The protective effect of phloretin in osteoarthritis: an in vitro and in vivo study
Wenhao
Zheng
,
Chunhui
Chen
,
Chuanxu
Zhang
,
Leyi
Cai
and
Hua
Chen
 *
*
Department of Orthopaedic Surgery, The Second Affiliated Hospital and Yuying Children's Hospital of Wenzhou Medical University, Wenzhou 325000, China. E-mail: chenhuafey@126.com; Fax: +86-577-88879123; Tel: +86-577-88879158
First published on 7th November 2017
Abstract
Osteoarthritis (OA) is a degenerative joint disease characterized by the degradation and inflammation of cartilage. Phloretin, a type of dihydrochalcone mainly found in apples and apple-derived products, has been reported to possess various potent biological effects such as antioxidant, anticancer, anti-inflammatory, and immunomodulatory. However, the anti-inflammatory effects of phloretin on OA have not been reported. This study was aimed at assessing the effects of phloretin on human OA chondrocytes. Human OA chondrocytes were pretreated with phloretin (10, 30, and 100 μM) for 2 h and subsequently stimulated with IL-1β for 24 h. The production of NO, PGE2, TNF-α, and IL-6 was determined using the Griess reagent and ELISAs. The mRNA expression of COX-2, iNOS, MMP-3, MMP-13, and ADAMTS-5 was measured by real-time PCR. Changes in the protein expression of COX-2, iNOS, MMPs, ADAMTS, aggrecan, collagen-II, NF-κB, and the PI3K/Akt signaling pathway were detected by western blotting. In this study, we found that phloretin significantly inhibited the IL-1β-induced production of NO, PGE2, TNF-α, and IL-6, the expression of COX-2, iNOS, MMP-3, MMP-13, and ADAMTS-5, and the degradation of aggrecan and collagen-II in human OA chondrocytes. Furthermore, phloretin dramatically suppressed the IL-1β-stimulated phosphorylation of PI3K/Akt and activation of NF-κB in human OA chondrocytes. In addition, treatment with phloretin not only prevented the destruction of cartilage and the thickening of subchondral bone but also relieved synovitis in a mouse model of OA. Moreover, immunohistochemical results showed that phloretin significantly decreased the expression of MMP-13 and increased the expression of collagen-II in OA in mice. In conclusion, these results suggest that phloretin may be a potential agent for the treatment of OA.
1. Introduction
Osteoarthritis (OA) is a multifactorial degenerative joint disorder associated with structural changes in joint tissues including the destruction of cartilage and remodeling of subchondral bone, as well as synovial inflammation in the elderly.1 The prevalence of OA increases with age.2 Previous studies showed that about 12% of the western aging population suffer from OA, and 25% of people aged over 55 have an episode of persistent knee pain.3 However, the etiology of OA remains unknown although inflammation and inflammatory cytokines have been reported to play critical roles in the initiation and progression of OA.4 It has been known that the excess production of inflammatory cytokines plays vital roles in the development of OA.5 Among these cytokines, interleukin-1β (IL-1β) has been shown to play a pivotal role in OA as it contributes to the degradation of cartilage matrix by inducing the expression of matrix metalloproteinases (MMPs) and a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS), which results in decreases in the synthesis of collagen and proteoglycans during the pathogenesis of OA.6 In addition, the stimulation of chondrocytes with IL-1β could induce the release of the inflammatory mediators nitric oxide (NO) and prostaglandin E2 (PGE2).7,8 The overproduction of NO and PGE2 has been considered to be closely related to the clinical manifestations of OA.9 Therefore, the inhibition of IL-1β and IL-1β-induced inflammatory mediators is suggested as a potential strategy for treating OA.Phloretin (PT), which is a naturally occurring phytochemical found in the Rosaceae family, is a dihydrochalcone or bicyclic flavonoid.10 It exists widely in the bark, leaves and fruit of apple trees.11 Phloretin has been reported to possess numerous potent beneficial biological effects such as antioxidant, anticancer, anti-inflammatory and immunomodulatory activities.12–14 Previous studies have reported important anti-inflammatory properties of phloretin. For example, phloretin reduced the levels of pro-inflammatory cytokines and mediators such as NO, PGE2, IL-6, TNF-α, inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) via the suppression of the activation of nuclear factor κB (NF-κB) and mitogen-activated protein kinase (MAPK) in RAW264.7 murine macrophages stimulated by lipopolysaccharide (LPS).15 In addition, phloretin inhibited the expression of IL-1β-induced COX-2 and intercellular adhesion molecule-1 (ICAM-1) via the inhibition of the MAPK/Akt/NF-κB signaling pathway in human lung epithelial cells.14 Phloretin was also found to promote lipolysis and inhibit inflammation in 3T3-L1 mouse cells and in macrophage-adipocyte co-cultures.16 Furthermore, in an in vivo study phloretin reduced the severity of rheumatoid arthritis and effectively mitigated joint inflammation and the destruction of cartilage and bone via reducing the production of pro-inflammatory cytokines (TNF-α, IL-6, IL-1β and IL-17).17 However, the anti-inflammatory effect of phloretin on OA is still unclear. Consequently, in the current study we investigated the anti-inflammatory effect and the potential mechanism of phloretin on IL-1β-stimulated human OA chondrocytes in vitro, as well as the protective role of phloretin in a mouse model of OA in vivo.
2. Materials and methods
2.1. Chemicals and reagents
Phloretin (purity >98%), recombinant human IL-1β, collagenase type II, and dimethyl sulfoxide (DMSO) were obtained from Sigma Chemical Co. (St Louis, MO, USA). Cell counting kit-8 (CCK-8) was purchased from Dojindo (Kumamoto, Japan). Primary antibodies against COX-2, iNOS, MMP-3, MMP-13, ADAMTS-5, collagen-II and aggrecan were purchased from Abcam (Cambridge, MA, USA). Primary antibodies against p65, p-p65, IκBα, p-IκBα, PI3K, p-PI3K, Akt and p-Akt were purchased from Cell Signaling Technology (Beverly, MA, USA). Goat anti-rabbit and goat anti-mouse horseradish peroxidase conjugates were purchased from Bio-Rad Laboratories (CA, USA). Fetal bovine serum (FBS), bovine serum albumin (BSA), Dulbecco's modified Eagle's medium (DMEM)/Ham's F12 medium and 0.25% trypsin-ethylenediaminetetraacetic acid (trypsin–EDTA) were purchased from Gibco (Life Technologies Corp., Carlsbad, CA, USA). TRIzol reagent was purchased from Invitrogen (Carlsbad, CA, USA). A QuantiTect reverse transcription kit was purchased from Qiagen (Valencia, CA). SYBR Green Master Mix was purchased from Bio-Rad Laboratories (CA, USA). ELISA kits for PGE2, TNF-α and IL-6 were purchased from R&D Systems (Minneapolis, MN, USA). Griess reagent was purchased from the Beyotime Institute of Biotechnology (Shanghai, China). All other chemicals were of reagent grade.2.2. Isolation and culture of primary human chondrocytes
The collection of samples of articular cartilage was performed according to the terms of the Medical Ethical Committee of the Second Affiliated Hospital of Wenzhou Medical University and followed the guidelines of the Declarations of Helsinki and Tokyo. OA cartilage tissues were obtained from ten OA patients (age 55 ± 10) who underwent total knee replacement surgery at the Second Affiliated Hospital of Wenzhou Medical University. Osteoarthritis was diagnosed according to the Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association.18 Full ethical consent was obtained from all patients. Primary chondrocytes were isolated from articular cartilage as described previously.19 Pieces of cartilage were digested with 0.2% collagenase type II for 5 h at 37 °C and then centrifuged at 1000 rpm for 5 min, and the supernatant was discarded. The inner cell mass was obtained and suspended in DMEM/F12 with 10% FBS and 1% antibiotic mixture (penicillin and streptomycin). Finally, cells were plated at a density of 1 × 105 cells per mL in a 6-well plate and incubated in a humidified atmosphere of 5% CO2 at 37 °C. The media were changed every 2–3 days. Cells were passaged when at 80% to 90% confluence using 0.25% trypsin–EDTA solution. Only passages 1 and 2 were used in our study to avoid the loss of phenotype.2.3. Cell viability
The effects of phloretin on the viability of cells were assessed by a CCK-8 assay according to the manufacturer's instructions. Human OA chondrocytes were cultured in 96-well plates at a density of 5 × 103 cells per well for 24 h. Then, cells were pretreated with different concentrations (0, 10, 30 and 100 μM) of phloretin for 24 h and 48 h. After that, 10 μL CCK-8 was added to each well and the mixture was incubated at 37 °C for 4 h. The optical density was read at a wavelength of 450 nm with a microplate reader (Leica Microsystems, Germany). The inter-assay and intra-assay coefficients of variability of the CCK-8 assay were 5.0% and 0.1–4.6%, respectively.2.4. Measurement of NO, PGE2, TNF-α and IL-6
The NO levels in the culture medium were determined by the Griess reaction as previously described.20 In brief, the production of NO was assayed by measuring the levels of the stable NO metabolite nitrite using sodium nitrite resuspended in distilled H2O as a standard. The culture supernatant (100 μL) was allowed to react with an equal volume of Griess reagent (one part 0.1% naphthylethylenediamine and one part 1% sulfanilamide in 5% H3PO4) in a flat-bottomed 96-well microplate for 10 min at room temperature in the dark. Nitrite levels were determined by measuring the absorbance at 540 nm using a spectrophotometer (SpectraMax 340; Molecular Devices, Sunnyvale, CA, USA). The levels of nitrite were normalized to standard values. The levels of PGE2, TNF-α and IL-6 in the culture medium were investigated using commercial ELISA kits according to the manufacturer's instructions (R&D Systems, Minneapolis, MN, USA). The inter-assay and intra-assay coefficients of variability of the ELISA assay were 6.6–7.5% and 2.1–3.4%, respectively.2.5. Quantitative real-time polymerase chain reaction
Total RNA was isolated from chondrocytes using TRIzol reagent according to the manufacturer's instructions. Its concentration was determined spectrophotometrically at 260 nm (Thermo Scientific NanoDrop 2000). The A260/A280 ratio was calculated to confirm its quality and purity. First-strand cDNA was synthesized using 1 μg of total RNA and a QuantiTect reverse transcription kit. Quantitative real-time PCR (Q-PCR) was performed using a CFX96 real-time PCR system (Bio-Rad Laboratories, CA, USA) under the following conditions: 10 min at 95 °C, followed by 40 cycles of 15 s at 95 °C and 1 min at 60 °C. The reaction was performed in a total volume of 10 μL containing 4.5 μL diluted cDNA, 0.25 μL forward primer, 0.25 μL reverse primer and 5 μL SYBR Green Master Mix. The level of the target mRNA was normalized to the level of GAPDH and compared with that of controls. Data were analyzed using the 2−ΔΔCT method.21 Each gene analysis was performed in triplicate. The primer sequences of the target genes are listed in Table 1. The inter-assay and intra-assay coefficients of variability of the Q-PCR assay were 4.3% and 1.3–4.9%, respectively.| Gene | Forward primer | Reverse primer | Origin |
|---|---|---|---|
| COX-2 | 5′-GAGAGATGTATCCTCCCACAGTCA-3′ | 5′-GACCAGGCACCAGACCAAAG-3′ | Human |
| iNOS | 5′-CCTTACGAGGCGAAGAAGGACAG-3′ | 5′-CAGTTTGAGAGAGGAGGCTCCG-3′ | Human |
| MMP-3 | 5′-CTGGCCTGCTGGCTCATGCTT-3′ | 5′-GCAGGGTCCTTGGAGTGGTCA-3′ | Human |
| MMP-13 | 5′-CCAGAACTTCCCAACCAT-3′ | 5′-ACCCTCCATAATGTCATACC-3′ | Human |
| ADAMTS-5 | 5′-GCAGAACATCGACCAACTCTACTC-3′ | 5′-CCAGCAATGCCCACCGAAC-3′ | Human |
| GAPDH | 5′-TCTCCTCTGACTTCAACAGCGAC-3′ | 5′-CCCTGTTGCTGTAGCCAAATTC-3′ | Human |
2.6. Western blot analysis
Proteins were extracted from chondrocytes with RIPA lysis buffer. The lysates were sonicated on ice and centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 30 min at 4 °C. The protein concentration of the supernatant was determined using a BCA protein assay kit. Equal amounts of protein (40 μg) were separated by 12% SDS-PAGE and transferred to PVDF membranes. The membranes were incubated with blocking buffer comprising 5% non-fat milk or 3–5% BSA (for phosphorylated antibodies) in TBS containing 0.1% Tween-20 (TBST) for 2 h at room temperature and then probed with primary antibodies against COX-2, iNOS, MMP-3, MMP-13, ADAMTS-5, collagen-II, aggrecan, p65, p-p65, IκBα, p-IκBα, PI3K, p-PI3K, Akt and p-Akt (dilution of 1
000 rpm for 30 min at 4 °C. The protein concentration of the supernatant was determined using a BCA protein assay kit. Equal amounts of protein (40 μg) were separated by 12% SDS-PAGE and transferred to PVDF membranes. The membranes were incubated with blocking buffer comprising 5% non-fat milk or 3–5% BSA (for phosphorylated antibodies) in TBS containing 0.1% Tween-20 (TBST) for 2 h at room temperature and then probed with primary antibodies against COX-2, iNOS, MMP-3, MMP-13, ADAMTS-5, collagen-II, aggrecan, p65, p-p65, IκBα, p-IκBα, PI3K, p-PI3K, Akt and p-Akt (dilution of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000) overnight at 4 °C. After being washed three times with TBS containing 0.1% Tween-20 for 5 min, the membranes were incubated with HRP-conjugated secondary antibodies (1
1000) overnight at 4 °C. After being washed three times with TBS containing 0.1% Tween-20 for 5 min, the membranes were incubated with HRP-conjugated secondary antibodies (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3000) for 2 h. Finally, the membranes were detected using an enhanced chemiluminescence (ECL) kit and quantified by Quantity One (Bio-Rad, Hercules, CA, USA) software. β-Actin was used as an internal control. The inter-assay and intra-assay coefficients of variability of the western blot assay were 2.5% and 0.5–7.5%, respectively.
3000) for 2 h. Finally, the membranes were detected using an enhanced chemiluminescence (ECL) kit and quantified by Quantity One (Bio-Rad, Hercules, CA, USA) software. β-Actin was used as an internal control. The inter-assay and intra-assay coefficients of variability of the western blot assay were 2.5% and 0.5–7.5%, respectively.
2.7. Immunofluorescence
Chondrocytes were seeded onto 6-well plates on glass coverslips and incubated for 24 h. Glass coverslips with chondrocyte monolayers were rinsed three times with PBS. Then, the cells were fixed with 4% paraformaldehyde for 15 min at room temperature and rinsed with PBS again. The cells and nuclear membranes were permeabilized with 0.1% Triton X-100 for 5 min at room temperature. Later, the cells were overlaid with 5% protease-free BSA for 1 h at room temperature, rinsed with PBS and incubated with a primary antibody against collagen-II and p65 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200) at 4 °C overnight. After being washed with PBS, the cells were incubated with fluorescein-conjugated goat anti-rabbit IgG antibody (1
200) at 4 °C overnight. After being washed with PBS, the cells were incubated with fluorescein-conjugated goat anti-rabbit IgG antibody (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500) for 1 h at room temperature. Finally, the cells were washed three times with PBS and mounted in a medium containing DAPI (Invitrogen). Slides were viewed with a confocal laser scanning microscope (Leica Microsystems, Germany).
500) for 1 h at room temperature. Finally, the cells were washed three times with PBS and mounted in a medium containing DAPI (Invitrogen). Slides were viewed with a confocal laser scanning microscope (Leica Microsystems, Germany).
2.8. Transient transfection and luciferase activity assay
A 1 μg sample of NF-κB promoter/luciferase DNA (Stratagene, Santa Clara, CA, USA) together with 20 ng of control pRL-TK DNA was transiently transfected into 1 × 105 chondrocytesper well in a 6-well plate using Lipofectamine Plus reagents for 24 h. Cells that had been pretreated with phloretin (10, 30, and 100 μM) were stimulated with IL-1β (10 ng mL−1) for 2 h. Each well was then washed twice with ice-cold PBS, harvested in 100 μL of lysis buffer (0.5 mM HEPES pH 7.8, 1% Triton N-101, 1 mM CaCl2, and 1 mM MgCl2) and used for the measurement of luciferase activity using a dual-luciferase assay kit. Luminescence was measured using a Top Counter microplate scintillation and luminescence counter in single-photon counting mode for 0.1 min per well after adaptation for 5 min in the dark. The luciferase activity was normalized to the expression of the control pRL-TK. The inter-assay and intra-assay coefficients of variability of the luciferase assay were 1.1% and 0.9–5.6%, respectively.2.9. Mouse model of OA
Eight-week-old male C57BL/6 wild-type (WT) mice were purchased from the Animal Center of the Chinese Academy of Sciences, Shanghai, China. The protocol for animal care and use conformed to the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and was approved by the Animal Care and Use Committee of Wenzhou Medical University. The experimental mice were subjected to surgically induced OA by destabilization of the medial meniscus (DMM), as previously described.22 In brief, after anaesthesia via intraperitoneal injection of 1% pentobarbital sodium, the cranial attachment of the medial meniscus to the tibial plateau (medial meniscotibial ligament) of the right knee was transected with a microsurgical knife. The lateral meniscotibial ligament was identified and protected during the surgery. A sham operation, which consisted of an arthrotomy without transection of the medial meniscotibial ligament, was performed in the right knee joint of mice in the sham control group.2.10. Experimental animal design
The mice were randomly divided into three groups of 10 mice to establish a sham control group (control), an osteoarthritis group (OA) and an osteoarthritis group treated with phloretin (phloretin). A mouse model of OA was created by DMM surgery. Phloretin was dissolved in 0.5% sodium carboxymethylcellulose (0.5% CMC-Na). Mice in the sham control group were subjected to a sham operation, which consisted of an arthrotomy without transection of the medial meniscotibial ligament. Mice in the phloretin group received a gavage of phloretin (100 mg kg−1) daily for 8 weeks after surgery, whereas mice in the OA group received a gavage of vehicle (0.5% CMC-Na). The dose of phloretin was chosen on the basis of a previous study17 and our preliminary experiment. Food and water were available ad libitum. Mice were maintained at a constant temperature of 20 ± 2 °C and a relative humidity of 50 ± 10% in a 12 h light/dark cycle. All animals were sacrificed by cervical dislocation at 8 weeks after surgery. Knee joint tissues were collected for further evaluation.2.11. Histological assessment and immunohistochemical studies
The collected joint samples were fixed in 4% paraformaldehyde for 24 hours at 4 °C and decalcified in 10% EDTA solution at 4 °C for two weeks. After that, the samples were dehydrated via an alcohol gradient, cleared, and embedded in paraffin blocks. Frontal serial sections (thickness of 5 μm) across entire joints were obtained, and 10 slides per joint at intervals of 50 μm were selected and stained with safranin O/Fast Green and hematoxylin–eosin to assess the destruction of cartilage. The stained sections were photographed digitally under a microscope. To determine the extent of the degeneration of cartilage, we used multiple separate systems for scoring the destruction of articular cartilage, synovitis and subchondral bone thickness. The destruction of articular cartilage was graded using the Osteoarthritis Research Society International (OARSI) scoring system for the medial femoral condyle and medial tibial plateau.23 The grade was assigned as follows (six OA grades): 0 = surface intact, cartilage intact; 1 = surface intact; 2 = surface discontinuity; 3 = vertical fissures; 4 = erosion; 5 = denudation; and 6 = deformation. Then, we used the summed OARSI score (0–12) for the medial femoral condyle and medial tibial plateau to assess the degree of destruction of articular cartilage. The severity of synovitis was graded using a scoring system that was previously described:24 enlargement of the synovial lining cell layer on a scale of 0–3 (0 = 1–2 cells, 1 = 2–4 cells, 2 = 4–9 cells, and 3 = 10 or more cells) and the density of cells in the synovial stroma on a scale of 0–3 (0 = normal cellularity, 1 = slightly increased cellularity, 2 = moderately increased cellularity, and 3 = greatly increased cellularity). We used AxioVision software to measure the thickness of the medial subchondral bone plate according to the sections stained with safranin O. Sections of mouse cartilage (5 μm) were prepared using a freezing microtome. After incubation overnight at 4 °C with primary antibodies against collagen-II and MMP-13, the histological sections were incubated with secondary antibodies (Beyotime Institute of Biotechnology, Inc., Jiangsu, China) for 2 h at room temperature. A DAB substrate system (ZsBio, Beijing, China) was used for color development. Hematoxylin staining was utilized to reveal the nuclei of the cells. The number of positively stained cells on the entire articular surface per specimen was counted, and the percentage of positive cells was calculated.252.12. Statistical analysis
All experiments were performed independently six times (n = 6). Data are presented as the mean ± standard deviation (SD). Comparisons between groups were analyzed using SPSS 17.0 software via ANOVA followed by Dunnett's test. A value of p < 0.05 was considered to be statistically significant.3. Results
3.1. Effect of phloretin on viability of human OA chondrocytes
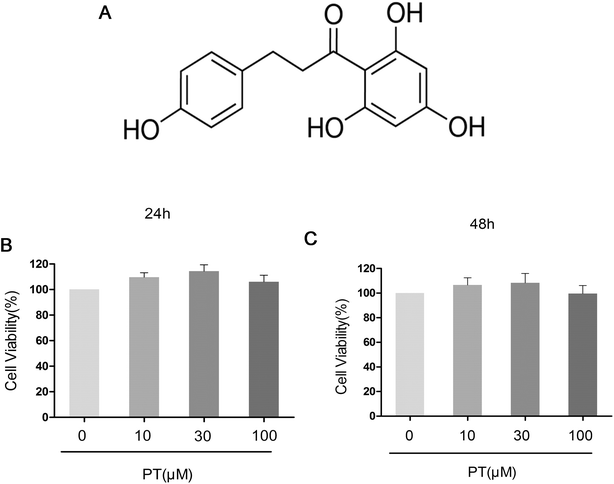
The effect of phloretin on the viability of chondrocytes was determined by a CCK-8 assay. Chondrocytes were cultured with increasing concentrations of phloretin (0, 10, 30, and 100 μM) for 24 h and 48 h, followed by CCK-8 analysis. The results showed that phloretin in the concentration range of 10–100 μM did not have cytotoxic effects on human OA chondrocytes (Fig. 1B and C). Consequently, phloretin (10, 30 and 100 μM) was used in the subsequent experiments.3.2. Effect of phloretin on IL-1β-induced production of NO, PGE2, TNF-α and IL-6 in human OA chondrocytes
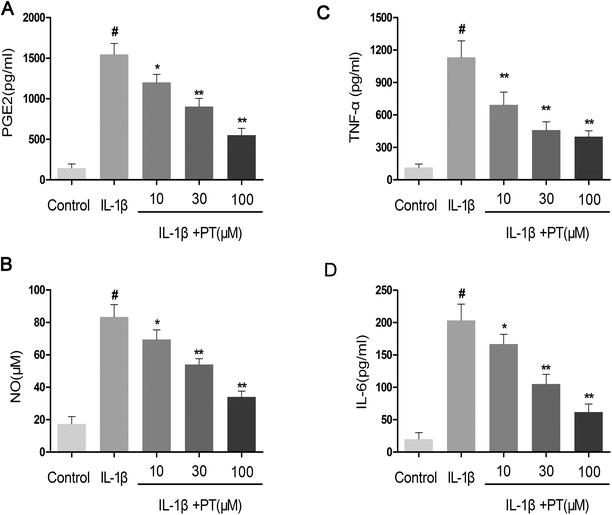
Firstly, the effect of phloretin on the IL-1β-induced production of NO, PGE2, TNF-α and IL-6 was determined. As shown in Fig. 2A–D, the production of NO (83.33 μM), PGE2 (1546.67 pg mL−1), TNF-α (995.14 pg mL−1) and IL-6 (203.33 pg mL−1) increased significantly in chondrocytes in comparison with the control group after treatment with IL-1β (p < 0.05). However, phloretin inhibited the IL-1β-induced production of NO, PGE2, TNF-α and IL-6 in a dose-dependent manner (p < 0.01).3.3. Effect of phloretin on IL-1β-induced expression of iNOS and COX-2 in human OA chondrocytes
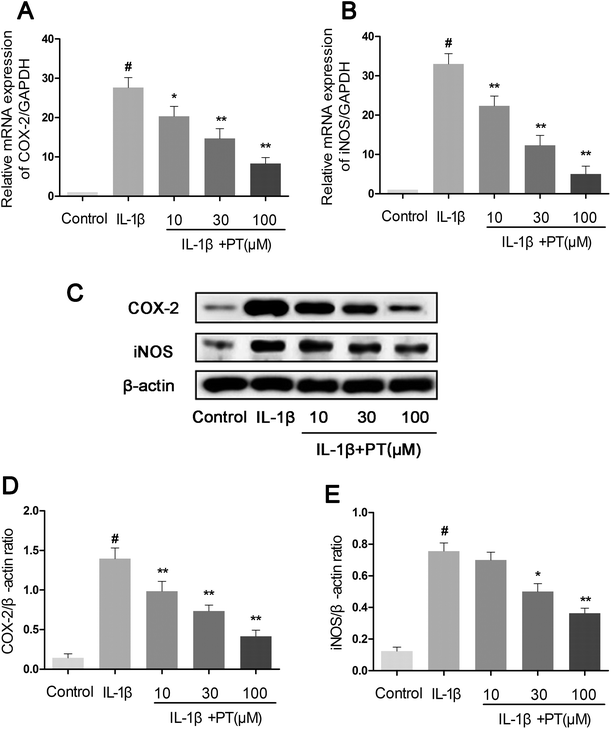
Subsequently, we investigated the effect of phloretin on the expression of iNOS and COX-2 in human chondrocytes. As shown in Fig. 3A and B, the stimulation of human OA chondrocytes by IL-1β resulted in the significant upregulation of the mRNA expression of iNOS (8.00-fold) and COX-2 (11.33-fold) in comparison with the control group (p < 0.05). However, phloretin dose-dependently inhibited the mRNA expression of iNOS and COX-2 induced by IL-1β (p < 0.01). In accordance with the results for mRNA expression, the protein expression of iNOS and COX-2 induced by IL-1β was also significantly suppressed by treatment with phloretin (p < 0.01, Fig. 3C–E).3.4. Effect of phloretin on IL-1β-induced expression of MMP-3, MMP-13, and ADAMTS-5 in human OA chondrocytes
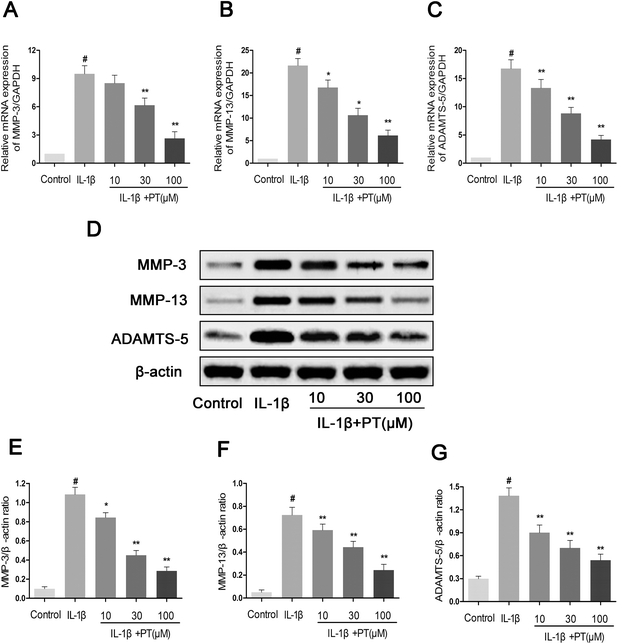
Next, we analyzed the effect of phloretin on the expression of MMP-3, MMP-13 and ADAMTS-5 in human OA chondrocytes. As shown in Fig. 4A–C, chondrocytes displayed an obvious upregulation of the mRNA expression of MMP-3 (8.10-fold), MMP-13 (13.13-fold) and ADAMTS-5 (22.00-fold) after stimulation with IL-1β in comparison with the control group (p < 0.05). However, treatment with phloretin markedly reduced the upregulation of the mRNA expression of MMP-3, MMP-13 and ADAMTS-5 (p < 0.01). In accordance with the results for mRNA expression, treatment with phloretin apparently blocked the protein expression of MMP-3, MMP-13 and ADAMTS-5 (p < 0.01, Fig. 4D–G).3.5. Effect of phloretin on IL-1β-induced degradation of aggrecan and collagen-II in human OA chondrocytes
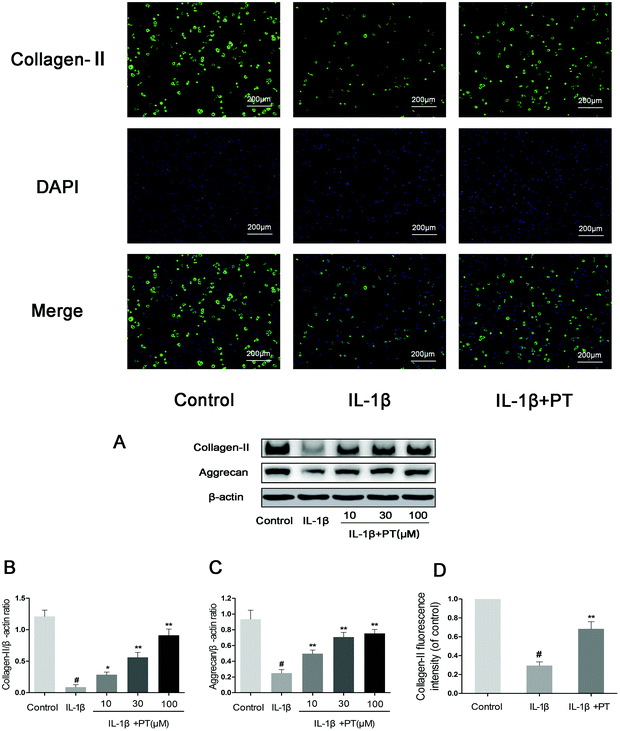
Then, we investigated the effect of phloretin on the IL-1β-induced degradation of aggrecan and collagen-II in human OA chondrocytes. As shown in Fig. 5A–C, chondrocytes displayed an obvious downregulation of the protein expression of aggrecan (0.27-fold) and collagen-II (0.072-fold) after stimulation with IL-1β (p < 0.05). In contrast, phloretin clearly inhibited the downregulation of the protein expression of aggrecan and collagen-II (p < 0.01). Moreover, the immunofluorescence results suggest that phloretin significantly suppressed the degradation of collagen-II, which is in accordance with the results of western blotting (Fig. 5D).3.6. Effect of phloretin on IL-1β-induced activation of NF-κB in human OA chondrocytes
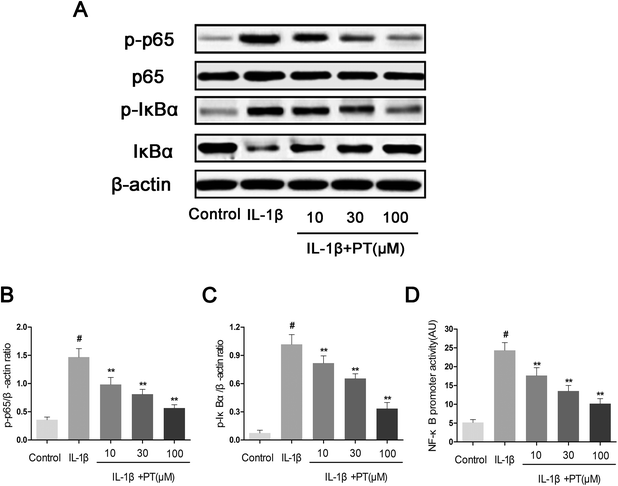
To further elucidate the mechanism underlying the anti-inflammatory effect of phloretin, western blotting and an NF-κB-dependent gene reporter assay were performed to study changes in the NF-κB signaling pathway. As shown in Fig. 6A–C, stimulation with IL-1β significantly induced the phosphorylation of NF-κB p65 (4.11-fold) and IκBα (13.86-fold) in chondrocytes (p < 0.05). Moreover, the stimulation of chondrocytes with IL-1β resulted in the significant degradation of IκBα. In contrast, phloretin exhibited a concentration-dependent inhibitory effect on the IL-1β-induced activation of NF-κB and degradation of IκBα in human OA chondrocytes (p < 0.01). On the other hand, the results of luciferase assays showed that phloretin significantly reduced the luciferase activity of the NF-κB promoter induced by IL-1β in a dose-dependent manner (p < 0.01, Fig. 6D).3.7. Phloretin inhibited IL-1β-induced nuclear translocation of NF-κB p65
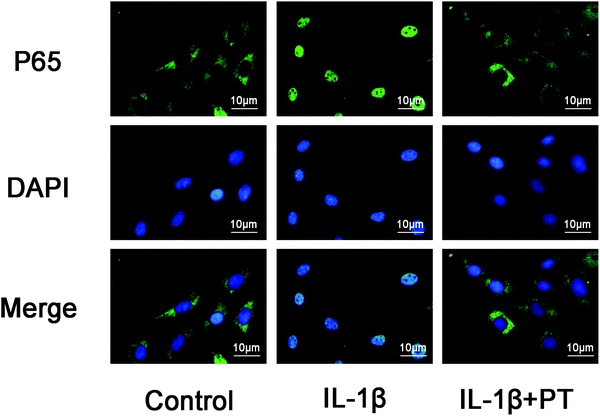
The effect of phloretin on the nuclear translocation of NF-κB p65 was examined via immunofluorescence in chondrocytes in response to the activation of NF-κB by IL-1β. Chondrocytes were not stimulated, treated with IL-1β alone for 30 min, or co-treated with IL-1β and 100 μM phloretin for 30 min. The control chondrocytes showed that the labeling of p65 was mostly restricted to the cytoplasm. After stimulation with IL-1β, chondrocytes displayed clear and enhanced nuclear staining of p65, which indicated the nuclear translocation of the NF-κB p65 subunit (Fig. 7). Nevertheless, phloretin succeeded in inhibiting the translocation of the p65 subunit into the nuclei and gave rise to a decrease in the activation of NF-κB. These findings of immunofluorescence studies suggested that phloretin is able to reverse the nuclear translocation of NF-κB p65 in chondrocytes stimulated with IL-1β, which is consistent with the inhibitory effect of phloretin on NF-κB observed by western blotting.3.8. Effect of phloretin on the IL-1β-induced phosphorylation of PI3K/Akt in human OA chondrocytes
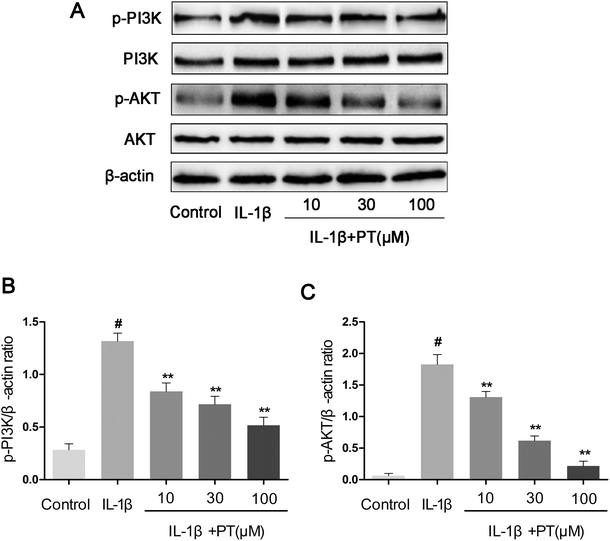
To further investigate the anti-inflammatory mechanism of phloretin, the effects of phloretin on the IL-1β-induced phosphorylation of PI3K/Akt were determined by western blotting. As shown in Fig. 8A–C, IL-1β significantly upregulated the phosphorylation of PI3K (4.62-fold) and Akt (28.75-fold) in comparison with the control group (p < 0.05). However, treatment with phloretin dose-dependently suppressed the IL-1β-induced phosphorylation of PI3K/Akt (p < 0.01).3.9. Effect of phloretin on OA induced by destabilization of the medial meniscus (DMM) in mice
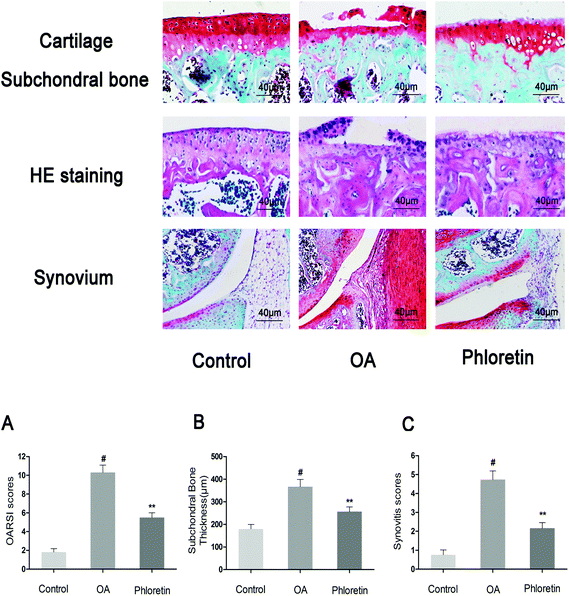
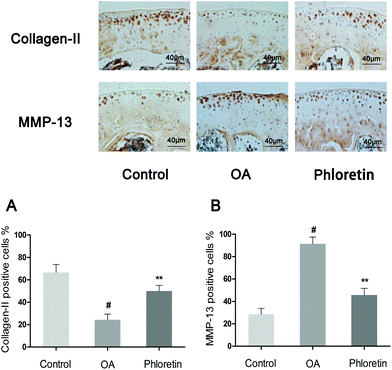
To determine whether phloretin has a preventive effect against the induction and progression of osteoarthritis in vivo, a DMM model of OA was established in mice, followed by a gavage of phloretin (100 mg kg−1) daily for 8 weeks after surgery. As shown in Fig. 9, staining with safranin O and hematoxylin–eosin showed that the cartilage surface was smooth and intact in the sham control group. However, the OA group exhibited superficial destruction of cartilage, massive loss of proteoglycans and apparent hypocellularity in comparison with the sham control group (p < 0.05). In contrast, treatment with phloretin significantly protected the structure of articular cartilage and maintained the proteoglycan levels in cartilage (p < 0.05). On the basis of the results of staining with safranin O, the OARSI scores of the OA group were manifestly higher (10.30 ± 0.46) than those of the sham control group (1.83 ± 0.20). In contrast, the phloretin group displayed markedly lower OARSI scores (5.50 ± 0.29) than the OA group. Moreover, it was found that phloretin significantly reduced the thickness of subchondral bone (Fig. 9B) and ameliorated inflammatory changes in the synovium (Fig. 9C) in comparison with the OA group (p < 0.05). We further investigated the expression of collagen-II and MMP-13 in the cartilage matrix using immunohistochemical studies. As shown in Fig. 10, quantitative analysis demonstrated that the OA group exhibited significantly fewer collagen-II-positive cells (24.44%) than the sham control group (p < 0.05). However, treatment with phloretin greatly increased the number of collagen-II-positive cells in comparison with the OA group (50.00%, p < 0.05, Fig. 10A). On the other hand, in the OA group the number of MMP-13-positive cells (91.33%) visibly increased in comparison with the sham control group but was observably decreased by treatment with phloretin (45.67%, p < 0.05, Fig. 10B). In summary, these results indicate that phloretin protected cartilage against degradation partly by inhibiting MMP-13 and enhancing the expression of collagen-II.4. Discussion
Currently, the treatment of OA has mainly aimed to relieve the chief complaints of OA patients, including joint pain, swelling and muscle tightness. Present pharmacological treatments for OA such as non-steroidal anti-inflammatory drugs (NSAIDs) are only temporarily effective and always bring about numerous serious side effects.26 Therefore, there is a considerable need to find safer and more effective therapeutic strategies for the treatment of OA. Accumulating evidence suggests that compounds extracted from natural plants have become potential reasonable agents of choice for OA patients because of their strong anti-inflammatory activities and low toxicity.19,27 Phloretin, which is a type of dihydrochalcone mainly found in apples and apple-derived products, has been reported to have potent anti-inflammatory effects.14 In the present study, we demonstrated that the effects of phloretin (0–100 μg mL−1) on human OA chondrocytes were not attributable to cytotoxic effects. We found that phloretin significantly inhibited IL-1β-induced inflammatory responses, including the expression of NO, PGE2, TNF-α, IL-6, iNOS, COX-2, MMP-3, MMP-13 and ADAMTS-5, as well as the degradation of collagen-II and aggrecan, in human OA chondrocytes. Besides, our results showed that phloretin significantly blocked the IL-1β-induced activation of NF-κB and phosphorylation of PI3K/Akt. Furthermore, phloretin exerted a protective effect against the degradation of cartilage in DMM-induced OA in mice.Inflammatory mediators such as NO and PGE2 have long been demonstrated to play critical roles in OA in that they accelerate the development of OA by inducing the synthesis of MMPs and inhibiting the synthesis of collagen and proteoglycans in chondrocytes.28,29 NO is an inflammatory mediator that is produced by the nitric oxide synthase (NOS) family of enzymes.30 It has been reported that NO promotes the production and activation of MMPs as well as other pro-inflammatory cytokines in OA.31,32 PGE2 is also an inflammatory mediator that is elevated by cyclooxygenase-2 (COX-2) and is involved in the degeneration of cartilage during the pathophysiology of OA owing to its potent excitation of MMPs and inhibition of the synthesis of extracellular matrix (ECM) in cartilage.33 Previous studies have shown that the inhibition of the production of inflammatory mediators such as NO and PGE2 was capable of attenuating the progression of OA.34 In this study, we demonstrated that phloretin significantly inhibited the IL-1β-induced production of NO and PGE2, as well as the expression of iNOS and COX-2 at the mRNA and protein levels, in human OA chondrocytes. Our findings agree with previous studies that reported that pretreatment with phloretin significantly reduced the levels of NO, PGE2, iNOS and COX-2 in mouse macrophages stimulated by LPS15 and that phloretin suppressed the thrombin-induced expression of COX-2 and production of PGE2 in THP-1 monocytes in a dose-dependent manner.35 TNF-α and IL-6 play pivotal roles in many inflammatory diseases, including OA. They can activate macrophages, which subsequently synthesize pro-inflammatory chemokines to maintain inflammation in the development of OA.36 In our study, we found that the induction of the production of TNF-α and IL-6 by IL-1β was reversed by phloretin. These results are consistent with the results of Chang et al.15 They found that pretreatment with phloretin had a dose-dependent inhibitory effect on IL-6 and TNF-α in RAW264.7 cells stimulated by LPS. From the above findings, our results suggest the feasibility of the use of phloretin in the treatment of OA on the basis of its strong inhibition of the overproduction of NO, PGE2, TNF-α and IL-6, as well as the overexpression of iNOS and COX-2, in the progression of OA.
MMPs comprise a family of enzymes that promote the turnover and breakdown of cartilage ECM in the pathophysiology of OA. Among all MMPs, MMP-13 has been shown to play a major role in the progression of OA because it strongly and irreversibly cleaves collagen-II, which constitutes the principal structure in cartilage ECM and accounts for the structural rigidity of cartilage.37,38 Thus, MMP-13 appears to be one of the most important candidates as a therapeutic target. It has been demonstrated that selective inhibitors of MMP-13 provided a potential therapy that could slow the progression of OA.39 MMP-3 has also been reported to break down a number of structural proteins in ECM such as collagen-II and proteoglycans. Apart from the degradation of ECM, MMP-3 also participates in activation cascades of MMP-13.40 Evidence is accumulating of the importance of the ADAMTS family of proteins in the degradation of cartilage in OA, in particular ADAMTS-5.41 It has been demonstrated that ADAMTS-5 is considered to be the primary aggrecanase responsible for the cleavage of aggrecan in the pathogenesis of OA, which has made it a potential therapeutic target in the treatment of OA.42 The inhibition of ADAMTS-5 by small interfering RNA (siRNA) decreased the loss of aggrecan from explants of human OA cartilage.43 It was found that inhibitors of ADAMTS could prevent the progression of OA.44 Interestingly, our results showed that the upregulation of the expression of MMP-3, MMP-13 and ADAMTS-5 in human OA chondrocytes stimulated with IL-1β was dramatically decreased by phloretin in a dose-dependent manner. Accordingly, we speculated that phloretin may exert its chondroprotective effects by blocking the expression and activation of MMPs and ADAMTS in the pathogenesis of OA.
Collagen-II and aggrecan constitute the major structures in ECM and maintain the normal physiological function of cartilage. The loss of collagen-II and aggrecan contributes to the acceleration of the progress of OA. Therefore, the inhibition of the degradation of collagen-II and aggrecan may represent a new therapeutic choice for OA. In our study, phloretin exhibited potent inhibition of the downregulation of the protein expression of aggrecan and collagen-II in human OA chondrocytes, which suggests that phloretin may suppress the degradation of cartilage by enhancing the expression of aggrecan and collagen-II.
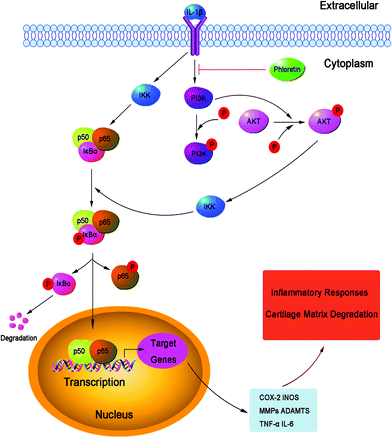
NF-κB is a transcription factor induced by inflammatory cytokines that plays a vital role in regulating the expression of iNOS, COX-2, TNF-α, IL-6, MMPs and other cytokines, as well as the regulation of many inducible inflammatory genes, in OA.45 Normally, NF-κB is present in the cytoplasm in an inactive form in combination with the inhibitory subunit IκBα. Once activated by IL-1β, IκBα is phosphorylated and degraded, and NF-κB p65 is translocated from the cytoplasm to the nucleus to trigger the expression of inflammation-related genes, including iNOS, COX-2, NO, PGE2, TNF-α, IL-6, MMPs and ADAMTS.46,47 Hence, blocking the activity of NF-κB may be an effective therapy in OA. Previous reports showed that the suppression of the activation of NF-κB had the ability to hinder the progression of OA.48 In addition, a study reported that NF-κB p65-specific siRNA inhibited the expression of COX-2, iNOS and MMP-9 in IL-1β-induced chondrocytes.49 Therefore, in our study, we also investigated molecular mechanisms by which phloretin inhibited inflammatory mediators in response to IL-1β in human OA chondrocytes. We found that phloretin significantly inhibited the IL-1β-induced activation of NF-κB and degradation of IκBα in human OA chondrocytes. Furthermore, phloretin reversed the translocation of NF-κB p65 from the cytoplasm to the nucleus when detected by immunofluorescence staining. Our results are partly supported by those of Huang et al., who found that phloretin suppressed the translocation of p65 into the nucleus and significantly inhibited the phosphorylation and degradation of IκBα to regulate the activity of NF-κB in human lung epithelial cells stimulated with IL-1β.14 Besides, phloretin inhibited the phosphorylation of IκBα and decreased the expression of the NF-κB subunit p65 in the nucleus in RAW 264.7 macrophages.16 The PI3K/Akt intracellular signaling pathway has also been demonstrated to be involved in alterations in both cells and ECM in the pathogenesis of OA.50 The activation of the PI3K/Akt pathway can increase the production of MMPs by chondrocytes via multiple downstream target proteins such as NF-κB. Moreover, the inhibition of the PI3K/Akt pathway has been considered as an option for the treatment of OA. Indeed, the inhibition of p-Akt and NF-κB by curcumin decreased the IL-1β-stimulated secretion MMPs and expression of COX-2 in chondrocytes.51 In our study, we found that phloretin greatly suppressed the IL-1β-induced phosphorylation PI3K/Akt in human OA chondrocytes, which was partly supported by a previous study that showed that treatment with phloretin significantly suppressed the phosphorylation of Akt in human lung epithelial cells activated by IL-1β.14 Therefore, previous studies, together with our findings, suggest that phloretin inhibited IL-1β-induced inflammatory responses by suppressing the phosphorylation of PI3K/Akt and activation of NF-κB in human OA chondrocytes, and the underlying mechanisms are illustrated specifically in Fig. 11. However, further studies are needed to clarify the exact mechanism of the regulatory effect of phloretin on the inflammatory process in human chondrocytes.
In addition to the in vitro study, we used the DMM model to assess the anti-inflammatory effect of phloretin in vivo. The DMM model has been widely employed to evaluate the efficacy of drugs for the treatment of OA because of its mechanical instability.52 Growing evidence suggests that the destruction of cartilage, remodeling of subchondral bone and synovitis are actively involved in the complex initiation and development of OA.53,54 As a result, agents that specifically block the mechanisms associated with the destruction of cartilage, remodeling of subchondral bone and synovitis may be beneficial for developing targeted therapy for OA. In the current study, our results showed that the mice in the OA group exhibited severe destruction of cartilage and extensive loss of proteoglycans, which were alleviated by treatment with phloretin. Moreover, phloretin reduced the OARSI scores and the thickness of the subchondral bone plate and ameliorated the severity of synovitis in mice in the OA group, which indicated that phloretin had the ability to hinder the progression of OA. Furthermore, treatment with phloretin significantly decreased the expression of MMP-13 and increased the expression of collagen-II in mice in the OA group. These results, together with the in vitro findings, provide evidence that phloretin exerts anti-inflammatory effects in OA both in vitro and in vivo. However, in the present study, we did not determine the potential toxicity in the in vivo study, and the exact mechanism of the regulatory effect of phloretin on the inflammatory response in vitro has not been clarified. This was a limitation of our research. Further work is needed to investigate the potential toxicity in vivo and clarify the exact mechanism of the regulatory effect of phloretin on the inflammatory process in human chondrocytes.
5. Conclusion
In conclusion, the present study demonstrated that phloretin inhibited IL-1β-induced inflammation via suppressing the phosphorylation of PI3K/Akt and activation of NF-κB in human OA chondrocytes. Furthermore, phloretin exerted a protective effect against the degradation of cartilage in DMM-induced OA in mice. Taken together, these findings suggest that phloretin may be a potential therapeutic choice for the treatment of OA in the future.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors thank all the staff in the Laboratory of Orthopedic Research Institute and Scientific Research Center of Second Affiliated Hospital of Wenzhou Medical University. This study was supported by Zhejiang Province Medical and Health Technology Project (2017KY480), Wenzhou Science and Technology Bureau (Y20160040) and Zhejiang Province College Students Science and Technology Innovation Program (2017R413079).References
- G. R. Squires, S. Okouneff, M. Ionescu and A. R. Poole, The pathobiology of focal lesion development in aging human articular cartilage and molecular matrix changes characteristic of osteoarthritis, Arthritis Rheum., 2003, 48, 1261–1270 CrossRef CAS PubMed.
- E. Halilaj, D. C. Moore, D. H. Laidlaw, C. J. Got, A. P. Weiss, A. L. Ladd and J. J. Crisco, The morphology of the thumb carpometacarpal joint does not differ between men and women, but changes with aging and early osteoarthritis, J. Biomech. Eng., 2014, 47, 2709–2714 CrossRef PubMed.
- D. J. Hunter and D. T. Felson, Osteoarthritis, Br. Med. J., 2006, 332, 639–642 CrossRef PubMed.
- C. S. Bonnet and D. A. Walsh, Osteoarthritis, angiogenesis and inflammation, Rheumatology, 2005, 44, 7–16 CrossRef CAS PubMed.
- J. P. Pelletier, J. Martel-Pelletier and S. B. Abramson, Osteoarthritis, an inflammatory disease: potential implication for the selection of new therapeutic targets, Arthritis Rheum., 2001, 44, 1237–1247 CrossRef CAS PubMed.
- M. Kobayashi, G. R. Squires, A. Mousa, M. Tanzer, D. J. Zukor, J. Antoniou, U. Feige and A. R. Poole, Role of interleukin-1 and tumor necrosis factor alpha in matrix degradation of human osteoarthritic cartilage, Arthritis Rheum., 2005, 52, 128–135 CrossRef CAS PubMed.
- S. B. Abramson, M. Attur, A. R. Amin and R. Clancy, Nitric oxide and inflammatory mediators in the perpetuation of osteoarthritis, Curr. Rheumatol. Rep., 2001, 3, 535–541 CrossRef CAS PubMed.
- S. Ahmed, A. Rahman, A. Hasnain, M. Lalonde, V. M. Goldberg and T. M. Haqqi, Green tea polyphenol epigallocatechin-3-gallate inhibits the IL-1 beta-induced activity and expression of cyclooxygenase-2 and nitric oxide synthase-2 in human chondrocytes, Free Radicals Biol. Med., 2002, 33, 1097–1105 CrossRef CAS PubMed.
- Y. J. Chen, K. S. Tsai, D. C. Chan, K. C. Lan, C. F. Chen, R. S. Yang and S. H. Liu, Honokiol, a low molecular weight natural product, prevents inflammatory response and cartilage matrix degradation in human osteoarthritis chondrocytes, J. Orthop. Res., 2014, 32, 573–580 CrossRef CAS PubMed.
- J. R. Ehrenkranz, N. G. Lewis, C. R. Kahn and J. Roth, Phlorizin: a review, Diabetes Metab. Res. Rev., 2005, 21, 31–38 CrossRef CAS PubMed.
- J. Gonzalez-Gallego, M. V. Garcia-Mediavilla, S. Sanchez-Campos and M. J. Tunon, Fruit polyphenols, immunity and inflammation, Br. J. Nutr., 2010, 104(Suppl 3), S15–S27 CrossRef CAS PubMed.
- B. M. Rezk, G. R. Haenen, W. J. van der Vijgh and A. Bast, The antioxidant activity of phloretin: the disclosure of a new antioxidant pharmacophore in flavonoids, Biochem. Biophys. Res. Commun., 2002, 295, 9–13 CrossRef CAS PubMed.
- C. C. Lin, C. L. Chu, C. S. Ng, C. Y. Lin, D. Y. Chen, I. H. Pan and K. J. Huang, Immunomodulation of phloretin by impairing dendritic cell activation and function, Food Funct., 2014, 5, 997–1006 CAS.
- W. C. Huang, S. J. Wu, R. S. Tu, Y. R. Lai and C. J. Liou, Phloretin inhibits interleukin-1beta-induced COX-2 and ICAM-1 expression through inhibition of MAPK, Akt and NF-kappaB signaling in human lung epithelial cells, Food Funct., 2015, 6, 1960–1967 CAS.
- W. T. Chang, W. C. Huang and C. J. Liou, Evaluation of the anti-inflammatory effects of phloretin and phlorizin in lipopolysaccharide-stimulated mouse macrophages, Food Chem., 2012, 134, 972–979 CrossRef CAS PubMed.
- W. C. Huang, W. T. Chang, S. J. Wu, P. Y. Xu, N. C. Ting and C. J. Liou, Phloretin and phlorizin promote lipolysis and inhibit inflammation in mouse 3 T3-L1 cells and in macrophage-adipocyte co-cultures, Mol. Nutr. Food Res., 2013, 57, 1803–1813 CAS.
- S. P. Wang, S. C. Lin, S. Li, Y. H. Chao, G. Y. Hwang and C. C. Lin, Potent Antiarthritic Properties of Phloretin in Murine Collagen-Induced Arthritis, J. Evidence-Based Complementary Altern. Med., 2016, 2016, 9831263 Search PubMed.
- R. Altman, E. Asch, D. Bloch, G. Bole, D. Borenstein, K. Brandt, W. Christy, T. D. Cooke, R. Greenwald and M. Hochberg, et al., Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association, Arthritis Rheum., 1986, 29, 1039–1049 CrossRef CAS PubMed.
- W. Zheng, H. Zhang, Y. Jin, Q. Wang, L. Chen, Z. Feng, H. Chen and Y. Wu, Butein inhibits IL-1beta-induced inflammatory response in human osteoarthritis chondrocytes and slows the progression of osteoarthritis in mice, Int. Immunopharmacol., 2017, 42, 1–10 CrossRef CAS PubMed.
- R. Y. Au, T. K. Al-Talib, A. Y. Au, P. V. Phan and C. G. Frondoza, Avocado soybean unsaponifiables (ASU) suppress TNF-alpha, IL-1beta, COX-2, iNOS gene expression, and prostaglandin E2 and nitric oxide production in articular chondrocytes and monocyte/macrophages, Osteoarthritis Cartilage, 2007, 15, 1249–1255 CrossRef CAS PubMed.
- M. W. Pfaffl, A new mathematical model for relative quantification in real-time RT-PCR, Nucleic Acids Res., 2001, 29, e45 CrossRef CAS PubMed.
- F. Vasheghani, Y. Zhang, Y. H. Li, M. Blati, H. Fahmi, B. Lussier, P. Roughley, D. Lagares, H. Endisha, B. Saffar, D. Lajeunesse, W. K. Marshall, Y. R. Rampersaud, N. N. Mahomed, R. Gandhi, J. P. Pelletier, J. Martel-Pelletier and M. Kapoor, PPARgamma deficiency results in severe, accelerated osteoarthritis associated with aberrant mTOR signalling in the articular cartilage, Ann. Rheum. Dis., 2015, 74, 569–578 CrossRef CAS PubMed.
- K. P. Pritzker, S. Gay, S. A. Jimenez, K. Ostergaard, J. P. Pelletier, P. A. Revell, D. Salter and W. B. van den Berg, Osteoarthritis cartilage histopathology: grading and staging, Osteoarthritis Cartilage, 2006, 14, 13–29 CrossRef CAS PubMed.
- J. S. Lewis, W. C. Hembree, B. D. Furman, L. Tippets, D. Cattel, J. L. Huebner, D. Little, L. E. DeFrate, V. B. Kraus, F. Guilak and S. A. Olson, Acute joint pathology and synovial inflammation is associated with increased intra-articular fracture severity in the mouse knee, Osteoarthritis Cartilage, 2011, 19, 864–873 CrossRef CAS PubMed.
- P. Chen, C. Xia, S. Mei, J. Wang, Z. Shan, X. Lin and S. Fan, Intra-articular delivery of sinomenium encapsulated by chitosan microspheres and photo-crosslinked GelMA hydrogel ameliorates osteoarthritis by effectively regulating autophagy, Biomaterials, 2016, 81, 1–13 CrossRef CAS PubMed.
- Q. H. Ding, Y. Cheng, W. P. Chen, H. M. Zhong and X. H. Wang, Celastrol, an inhibitor of heat shock protein 90beta potently suppresses the expression of matrix metalloproteinases, inducible nitric oxide synthase and cyclooxygenase-2 in primary human osteoarthritic chondrocytes, Eur. J. Pharmacol., 2013, 708, 1–7 CrossRef CAS PubMed.
- W. Zheng, Z. Feng, S. You, H. Zhang, Z. Tao, Q. Wang, H. Chen and Y. Wu, Fisetin inhibits IL-1beta-induced inflammatory response in human osteoarthritis chondrocytes through activating SIRT1 and attenuates the progression of osteoarthritis in mice, Int. Immunopharmacol., 2017, 45, 135–147 CrossRef CAS PubMed.
- A. R. Amin and S. B. Abramson, The role of nitric oxide in articular cartilage breakdown in osteoarthritis, Curr. Opin. Rheumatol., 1998, 10, 263–268 CrossRef CAS PubMed.
- M. B. Goldring, J. Birkhead, L. J. Sandell, T. Kimura and S. M. Krane, Interleukin 1 suppresses expression of cartilage-specific types II and IX collagens and increases types I and III collagens in human chondrocytes, J. Clin. Invest., 1988, 82, 2026–2037 CrossRef CAS PubMed.
- X. Q. Wei, I. G. Charles, A. Smith, J. Ure, G. J. Feng, F. P. Huang, D. Xu, W. Muller, S. Moncada and F. Y. Liew, Altered immune responses in mice lacking inducible nitric oxide synthase, Nature, 1995, 375, 408–411 CrossRef CAS PubMed.
- K. Sasaki, T. Hattori, T. Fujisawa, K. Takahashi, H. Inoue and M. Takigawa, Nitric oxide mediates interleukin-1-induced gene expression of matrix metalloproteinases and basic fibroblast growth factor in cultured rabbit articular chondrocytes, J. Biochem., 1998, 123, 431–439 CrossRef CAS PubMed.
- W. Zheng, Z. Tao, C. Chen, C. Zhang, H. Zhang, X. Ying and H. Chen, Plumbagin Prevents IL-1beta-Induced Inflammatory Response in Human Osteoarthritis Chondrocytes and Prevents the Progression of Osteoarthritis in Mice, Inflammation, 2017, 40, 849–860 CrossRef CAS PubMed.
- H. Futani, A. Okayama, K. Matsui, S. Kashiwamura, T. Sasaki, T. Hada, K. Nakanishi, H. Tateishi, S. Maruo and H. Okamura, Relation between interleukin-18 and PGE2 in synovial fluid of osteoarthritis: a potential therapeutic target of cartilage degradation, J. Immunother., 2002, 25(Suppl 1), S61–S64 CrossRef CAS PubMed.
- W. Zheng, Z. Tao, L. Cai, C. Chen, C. Zhang, Q. Wang, X. Ying, W. Hu and H. Chen, Chrysin Attenuates IL-1beta-Induced Expression of Inflammatory Mediators by Suppressing NF-kappaB in Human Osteoarthritis Chondrocytes, Inflammation, 2017, 40, 1143–1154 CrossRef CAS PubMed.
- M. S. Kim, S. H. Park, S. Y. Han, Y. H. Kim, E. J. Lee, J. H. Yoon Park and Y. H. Kang, Phloretin suppresses thrombin-mediated leukocyte-platelet-endothelial interactions, Mol. Nutr. Food Res., 2014, 58, 698–708 CAS.
- M. Feldmann, F. M. Brennan and R. N. Maini, Role of cytokines in rheumatoid arthritis, Annu. Rev. Immunol., 1996, 14, 397–440 CrossRef CAS PubMed.
- P. S. Burrage, K. S. Mix and C. E. Brinckerhoff, Matrix metalloproteinases: role in arthritis, Front. Biosci., 2006, 11, 529–543 CrossRef CAS.
- P. G. Mitchell, H. A. Magna, L. M. Reeves, L. L. Lopresti-Morrow, S. A. Yocum, P. J. Rosner, K. F. Geoghegan and J. E. Hambor, Cloning, expression and type II collagenolytic activity of matrix metalloproteinase-13 from human osteoarthritic cartilage, J. Clin. Invest., 1996, 97, 761–768 CrossRef CAS PubMed.
- Y. Hu, J. S. Xiang, M. J. DiGrandi, X. Du, M. Ipek, L. M. Laakso, J. Li, W. Li, T. S. Rush, J. Schmid, J. S. Skotnicki, S. Tam, J. R. Thomason, Q. Wang and J. I. Levin, Potent, selective, and orally bioavailable matrix metalloproteinase-13 inhibitors for the treatment of osteoarthritis, Bioorg. Med. Chem., 2005, 13, 6629–6644 CrossRef CAS PubMed.
- D. Q. Wu, H. M. Zhong, Q. H. Ding and L. Ba, Protective effects of biochanin A on articular cartilage: in vitro and in vivo studies, BMC Complementary Altern. Med., 2014, 14, 444 CrossRef PubMed.
- M. Kapoor, J. Martel-Pelletier, D. Lajeunesse, J. P. Pelletier and H. Fahmi, Role of proinflammatory cytokines in the pathophysiology of osteoarthritis, Nat. Rev. Rheumatol., 2011, 7, 33–42 CrossRef CAS PubMed.
- M. K. Majumdar, R. Askew, S. Schelling, N. Stedman, T. Blanchet, B. Hopkins, E. A. Morris and S. S. Glasson, Double-knockout of ADAMTS-4 and ADAMTS-5 in mice results in physiologically normal animals and prevents the progression of osteoarthritis, Arthritis Rheum., 2007, 56, 3670–3674 CrossRef CAS PubMed.
- R. H. Song, M. D. Tortorella, A. M. Malfait, J. T. Alston, Z. Yang, E. C. Arner and D. W. Griggs, Aggrecan degradation in human articular cartilage explants is mediated by both ADAMTS-4 and ADAMTS-5, Arthritis Rheum., 2007, 56, 575–585 CrossRef CAS PubMed.
- C. Cooper, S. Snow, T. E. McAlindon, S. Kellingray, B. Stuart, D. Coggon and P. A. Dieppe, Risk factors for the incidence and progression of radiographic knee osteoarthritis, Arthritis Rheum., 2000, 43, 995–1000 CrossRef CAS PubMed.
- P. P. Tak and G. S. Firestein, NF-kappaB: a key role in inflammatory diseases, J. Clin. Invest., 2001, 107, 7–11 CrossRef CAS PubMed.
- A. V. Miagkov, D. V. Kovalenko, C. E. Brown, J. R. Didsbury, J. P. Cogswell, S. A. Stimpson, A. S. Baldwin and S. S. Makarov, NF-kappaB activation provides the potential link between inflammation and hyperplasia in the arthritic joint, Proc. Natl. Acad. Sci. U. S. A., 1998, 95, 13859–13864 CrossRef CAS.
- K. B. Marcu, M. Otero, E. Olivotto, R. M. Borzi and M. B. Goldring, NF-kappaB signaling: multiple angles to target OA, Curr. Drug Targets, 2010, 11, 599–613 CrossRef CAS PubMed.
- J. A. Roman-Blas and S. A. Jimenez, NF-kappaB as a potential therapeutic target in osteoarthritis and rheumatoid arthritis, Osteoarthritis Cartilage, 2006, 14, 839–848 CrossRef CAS PubMed.
- C. Lianxu, J. Hongti and Y. Changlong, NF-kappaBp65-specific siRNA inhibits expression of genes of COX-2, NOS-2 and MMP-9 in rat IL-1beta-induced and TNF-alpha-induced chondrocytes, Osteoarthritis Cartilage, 2006, 14, 367–376 CrossRef CAS PubMed.
- J. Chen, R. Crawford and Y. Xiao, Vertical inhibition of the PI3K/Akt/mTOR pathway for the treatment of osteoarthritis, J. Cell. Biochem., 2013, 114, 245–249 CrossRef CAS PubMed.
- M. Shakibaei, T. John, G. Schulze-Tanzil, I. Lehmann and A. Mobasheri, Suppression of NF-kappaB activation by curcumin leads to inhibition of expression of cyclo-oxygenase-2 and matrix metalloproteinase-9 in human articular chondrocytes: Implications for the treatment of osteoarthritis, Biochem. Pharmacol., 2007, 73, 1434–1445 CrossRef CAS PubMed.
- S. S. Glasson, T. J. Blanchet and E. A. Morris, The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse, Osteoarthritis Cartilage, 2007, 15, 1061–1069 CrossRef CAS PubMed.
- Y. Wei and L. Bai, Recent advances in the understanding of molecular mechanisms of cartilage degeneration, synovitis and subchondral bone changes in osteoarthritis, Connect. Tissue Res., 2016, 57, 245–261 CrossRef CAS PubMed.
- T. Hugle and J. Geurts, What drives osteoarthritis?-synovial versus subchondral bone pathology, Rheumatology, 2017, 56, 1461–1471 Search PubMed.
| This journal is © The Royal Society of Chemistry 2018 |