Metabolic fate of strawberry polyphenols after chronic intake in healthy older adults
Amandeep K.
Sandhu
 a,
Marshall G.
Miller
b,
Nopporn
Thangthaeng
b,
Tammy M.
Scott
b,
Barbara
Shukitt-Hale
a,
Marshall G.
Miller
b,
Nopporn
Thangthaeng
b,
Tammy M.
Scott
b,
Barbara
Shukitt-Hale
 b,
Indika
Edirisinghe
a and
Britt
Burton-Freeman
b,
Indika
Edirisinghe
a and
Britt
Burton-Freeman
 *ac
*ac
aCenter for Nutrition Research, Institute for Food Safety and Health, Illinois Institute of Technology, IL, USA. E-mail: bburton@iit.edu; Fax: +708-341-7078
bUSDA-ARS Human Nutrition Research Center on Aging at Tufts University, Boston, MA, USA
cDepartment of Nutrition, University of California, Davis, CA, USA
First published on 19th December 2017
Abstract
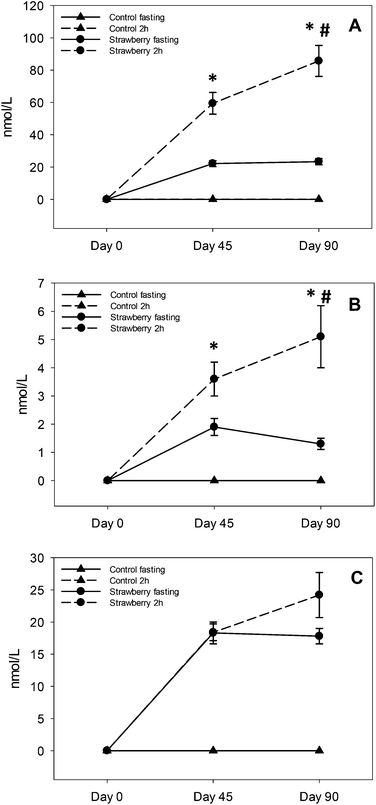
Strawberries contain a wide array of nutrients and phytochemicals including polyphenols such as anthocyanins, proanthocyanidins and ellagitannins. These polyphenols are absorbed and metabolized to various phenolic metabolites/conjugates in the body, which may play a role in disease risk reduction. In the present study, we investigated the metabolic fate of strawberry polyphenols after chronic (90 days) supplementation of freeze-dried strawberry (24 g d−1, equivalent to 2 cups of fresh strawberries) vs. control powder in 19 healthy older adults. Blood samples were collected at two time-points i.e., fasting (t = 0 h) and 2 h after the breakfast meal. On days 45 and 90 breakfast also included a control or strawberry drink consistent with their treatment randomization. A total of 21 polyphenolic metabolites were quantified in plasma consisting of 3 anthocyanins/metabolites, 3 urolithin metabolites and 15 phenolic acid metabolites. Among anthocyanins/metabolite, pelargonidin glucuronide (85.7 ± 9.0 nmol L−1, t = 2 h, day 90) was present in the highest concentration. Persistent concentrations of anthocyanins/metabolites, urolithins and some phenolic acids were observed in fasting (t = 0 h) plasma samples on day 45 and 90 after strawberry drink consumption suggesting a role of enteric, enterohepatic recycling or upregulation of gut microbial and/or human metabolism of these compounds. Our results suggest that strawberry polyphenols are absorbed and extensively metabolized, and can persist in the circulation.
Introduction
Among different kinds of berries, strawberries are one of the most important commercial berry crops in many parts of the world.1 Strawberries are recognized for having a protective role in chronic disease risk reduction such as diabetes,2 obesity,3 various types of cancers,4–6 cardiovascular diseases,7 and neurological disorders,8 which appears to go beyond the biological activity of their essential nutrient composition.Strawberries vary from pink to red in color, have sour to sweet flavor and possess a unique polyphenol profile. The major strawberry polyphenols are anthocyanins, ellagitannins and proanthocyanidins.9 Anthocyanins account for more than 75% of total polyphenols in strawberries.10 The total anthocyanin content in strawberries varies from 20 to 60 mg per 100 g fresh weight11 while the total ellagitannin content varies from 8 to 23 mg per 100 g fresh weight,9,12 depending upon the cultivar.
It is well documented that anthocyanins are rapidly absorbed in the body in their native form (glycoside attached).13 Following ingestion, they enter the stomach, small intestine and finally the colon where they can undergo structural modification (deglycolysation, degradation, hydroxylation etc.) at various points due to changing physiological conditions in the intestine. They can also be glucuronidated, methylated and sulfated by phase II enzymes in cells of the small intestine, liver and kidney before entering hepatic or systemic circulation or before excretion.14 Previous work on anthocyanin metabolism was focused on parent anthocyanins/metabolites15,16 limiting estimates of the array of metabolites likely generated from ingesting strawberries; both from the perspective of other polyphenols present in strawberries, but also the phenolic metabolites formed from degradation and or microbial action in the colon.17,18 Similar to anthocyanins, ellagitannins are stable in the conditions of the stomach and some could be hydrolyzed in small intestine due to pH changes, while others remain in their intact form.19,20 Ellagic acid is poorly absorbed, so it moves to the colon where it is further metabolized by gut bacteria to urolithins, which are considered the major products and bioactive forms of ellagitanins and ellagic acid.21
The bioavailability and pharmacokinetic parameters of strawberry anthocyanins have been studied by our group over time frames of 6–10 h.16,22,23 Peak concentrations of strawberry anthocyanins in plasma are reached between 1 and 3 h depending on the amount consumed and whether eaten with or without a meal. The present study aimed to explore changes in the concentrations of anthocyanins, urolithins and phenolic acid metabolites after chronic (45 and 90 days) intake by healthy older adults. The analyzed samples are from a United States Department of Agriculture (USDA) project investigating the effects of strawberry consumption on mobility and cognition.
Methods
Study design and procedure
The Tufts medical institutional review board approved the study. All study participants provided written informed consent before any study procedure were performed. The trial was registered on clinicaltrials.gov (clincaltrials.gov identifier: NCT02051140). This is a double-blinded, 2-arm, placebo controlled 90-day feeding trial. Screened participants visited the USDA Jean Mayer Human Nutrition Research Center on Aging (HNRCA) at Tufts University on 4 different occasions (Fig. 1). Visit 1 was a pre-study visit to familiarize participants with the study procedures.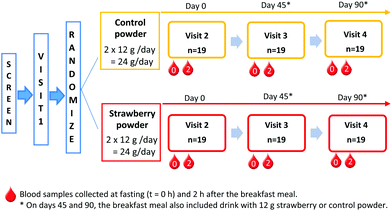 | ||
Fig. 1 Study design. Blood samples collected at fasting (t = 0 h) and 2 h after the breakfast meal. *![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) On days 45 and 90, the breakfast meal also included drink with 12 g strawberry or control powder. On days 45 and 90, the breakfast meal also included drink with 12 g strawberry or control powder. | ||
Upon arrival for visit 2 (day 0), anthropometric measurements, vital signs, and fasting blood samples were collected by a registered nurse. Thereafter, participants ate a standard breakfast meal and the second blood sample was collected at 2 h after the breakfast. At the end of the visit, participants consumed their first supplement packet and given powder packets (strawberry or control) for at least 45 days until their next visit. During the approximately 45 days between HNRCA visits, an investigator telephoned participants once each week. This phone call served as a reminder to participants and allowed the researchers to track compliance and assess supplement tolerability.
During study visit 3 (day 45), participants provided a fasting blood sample before and then 2 h after consuming the standard breakfast with either a strawberry or control drink, according to their randomly assigned daily intake of treatment. Breakfast was the same as in visit 2 (day 0); however, participants were only given as much of each item as they consumed during the previous visit. Participants returned all their used powder packets and compliance calendar from the first 45 days of the intervention and received another set of packets and compliance calendar, sufficient to complete the study.
During visit 4 (day 90), participants consumed their final strawberry or control packet with the study breakfast following the same procedures as visit 3 (day 45).
Study treatments
The strawberry drink was prepared from 12 g of freeze-dried strawberry powder (California Strawberry Commission, Watsonville, CA), which is equivalent to ∼1 cup of fresh strawberries (approximately 132 g). Participants consumed the drink two times per day (equivalent to approx. 2 cups per day fresh strawberries), 30 min prior to lunch and dinner. The control drink was prepared from a control powder (California Strawberry Commission, Watsonville, CA) that did not contain strawberry but was matched for color, flavor, consistency, and caloric content of the strawberry powders. Drinks were prepared by combining the strawberry or control powders (12 g per packet) with water (1 cup).The standardized breakfast provided on day 0, 45 and 90 consisted of a corn muffin, banana, apple juice, and coffee. Participants were given the same amount of breakfast at every visit during the study.
Study participants
Inclusion criteria: men and women who were between 60 and 75 years old, body mass index (BMI) between 18.5–29.9 kg m−2, >12 months post-menopausal (women), English fluency, adequate visual acuity for computer use, and nonsmoker, and in relatively good health (i.e., no previous history or current clinical evidence of cardiovascular, metabolic, respiratory, renal, gastrointestinal or hepatic diseases). Subjects taking medications or dietary supplements that would interfere with the outcomes of the study, anyone reporting allergies or sensitivity to berry products, vegetarians or vegans, consuming alcohol >2 drinks per day, presenting with cognitive deficits, neurological disorder, impaired mobility, at risk for falls, illicit drug use, or mini mental status exam score <24 were not eligible to participate in the study.Diet assessment and compliance
The National Cancer Institute's (NCI) online Diet History Questionnaire II (DHQ-II) was used to assess participants’ usual diet over the previous 12 months during study visit 1. The DHQ-II is a comprehensive, 124-item inventory that collects data on specific foods commonly consumed in the United States. Participants also completed an additional questionnaire on overall berry consumption because many berries are not assessed in the DHQ-II.To track compliance, participants completed supplement logs specifying the time of packet consumption twice daily and any adverse reactions. Participants were also contacted by telephone each week by one of the investigators to check compliance, monitor for any adverse events, and remind participants to continue consuming the study packets. Lastly, participants returned the empty and/or unused powder packets to the investigators at each study visit for compliance monitoring.
Blood analysis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 514g for 10 min at 4 °C. Samples were transferred to amber HPLC vials and were analyzed using an Agilent 1290 Infinity ultra-high performance liquid chromatography (UHPLC) system with an Agilent 6460 Triple Quadrupole Mass Spectrometer (Agilent Technologies, Santa Clara, CA). The system was equipped with a binary pump with an integrated vacuum degasser, an autosampler with a thermostat, and a column compartment with a thermostat. Standards were optimized for collision energies and multiple reaction monitoring (MRM) transitions using Mass Hunter Optimizer. The MRM transition for PG was based on an UHPLC accurate mass quadrupole time-of-flight mass spectrometry with electrospray ionization analysis conducted in our previous study.16 MRM transitions for phenolic acid metabolites without standards were based on previous literature reports.24 Standards were prepared in blank plasma. Two separate dynamic MRM methods were used for the quantification of anthocyanins (including their conjugated metabolites) and urolithin derivatives, and phenolic acid metabolites, respectively.
514g for 10 min at 4 °C. Samples were transferred to amber HPLC vials and were analyzed using an Agilent 1290 Infinity ultra-high performance liquid chromatography (UHPLC) system with an Agilent 6460 Triple Quadrupole Mass Spectrometer (Agilent Technologies, Santa Clara, CA). The system was equipped with a binary pump with an integrated vacuum degasser, an autosampler with a thermostat, and a column compartment with a thermostat. Standards were optimized for collision energies and multiple reaction monitoring (MRM) transitions using Mass Hunter Optimizer. The MRM transition for PG was based on an UHPLC accurate mass quadrupole time-of-flight mass spectrometry with electrospray ionization analysis conducted in our previous study.16 MRM transitions for phenolic acid metabolites without standards were based on previous literature reports.24 Standards were prepared in blank plasma. Two separate dynamic MRM methods were used for the quantification of anthocyanins (including their conjugated metabolites) and urolithin derivatives, and phenolic acid metabolites, respectively.
Statistical analysis
The anthocyanin and urolithin metabolites in plasma were analyzed using paired Student's t-test between the visits (day 45 fasting vs. day 90 fasting, day 45 2 h vs. day 90 2 h, day 45 fasting vs. 2 h and day 90 fasting vs. 2 h). For phenolic acid metabolites, Student's t-test was used for comparison of control and strawberry samples and paired Student's t-test was used for comparison between the visits. Statistical analysis was conducted using Microsoft Excel 2013 v 15. The data are reported as mean ± standard error and considered statistically significant at p ≤ 0.05.Results
Subject demographics
A total of 38 subjects finished the study (strawberry group = 19 and control group = 19). The average age of the strawberry group was 66.7 ± 4.4 years with average BMI of 23.8 ± 2.4 kg m−2. The average age and BMI of participants in the control group was 68.5 ± 4.3 years and 25.9 ± 2.6 kg m−2, respectively (Table 1).| Control | Strawberry | |
|---|---|---|
| Values are presented as group mean ± standard deviation. | ||
| Number of subjects (N) | 19 | 19 |
| Age (y) | 68.5 ± 4.3 | 66.7 ± 4.4 |
| BMI (kg m−2) | 25.9 ± 2.6 | 23.8 ± 2.4 |
| Compliance | 97.4% | 99.6% |
Diet assessment and compliance
Analysis of food intake data from the DHQ-II revealed only one dietary variable on which the control and strawberry groups significantly differed. Participants in the strawberry group consumed significantly less folic acid than did participants in the control group, F (1, 35) = 11.263, p = 0.002 (104.9 vs. 188.7 estimated μg per day, respectively). No significant differences were observed among the other dietary intake variables. Importantly, analysis of estimated annual fruit intake showed that the strawberry group did not differ from the control group (p = 0.433; 1.4 vs. 1.7 cups per day, respectively).On average, 2.7 packets per person were missed during the course of the study resulting in supplement compliance for both groups at >95% (see Table 1). No difference in the number of missed packets was observed between participants in the control and strawberry groups (p = 0.09).
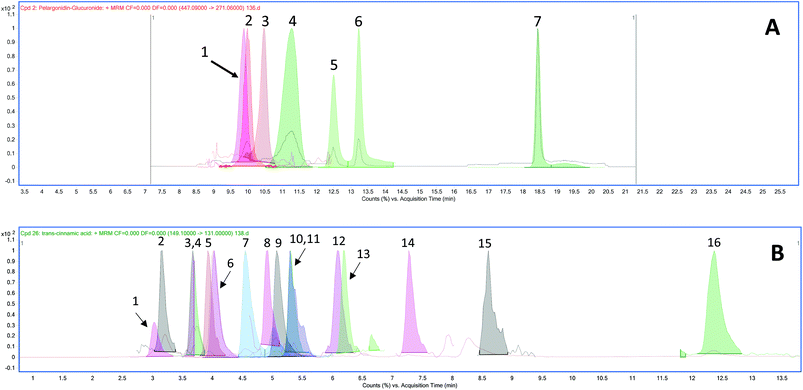
Metabolites in plasma
The chromatograms of the ion transitions of anthocyanins and their conjugated metabolites, urolithin derivatives and phenolic acid metabolites are shown in Fig. 2.| Phenolic acid metabolites | Group | Plasma concentrations (nmol L−1) | |||
|---|---|---|---|---|---|
| Day 45 | Day 90 | ||||
| 0 h | 2 h | 0 h | 2 h | ||
| Values are presented as mean ± standard error.a Statistically significant differences in plasma concentrations when comparing fasting (t = 0 h) samples after strawberry and control drink intake.b Statistical significance at 2 h. Statistical significance, p ≤ 0.05. | |||||
| Vanillic acid glucuronide | Control | 689.0 ± 21.9 | 2403.3 ± 125.3 | 716.9 ± 43.1 | 2650.3 ± 324.7 |
| Strawberry | 807.0 ± 41.1a | 2694.0 ± 334.0 | 783.0 ± 25.7 | 2599.3 ± 241.4 | |
| 3,4-dihydroxybenzoic acid | Control | 21.5 ± 0.7 | 29.1 ± 6.2 | 29.4 ± 5.4 | 30.5 ± 5.4 |
| Strawberry | 22.9 ± 2.2 | 29.1 ± 3.7 | 25.2 ± 4.3 | 25.7 ± 1.9 | |
| Isovanillic acid glucuronide | Control | 1030.2 ± 51.7 | 6039.6 ± 472.0 | 1063.4 ± 89.6 | 7035.0 ± 1163.9 |
| Strawberry | 1145.3 ± 73.2 | 5251.5 ± 699.0 | 1061.9.0 ± 40.8 | 4735.4 ± 466.7 | |
| 3,4-dihydroxybenzaldehyde | Control | 22.0 ± 0.7 | 28.7 ± 5.9 | 22.3 ± 1.1 | 24.6 ± 1.5 |
| Strawberry | 26.7 ± 1.3a | 33 ± 4.5 | 26.7 ± 1.3a | 29.9 ± 2.6 | |
| Hippuric acid (μmol L−1) | Control | 8.6 ± 1.4 | 8.5 ± 0.8 | 12.0 ± 1.6 | 10.7 ± 1.4 |
| Strawberry | 22.9 ± 5.0a | 25.7 ± 5.0b | 20.9 ± 4.1a | 20.3 ± 3.1b | |
| 4-hydroxybenzaldehyde | Control | 206.5 ± 21.0 | 212.1 ± 15.6 | 221.5 ± 14.1 | 252.0 ± 5.4 |
| Strawberry | 270.0 ± 24.9a | 415.5 ± 91.2b | 300.9 ± 22.6a | 409.3 ± 56.3b | |
| 3-hydroxybenzoic acid | Control | 642.3 ± 24.4 | 728.3 ± 40.6 | 635.0 ± 29.1 | 774.2 ± 89.3 |
| Strawberry | 776.5 ± 4.4a | 1030.0 ± 138.0b | 772.9 ± 32.6a | 938.7 ± 71.0 | |
| 2,3-dihydroxybenzoic acid | Control | 45.4 ± 10.4 | 70.1 ± 6.7 | 40.6 ± 9.8 | 85.4 ± 10.3 |
| Strawberry | 23.3 ± 3.8 | 53.9 ± 8.2 | 25.3 ± 5.8 | 67 ± 10.6 | |
| 4-hydroxyphenylacetic acid | Control | 1080.6 ± 170.6 | 1143.4 ± 181.7 | 1047.9 ± 146.6 | 1288.3 ± 158.4 |
| Strawberry | 704.1 ± 119.2 | 919.0 ± 164.8 | 1023.2 ± 224.6 | 1400.1 ± 350.1 | |
| Vanillic acid | Control | 0.0 ± 0.0 | 850.0 ± 37.3 | 0.0 ± 0.0 | 916.1 ± 87.9 |
| Strawberry | 0.0 ± 0.0 | 1087.6 ± 123.6 | 0.0 ± 0.0 | 975.3 ± 54.6 | |
| Isovanillic acid | Control | 0.0 ± 0.0 | 129.1 ± 10.0 | 0.0 ± 0.0 | 148.6 ± 22.6 |
| Strawberry | 0.0 ± 0.0 | 192.1 ± 23.2b | 0.0 ± 0.0 | 167.2 ± 9.8 | |
| Isoferulic acid glucuronide | Control | 49.1 ± 7.0 | 174.8 ± 48.3 | 53.1 ± 7.5 | 136.2 ± 15.7 |
| Strawberry | 51.5 ± 4.4 | 144.8 ± 15.4 | 43.9 ± 2.0 | 150.6 ± 16.0 | |
| p-coumaric acid | Control | 4.8 ± 0.2 | 8.1 ± 0.7 | 5.3 ± 0.6 | 9.0 ± 0.9 |
| Strawberry | 6.7 ± 0.9 | 39.3 ± 7.6b | 7.8 ± 1.0a | 36.3 ± 3.8b | |
| Ferulic acid | Control | 0.0 ± 0.0 | 48.0 ± 4.0 (n = 14) | 0.0 ± 0.0 | 44.6 ± 6.1 (n = 15) |
| Strawberry | 0.0 ± 0.0 | 51.7 ± 3.7 (n = 5) | 0.0 ± 0.0 | 51.4 ± 5.1 (n = 9) | |
| trans-cinnamic acid | Control | 12.7 ± 1.7 | 45.8 ± 5.1 | 17.4 ± 5.2 | 44.6 ± 6.1 |
| Strawberry | 19.5 ± 2.8a | 88.8 ± 8.8b | 23.5 ± 4.5 | 75.5 ± 7.1b | |
Discussion
Several polyphenolic compounds and their conjugated metabolites were detected and quantified in plasma samples of older adults consuming freeze-dried strawberry powder for 90 days. Parent anthocyanins, including the primary glucuronide conjugate of pelargonidin, three urolithin derivatives and fifteen phenolic acid metabolites were quantified. Important findings included: evidence for persistence of anthocyanins and some phenolic acid metabolites in circulation with chronic strawberry intake, enhancement in 2 h anthocyanin/metabolite and phenolic acids (4-hydroxybenzaldehyde, trans-cinnamic acid, hippuric acid and p-coumaric acid) concentrations after a strawberry drink supplementation, and urolithin metabolites were observed only in 68% of subjects with large inter individual variability.In acute pharmacokinetic studies conducted by our group22,23 and others,15 strawberry anthocyanins peaked within 1–3 hours and were back to baseline concentrations by 8–10 hours after consumption.22 However, in the present study, subjects consumed strawberry powder twice per day (morning and evening) and after an overnight fast (∼12–15 h since last intake of strawberry), anthocyanins were detected in plasma at both 45 and 90 day assessment, suggesting a relative retention/persistence of some compounds over a longer period than observed in our previous acute studies.22,23 This may have been due to metabolic recycling (enterohepatic and enteric) of the strawberry anthocyanins which can prolong systemic exposure to various metabolites25 or be due to excretion kinetics, which could be less efficient in an older population.26 It is also possible that many metabolic processes are altered with prolonged exposure to strawberry polyphenols, such as up-regulation of key metabolizing enzymes (phase I and II), changes in the activities of efflux transporters to maintain equilibrium and changes in the gut microbiome, all together contributing to increased and sustained production of various metabolites.
This is the first study suggesting sustained retention of anthocyanins in plasma after chronic supplementation of strawberries. Kalt and co-workers reported that anthocyanins are abundant and persistent in the gastrointestinal tract based on their observation of anthocyanin metabolites in urine after the 5 day anthocyanin free run in period27 and in baseline urine samples (0 h void) before the start of a blueberry juice intervention.28 Contrary to their study, we did not detect anthocyanins in fasting (t = 0 h) or 2 h control samples at day 0, 45 and 90 or on day 0 of samples form the strawberry group, which rules out the possibility that anthocyanins were present in the circulation before the start of the study. However, repetitive intake of strawberries resulted in elevated but maintained fasting concentrations of P3G, P3R and PG over time on day 45 and 90, suggesting a relative equilibrium. Future studies will be required to determine if these findings are unique to strawberry pelargonidin compounds, an age-related phenomenon or due to the many other mechanisms already suggested.
Elevated concentrations of PG, P3G and P3R at 2 h after strawberry intake are not new findings; however, enhancement in PG and P3R concentrations at 2 h from day 45 to day 90 chronic intake is a new finding. Fasting concentrations did not change from day 45 to 90, excluding this as a possible explanation. The study was not designed to determine why this occurred, however, we can speculate this may have been due to metabolic processing mechanisms that were upregulated with chronic strawberry intake exposure allowing for higher concentrations to be achieved with a standardized dose. Alternatively, greater retention of anthocyanins in bile with chronic intake may have occurred, such that the combination of intake plus bile excretion with meal ingestion resulted in higher circulating concentrations. However, future studies are required to determine the mechanism underlying this observation. From a practical standpoint, regular inclusion of strawberries in the diet may prime metabolic processing to achieve higher blood anthocyanin concentrations with smaller single servings favoring long-term adherence to regular intake.
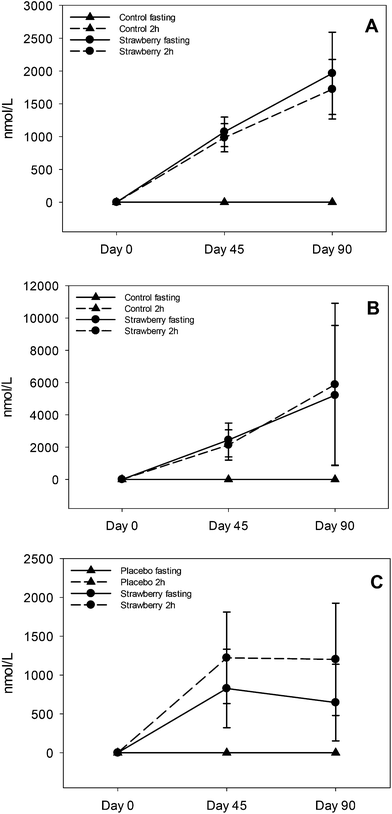
Ellagitannins are abundant in strawberries29 and are degraded to their monomer ellagic acid. Ellagic acid is poorly absorbed, therefore it is passed to the jejunum and colon where it is metabolized to various urolithins by specific bacterial species.21 These urolithins can be further conjugated to glucuronides and sulfates in the small intestine and liver before entering the systemic circulation. The predominant metabolites in plasma were urolithin A and B glucuronides which is consistent with a previous study on strawberry ellagitannins.30 Additionally, we found 3 main urolithin phenotypes: 37% were phenotype A (produced only urolithin A glucuronide), 26% were phenotype B (produced urolithin A and B glucuronides) and 32% were phenotype 0 (no urolithins detected).31 One subject (5%) produced only urolithin B glucuronide. The present study had more non-producers (i.e., phenotype 0) than previously reported and a more even ratio of phenotype A and B, which has been suggested to be associated with more individuals with gut dysbiosis.31 Consistent with other studies,32,33 and summarized in comprehensive review on urolithins,34 there was remarkable inter-individual variability in the concentration of urolithin metabolites. For urolithin A glucuronide the concentration ranged from 120–7676 nmoL L−1 in fasting samples (day 45 and 90) and 142–5958 nmoL L−1 in 2 h post strawberry drink samples (day 45 and 90). Similar inter-individual variability was observed in the concentrations of urolithin B glucuronide and isourolithin A glucuronide, which could be explained by differences in the gut microbial population resulting in low or high production of urolithin metabolites. Chronic intake of strawberries did not induce production of urolithins in non-producers which may be due to the absence of particular bacterial species required for the production of urolithins in non-producers.31 We also speculate that age could be an important factor in these findings that could be addressed in future studies. In addition to persistence of urolithin A and B glucuronides in circulation, continuous intake of ellagitannins could also enhance existent bacterial species or genomic capacity of certain species increasing urolithin production and establishing a two way interaction between urolithins and microbiota.35
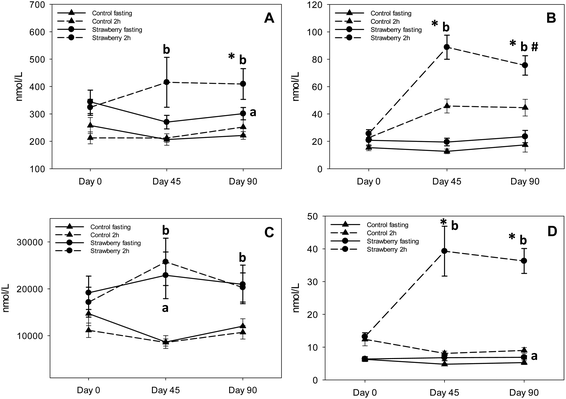
Recent research suggests that anthocyanins are rapidly absorbed from gastrointestinal lumen and can undergo presystemic metabolism in the intestinal wall or liver, or they can reach the colon where they are further metabolized by bacteria to various phenolic acid derivatives.36–38,17 The notion of low bioavailability of anthocyanins of parent structures is clear, however, we now know that much of the remaining parent structures are degraded or metabolized to phenolic acid derivatives and are present at much higher concentrations than the parent anthocyanins. A significant increase in the concentrations of 5 phenolic acids (3,4-dihydroxybenzaldehyde, hippuric acid, 4-hydroxybenzaldehyde, p-coumaric acid and 3-hydroxybenzoic acid) was observed in fasting samples after strawberry consumption for 90 days, compared to control group, suggesting persistence of these metabolites over time. Azzini et al., reported the appearance of 4-hydroxybenzoic acid, a metabolite of P3G in plasma 8 h after strawberry consumption.39 Interestingly, we detected 3-hydroxybenzoic acid instead of 4-hydroxybenzoic acid, which could be explained by the absence of cyandin glycosides (accounts for 3–10% of strawberry anthocyanins) in plasma and it is possible that cyanidin based anthocyanins underwent metabolic degradation or reached the colon where they were further metabolized to 3-hydroxybenzoic acid by bacteria. Also, 3,4-dihydroxybenzaldehyde and 4-hydroxybenzaldehyde are reported as metabolites of cyanidin-3-O-glucoside.17,24,39,40 Interconversion of compounds is also a possibility i.e., 4-hydroxybenzoic acid converted to 3-hydroxybenzoic or 4-hydroxybenzoic acid transformed to its aldehyde form. p-coumaric acid and cinnamic acid peaked at 2 h post strawberry drink intake because they are present in strawberries as free and bound phenolic acids.41 Hippuric acid was present in the highest concentration in plasma samples of both strawberry and control groups; however, concentrations were significantly higher in fasting (2 to 2.5 times higher than control) and 2 h (approximately 2 times higher than control) plasma samples of the strawberry group. These results are in agreement with previous berry intervention studies.42,43 Hippuric acid is a common metabolite of many flavonoids44 and is formed by glycination of benzoic acid in liver.45 It is also generated from protein and amino acid metabolism contributing to elevated background levels.24 Continuous intake of strawberry polyphenols may be modulating phase II metabolism, possibly explaining the significant increase in vanillic acid glucuronide in the fasting samples of the strawberry group on day 45 compared to the control group. We did not observe any significant differences between intervention groups in the remaining phenolic acid derivatives. Some of these phenolic metabolites may have been derived from the background diet, which is consistent with the elevated phenolic acid derivatives observed in baseline and control samples. Therefore, slight changes occurring in their concentration after strawberry intake were likely masked.
To the best of our knowledge, this is the first study reporting the metabolic fate of strawberry polyphenols in plasma after chronic supplementation. The study had strengths and limitations. First, the study design was parallel, which limited our ability to compare treatment effects within subjects; however, this was also a strength because the design minimized issues with carryover. Second, plasma samples were collected only at two time-points (0 and 2 h) after chronic feeding. Collecting samples over a longer time frame postprandially would have allowed a more comprehensive pharmacokinetic analysis of acute polyphenol metabolism on the supplemented background diets. Third, pure standards were not available for some metabolites so they were quantified using parent compounds, and lastly proanthocyanidins metabolites were not evaluated in the plasma samples which could have resulted in more comprehensive analysis of strawberry metabolites. Strengths of the study included analysis of a wide array of metabolites in plasma including anthocyanins, urolithins and phenolic acid metabolites after chronic intake and using a modified acute sampling paradigm. The persistence of these metabolites in circulation is reported for the first time after chronic strawberry intake.
Overall, the results from this study suggest that polyphenols from strawberry are extensively metabolized by the host and they remain persistent in circulation. Whether this persistence is part of delivering the beneficial effects of strawberries requires further investigation.
Conflicts of interest
The authors declared no potential conflicts of interest.Acknowledgements
The study was funded by the California Strawberry Commission and by USDA Intramural Funds. Freeze dried strawberry and control powders were provided by the California Strawberry Commission. We would like to thank Dr Francisco A. Tomás Barberán (CEBAS Institute, Murcia, Spain) for providing the urolithin standards.References
- F. Wu, Z. Guan and A. Whidden, Strawberry Industry Overview and Outlook, University of Florida, http://www.fred.ifas.ufl.edu/pdf/webinar/Strawberry.pdf, 2012 Search PubMed.
- R. Amani, S. Moazen, H. Shahbazian, K. Ahmadi and M. T. Jalali, Flavonoid-rich beverage effects on lipid profile and blood pressure in diabetic patients, World J Diabetes, 2014, 5, 962–968 CrossRef PubMed.
- A. Basu, S. Morris, A. Nguyen, N. M. Betts, D. Fu and T. J. Lyons, Effects of Dietary Strawberry Supplementation on Antioxidant Biomarkers in Obese Adults with Above Optimal Serum Lipids, J. Nutr. Metab., 2016, 2016, 9 CrossRef PubMed.
- N. D. Freedman, Y. Park, A. F. Subar, A. R. Hollenbeck, M. F. Leitzmann, A. Schatzkin and C. C. Abnet, Fruit and vegetable intake and esophageal cancer in a large prospective cohort study, Int. J. Cancer, 2007, 121, 2753–2760 CrossRef CAS PubMed.
- N. D. Freedman, Y. Park, A. F. Subar, A. R. Hollenbeck, M. F. Leitzmann, A. Schatzkin and C. C. Abnet, Fruit and vegetable intake and head and neck cancer risk in a large United States prospective cohort study, Int. J. Cancer, 2008, 122, 2330–2336 CrossRef CAS PubMed.
- N. D. Freedman, A. F. Subar, A. R. Hollenbeck, M. F. Leitzmann, A. Schatzkin and C. C. Abnet, Fruit and vegetable intake and gastric cancer risk in a large United States prospective cohort study, Cancer Causes Control, 2008, 19, 459–467 CrossRef PubMed.
- J. M. Alvarez-Suarez, F. Giampieri, S. Tulipani, T. Casoli, G. Di Stefano, A. M. Gonzalez-Paramas, C. Santos-Buelga, F. Busco, J. L. Quiles, M. D. Cordero, S. Bompadre, B. Mezzetti and M. Battino, One-month strawberry-rich anthocyanin supplementation ameliorates cardiovascular risk, oxidative stress markers and platelet activation in humans, J. Nutr. Biochem., 2014, 25, 289–294 CrossRef CAS PubMed.
- E. E. Devore, J. H. Kang, M. M. Breteler and F. Grodstein, Dietary intakes of berries and flavonoids in relation to cognitive decline, Ann. Neurol., 2012, 72, 135–143 CrossRef CAS PubMed.
- B. Buendia, M. I. Gil, J. A. Tudela, A. L. Gady, J. J. Medina, C. Soria, J. M. Lopez and F. A. Tomas-Barberan, HPLC-MS analysis of proanthocyanidin oligomers and other phenolics in 15 strawberry cultivars, J. Agric. Food Chem., 2010, 58, 3916–3926 CrossRef CAS PubMed.
- V. Neveu J. Perez-Jiménez, F. Vos, V. Crespy, L. du Chaffaut, L. Mennen, C. Knox, R. Eisner, J. Cruz, D. Wishart and A. Scalbert, Phenol-Explorer: an online comprehensive database on polyphenol contents in foods, Database 2010, http://phenol-explorer.eu/contents/food/69 Search PubMed.
- F. L. da Silva, M. T. Escribano-Bailón, J. J. Pérez Alonso, J. C. Rivas-Gonzalo and C. Santos-Buelga, Anthocyanin pigments in strawberry, LWT–Food Sci. Technol., 2007, 40, 374–382 CrossRef.
- K. Aaby, S. Mazur, A. Nes and G. Skrede, Phenolic compounds in strawberry (Fragaria x ananassa Duch.) fruits: Composition in 27 cultivars and changes during ripening, Food Chem., 2012, 132, 86–97 CrossRef CAS PubMed.
- G. Cao, H. U. Muccitelli, C. Sanchez-Moreno and R. L. Prior, Anthocyanins are absorbed in glycated forms in elderly women: a pharmacokinetic study, Am. J. Clin. Nutr., 2001, 73, 920–926 CAS.
- X. Wu, G. Cao and R. L. Prior, Absorption and metabolism of anthocyanins in elderly women after consumption of elderberry or blueberry, J. Nutr., 2002, 132, 1865–1871 CAS.
- W. Mullen, C. A. Edwards, M. Serafini and A. Crozier, Bioavailability of pelargonidin-3-O-glucoside and its metabolites in humans following the ingestion of strawberries with and without cream, J. Agric. Food Chem., 2008, 56, 713–719 CrossRef CAS PubMed.
- K. Banaszewski, E. Park, I. Edirisinghe, J. C. Cappozzo and B. M. Burton-Freeman, A pilot study to investigate bioavailability of strawberry anthocyanins and characterize postprandial plasma polyphenols absorption patterns by Q-TOF LC/MS in humans, J. Berry Res., 2013, 3, 113–126 CAS.
- C. Czank, A. Cassidy, Q. Zhang, D. J. Morrison, T. Preston, P. A. Kroon, N. P. Botting and C. D. Kay, Human metabolism and elimination of the anthocyanin, cyanidin-3-glucoside: a 13C-tracer study, Am. J. Clin. Nutr., 2013, 97, 995–1003 CrossRef CAS PubMed.
- R. Feliciano, G. Istas, C. Heiss and A. Rodriguez-Mateos, Plasma and Urinary Phenolic Profiles after Acute and Repetitive Intake of Wild Blueberry, Molecules, 2016, 21, 1120 CrossRef PubMed.
- E. M. Daniel, S. Ratnayake, T. Kinstle and G. D. Stoner, The Effects of pH and Rat Intestinal Contents on the Liberation of Ellagic Acid from Purified and Crude Ellagitannins, J. Nat. Prod., 1991, 54, 946–952 CrossRef CAS.
- A. González-Sarrías, R. García-Villalba, M. Á. Núñez-Sánchez, J. Tomé-Carneiro, P. Zafrilla, J. Mulero, F. A. Tomás-Barberán and J. C. Espín, Identifying the limits for ellagic acid bioavailability: A crossover pharmacokinetic study in healthy volunteers after consumption of pomegranate extracts, J. Funct. Foods, 2015, 19, 225–235 CrossRef.
- J. C. Espin, M. Larrosa, M. T. Garcia-Conesa and F. Tomas-Barberan, Biological Significance of Urolithins, the Gut Microbial Ellagic Acid-Derived Metabolites: The Evidence So Far, Evidence-Based Complementary Altern. Med., 2013, 2013, 15 Search PubMed.
- A. K. Sandhu, Y. Huang, D. Xiao, E. Park, I. Edirisinghe and B. Burton-Freeman, Pharmacokinetic Characterization and Bioavailability of Strawberry Anthocyanins Relative to Meal Intake, J. Agric. Food Chem., 2016, 64, 4891–4899 CrossRef CAS PubMed.
- E. Park, I. Edirisinghe, H. Wei, L. P. Vijayakumar, K. Banaszewski, J. C. Cappozzo and B. Burton-Freeman, A dose–response evaluation of freeze-dried strawberries independent of fiber content on metabolic indices in abdominally obese individuals with insulin resistance in a randomized, single-blinded, diet-controlled crossover trial, Mol. Nutr. Food Res., 2016, 60, 1099–1109 CAS.
- R. M. de Ferrars, C. Czank, Q. Zhang, N. P. Botting, P. A. Kroon, A. Cassidy and C. D. Kay, The pharmacokinetics of anthocyanins and their metabolites in humans, Br. J. Pharmacol., 2014, 171, 3268–3282 CrossRef CAS PubMed.
- Z. Liu and M. Hu, Natural polyphenol disposition via coupled metabolic pathways, Expert Opin. Drug Metab. Toxicol., 2007, 3, 389–406 CrossRef CAS PubMed.
- A. Rodriguez-Mateos, T. Cifuentes-Gomez, I. Gonzalez-Salvador, J. I. Ottaviani, H. Schroeter, M. Kelm, C. Heiss and J. P. E. Spencer, Influence of age on the absorption, metabolism, and excretion of cocoa flavanols in healthy subjects, Mol. Nutr. Food Res., 2015, 59, 1504–1512 CAS.
- W. Kalt, Y. Liu, J. E. McDonald, M. R. Vinqvist-Tymchuk and S. A. Fillmore, Anthocyanin metabolites are abundant and persistent in human urine, J. Agric. Food Chem., 2014, 62, 3926–3934 CrossRef CAS PubMed.
- W. Kalt, J. E. McDonald, Y. Liu and S. A. Fillmore, Flavonoid Metabolites in Human Urine during Blueberry Anthocyanin Intake, J. Agric. Food Chem., 2017, 65, 1582–1591 CrossRef CAS PubMed.
- N. P. Seeram, R. Lee, H. S. Scheuller and D. Heber, Identification of phenolic compounds in strawberries by liquid chromatography electrospray ionization mass spectroscopy, Food Chem., 2006, 97, 1–11 CrossRef CAS.
- P. Truchado, M. Larrosa, M. T. Garcia-Conesa, B. Cerda, M. L. Vidal-Guevara, F. A. Tomas-Barberan and J. C. Espin, Strawberry processing does not affect the production and urinary excretion of urolithins, ellagic acid metabolites, in humans, J. Agric. Food Chem., 2012, 60, 5749–5754 CrossRef CAS PubMed.
- F. A. Tomás-Barberán, R. García-Villalba, A. González-Sarrías, M. V. Selma and J. C. Espín, Ellagic Acid Metabolism by Human Gut Microbiota: Consistent Observation of Three Urolithin Phenotypes in Intervention Trials, Independent of Food Source, Age, and Health Status, J. Agric. Food Chem., 2014, 62, 6535–6538 CrossRef PubMed.
- B. Cerdá, F. A. Tomás-Barberán and J. C. Espín, Metabolism of Antioxidant and Chemopreventive Ellagitannins from Strawberries, Raspberries, Walnuts, and Oak-Aged Wine in Humans: Identification of Biomarkers and Individual Variability, J. Agric. Food Chem., 2005, 53, 227–235 CrossRef PubMed.
- A. Gonzalez-Sarrias, J. A. Gimenez-Bastida, M. T. Garcia-Conesa, M. B. Gomez-Sanchez, N. V. Garcia-Talavera, A. Gil-Izquierdo, C. Sanchez-Alvarez, L. O. Fontana-Compiano, J. P. Morga-Egea, F. A. Pastor-Quirante, F. Martinez-Diaz, F. A. Tomas-Barberan and J. C. Espin, Occurrence of urolithins, gut microbiota ellagic acid metabolites and proliferation markers expression response in the human prostate gland upon consumption of walnuts and pomegranate juice, Mol. Nutr. Food Res., 2010, 54, 311–322 CAS.
- F. A. Tomás-Barberán, A. González-Sarrías, R. García-Villalba, M. A. Núñez-Sánchez, M. V. Selma, M. T. García-Conesa and J. C. Espín, Urolithins, the rescue of “old” metabolites to understand a “new” concept: Metabotypes as a nexus among phenolic metabolism, microbiota dysbiosis, and host health status, Mol. Nutr. Food Res., 2017, 61, 1500901 Search PubMed.
- D. Bialonska, P. Ramnani, S. G. Kasimsetty, K. R. Muntha, G. R. Gibson and D. Ferreira, The influence of pomegranate by-product and punicalagins on selected groups of human intestinal microbiota, Int. J. Food Microbiol., 2010, 140, 175–182 CrossRef CAS PubMed.
- J. Fang, Some anthocyanins could be efficiently absorbed across the gastrointestinal mucosa: extensive presystemic metabolism reduces apparent bioavailability, J. Agric. Food Chem., 2014, 62, 3904–3911 CrossRef CAS PubMed.
- J. Fang, Bioavailability of anthocyanins, Drug Metab. Rev., 2014, 46, 508–520 CrossRef CAS PubMed.
- I. A. Ludwig, P. Mena, L. Calani, G. Borges, G. Pereira-Caro, L. Bresciani, D. Del Rio, M. E. Lean and A. Crozier, New insights into the bioavailability of red raspberry anthocyanins and ellagitannins, Free Radical Biol. Med., 2015, 89, 758–769 CrossRef CAS PubMed.
- E. Azzini, P. Vitaglione, F. Intorre, A. Napolitano, A. Durazzo, M. S. Foddai, A. Fumagalli, G. Catasta, L. Rossi, E. Venneria, A. Raguzzini, L. Palomba, V. Fogliano and G. Maiani, Bioavailability of strawberry antioxidants in human subjects, Br. J. Nutr., 2010, 104, 1165–1173 CrossRef CAS PubMed.
- C. Felgines, S. Talavera, M. P. Gonthier, O. Texier, A. Scalbert, J. L. Lamaison and C. Remesy, Strawberry anthocyanins are recovered in urine as glucuro- and sulfoconjugates in humans, J. Nutr., 2003, 133, 1296–1301 CAS.
- P. Mattila, J. Hellström and R. Törrönen, Phenolic Acids in Berries, Fruits, and Beverages, J. Agric. Food Chem., 2006, 54, 7193–7199 CrossRef CAS PubMed.
- K. Hanhineva, M. A. Lankinen, A. Pedret, U. Schwab, M. Kolehmainen, J. Paananen, V. de Mello, R. Sola, M. Lehtonen, K. Poutanen, M. Uusitupa and H. Mykkanen, Nontargeted metabolite profiling discriminates diet-specific biomarkers for consumption of whole grains, fatty fish, and bilberries in a randomized controlled trial, J. Nutr., 2015, 145, 7–17 CrossRef CAS PubMed.
- C. Vetrani, A. A. Rivellese, G. Annuzzi, M. Adiels, J. Boren, I. Mattila, M. Oresic and A. M. Aura, Metabolic transformations of dietary polyphenols: comparison between in vitro colonic and hepatic models and in vivo urinary metabolites, J. Nutr. Biochem., 2016, 33, 111–118 CrossRef CAS PubMed.
- R. W. Pero, Health consequences of catabolic synthesis of hippuric acid in humans, Curr. Clin. Pharmacol., 2010, 5, 67–73 CrossRef CAS PubMed.
- J. L. Simkin and K. White, The formation of hippuric acid. The influence of benzoate administration on tissue glycine levels, Biochem. J., 1957, 65, 574–582 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2018 |