1968–1978 CrossRef CAS PubMed.
- A. E. van der Velde, C. L. Vrins, K. van der Oever, C. Kunne, R. P. Oude-Elferink, F. Kuipers and A. K. Groen, Direct intestinal cholesterol secretion contributes significantly to total fecal natural sterol excretion in mice, Gastroenterology, 2007, 133, 967–975 CrossRef CAS PubMed.
- W. Tang, L. Jia, Y. Ma, P. Xie, J. Haywood, P. A. Dawson, J. Li and L. Yu, Ezetimibe restores biliary cholesterol excretion in mice expressing Niemann-Pick C1-Like 1 only in liver, Biochim. Biophys. Acta, 2011, 1811, 549–555 CrossRef CAS PubMed.
- G. Brufau, A. K. Groen and F. Kuipers, Reverse cholesterol transport revisited: Contribution of biliary versus intestinal cholesterol excretion, Arterioscler., Thromb., Vasc. Biol., 2011, 31, 1726–1733 CrossRef CAS PubMed.
- R. E. Temel and J. M. Brown, A new framework for reverse cholesterol transport: Non-biliary contributions to reverse cholesterol transport, World J. Gastroenterol., 2010, 16, 5946–5952 CAS.
- K. S. Bura, C. Lord, S. Marshall, A. McDaniel, G. Thomas, M. Warrier, J. Zhang, M. A. Davis, J. K. Sawyer, R. Shah, M. D. Wilson, A. Dikkers, U. J. Tietge, X. Collet, L. L. Rudel, R. E. Temel and J. M. Brown, Intestinal SR-BI does not impact cholesterol absorption or transintestinal cholesterol efflux in mice, J. Lipid Res., 2013, 54, 1567–1577 CrossRef CAS PubMed.
- J. F. de Boer, G. Brufau, M. Schonewille, A. Dikkers, H. Wolters, U. J. Tietg and A. K. Groen, Inhibition of NPC1L1 increases transintestinal cholesterol excretion (tice) dependent on Abcg5/g8 but independent of plasma Apob-containing lipoproteins, Atherosclerosis, 2014, 235, e47 CrossRef.
- E. M. Danielsen, G. H. Hansen, K. Rasmussen, L. L. Niels-Christiansen and F. Frenzel, Apoplipoprotein A-1 (apoA-1) deposition in, and release from, the enterocyte brush border: a possible role in transintestinal choltesterol efflux (TICE)?, Biochim. Biophys. Acta, 2012, 1813, 530–536 CrossRef PubMed.
- C. L. J. Vrins, From blood to gut: Direct secretion of cholesterol via transintestinal cholesterol efflux, World J. Gastroenterol., 2010, 16, 5953–5957 CAS.
- A. E. van der Velde, G. Brufau and A. K. Groen, Transintestinal colesterol efflux, Curr. Opin. Lipidol., 2010, 21, 167–171 CrossRef CAS PubMed.
- H. R. Davis Jr., K. K. Pula, K. B. Alton, R. E. Burrier and R. W. Watkins, The synergistic hypocholesterolemic activity of the potent cholesterol absorption inhibitor, ezetimibe, in combination with 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors in dogs, Metabolism, 2001, 50, 1234–1241 CrossRef CAS PubMed.
- G. G. Fontanari, J. P. Batistuti, R. J. da Cruz, P. H. N. Saldiva and J. A. G. Areas, Cholesterol-lowering effect of whole lupin (Lupinus albus) seed and its protein isolate, Food Chem., 2012, 132, 1521–1526 CrossRef CAS.
- A. Gil-Ramirez, A. Ruiz-Rodriguez, F. R. Marin, G. Reglero and C. Soler-Rivas, Effect of ergosterol-enriched extracts obtained from Agaricus bisporus on cholesterol absorption using an in vitro digestion model, J. Funct. Foods, 2014, 11, 589–597 CrossRef CAS.
- C. Zacherl, P. Eisner and K. H. Engel, In vitro model to correlate viscosity and bile acid-binding capacity of digested water-soluble and insoluble dietary fibers, Food Chem., 2011, 126, 423–428 CrossRef CAS.
- M. Skoog, N. Xu, M. Berggren-Soderlund, J. A. Lovegrove and P. Nilsson-Ehle, ACTH reduces the rise in ApoB-48 levels after fat intake, Atherosclerosis, 2007, 191, 433–439 CrossRef CAS PubMed.
- L. A. Drozdowski, R. A. Reimer, F. Temelli, R. C. Bell, T. Vasanthan and A. B. Thomson, Beta-glucan extracts inhibit the in vitro intestinal uptake of long-chain fatty acids and cholesterol and down-regulate genes involved in lipogenesis and lipid transport in rats, J. Nutr. Biochem., 2010, 21, 695–701 CrossRef CAS PubMed.
- K. Matsuoka, E. Rie, S. Yui, C. Honda and K. Endo, Competitive solubilization of cholesterol and β-sitosterol with changing biliary lipid compositions in model intestinal solution, Chem. Phys. Lipids, 2012, 165, 7–14 CrossRef CAS PubMed.
- S. M. Melnikov, J. W. M. Seijen ten Hoorn and A. P. Eijkelenboom, Effect of phytosterols and phytostanols on the solubilization of cholesterol by dietary mixed micelles: an in vitro study, Chem. Phys. Lipids, 2004, 127, 121–141 CrossRef CAS.
- E. A. Trautwein, G. S. M. J. E. Duchateau, Y. Lin, S. M. Melnikov, H. O. F. Molhuizen and F. Y. Ntanios, Proposed mechanisms of cholesterol-lowering action of plant sterols, Eur. J. Lipid Sci. Technol., 2003, 105, 171–185 CrossRef CAS.
- T. Drazic, K. Molcanov, V. Sachdev, M. Malnar, S. Hecimovic, J. V. Patankar, S. Obrowsky, S. Levak-Frank, I. Habus and D. Kratky, Novel amino-β-lactam derivatives as potent cholesterol absorption inhibitors, Eur. J. Med. Chem., 2014, 87, 722–734 CrossRef CAS PubMed.
- X. H. Yu, K. Qian, N. Jiang, X. L. Zheng, F. S. Cayabyab and C. K. Tang, ABCG5/ABCG8 in cholesterol excretion and atherosclerosis, Clin. Chim. Acta, 2014, 139, 209–218 Search PubMed.
- M. Z. Dieter, J. M. Maher, X. Cheng and C. D. Klaasen, Expression and regulation of the sterol half-transporter genes ABCG5 and ABCG8 in rats, Comp. Biochem. Physiol., C: Comp. Pharmacol., 2004, 139, 209–218 CrossRef PubMed.
- A. P. Kourounakis, A. N. Matralis and A. Nikitakis, Design of more potent squealene synthase inhibitors with multiple activities, Bioorg. Med. Chem., 2010, 18, 7402–7412 CrossRef CAS PubMed.
- S. An, Y. D. Park, Y. K. Paik, T. S. Jeong and W. S. Lee, Human ACAT inhibitory effects of shikonin derivatives from Lithospermum erythrorhizon, Bioorg. Med. Chem. Lett., 2007, 17, 1112–1116 CrossRef CAS PubMed.
- J. H. Choi, M. C. Rho, S. W. Lee, O. E. Kwon, H. R. Park, J. Y. Kang, S. H. Lee, H. S. Lee, K. H. Bae and Y. K. Kim, Glabrol, an acyl-coenzyme A: cholesterol acyltransferase inhibitor from licorice roots, J. Ethnopharmacol., 2007, 110, 563–566 CrossRef CAS PubMed.
- Y. Jia, M. J. Bhuiyan, H. J. Jun, J. H. Lee, M. H. Hoang, H. J. Lee, N. Kim, D. Lee, K. Y. Hwang, B. Y. Hwang, D. W. Choi and S. J. Lee, Ursolic acid is a PPAR-α agonist that regulates hepatic lipid metabolism, Bioorg. Med. Chem. Lett., 2011, 21, 5876–5880 CrossRef CAS PubMed.
- M. N. Woo, S. M. Jeon, H. J. Kim, M. K. Lee, S. K. Shin, Y. C. Shin, Y. B. Park and M. S. Choi, Fucoxanthin supplementation improves plasma and hepatic lipid metabolism and blood glucose concentration in high-fat fed C57BL/6N mice, Chem.-Biol. Interact., 2010, 186, 316–322 CrossRef CAS PubMed.
- A. Teichmann, P. C. Dutta, A. Staffas and M. Jägerstad, Sterol and vitamin D2 concentrations in cultivated and wild grown mushrooms: Effects of UV irradiation, LWT–Food Sci. Technol., 2007, 40, 815–822 CrossRef CAS.
- A. Gil-Ramirez, C. Clavijo, M. Palanisamy, A. Ruiz-Rodriguez, M. Navarro-Rubio, M. Perez, F. R. Marin, G. Reglero and C. Soler-Rivas, Study on the 3-hydroxy-3-methyl-glutaryl CoA reductase inhibitory properties of Agaricus bisporus and extraction of bioactive fractions using pressurized solvent technologies, J. Sci. Food. Agric., 2013, 93, 2789–2796 CrossRef CAS PubMed.
- C. Fernandez, Y. Suarez, A. J. Ferruelo, D. Gomez-Coronado and M. A. Laseunción, Inhibition of cholesterol biosynthesis by Delta22-unsaturated phytosterols via competitive inhibition of sterol Delta24-reductase in mammalian cells, Biochem. J., 2002, 366, 109–119 CrossRef CAS PubMed.
- F. M. N. A. Aida, M. Shuhaimi, M. Yazid and A. G. Maaruf, Mushroom as a potential source of prebiotics: a review, Trends Food Sci. Technol., 2009, 20, 567–575 CrossRef CAS.
- P. C. K. Cheung, The hypocholesterolemic effect of two edible mushrooms: Auricularia auricular (three-ear) and Tremella fuciformis (white jelly-leaf) in hypercholesterolemic rats, Nutr. Res., 1996, 16, 1721–1725 CrossRef.
- J. E. Ramberg, E. D. Nelson and R. A. Sinnot, Immunomodulatory dietary polysaccharides: a systematic review of the literature, Nutr. J., 2010, 9, 54 CrossRef PubMed.
- M. L. C. Gonzaga, N. M. P. S. Ricardo, F. Heatley and S. A. Soares, Isolation and characterization of polysaccharides from Agaricus blazei Murill, Carbohydr. Polym., 2005, 60, 43–49 CrossRef CAS.
- M. Zhang, P. C. K. Cheung and L. Zhang, Evaluation of mushroom dietary fiber (nonstarch polysaccharides) from sclerotia of Pleurotus tuber-regium (Fries) singer as a potential antitumor agent, J. Agric. Food Chem., 2001, 49, 5059–5062 CrossRef CAS PubMed.
- M. Palanisamy, L. Aldars-Garcia, A. Gil-Ramirez, A. Ruiz-Rodriguez, F. R. Marin, G. Reglero and C. Soler-Rivas, Pressurized water extraction of β-glucan enriched fractions with bile acids-binding capacities obtained from edible mushrooms, Biotechnol. Prog., 2014, 30, 391–400 CrossRef CAS PubMed.
- N. Gunde-Cimerman, A. Plemenitas and A. Cimerman, Pleurotus fungi produce mevinolin, an inhibitor of HMG CoA reductase, FEMS Microbiol. Lett., 1993, 113, 333–337 CrossRef CAS PubMed.
- N. Gunde-Cimeman, A. Plemenitas and A. Cimerman, A hydroxymethylglutaryl-CoA reductase inhibitor synthesized by yeasts, FEMS Microbiol. Lett., 1995, 132, 39–43 CrossRef.
- D. K. Singh, S. Banerjee and T. D. Porter, Green and black tea extracts inhibit HMG-CoA reductase and activate AMP kinase to decrease cholesterol synthesis in hepatoma cells, J. Nutr. Biochem., 2009, 20, 816–822 CrossRef CAS PubMed.
- M. Zang, S. Xu, K. A. Maitland-Toolan, A. Zucollo, X. Hou, B. Jiang, M. Wierzbicki, T. J. Verbeuren and R. A. Cohen, Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor-deficient mice, Diabetes, 2006, 55, 2180–2191 CrossRef CAS PubMed.
- B. P. Laden and T. D. Porter, Resveratrol inhibits human squealene monooxygenase, Nutr. Res., 2001, 21, 747–753 CrossRef CAS.
- T. Hosoya, T. Tanimoto, K. Onodera, Y. Kurihara, Y. Takamatsu and Y. Tsujita, Zaragozic acids production from discomycetes, Mycoscience, 1997, 38, 305–311 CrossRef CAS.
- E. J. Zerenturk, I. Kristiana, S. Gill and A. J. Brown, The endogenous regulator 24(S),25-epoxycholesterol inhibits cholesterol synthesis at DHCR24 (Seladin-1), Biochim. Biophys. Acta, 2012, 1821, 1269–1277 CrossRef CAS PubMed.
- M. Sato, Y. Tokuji, S. Yoneyama, K. Fujii-Akiyama, M. Kinoshita and M. Ohnish, Profiling of hepatic gene expression of mice fed with edible Japanese mushrooms by DNA microarray analysis: comparison among Pleurotus ostreatus, Grifola frondosa, and Hypsizigus marmoreus, J. Agric. Food Chem., 2011, 59, 10723–10731 CrossRef CAS PubMed.
- A. M. de Miranda, J. V. Rossoni Junior, L. Souza E Silva, R. C. Dos Santos, M. E. Silva and M. L. Pedrosa, Agaricus brasiliensis (sun mushroom) affects the expression of genes related to cholesterol homeostasis, Eur. J. Nutr., 2016, 56, 1707–1717 CrossRef PubMed.
- T. A. Ismail, M. M. Soliman, M. A. Nassan and D. I. Mohamed, Antihypercholesterolemic effects of mushroom, chrysin, curcumin and omega-3 in experimental hypercholesterolemic rats, J. Food Nutr. Res., 2015, 3, 77–87 CrossRef.
- K. Hiwatashi, Y. Kosaka, N. Suzuki, K. Hata, T. Mukaiyama, K. Sakamoto, H. Shirakawa and M. Komai, Yamabushitake mushroom (Hericium erinaceus) improved lipid metabolism in mice fed a high-fat diet, Biosci., Biotechnol., Biochem., 2010, 74, 1447–1451 CrossRef CAS PubMed.
- K. B. Hong, S. Y. Hong, E. Y. Joung, B. H. Kim, S. H. Bae, Y. Park and H. J. Sun, Hypocholesterolemic effects of the cauliflower culinary-medicinal mushroom, Sparassis crispa (higher basidiomycetes) in diet-induced hypercholesterolemic rats, Int. J. Med. Mushrooms, 2015, 17, 965–975 CrossRef PubMed.
- J. R. Porter, J. S. Burg, P. J. Espenshade and P. A. Iglesias, Ergosterol regulates sterol regulatory element binding protein (SREBP) cleavage in fission yeast, J. Biol. Chem., 2010, 285, 41051–41061 CrossRef CAS PubMed.
- M. Miyata, T. Hata, Y. Yamazoe and K. Yoshinari, SREBP-2 negativeley regulates FXR-dependent transcription of FGF19 in human intestinal cells, Biochem. Biophys. Res. Commun., 2014, 443, 447–482 CrossRef PubMed.
- T. Matsubara, F. Li and F. J. Gonzalez, FXR signaling in the enterohepatic system, Mol. Cell. Endocrinol., 2013, 368, 17–29 CrossRef CAS PubMed.
- A. Gil-Ramirez, V. Caz, R. Martin-Hernandez, F. R. Marin, C. Largo, A. Rodriguez-Casado, M. Tabernero, G. Reglero and C. Soler-Rivas, Modulation of Dio1 gene expression by edible mushrooms extracts in normo- and hypocholesterolaemic mice. ScientificTracks abstracts, J. Food Process. Technol., 2015 Search PubMed , 5th Euro-Global Summit and Expo on Food & Beverages, at Alicante, Spain, Volume 6.
- V. Caz, A. Gil-Ramirez, M. Santamaria, M. Tabernero, C. Soler-Rivas, R. Martin-Hernandez, F. R. Marin, G. Reglero and C. Largo, Plasma cholesterol-lowering activity of lard functionalized with mushroom extracts is independent of Niemann-Pick C1-like protein and ABC sterol transporter gene expression in hypercholesterolemic mice, J. Agric. Food Chem., 2016, 64, 1686–1694 CrossRef CAS PubMed.
- G. Garcia-Llatas and M. T. Rodriguez-Estrada, Current and new insights on phytosterol oxides in plant sterol-enriched food, Chem. Phys. Lipids, 2011, 164, 607–624 CrossRef CAS PubMed.
- A. Berger, D. Rein, E. Kratky, I. Monnard, H. Hajjaj, I. Meirim, C. Piquet-Welsch, J. Hauser, K. Mace and P. Niederberger, Cholesterol-lowering properties of Ganoderma lucidum in vitro, ex vivo, and in hamsters and minipigs, Lipids Health Dis., 2004, 3, 2 CrossRef CAS PubMed.
- S. W. Altmann, H. R. Davis Jr., L. J. Zhu, X. Yao, L. M. Hoos, G. Tetzloff, S. P. Iyer, M. Maquire, A. Golovko, M. Zeng, L. Wang, N. Murgolo and M. P. Graziano, Niemann-Pick C1 Like 1 protein is critical for intestinal cholesterol absorption, Science, 2004, 303, 1201–1204 CrossRef CAS PubMed.
- H. R. Davis Jr., L. J. Zhu, L. M. Hoos, G. Tetzloff, M. Maquire, J. Liu, X. Yao, S. P. Iyer, M. H. Lam, E. G. Lund, P. A. Detmers, M. P. Graziano and S. W. Altmann, Niemann-Pick C1 Like 1 (NPC1L1) is the intestinal phytosterol and cholesterol transporter and a key modulator of whole-body cholesterol homeostasis, J. Biol. Chem., 2004, 279, 33586–33592 CrossRef PubMed.
- L. B. Nguyen, G. Salen, S. Shefer, J. Bullock, T. Chen, G. S. Tint, I. R. Chowdhary and S. Lerner, Deficient ileal 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in sitosterolemia: sitosterol is not a feedback inhibitor of intestinal cholesterol biosynthesis, Metabolism, 1994, 43, 855–859 CrossRef CAS PubMed.
- M. Fukushima, M. Nakano, Y. Morii, T. Ohashi, Y. Fujiwara and K. Sonoyama, Hepatic LDL receptor mRNA in rats is increased by dietary mushroom (Agaricus bisporus) fiber and sugar beet fiber, J. Nutr., 2000, 130, 2151–2156 CAS.
- M. Fukushima, T. Ohashi, Y. Fujiwara, K. Sonoyama and M. Nakano, Cholesterol.-lowering effects of maitake (Grifola frondosa) fiber, shiitake (Lentinus edodes) fiber, and enokitake (Flammulina velutipes) fiber in rats, Exp. Biol. Med., 2001, 226, 758–765 CrossRef CAS.
- P. V. Jeurink, C. L. Noguera, H. F. Savelkoul and H. J. Wichers, Immunomodulatory capacity of fungal proteins on the cytokine production of human peripheral blood mononuclear cells, Int. Immunopharmacol., 2008, 8, 1124–1133 CrossRef CAS PubMed.
- V. Caz, A. Gil-Ramirez, C. Largo, M. Tabernero, M. Santamaria, R. Martin-Hernandez, F. R. Marin, G. Reglero and C. Soler-Rivas, Modulation of cholesterol-related gene expression by dietary fiber fractions from edible mushrooms, J. Agric. Food Chem., 2015, 63, 7371–7380 CrossRef CAS PubMed.
- Y. B. Hu, Z. Wang and S. Y. Xu, Corn bran dietary fibre modified by xylanase improves the mRNA expression of genes involved in lipids metabolism in rats, Food Chem., 2008, 109, 499–505 CrossRef CAS.
- G. E. Bartley, W. Yokoyama, S. A. Young, W. H. Anderson, S. C. Hung, D. R. Albers, M. L. Langhorst and H. Kim, Hypocholesterolemic effects of hydroxypropyl methylcellulose are mediated by altered gene expression in hepatic bile and cholesterol pathways of male hamsters, J. Nutr., 2010, 140, 1255–1260 CrossRef CAS PubMed.
- J. A. Parnell and R. A. Reimer, Effect of prebiotic fibre supplementation on hepatic gene expression and serum lipids: a dose-response study in JCR:LA-cp rats, Br. J. Nutr., 2010, 103, 1577–1584 CrossRef CAS PubMed.
- P. J. H. Jones, Dietary agents that target gastrointestinal and hepatic handling of bile acids and cholesterol, J. Clin. Lipidol., 2008, 2, S4–S10 CrossRef PubMed.
- J. Chen and X. F. Huang, The effects of diets enriched in beta-glucans on blood lipoprotein concentrations, J. Clin. Lipidol., 2009, 3, 154–158 CrossRef PubMed.
- M. M. Kaczmarczyk, M. J. Miller and G. G. Freund, The health benefits of dietary fiber: Beyond the usual suspects of type 2 diabetes mellitus, cardiovascular disease and colon cancer, Metabolism, 2012, 61, 1058–1066 CrossRef CAS PubMed.
- A. Gil-Ramirez, V. Caz, F. R. Smiderle, R. Martin-Hernandez, C. Largo, M. Tabenero, F. R. Marin, M. Iacomini, G. Reglero and C. Soler-Rivas, Water-soluble compounds from Lentinula edodes influencing the HMG-CoA reductase activity and the expression of genes involved in the cholesterol metabolism, J. Agric. Food Chem., 2016, 64, 1910–1920 CrossRef CAS PubMed.
- H. Y. Huang, M. Korivi, H. T. Yang, C. C. Huang, Y. Y. Chaing and Y. C. Tsai, Effect of Pleurotus tuber-regium polysaccharides supplementation on the preogression of diabetes complications in obese-diabetic rats, Chin. J. Physiol., 2014, 57, 198–208 CrossRef CAS PubMed.
- K. Sugiyama, T. Akachi and A. Yamakawa, Hypocholesterolemic action of eritadenine is mediated by a modification of hepatic phospholipid-metabolism in rats, J. Nutr., 1995, 125, 2134–2144 CAS.
- T. Yamada, J. Komoto, K. Lou, A. Ueki, D. H. Hua, K. Sugiyama, Y. Takata, H. Ogawa and F. Takusagawa, Structure and function of eritadenine and its 3-deaza analogues: Potent inhibitors of S-adenosylhomocysteine hydrolase and hypocholesterolemic agents, Biochem. Pharmacol., 2007, 73, 981–989 CrossRef CAS PubMed.
- Y. Shimada, A. Yamakawa, T. Morita and K. Sugiyama, Effects of dietary eritadenine on the liver microsomal Delta6-desaturase activity and its mRNA in rats, Biosci., Biotechnol., Biochem., 2003, 67, 1258–1266 CrossRef CAS PubMed.
- H. Yang, I. Hwang, S. Kim, E. J. Hong and E. B. Jeung, Lentinus edodes promotes fat removal in hypercholesterolemic mice, Exp. Ther. Med., 2013, 6, 1409–1413 CrossRef CAS PubMed.
- A. Gil-Ramirez, C. Soler-Rivas, A. Rodriguez-Casado, A. Ruiz-Rodriguez, G. Reglero and F. R. Marin, Effect of selenium-enriched Agaricus bisporus (Higher Basidiomycetes) extracts, obtained by pressurized water extraction, on the expression of cholesterol homeostasis related genes by Low-Density Array, Int. J. Med. Mushrooms, 2015, 17, 105–116 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2018 |