Colloidal particles for the delivery of steroid glycosides
Krassimir P.
Velikov
 *abc,
Marjolein
van Ruijven
a,
Alois K.
Popp
a,
Ashok R.
Patel†
*abc,
Marjolein
van Ruijven
a,
Alois K.
Popp
a,
Ashok R.
Patel†
 a,
Leonard M.
Flendrig
a and
Sergey M.
Melnikov‡
a
a,
Leonard M.
Flendrig
a and
Sergey M.
Melnikov‡
a
aUnilever R&D Vlaardingen, Olivier van Noortlaan 120, 3133 AT Vlaardingen, The Netherlands. E-mail: krassimir.velikov@unilever.com
bvan der Waals-Zeeman Institute, Institute of Physics, University of Amsterdam, 1098XH Amsterdam, The Netherlands
cSoft Condensed Matter, Debye Institute for Nanomaterials Science, Utrecht University, Princetonplein 5, 3584 CC Utrecht, The Netherlands
First published on 13th November 2017
Abstract
Water insoluble bioactive molecules with very high melting temperature and low solubility in water are difficult to formulate in food products. We demonstrate the synthesis of nanoscale particles from steroid glycosides using a facile liquid antisolvent precipitation method in the presence of various food grade stabilizers. Colloidal particles with sizes well below 200 nm are prepared from steroid glycosides containing extracts, as well as mixtures with phytosterol. In the mixtures, the formation of the typical for the phytosterol rod-like particles is suppressed. Particle size and structure are investigated by electron microscopy and dynamic light scattering. Due to the presence of surface charge and steric stabilization, colloidal particles do not display aggregation and are stable for a period of longer than one year. The results of this study are important for the formulation and delivery of steroid glycoside and phytosterol bioactive molecules in the fields of food, nutraceuticals, and medical applications.
Introduction
The formulation of functional ingredients derived from plants (e.g. extracts) in the form of stable and palatable food products is often a technological challenge. Difficulties are often linked to the complex compositions of the extracts, which often contain sensitive or difficult to process compounds. Such an example are the steroid glycosides (StGly) from Hoodia gordonii (H.g.) plants. Several in vivo studies have shown that the oral administration of extracts rich in these StGly significantly and reversibly decreased food intake and body weight and/or weight gain.1–3 StGly belong to a class of compounds found in plants and have a wide spectrum of bioactivity (e.g. anti-hyperglycaemic activity, appetite suppression, anti-obesity, improved immune function) with potential for application in functional foods or food supplements.1,4–8The main challenge in the formulation of delivery systems for StGly is the poor solubility in common triglycerides and very low solubility in water. In addition, StGly extract has a high melting temperature (>100 °C), which makes it difficult and commercially unattractive to produce stable bio-accessible formulations using the combination of melting and emulsification.9,10 The use of StGly in powder form can potentially negatively affect the organoleptic properties of the product by giving rise to a sandy texture.11 Larger particles will be also affected by gravity, leading to sedimentation which can compromise the physical stability of the product and can render the incorporated bioactive compound unavailable (i.e. retained in the product container). The sedimentation of particles in a product also creates problems with the cleaning of the processing equipment, leading to possible cross-contamination and/or losses of expensive ingredients. Importantly, even if the sedimentation is eliminated by increasing the viscosity and/or creating a yield stress in the continuous phase of the product, the bioaccessibility of the bioactive compounds may not be optimal due to the slow solubilisation of the larger particles.12 This issue has been recognised for a while and several formulation approaches have been developed for bioactive compounds based on oil soluble digestible derivatives (e.g. phytosterol esters in the case of phytosterols) or solubilisation using surfactants.13 While the use of chemical modification has been successful for some functional ingredients, this approach typically requires a long time to establish that the new formulation is safe and effective.
Colloidal particles, which can be fabricated with tuneable size, composition, and surface properties, can provide another highly adaptable solution for the formulation of water insoluble bioactive ingredients. Colloidal delivery systems can play an important role in the design of functional products with advanced control on product functionality.14 Due to their dimensions, when formulated with sufficient compatibility with the targeted product, colloidal delivery systems can be used to control/improve the structure, stability, taste, and appearance of (food) products. Colloidal particles in particular have been explored as delivery systems for various functional ingredients such as minerals and nutraceuticals.15–18 Colloidal particles in particular offer the possibility of blending bioactives in their solid body15 to form multiple delivery systems. They can also be used in combination with foams and emulsions.19 Next to colloidal particles, emulsions and mesophases are other typical examples of colloidal delivery systems that find wide applications in the formulation of functional ingredients.20–22
The synthesis of organic colloidal particles is often based on liquid antisolvent precipitation. This process was pioneered by Fessi et al.23 and subsequently widely applied in various industries to prepare colloidal particles of various compounds such as nutrient mimetics,24 colourants,25 drugs26,27 and nutraceuticals.28–32 The precipitation of the particles is caused by a change in the solvent quality of the fluid phase, in which the material to be precipitated is initially dissolved.33 For food grade applications, low molecular weight alcohols such as ethanol or isopropanol are often suitable and preferred solvents. This requires good solubility of the bioactives in these solvents. Although the general principles are well established, applying the method to new molecules with complex structures such as many phytochemicals may not be straightforward. The process can be affected by the compositional complexity of natural extracts containing the targeted bioactives, the solubility in such multicomponent systems not being well established, and unknown interfacial activities of the molecules present.
In this article, we demonstrate the synthesis and characterization of stable colloidal particles of steroid glycosides and their mixtures with phytosterol. We demonstrate an exceptional colloidal stability by monitoring their particle size over long time storage.
Experimental
Materials
Steroid glycoside (from H. gordonii) extract (79.3 wt%) was provided by Cognis Iberia S.A.U. (Barcelona, Spain). The analytical characterization of the StGly extract was reported by Russell and Swindells.34 Phytosterol (PS) was obtained from Acros Organics: total sterols >95.0%. Typical composition: β-sitosterol: 75 to 80%, β-sitostanol: 10 to 14%, campesterol: 6 to 10%, campestanol: <2.0%, others: <3.0% (loss on drying ∼3.5 wt%). Poly(vinylpyrrolidone) with an average molar mass of 40 kg mol−1 (PVP-40) was purchased from Sigma-Aldrich, and polyoxyethylene sorbitan monooleate (Tween® 80, no. 822187) was obtained from Merck Schuchardt. Gum arabic, anhydrous citric acid, and potassium sorbate were purchased from Sigma Aldrich. Cold-water soluble octenyl succinic anhydrite-grafted (OSA) starch HI-CAP 100 (E1450) was obtained from National Starch (Manchester, UK; Batch MCI 6569). Ethanol used for synthesis (Royal Nedalco) was of analytical reagent quality. Deionized water purified by a Millipore purification system (MILLIPORE Synergy 185) was used in all reactions. All chemicals were used as received.Formation of colloidal particles
Colloidal particles were prepared by liquid anti-solvent precipitation.23 In a typical experiment, a StGly extract was combined with an ethanol soluble stabiliser. Ethanol was added and the mixture was sonicated in a Bransonic 211 bath operating at maximum power until a transparent solution was obtained. If the stabiliser is only soluble in water, it is dissolved in the aqueous antisolvent phase. The ethanolic solution was quickly (for less than 5 s) added to demineralized water or the solution of the water soluble stabilizer under vigorous mixing using a Silverson high-shear mixer equipped with an emulsor fine screen set at 5000 rpm for 2 min. After the particle formation, ethanol was removed by slow evaporation under reduced pressure using a rotary evaporator (Rotavapor R-114, Buchi, Switzerland). Optionally, for preservation purposes the formulation was adjusted to pH 3.9 by adding 10 mL of 0.1 M anhydrous citric acid and 0.067 M potassium sorbate.In the case of Tween 80 stabilised particles, the solvent phase was 5.0 g ethanolic solution of 5.0 wt% StGly. The anti-solvent phase was 200 g aqueous solution of 0.5 wt% Tween 80. PVP (both water and ethanol soluble) was added to the solvent phase. Particles were synthesised using two solvent phases containing 2.4 g or 4.8 g PVP in a solution containing 2.4 g StGly in 40.0 g ethanol. The antisolvent phase in all cases was 200 g water. To prepare particles stabilised with OSA starch and gum arabic, because these stabilisers are not soluble in ethanol, they were dissolved in the antisolvent phase (i.e. aqueous phase). To prepare the particles 5.0 g of 5.0 wt% solution of StGly in ethanol was rapidly mixed into 200 g aqueous phase containing 1.0 wt% OSA starch or 0.5 wt% gum arabic.
Mixed StGly-PS particles were prepared by first dissolving both PS and StGly in the solvent phase (forming 5 g of ethanolic solution containing 5.0 wt% StGly and 5.0 wt% PS). The stabiliser, Tween 80, was dissolved in the aqueous phase (200 g of 0.5 wt% Tween 80) which was used as the antisolvent phase.
Characterisation
The particle size and morphology were investigated by transmission electron microscopy (TEM) using a FEI TECNAI-20 operating at 200 kV. TEM samples were prepared by drying a drop of diluted suspension on a carbon-coated copper grid. In order to minimize artefacts from drying, the TEM samples were first vitrified by a rapid temperature quench in liquid ethane and then freeze-dried under vacuum.Particle size measurements in dispersions were performed at 25 °C using a dynamic light scattering (DLS) based particle size analyser (Zetasizer Nano ZS, Malvern Instruments Ltd). All the measurements were performed in disposable cuvettes (DTS0012) on diluted dust free suspensions that were filtered through a 5 μm Acrodisc 32 mm Syringe Filter with Supor® Membrane (Pall).
ζ-Potentials were measured using a Zetasizer Nano ZS (Malvern Instruments Ltd). The measured distribution of the electrophoretic mobility was converted to ζ-potentials using the Smoluchowski or Hückel theory depending on the conductivity of the sample. The samples were measured at a temperature of 25 °C. All the measurements were performed in disposable sizing cuvettes on diluted dust free suspensions that were filtered through the 5 μm Acrodisc 32 mm Syringe Filter with Supor® Membrane (Pall).
Results and discussion
To synthesise colloidal particles from StGly using representative food grade stabilisers, we used a one-step process using water as the antisolvent. The precipitation of StGly is caused by a change in the solvent quality, because of the mixing of water with the organic solvent (i.e. ethanol). This is accomplished by the rapid mixing of the StGly solution in the organic solvent into the aqueous phase. We employed a bench top setup using a high speed mechanical stirrer. The mixing conditions (i.e. mixing speed, mixing volume, mixer head) were kept constant for all the syntheses. The possible effects of the mixing conditions on particle size and size distribution are outside the scope of this study. The mixing speed was selected to minimise the incorporation of air bubbles, which may act as an interface for particle nucleation. The supersaturation triggers a primary nucleation process with a nucleation rate ∝![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(γ3/(ln
exp(γ3/(ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S)2), where γ is the solid–liquid interfacial energy and S is the supersaturation. The supersaturation S = c(r)/c* is linked to the particle radius r; ln(S) = 2γ/rkT. c(r) denotes the solubility of a particle with radius r, and c* is the equilibrium solubility. Once the nucleation process starts, a particle can grow in size by condensation, caused by the diffusing molecules, and become incorporated into the particle phase. Particle aggregation can take place if stabilisation is not sufficient. Hence, the choice of a stabilisation mechanism is important for controlling the particle size. The choice of the stabiliser also can affect the compatibility of the particles with the product where they will be incorporated.
S)2), where γ is the solid–liquid interfacial energy and S is the supersaturation. The supersaturation S = c(r)/c* is linked to the particle radius r; ln(S) = 2γ/rkT. c(r) denotes the solubility of a particle with radius r, and c* is the equilibrium solubility. Once the nucleation process starts, a particle can grow in size by condensation, caused by the diffusing molecules, and become incorporated into the particle phase. Particle aggregation can take place if stabilisation is not sufficient. Hence, the choice of a stabilisation mechanism is important for controlling the particle size. The choice of the stabiliser also can affect the compatibility of the particles with the product where they will be incorporated.
In order to ensure stabilization against aggregation several food grade stabilizers representing the different classes of food ingredients were used. In our examples, we used a food grade emulsifier (i.e. Tween 80), polysaccharides (i.e. OSA starch, gum arabic), and a synthetic food-grade polymer (i.e. PVP). TEM images showing examples of colloidal particles comprising steroid glycosides and stabilised by Tween 80 and PVP are shown in Fig. 1. The range of particle sizes observed is similar because we used similar supersaturation S. There seems to an interesting difference between Tween and PVP stabilised particles related to the particle shape. A portion of the particles stabilised by the low molecular surfactant seems to have some small shape-anisotropy – they appear to have a rod like shape. The behaviour is similar to that of the rod-like phytosterol particles stabilised by Tween 80.12 When the polymer is used as a stabiliser the particle appears to have a spherical shape. Although understanding this difference is outside the scope of this article, such a difference may be attributed to the high mobility of the low molecular weight surfactant on the particle interface which in principle can allow certain crystal planes to grow faster.
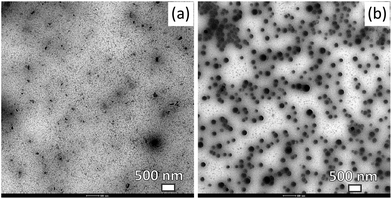 | ||
| Fig. 1 TEM micrographs of colloidal particles comprising StGly extract stabilized with (a) Tween 80 and (b) PVP. Scale bars = 500 nm. | ||
Although Tween 80 is soluble in both water and ethanol, it was initially dissolved in the ethanol phase together with the StGly. The location of the stabiliser with the bioactive could be considered as advantageous to ensure its fast adsorption on the surface of the newly formed particles. Being a non-anionic surfactant, Tween provides mostly steric stabilisation. The StGly molecule does not have groups which can deprotonate; based on the molecular structure it is expected to have some amphiphilic character and can potentially contribute to steric repulsion. This can be expected from the presence of a polar sugar-like group and a hydrophobic steroidal part.34 Nevertheless the particle from organic non-polar compounds can acquire a negative surface charge.12 The negative surface charge for example can be due to the polar hydroxyl groups present at the crystal surface. Similar surface properties were found in the cases of both cholesterol30,35 and phytosterols.12 The electrophoretic mobility of the Tween-stabilised StGly particles was measured in water (pH 7) and the result reveals that the particles have a large negative zeta potential of −50 mV. The surface charge is a fundamental parameter contributing to the colloidal stability and it is often important to understand the nature of the particle stability. The contribution to the negative surface charge in the case of plant extracts containing phytochemicals could also be caused by the naturally present minor quantities of organic molecules that have ionisable groups (e.g. organic acids).
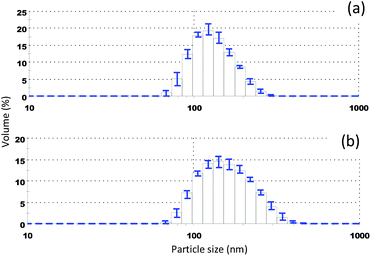
While the non-ionic surfactant can be a very good stabiliser, typically surfactants can affect the product taste or interfere with the stabilisation of other dispersed phases such as foams and emulsions. Taste is usually absent for very large molecules such as polymers. It is also known that adsorbing polymers are able to provide very good steric stabilisation of colloidal dispersions. Depending on its solubility characteristics, the stabilising polymer can be introduced in the reaction through the solvent or the antisolvent phase, and this can be used to provide even better control on colloidal stability. We demonstrate this by using PVP which is soluble in both ethanol and water. The particle size can be well controlled by using different concentrations of StGly in the initial solution, which creates different levels of supersaturation. Fig. 2 shows examples of particle size distributions of PVP stabilized StGly colloidal particles. The average particle size can be tuned from below 100 nm, preferred for optically transparent formulations, to larger sizes within the colloidal range.
For food products, the use of natural stabilisers is preferred. To demonstrate this possibility, we will use two water soluble biopolymers: OSA starch and gum arabic. Both biopolymers are known for their ability to provide colloidal stability through steric and electrosteric stabilisation and have been used in the synthesis of colloidal particles.36Fig. 3 shows electron micrographs of StGly colloidal particles stabilised by gum arabic and OSA starch respectively. In both cases, well defined spherical particles are formed.
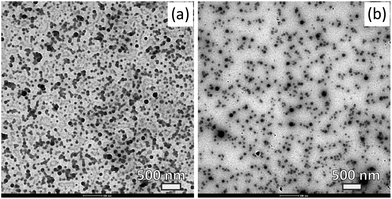 | ||
| Fig. 3 TEM micrographs of colloidal particles comprising the StGly extract stabilised with (a) gum arabic and (b) OSA starch. Scale bars = 500 nm. | ||
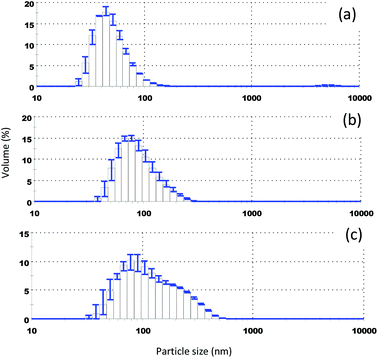
One of the main advantages of using colloidal particles as the delivery system is their ability to alter the particle composition without the need for a solvent (as for example in the case of emulsions) or deliver more than one functional/bioactive ingredient. The colloidal particles could be of pure StGly or could be a multiple delivery system which combines StGly with other bioactives such as PS, phytosterol esters, or other fat soluble (or water insoluble) compounds can be combined. To demonstrate the ability to blend via co-precipitation of different bioactive compounds, we select PS which is also known for its notoriously low solubility in water and limited solubility in edible oils. PS formulations have been prepared using colloidal delivery systems such as microemulsions,13 nanoscale emulsions,18 and colloidal particles.12 We synthesized mixed StGly–PS colloidal particles (Fig. 4) using Tween 80 as a non-ionic stabiliser. One particular advantage of this approach is its ability to suppress the crystallization of PS, which typically forms rod- or needle like particles.12 Control of the shape of the delivery systems has being found to be important in their interactions with cells. StGly extract owing to its complex composition and large molecule weight components is unable to crystallise and may act as a matrix which can suppress the crystallization of PS. The presence of a mixture of various ingredients can suppress the crystallization of PS leading to a loss in shape anisotropy. Similarly, suppression of quercetin needle-like crystal formation was recently observed in colloidal particles from water insoluble proteins.15
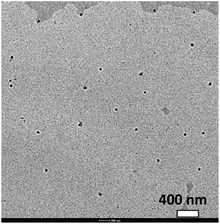 | ||
| Fig. 4 TEM micrograph of composite colloidal particles comprising 50 wt% StGly extract and 50 wt% PS. The particles are stabilized by using non-ionic surfactants (Tween 80). Scale bar = 400 nm. | ||
Very stable dispersions (no sedimentation, aggregation) with sufficiently small sizes are easily obtained. Fig. 5 shows the volume averaged size distribution of PVP stabilized StGly colloidal particles measured at intervals of 6 months. Although there is a small shift in the particle size distribution, the cut off size of the largest particles remains in the same range. Although not studied in detail, the change in the stabilizer can improve the dispersion stability against aggregation or Ostwald ripening.
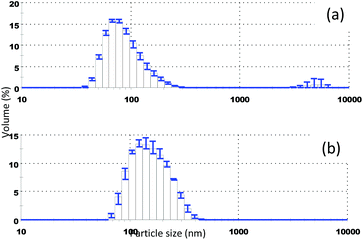
Fig. 6 shows the volume averaged particle size distribution of Tween 80 stabilized StGly measured during a period of 33 months. Although the particles are very small in size and 1 wt% ethanol is present in the suspension, residual from the precipitation process, only a small change in the size distribution was observed during the period of 33 months. Such a small change in the size distribution could be a result of the small but not negligible solubility of StGly in water which can be further increased in the presence of some ethanol. The solubility in water–ethanol mixtures was not quantified, as such small changes in particle size distribution are acceptable in terms of product stability.
The small change in particle size upon very long storage at ambient temperature is indicative of the very good stability of these colloidal systems. Long term colloidal stability is critical for the application as delivery systems, which combined with the increase in surface area is a good prerequisite for good bioavailability. The use of StGly (from H. Gordonii) comprising colloidal particles in an animal study has confirmed that slow or incomplete gastrointestinal dissolution was not a limiting factor for systemic bioavailability.37
Conclusions
In conclusion, we demonstrated how colloidal delivery systems based on colloidal particles can be used for the formulation of steroid glycosides. We demonstrated the synthesis of colloidal particles containing steroid glycosides using a facile food grade method. Liquid antisolvent precipitation was used in the presence of various food compatible stabilizers such as polymers and surfactants, owing to their advantages and limitations. The sufficiently small size of the colloidal particles led to excellent stability against sedimentation, as a result of their fully Brownian character. Such colloidal delivery systems could find several applications in food and pharmaceutical products for the delivery of StGly. Owing to their ability to use different stabilisers, such colloidal particles can be compatible with a wide range of product formats. The encapsulation of such plant bioactive molecules could also potentially find applications in crop protection.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank B. A. Graf, S. L. Abrahamse, and G. S. M. J. E. Duchateau for stimulating discussions. This research work was partially funded by the Dutch Ministry of Economic Affairs through the Food & Nutrition Delta 2 Program (grant DFN0642300) and by NanoNextNL (consortium of Dutch government and 130 other partners).Notes and references
- B. Avula, Y. H. Wang, R. S. Pawar, Y. J. Shukla, T. J. Smillie and I. A. Khan, J. Pharm. Biomed. Anal., 2008, 48, 722–731 CrossRef CAS PubMed.
- C. A. Pereira, L. L. S. Pereira and A. D. Corrêa, J. Med. Plants Res., 2010, 4, 2305–2312 Search PubMed.
- C. Smith and A. Krygsman, J. Ethnopharmacol., 2014, 155, 1284–1290 CrossRef PubMed.
- A. Esteghamati, T. Mazaheri, M. V. Rad and S. Noshad, Int. J. Endocrinol. Metab., 2015, 13, e19678 Search PubMed.
- M. Zhu, J. Luo, H. Lv and L. Kong, J. Funct. Foods, 2014, 6, 585–597 CrossRef CAS.
- H. C. Dutt, S. Singh, B. Avula, I. A. Khan and Y. S. Bedi, J. Med. Food, 2012, 15, 108–119 CrossRef CAS PubMed.
- J. P. Munafo Jr. and T. J. Gianfagna, Nat. Prod. Rep., 2015, 32, 454–477 RSC.
- P. J. D. Bouic, A. Clark, J. Lamprecht, M. Freestone, E. J. Pool, R. W. Liebenberg, D. Kotze and P. P. Van Jaarsveld, Int. J. Sports Med., 1999, 20, 258–262 CrossRef CAS PubMed.
- (a) T. Helgason, H. Salminen, K. Kristbergsson, D. J. McClements and J. Weiss, J. Colloid Interface Sci., 2015, 448, 114 CrossRef CAS PubMed; (b) C. Qian, E. A. Decker, H. Xiao and D. J. McClements, J. Am. Oil Chem. Soc., 2012, 89, 17 CrossRef CAS; (c) R. H. Müller, K. Mäder and S. Gohla, Eur. J. Pharm. Biopharm., 2000, 50, 161 CrossRef.
- (a) S. Gramdorf, S. Hermann, A. Hentschel, K. Schrader, R. H. Müller, M. Kumpugdee-Vollrath and M. Kraume, Colloids Surf., A, 2008, 331, 108 CrossRef CAS; (b) Y. Yang, A. Corona, B. Schubert, R. Reeder and M. A. Henson, J. Colloid Interface Sci., 2014, 418, 261 CrossRef CAS PubMed; (c) M. A. Schubert and C. C. Müller-Goymann, Eur. J. Pharm. Biopharm., 2005, 61, 77 CrossRef CAS PubMed.
- E. Imai, Y. Shimichi, I. Maruyama, A. Inoue, S. Ogawa, K. Hatae and A. Shimada, J. Texture Stud., 1997, 28, 257–272 CrossRef.
- L. Rossi, J. W. M. Seijen Ten Hoorn, S. M. Melnikov and K. P. Velikov, Soft Matter, 2010, 6, 928–936 RSC.
- N. Garti, A. Spernath, A. Aserin and R. Lutz, Soft Matter, 2005, 1, 206–218 RSC.
- K. P. Velikov and E. Pelan, Soft Matter, 2008, 4, 1964–1980 RSC.
- A. R. Patel, P. C. M. Heussen, J. Hazekamp, E. Drost and K. P. Velikov, Food Chem., 2012, 133, 423–429 CrossRef CAS PubMed.
- A. R. Patel, J. Seijen-ten-Hoorn, P. C. M. Heussen, E. Drost, J. Hazekamp and K. P. Velikov, J. Colloid Interface Sci., 2012, 374, 150–156 CrossRef CAS PubMed.
- L. Rossi, K. P. Velikov and A. P. Philipse, Food Chem., 2014, 151, 243–247 CrossRef CAS PubMed.
- H. S. Ribeiro, R. Gupta, K. W. Smith, K. F. van Malssen, A. K. Popp and K. P. Velikov, Soft Matter, 2016, 12, 5835–5846 RSC.
- A. R. Patel, E. Drost, T. B. J. Blijdenstein and K. P. Velikov, ChemPhysChem, 2012, 13, 3777–3781 CrossRef CAS PubMed.
- D. J. McClements, E. A. Decker and J. Weiss, J. Food Sci., 2007, 72, R109–R124 CrossRef CAS PubMed.
- D. J. McClements and Y. Li, Adv. Colloid Interface Sci., 2010, 159, 213–228 CrossRef CAS PubMed.
- D. J. McClements and J. Rao, Crit. Rev. Food Sci. Nutr., 2011, 51, 285–330 CrossRef CAS PubMed.
- H. Fessi, F. Puisieux, J. P. Devissaguet, N. Ammoury and S. Benita, Int. J. Pharm., 1989, 55, R1–R4 CrossRef CAS.
- D. J. McClements, C. Chung and B. C. Wu, Food Funct., 2017, 8, 498–510 CAS.
- H. Auweter, H. Haberkorn, W. Heckmann, D. Horn, E. Luddecke, J. Rieger and H. Weiss, Angew. Chem., Int. Ed., 1999, 38, 2188–2191 CrossRef CAS PubMed.
- D. Horn and J. Rieger, Angew. Chem., Int. Ed., 2001, 40, 4330–4361 CrossRef CAS PubMed.
- M. E. Matteucci, M. A. Hotze, K. P. Johnston and R. O. Williams, Langmuir, 2006, 22, 8951–8959 CrossRef CAS PubMed.
- V. Uskokovic and E. Matijevic, J. Colloid Interface Sci., 2007, 315, 500–511 CrossRef CAS PubMed.
- V. Uskokovic, Steroids, 2008, 73, 356–369 CrossRef CAS PubMed.
- L. Mukhopadhyay, P. K. Bhattacharyya, A. R. Das and S. P. Moulik, Colloid Polym. Sci., 1993, 271, 793–798 CAS.
- A. R. Patel, P. C. M. Heussen, E. Dorst, J. Hazekamp and K. P. Velikov, Food Chem., 2013, 141, 1466–1471 CrossRef CAS PubMed.
- I. J. Joye and D. J. McClements, Trends Food Sci. Technol., 2013, 34, 109–123 CrossRef CAS.
- A. A. Thorat and S. V. Dalvi, Chem. Eng. J., 2012, 181–182, 1–34 CrossRef CAS.
- P. J. Russell and C. Swindells, Food Chem. Toxicol., 2012, 50, S6–S13 CrossRef CAS PubMed.
- R. Shabd, B. M. Upadhyay and S. B. Singh, Carbohydr. Res., 1981, 98, 123–126 CrossRef CAS.
- C. C. Chen and G. Wagner, Chem. Eng. Res. Des., 2004, 82(A11), 1432–1437 CrossRef CAS.
- B. A. Graf, C. J. van Platerink, G. A. M. Ten Have, N. E. P. Deutz, K. P. Velikov, L. M. Flendrig, S. M. Melnikov, U. M. Garczarek, S. L. Abrahamse and G. S. M. J. E. Duchateau, J. Funct. Foods, 2011, 3, 135–143 CrossRef CAS.
Footnotes |
| † Present address: International Iberian Nanotechnology Laboratory, Avda. Mestre José Veiga s/n, 4715 Braga, Portugal. |
| ‡ Present address: IOI Loders Croklaan, Hogeweg 1, 1521AZ Wormerveer, The Netherlands. |
| This journal is © The Royal Society of Chemistry 2018 |