Towards sustainable kinetic resolution, a combination of bio-catalysis, flow chemistry and bio-based solvents†
Andree
Iemhoff
ab,
James
Sherwood
 a,
Con R.
McElroy
a and
Andrew J.
Hunt
a,
Con R.
McElroy
a and
Andrew J.
Hunt
 *ac
*ac
aDepartment of Chemistry, University of York, Heslington, York, YO10 5DD, UK
bInstitut für Technische und Makromolekulare Chemie, RWTH Aachen University, Worringerweg 1, 52074 Aachen, Germany
cMaterials Chemistry Research Center, Department of Chemistry, Faculty of Science, Khon Kaen University, Khon Kaen, 40002, Thailand. E-mail: andrew@kku.ac.th
First published on 1st December 2017
Abstract
The esterification of 2-phenylpropionic acid was investigated as a model system for enzyme catalysed (CALB, Novozyme 435) reactions in bio-based solvents. A multi-parameter correlation taking into account solvent parameters was developed to explain experimental observations. A continuous flow process using p-cymene as the solvent was operated over several weeks, thus combining a sustainable solvent and flow chemistry for kinetic resolution.
Arylpropionic acid derivatives such as ibuprofen, ketoprofen and flurbiprofen represent an important class of non-steroidal anti-inflammatory drugs.1 They are widely used in medicines as the racemic mixture, however, only the S-enantiomer is biologically active.2 Therefore, researchers have taken great interest in resolving the enantiomers of these compounds via enzyme catalysis using lipases, specifically the industrially established Novozyme 435 (supported lipase B from Candida Antarctica), which has been the focus of many publications.3–6
Chiral resolution coupled with direct esterification with an alcohol is considered more desirable than transesterification or hydrolysis as the number of processing steps can be reduced.3 However, this requires the use of organic solvents as the reaction medium instead of water. Generally toluene, isooctane or halogenated solvents like 1,2-dichloropropane are chosen as the reaction medium.4–6
Organic solvents make up 80–90% of the non-aqueous mass in these synthetic transformations.7 However, many traditional solvents face legislative restrictions on safety grounds or are unsustainable, being derived from fossil resources.8 Many of these solvents have restricted industrial use because of their safety and sustainability issues.9 Green solvents are increasingly being shown to be viable alternatives to conventional solvents in various existing processes.8
Besides reducing the number of transformations, process intensification through the use of flow systems has gained significant attention in recent years as a safe way to increase productivity.10,11 A number of reviews have focused on advances in the use of lipases for synthesis, including continuous flow enzymatic catalysis.12 In addition, several research articles have demonstrated the effective use of lipases for the kinetic resolution of target compounds within continuous flow processes.13
Herein, a sustainable two step kinetic resolution by direct esterification of 2-arylpropionic acids was undertaken. 2-Phenylpropionic acid (2PPA) has been used as a model compound. The performance of 17 solvents, both petroleum- and bio-based, in the esterification of 2PPA with ethanol was studied. Furthermore, the process has been transferred from batch synthesis to a more productive flow process utilising bio-derived p-cymene.
Fig. 1 shows the conversion over time for the esterification of 2PPA, catalysed by Novozyme 435 in different solvents. The highest conversion was achieved with non-polar hydrocarbon solvents. n-Hexane and toluene are high performance solvents, but are considered ‘hazardous’ and ‘problematic’ respectively in a recent green chemistry solvent selection guide for the pharmaceutical industry, rendering them unattractive in a green context.9 Anisole, which is listed as ‘recommended’,9 leads to 50% conversion after 24 hours (Table 1), which is lower than the hydrocarbons but significantly better than other ethers like THF and its biomass derived equivalent 2-MeTHF, for which the conversion was no more than 5% after 24 hours (Table 1). Limonene is a terpene that can be obtained from waste biomass such as orange peel.14 Limonene and its aromatised analogue p-cymene were found to give the best conversions for this reaction after 24 hours.
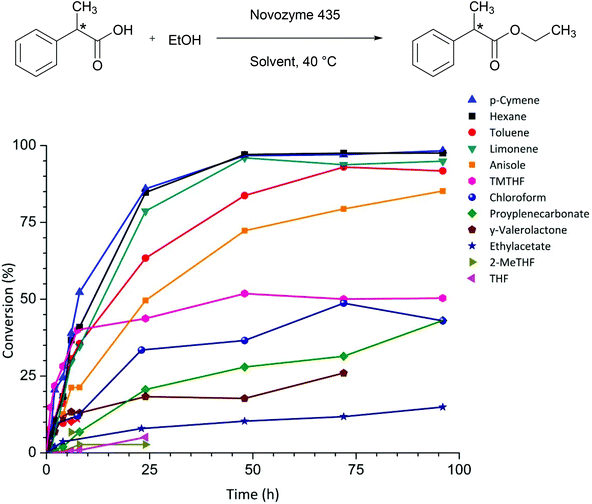 | ||
| Fig. 1 Conversion over time for 12 different organic solvents in the esterification of 2-phenylpropionic acid with ethanol. | ||
| Solvent | β , |
V
m![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b/mL mol−1 b/mL mol−1 |
log(P)c | r /μmol h−1 | C |
|---|---|---|---|---|---|
| a Ref. 17 and 29. b Molecular volumes of the respective solvents were calculated by dividing its molar mass by its density. c Ref. 30. d Initial rate of ester formation. e Conversion after 24 hours. | |||||
| p-Cymene | 0.13 | 157 | 4.10 | 13.53 | 86% |
| n-Hexane | 0.00 | 132 | 3.50 | 11.97 | 84% |
| Limonene | 0.00 | 162 | 4.58 | 10.18 | 86% |
| Toluene | 0.11 | 106 | 2.50 | 6.56 | 63% |
| TMTHF | 0.67 | 160 | 2.32 | 3.46 | 44% |
| Chloroform | 0.10 | 80.1 | 1.97 | 2.17 | 34% |
| Propylene carbonate | 0.40 | 85.1 | −0.41 | 2.08 | 21% |
| Anisole | 0.32 | 109 | 2.62 | 1.98 | 50% |
| γ-Valerolactone | 0.60 | 95.4 | −0.13 | 1.36 | 18% |
| 2-MeTHF | 0.58 | 101 | 1.26 | 0.77 | 3% |
| Ethyl acetate | 0.45 | 98.2 | 0.68 | 0.21 | 8% |
| THF | 0.55 | 81.1 | 0.49 | 0.08 | 5% |
| DMF | 0.69 | 77.4 | −1.00 | — | — |
| NMP | 0.77 | 96.2 | −0.46 | — | — |
| Diethyl carbonate | 0.40 | 121 | 1.21 | — | — |
| 2-Butanone | 0.48 | 89.6 | 0.29 | — | — |
| Cyrene™ | 0.61 | 84.3 | −1.52 | — | — |
2,2,5,5-Tetramethyltetrahydrofuran (TMTHF) has previously been demonstrated as a green and potentially bio-based alternative to toluene.15 The absence of a proton at the alpha-position to the oxygen of the ether eliminates the potential to form peroxides making it safer than traditional ethers. TMTHF shows promising initial rates but reaches a plateau and does not exceed 50% conversion. Ester solvents, which are typically non-toxic, give rise to poor conversions. No product formation was observed after 24 hours in the polar aprotic solvents NMP and DMF, which are classified as highly hazardous because of their reprotoxicity. Similarly no conversion was observed with more desirable solvents such as dihydrolevoglucosenone (Cyrene™), diethyl carbonate, and butanone (Table 1).
Much work has been conducted to evaluate the influence of solvents on enzyme catalysed reactions, focusing on the octanol–water partition coefficient log(P), an intuitively relevant variable.5 However, a growing number of publications have indicated that a more intricate relationship governs the influence of the solvent on enzymatic reactions.16–18 The multi-parameter Kamlet–Taft solvatochromic scales of solvent polarity have been shown to provide more a detailed and specific description of solvent effects.19 A generalised equation relating chemical phenomena to solvent polarity, where XYZ is proportional to free energy, can be found in eqn (1), including contributions for solvent hydrogen bond donating ability (α), hydrogen bond accepting ability (β), and dipolarity/polarisability (π*).
| XYZ = XYZ0 + aα + bβ + sπ* | (1) |
Furthermore, Kamlet et al. described the partition coefficient log(P) of solvent molecules as a function of their solvatochromic parameters.20 This required introducing the molar volume (Vm) to adequately describe log(P) as dependent on the size and hydrogen bond accepting ability (β) of the solvent. In this present work, log(P) and solvatochromic parameters were used to construct separate correlations with the natural logarithm of the initial reaction rate, ln(r). From the reaction of 2PPA in different solvents, the initial rate of ester formation was obtained (see Fig. S3, ESI†). Correlations describing ln(r) were arrived at by evaluating the statistical relevance of each variable and discarding insignificant parameters (i.e. α, β, π*, and Vm). The plot of experimental versus predicted ln(r) gives a visual indication of the quality of these correlations (Fig. 2). In five solvents essentially no reaction occurred, so these solvents are absent from the correlations.
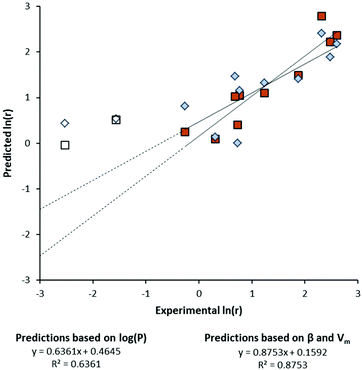 | ||
| Fig. 2 Correlations using different sets of solvent parameters to calculate the initial rate of the esterification of 2PPA. | ||
A multi-parameter approach employing β and Vm (Fig. 2, square data points) yields a stronger correlation than the partition coefficient log(P) alone (Fig. 2, diamond data points). Solvent performance is overestimated for ethyl acetate and tetrahydrofuran when the reaction is slow (Fig. 2, unshaded data points). However, the rate of reaction remains solvent dependent.
The description of reaction rate based on β and Vm is in agreement with previously published work on the bio-catalysed synthesis of hexyl laurate.21 The fact that β and Vm underpin both log(P),20 and the rate of lipase catalysed esterification reactions may be coincidence, or a similar principle relating log(P) and ln(r) is perhaps in operation. Therefore, when log(P) is used to correlate rates of reaction to solvent properties, it is a helpful approximation but not a true explanation of the fundamental solvent effects in operation.
In order to maintain their specific structure and activity in non-polar media, a water layer around the enzyme has been found to be crucial.22 Previous studies have concluded that the concentration of an organic substrate is in equilibrium between the bulk organic phase and the aqueous phase near the enzyme and therefore the catalytic active site.16 Solvents with high polarity were shown to disrupt the water layer, which is generally referred to as water stripping.23 This phenomenon will decrease the activity of the enzyme. Previous studies indicate the solvent does not directly interact with the active site of enzymes.24 As such, the mechanism of the enzymatic reaction is not affected by solvents of different polarity.25 Generally, the rate of carbonyl additions (including esterifications and amidations) are all inversely proportional to β.17 However, the exclusion of the solvent from the enzyme active site means the same solvent effect is not operational when the esterification is bio-catalysed, despite the similar relationship between solvent polarity and rate of reaction.
The highest initial rates (>10 μmol h−1) are found for solvents which are large (bulky) with low β values, such as p-cymene and n-hexane. Given the reactive substrates are a protic organic acid and an alcohol, a solvent with low hydrogen bond accepting ability may promote partition into the more hydrophilic enzyme environment through lack of intermolecular bonding. Furthermore, if the solvent is bulky this will hinder the formation of a structured solvation environment for the substrate, which is also advantageous to encourage the substrate to migrate into the enzyme environment instead. The reaction failed in dipolar aprotic solvents, which have the highest hydrogen bond acceptor strength and small molar volumes.
Revisiting water stripping data for the transesterifying subtilisin enzyme,23 an equivalent relationship (eqn (2), where Wd is fraction of desorbed water and Wa is the fraction of absorbed water) to the observed rates of esterification (eqn (3)) was found. This means the origin of decreased enzyme water stripping and increased rate of reaction can be traced back to the same fundamental solvent properties: β and Vm. This is an indication that the ability of the organic solvent to preserve the enzymatic function is the origin of the solvent effect. Larger, non-hydrogen bonding solvents have a lower affinity for water and as such are ideal for enzymatic catalysis.
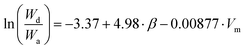 | (2) |
| ln(r) = −0.18 − 2.44·β + 0.0183·Vm | (3) |
The rates of reaction in ethyl acetate and THF are predicted to be slow (according to eqn (2)), and in reality are much slower still. The water stripping abilities of these two solvents are significantly greater than hydrocarbon solvents, and more than predicted by eqn (2). This may explain the poor prediction of rates for ethyl acetate and THF, but only by deferring the discrepancy onto the modelling of water stripping, which is also unexplained. It was assumed that diethyl carbonate and butanone were also strongly water stripping after the reaction failed to occur in these solvents.
Based on the kinetic studies in batch, p-cymene was identified as the green solvent which gave the best activity in the conversion of 2PPA (Table 1) and was therefore selected for application in a continuous flow process. Novozyme 435 can be transferred from batch systems to continuous flow processes.26 Specifically, the lipase catalysed kinetic resolution of arylpropionic acid derivatives like ibuprofen and flurbiprofen has been demonstrated in continuous flow systems before, albeit in toluene or cyclohexane.27
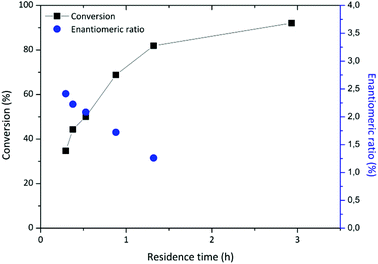
A HPLC column (150 mm length × 4.6 mm diameter) was used as a packed bed reactor and equipped with Novozyme 435. For the system at hand, flow rates between 9 μL min−1 and 90 μL min−1 were tested. This corresponds to residence times between 20 min and 3 hours. Longer residence times increased the conversion up to 93% (Fig. 3). Batch reaction conditions required 40 hours rather than 3 hours to obtain similar results. The data is tabulated in Table S2 of the ESI.†
The enantiomeric excess of 2-phenylpropionic acid ethyl ester was determined by chiral HPLC analysis. The enantiomeric ratio, E, was calculated following an established methodology.28 The best resolution (E = 2.41) was obtained at 35% conversion, while E = 2.23 at 44% conversion. These values are well below what is considered acceptable resolution,6 but agree with other results for the enantioselective esterification of 2PPA,5 or ketoprofen, catalysed by Novozym 435 at similar conversions.4 However, these previous literature results were obtained by batch reaction after 10 hours with an excess of immobilised enzyme in isobutyl methyl ketone and limonene respectively.
In the batch experiments, a substrate to catalyst ratio of 6 mg mg−1 was employed. In flow, the system could be operated for three weeks (with no loss in activity), converting 15 g of 2PPA. This leads to a substrate to catalyst ratio of 21 mg mg−1, which is a great improvement in the stability of the system. Monitoring 9 μL min−1 and 70 μL min−1 flow rates over an extended period of time indicated stable conversion of 2PPA (see Fig. S5, ESI†). Moreover, in batch experiments it was observed that the polymer support was mechanically ground by stirring over the course of the reaction. The physical appearance was greatly altered from the original polymer beads (see Fig. S6 and S7†). This had a marked impact upon catalyst recycling. Although the optimum batch system of p-cymene running for 24 hours gave a conversion of 86%, reuse of the supported enzyme almost halved conversion to 48%, dropping to 25% in the third reaction (see Fig. S4†). The catalyst in the packed bed reactor showed only minor changes in physical appearance and had mostly retained its structure even after three weeks of use. From images obtained via visual microscopy, swelling of the polymer can be observed but not the degradation and agglomeration seen in batch reactions (see Fig. S8†). Infra-red spectroscopy of p-cymene flowed through the catalyst bed for pre-treatment showed no evidence of any dissolved polymer (see Fig. S10†).
In conclusion, more sustainable solvents were successfully evaluated in the esterification of 2-phenylpropionic acid. A multi-parameter correlation, comprising β and Vm, showed an improved prediction of the experimentally obtained initial rates compared to correlations with log(P). A continuous flow process using p-cymene as the solvent was implemented and operated over several weeks, testing a range of flow rates and demonstrating resolution of the racemic substrate. This work lays the foundation for the use of alternative solvents combined with flow processes for the kinetic resolution of arylpropionic acid derivatives.
Conflicts of interest
There are no conflicts of interest to declare.Notes and references
- A. Burke, E. M. Smyth and G. A. Fitzgerald, Analgesic-antipyretic agents; Pharmacotherapy of gout, in Goodman and Gilman's The Pharmacological basis of therapeutics, ed. L. L. Brunton, J. S. Lazo and K. L. Parker, Mcgraw-Hill, New York, 11th edn, 2006, pp. 671–716 Search PubMed.
- J. Caldwell, A. Hutt and S. J. Fournel-Gigleux, Biochem. Phamacol., 1998, 1, 105–114 Search PubMed.
- H. J. Park, W. J. Choi, E. C. Huh, E. Y. Lee and C. Y. Choi, J. Biosci. Bioeng., 1999, 87(4), 545–547 CrossRef CAS PubMed.
- N. D'Antona, P. Lombardi, G. Nicolosi and G. Salvo, Process Biochem., 2002, 38(3), 373–377 CrossRef.
- M. Arroyo and J. V. Sinisterra, J. Org. Chem., 1994, 59, 4410–4417 CrossRef CAS.
- T. Siódmiak, D. Mangelings, Y. Vander Heyden, M. Ziegler-Borowska and M. P. Marszall, Appl. Biochem. Biotechnol., 2015, 175, 2769–2785 CrossRef PubMed.
- D. J. C. Constable, C. Jimenez-Gonzales and R. K. Henderson, Org. Process Res. Dev., 2007, 11, 133–137 CrossRef CAS.
- (a) J. H. Clark, A. J. Hunt, C. Topi, G. Paggiola and J. Sherwood, in Sustainable Solvents: Perspectives from Research, Business and International Policy, RSC Publishing, Cambridge, 2017, pp. 1–358 Search PubMed; (b) S. Jin, F. Byrne, C. R. McElroy, J. Sherwood, J. H. Clark and A. J. Hunt, Faraday Discuss., 2017, 202, 157–173 RSC.
- D. Prat, A. Wells, J. Hayler, H. Sneddon, C. R. McElroy, S. Abou-Shehada and P. J. Dunn, Green Chem., 2016, 18, 288 RSC.
- K. Booker-Milburn, Nat. Chem., 2012, 4, 433–435 CrossRef CAS PubMed.
- R. Porta, M. Benaglia and A. Puglisi, Org. Process Res. Dev., 2016, 20, 2–25 CrossRef CAS.
- (a) I. Itabaiana Jr., L. S. M. Miranda and R. O. M. A. de Souza, J. Mol. Catal. B: Enzym., 2013, 86, 1–9 CrossRef; (b) A. S. de Miranda, L. S. M. Miranda and R. O. M. A. de Souza, Biotechnol. Adv., 2015, 33, 372–393 CrossRef CAS PubMed; (c) R. Wohlgemuth, I. Plazl, P. Žnidaršič-Plazl, K. V. Gernaey and J. M. Woodley, Trends Biotechnol., 2015, 33, 302–314 CrossRef CAS PubMed.
- (a) H. M. Salvi, M. P. Kamble and G. D. Yadav, Appl. Biochem. Biotechnol., 2017 DOI:10.1007/s12010-017-2572-7; (b) A. Cimporescu, A. Todea, V. Badea, C. Paul and F. Peter, Process Biochem., 2016, 51, 2076–2208 CrossRef CAS; (c) M. V. M. Silva, J. F. Bassut, I. I. Junior, S. P. de Souza, M. L. G. Estrada, L. S. M. Miranda and R. O. M. A. de Souza, RSC Adv., 2015, 5, 102409–102415 RSC; (d) S. Kataok, Y. Takeuchi, A. Harada, M. Yamada and A. Endo, Green Chem., 2010, 12, 331–337 RSC.
- A. Farhat, A. S. Fabiano-Tixier, M. E. Maataoui, J. F. Maingonnat, M. Romdhane and F. Chemat, Food Chem., 2011, 125, 255–261 CrossRef CAS.
- F. Byrne, B. Forier, G. Bossaert, C. Hoebers, T. J. Farmer, J. H. Clark and A. J. Hunt, Green Chem., 2017, 19, 3671–3678 RSC.
- P. J. Halling, Enzyme Microb. Technol., 1994, 16, 178–206 CrossRef CAS PubMed.
- J. H. Clark, D. J. Macquarrie and J. Sherwood, Green Chem., 2012, 14, 90–93 RSC.
- A. G. Lanctôt, T. M. Attard, J. Sherwood, C. R. McElroy and A. J. Hunt, RSC Adv., 2016, 6, 48753–48756 RSC.
- M. J. Kamlet, J.-L. M. Abboud, M. H. Abraham and R. W. Taft, J. Org. Chem., 1983, 48, 2877–2887 CrossRef CAS.
- M. J. Kamlet, M. H. Abraham, R. M. Doherty and R. W. Taft, J. Am. Chem. Soc., 1984, 106, 464–466 CrossRef CAS.
- G. Paggiola, A. J. Hunt, C. R. McElroy, J. Sherwood and J. H. Clark, Green Chem., 2014, 16, 2107–2110 RSC.
- A. Zaks and A. M. Klibanov, Biochemistry, 1985, 82, 3192–3196 CAS.
- L. A. S. Gorman and J. S. Dordick, Biotechnol. Bioeng., 1992, 39, 392–397 CrossRef CAS PubMed.
- P. C. Michels, J. S. Dordick and D. S. Clark, J. Am. Chem. Soc., 1997, 119, 9331–9335 CrossRef CAS.
- J. Kim, D. S. Clark and J. S. Dordick, Biotechnol. Bioeng., 2000, 67, 112–116 CrossRef CAS PubMed.
- (a) Z. Boros, P. Falus, M. Márkus, D. Weiser, M. Oláh, G. Hornyánszky, J. Nagy and L. Poppe, J. Mol. Catal. B: Enzym., 2013, 85–86, 119–125 CrossRef CAS; (b) E. A. Manoel, K. C. Pais, M. C. Flores, L. S. M. Miranda, M. A. Z. Coelho, A. B. C. Simas, D. M. G. Freire and R. O. M. A. De Souza, J. Mol. Catal. B: Enzym., 2013, 87, 139–143 CrossRef CAS.
- (a) L. Tamborini, D. Romano, A. Pinto, A. Bertolani, F. Molinari and P. Conti, J. Mol. Catal. B: Enzym., 2012, 84, 78–82 CrossRef CAS; (b) J. Chen and S. Tsai, Biotechnol. Prog., 2000, 16, 986–992 CrossRef CAS PubMed.
- C.-S. Chen, Y. Fujimoto, G. Girdaukas and C. J. Sih, J. Am. Chem. Soc., 1982, 104, 7294–7299 CrossRef CAS.
- (a) Y. Marcus, Chem. Soc. Rev., 1993, 22, 409 RSC; (b) J. Sherwood, M. De bruyn, A. Constantinou, L. Moity, C. R. McElroy, T. J. Farmer, T. Duncan, W. Raverty, A. J. Hunt and J. H. Clark, Chem. Commun., 2014, 50, 9650–9652 RSC; (c) P. G. Jessop, D. A. Jessop, D. Fu and L. Phan, Green Chem., 2012, 14, 1245 RSC.
- (a) C. Laane, S. Boeren, K. Vos and C. Veeger, Biotechnol. Bioeng., 1987, 30, 81–87 CrossRef CAS PubMed; (b) Material Safety Data Sheet, Sigma Aldrich, accessed 06/2016; ; (c) TOXNET Databases, http://toxnet.nlm.nih.gov/index.html, accessed 06/2016; (d) Data kindly provided by Circa Group Ltd, the manufacturer of cyrene, by F. Hoffmann La Roche Ltd, hereby acknowledged as the source of the data and corresponding study; ; (e) J. Zhang, G. White, M. Ryan, A. J. Hunt and M. J. Katz, ACS Sustainable Chem. Eng., 2016, 4, 7186–7192 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7gc03177g |
| This journal is © The Royal Society of Chemistry 2018 |