A novel approach to 5H-pyrazino[2,3-b]indoles via annulation of 3-diazoindolin-2-imines with 2H-azirines or 5-alkoxyisoxazoles under Rh(II) catalysis†
Julia O.
Ruvinskaya
,
Nikolai V.
Rostovskii
 *,
Ilya P.
Filippov
*,
Ilya P.
Filippov
 ,
Alexander F.
Khlebnikov
,
Alexander F.
Khlebnikov
 and
Mikhail S.
Novikov
and
Mikhail S.
Novikov
 *
*
St. Petersburg State University, Institute of Chemistry, 7/9 Universitetskaya nab., St. Petersburg, 199034, Russia. E-mail: m.novikov@spbu.ru; Fax: +7 812-428-6939; Tel: +7 812-428-9344
First published on 30th November 2017
Abstract
A novel one-step method for the preparation of 5H-pyrazino[2,3-b]indoles with different substitution patterns in all rings of the tricyclic system via the Rh2(OAc)4-catalyzed reaction of 2H-azirines with 3-diazoindolin-2-imines is reported. Alkyl 5H-pyrazino[2,3-b]indole-3-carboxylates were also prepared by a one-pot procedure from synthetic equivalents of alkyl 2H-azirine-2-carboxylates, 5-alkoxyisoxazoles. The reactions provide the first examples of the use of Rh(II) catalysis for intermolecular annulations with 2H-azirines and isoxazoles.
Indole frameworks are important motifs in naturally occurring compounds,1 man-made pharmaceuticals,2 and optoelectronics materials.3 The unique properties of indole-fused compounds maintain interest in the development of effective methods for their preparation, including both the construction of indole-based skeletons and their functionalization.4 5H-Pyrazino[2,3-b]indole derivatives have received much attention as highly selective agonists, antagonists or inverse agonists for GABA brain receptors and are useful in the diagnosis and treatment of anxiety, sleep, and seizure disorders.5 Besides, these compounds are of interest as electron-acceptor units in bipolar host materials for organic light emitting devices.6
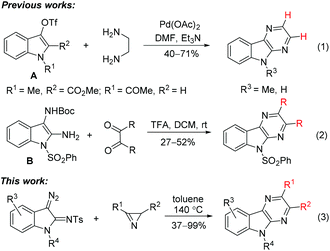
There are two general approaches for the construction of the pyrazino[2,3-b]indole skeleton. The synthetic scheme with the final formation of the pyrrole ring of the tricyclic pyrazino[2,3-b]indole system was accomplished starting from pyrazinylhydrazones of cyclohexanone, which were subjected to cyclization followed by aromatization of the cyclohexane fragment.5,7 The method has limitations related to modification of benzene ring of the target compound. The alternative scheme within the same approach, which involves cyclization of (2-aminophenyl)pyrazinones, is free of this disadvantage, but it has the problems with selective introduction of substituents in the pyrazine ring.8 The same limitation is inherent in more recent methods representing the second approach to the pyrazino[2,3-b]indole skeleton, specifically, pyrazino annulation of the indole system. Two examples of the annulation of indol-3-yl triflates A with ethylenediamine under Pd(OAc)2-catalysis were reported (Scheme 1, reaction (1)).9 The non-catalytic version of a similar reaction of ethylenediamine using N-acyl-2-bromoindol-3-one as a 1,2-bielectrophile is also known.10 Recently an alternative annulation method involving the reaction of Boc-protected diaminoindole B with 1,2-dicarbonyl compounds was developed (Scheme 1, reaction (2)).11 Unfortunately, the use of non-symmetrical 1,2-diamines and non-symmetrical 1,2-dicarbonyl compounds as annulation agents often leads to the formation of mixtures of regioisomeric annulation products.
 | ||
| Scheme 1 Synthesis of 5H-pyrazino[2,3-b]indoles by annulation of the pyrazine ring to the indole system. | ||
Herein, we report a high-yield synthesis of substituted pyrazino[2,3-b]indoles by the Rh(II)-catalyzed annulation of 3-diazoindolin-2-imines with 2H-azirines or 5-alkoxyisoxazoles (Scheme 1, reaction (3)). The scope and limitations of the method, related to selective introduction of various substituents into all rings of the pyrazino[2,3-b]indole system, as well as the interchangeability of annulation agents, azirines and isoxazoles, are discussed.
2H-Azirines are widely used for annulations of various carbo- and heterocycles.12 These reactions can occur under heating,13 photolysis,14 and catalysis by bases15 or transition metal compounds. Despite the fact that the reactions of the latter type are most promising for solving problems of stereo- and regio-control of azacycle formation, their range is practically limited by pyrrolo annulation reactions under Cu(I),16 Cu(II),17 Pd(II)18 and Y(III)19 catalysis. Pyrazino annulation with 2H-azirines has only been represented in the literature by the acid-catalyzed reaction with cyclic enaminoketones.20 The reaction presented in this work is the first example of the use of Rh(II) catalysis for intermolecular annulation with azirines.
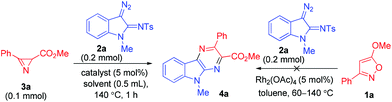
As a part of our ongoing research into the synthetic utility of isoxazoles21 and azirines22 as building blocks for heterocyclizations, we recently reported a switchable synthesis of pyrroles and pyrazines via the Rh(II)-catalyzed reaction of 1-sulfonyl-1,2,3-triazoles with isoxazoles.23 Earlier Lei et al.24 showed that the use of 5-aryl- or 5-alkylisoxazoles in the reactions of rhodium α-iminocarbenoids, derived from 1-sulfonyl-1,2,3-triazoles,25 results in the formation of pyrrole derivatives exclusively. When changing from isoxazoles to their synthetic equivalents, 2H-azirines, the outcome of the reaction, viz. pyrrole or pyrazine formation, becomes strongly dependent on the structure of both the azirine26 and iminocarbenoid precursor,27 but 2H-azirine-2-carboxylates again provide pyrrole derivatives as the main products.26b,c We showed that in contrast to the azirine-2-carboxylates 5-alkoxyisoxazoles are much more favorable reaction partners for acyclic rhodium α-iminocarbenoids in the synthesis of pyrazine derivatives.23 At the same time, the reactions of cyclic rhodium carbenoids with isoxazoles and azirines are unknown. We suggested that 5-alkoxyisoxazoles could also be used for pyrazino annulation to the C2–C3 bond of the indole system of 3-diazoindolin-2-imines,28 a source of cyclic rhodium α-iminocarbenoids, in the synthesis of pyrazino[2,3-b]indole derivatives. Unfortunately, the very first experiments with the use of isoxazole 1a, diazoindoline 2a, and Rh2(OAc)4 as a catalyst revealed that isoxazole 1a is inactive in the reaction (Table 1). Heating the mixture of these compounds in different solvents at temperatures of 60–140 °C resulted in complete decomposition of the diazo compound without any conversion of the isoxazole. When isoxazole 1a was replaced by its synthetic equivalent, azirine 3a, the reaction proceeded smoothly at 140 °C to form pyrazinoindole 4a as a single product. It was isolated by column chromatography in 79% yield.
| Entry | Catalysta | Solvent | Temperature, °C | Yieldb of 4a, % | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a Piv = pivalate, Oct = octanoate, esp = α,α,α′,α′-tetramethyl-1,3-benzenedipropanoate, tfa = trifluoroacetate. b 1H NMR yields using dibromomethane as an internal standard. c Isolated yield. d 2.5 mol% of Rh2(OAc)4. e 0.3 mmol of 2a. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1 | Rh2(OAc)4 | Toluene | 140 | 85 (79)c | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2 | Rh2(tfa)4 | Toluene | 140 | 36 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3 | Rh2(Oct)4 | Toluene | 140 | 69 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4 | Rh2(Piv)4 | Toluene | 140 | 22 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5 | Rh2(esp)2 | Toluene | 140 | 19 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6 | Rh2(OAc)4 | Toluene | 110 | 66 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 7 | Rh2(OAc)4 | Toluene | 160 | 82 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 8 | Rh2(OAc)4 | o-Xylene | 140 | 73 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 9 | Rh2(OAc)4 | DCE | 140 | 74 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 10 | Rh2(OAc)4 | Toluene | 140 | 80d | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 11 | Rh2(OAc)4 | Toluene | 140 | 91 (87)c | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Further optimization of the reaction conditions (Table 1) showed that the highest yield of 4a is observed in the reaction catalyzed by Rh2(OAc)4 (entry 1), while Rh2(tfa)4 (entry 2) and the catalysts with bulkier ligands, Rh2(Piv)4, Rh2(Oct)4, Rh2(esp)2 (entries 3–5), give less satisfactory results. The decrease in the reaction temperature results in a decrease in product yield, but the increase in temperature above 140 °C does not improve the yield (entries 6 and 7). The reaction is relatively insensitive to solvent changes (entries 8 and 9). It was also found that when the catalyst loading is halved (entry 10), the yield of 4a is reduced. The use of 3 equiv. of the diazo compound allowed increasing the yield up to 91% (entry 11). Thus, the optimal conditions were found to be heating of a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of 2a and 3a at 140 °C in toluene in the presence of Rh2(OAc)4 (5 mol%), and they were used in further experiments.
1 mixture of 2a and 3a at 140 °C in toluene in the presence of Rh2(OAc)4 (5 mol%), and they were used in further experiments.
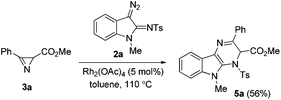
In all the experiments mentioned above, the reaction mixture gradually acquired a yellow color, which disappeared after some time. The intermediate formation of the yellow compound, which we assumed to be dihydropyrazinoindole 5a (Scheme 2), was also detected by TLC. Intermediate 5a could be isolated by column chromatography in 56% yield, when the reaction was carried out at a lower temperature (Table 1, entry 7) and stopped once the content of this compound reached a maximum (after ca. 5 min).
The structure of the isolated product was confirmed by standard spectroscopic methods. Dihydropyrazinoindole 5a is rather unstable and completely converts to pyrazine 4a in CDCl3 in 48 h.
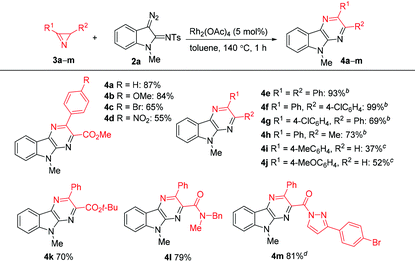
The scope of the reaction was explored by reacting a series of azirines 3b–m with diazo compound 2a under the optimized conditions (Table 2). It was shown that the reaction tolerates with both electron-donor and electron-acceptor aryl substituents in 3-arylazirine-2-carboxylates (compounds 4a–d), as well as with substituted carbamoyl group at the C2 atom of azirine ring (compound 4l). Furthermore, we succeeded to prepare pyrazinoindole 4m with a pyrazolylcarbonyl substituent in 81% yield, even though 4 equiv. of 2a were required for full conversion of the azirine. The reactions of 2,3-diaryl- and 2-alkyl-3-aryl-substituted azirines gave high yields of pyrazinoindoles 4e–h with only 2 equiv. of the diazo compound, but in these cases additional heating of the reaction mixtures at 140 °C in the presence of p-toluenesulfonic acid (TsOH) (40 mol%) was required for the elimination of p-toluenesulfinic acid from the corresponding dihydropyrazinoindoles. Additional experiments showed that the best results can be achieved, when TsOH is added two minutes after the reaction has began. The reactions of 2a with monosubstituted 2H-azirines are complicated by the formation of a number of by-products, which significantly decreases the yield of the corresponding pyrazines (compounds 4i, j).
| Reaction conditions:a 3 (0.2 mmol), 2a (0.6 mmol), Rh2(OAc)4 (5 mol%), toluene (1 mL), 140 °C, 1 h.b 3 (0.2 mmol), 2a (0.4 mmol), Rh2(OAc)4 (5 mol%), toluene (1 mL), 140 °C, 2 min, then addition of TsOH (40 mol%), 140 °C, 1 h.c 3 (0.2 mmol), 2a (0.24 mmol), Rh2(OAc)4 (5 mol%), DCE (1 mL), 115 °C, 1 h.d 2a (0.8 mmol), 5 min. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
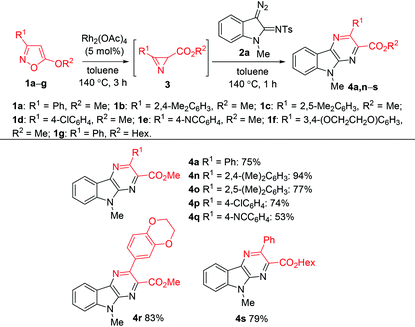
Alkyl azirine-2-carboxylates are known to be prepared by Fe(II)-29 or Cu(II)-catalyzed30 isomerization of 5-alkoxyisoxazoles. Very recently we have shown that dirhodium tetracarboxylates effectively catalyze this isomerization to provide azirines in nearly quantitative yields.31 As the formation of azirine-2-carboxylates from 5-alkoxyisoxazoles and their conversion to pyrazinoindoles can be promoted by the same catalyst, we tried to use a one-pot procedure for the preparation of pyrazinoindole 4a from isoxazole 1a without isolation of azirine 3a (Table 3). At the first stage isoxazole 1a was heated in the presence of Rh2(OAc)4 (5 mol%) at 140 °C in toluene for 3 h to form azirine 3a. Diazo compound 2a was then added, and the mixture was heated at 140 °C for an additional 1 h to give pyrazinoindole 4a in 75% yield. Using this procedure pyrazinoindoles 4n–s were synthesized in good yields. The decreased yield of compound 4q is related to the appreciable reactivity of the cyano group toward rhodium carbenoids.
| a Reaction conditions: 1 (0.2 mmol), Rh2(OAc)4 (5 mol%), toluene (1 mL), 140 °C, 3 h, then addition of 2a (0.6 mmol), 140 °C, 1 h. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
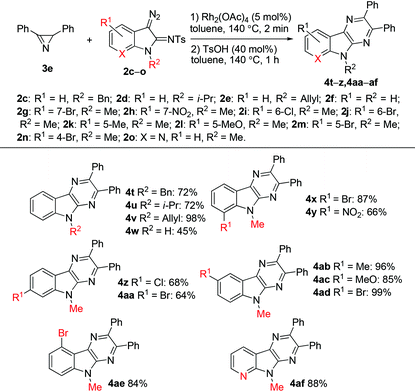
The scope of the reaction with respect to diazo compounds was studied using 3-diazoindolin-2-imines 2c–o and azirine 3e. We chose N-tosylimino-substituted diazo compounds for this study in view of the result of the reaction of azirine 3e with N-mesylimino-substituted diazo compound 2b. It was found that the elimination of methanesulfinic acid requires the same harsh reaction conditions and provides a lower yield of the pyrazinoindole 4e (60% from 2bvs. 93% from 2a). All tested diazoindolines with an N-substituted indole system gave pyrazinoindoles in good to excellent yields (Table 4). Notably, N-unprotected 3-diazoindolin-2-imine 2f was also suitable for this transformation albeit with a lower efficiency. It was found that electron-donating and halogen substituents at the 4-, 5-, 6-, and 7-positions of the 3-diazoindolin-2-imines are tolerated under the reaction conditions. Notably, the pyridine-type nitrogen atom in diazo compound 2o does not inhibit the reaction, and 6-aza analog 4af was synthesized in 88% yield.
| a Reaction conditions: 3e (0.2 mmol), Rh2(OAc)4 (5 mol%), toluene (1 mL), 140 °C, 2 min, then addition of 2 (0.6 mmol), 140 °C, 1 h. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The structures of synthesized compounds 4a–z, 4aa–af were verified by 1H and 13C NMR spectroscopy and HRMS, and the structure of pyrazinoindole 4j was confirmed by X-ray diffraction analysis.32
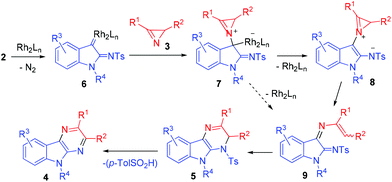
The most probable pathway for the formation of pyrazinoindole 4 is outlined in Scheme 3. α-Imino rhodium carbene complex 6 generated from diazo compound 2 and dirhodium tetraacetate reacts with azirine 3 to give metal-bound ylide 7.33 The latter loses the catalyst with the formation of zwitterionic intermediate 8 (metal-free azirinium ylide) which undergoes azirine ring-opening to give diiminoindoline 9. As far as we know, this reaction is the first example of the generation of azirinium ylides from 2H-azirines and cyclic rhodium carbenoids. Intermediate 9 also can form from 7 in one step via concerted azirine N–C2 bond and C–Rh bond cleavage. 1,6-Cyclization of diiminoindoline 9 to dihydropyrazinoindole 5 followed by elimination of toluenesulfinic acid provides pyrazinoindole 4.
In conclusion, we have developed an efficient one-step method for the preparation of 5H-pyrazino[2,3-b]indole derivatives from 2H-azirines and 3-diazoindolin-2-imines. The reaction proceeds under Rh2(OAc)4 catalysis via successive formation of rhodium carbenoids, azirinium ylides, 2,3-diiminoindolines and 4,5-dihydro-3H-pyrazino[2,3-b]indoles. The latter undergo elimination of p-toluenesulfinic acid to give 5H-pyrazino[2,3-b]indoles in good overall yields. To accelerate the last stage of the reaction with 2-alkyl and 2-aryl-substituted 2H-azirines, catalytic amounts of TsOH were added. The developed method allows wide variation of substituents in all the three rings of pyrazino[2,3-b]indole system. The reaction also tolerates aza substitution of carbon atoms in the benzene part of the indole fragment. Alkyl 5H-pyrazino[2,3-b]indole-3-carboxylates can also be prepared in good yields by a one-pot procedure from synthetic equivalents of 2H-azirine-2-carboxylates, 5-alkoxyisoxazoles. The reactions provide the first examples of the use of Rh(II) catalysis for intermolecular annulations with 2H-azirines and isoxazoles.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge the financial support of the Russian Foundation for Basic Research (grant no. 16-03-00596, 16-33-60130) and Saint Petersburg State University (grant no. 12.40.1427.2017). This research used resources of ‘Magnetic Resonance Research Centre’, ‘Chemical Analysis and Materials Research Centre’, ‘Centre for X-ray Diffraction Studies’, and ‘Chemistry Educational Centre’ of Research Park of Saint Petersburg State University.Notes and references
- (a) M. Ishikura, T. Abe, T. Choshi and S. Hibino, Nat. Prod. Rep., 2010, 27, 1630 RSC; (b) D. Crich and A. Banerjee, Acc. Chem. Res., 2007, 40, 151 CrossRef CAS PubMed.
- (a) A. J. Kochanowska-Karamyan and M. T. Hamann, Chem. Rev., 2010, 110, 4489 CrossRef CAS PubMed; (b) R. J. Sundberg, The Chemistry of Indoles, Academic Press, New York, 1970 Search PubMed; (c) J. L. Liang, S. E. Park, Y. J. Kwon and Y. D. Jahng, Bioorg. Med. Chem., 2012, 20, 4962 CrossRef CAS PubMed.
- (a) G. M. Nie, X. J. Han, J. Hou and S. S. Zhang, J. Electroanal. Chem., 2007, 604, 125 CrossRef CAS; (b) J. Roy, A. K. Jana and D. Mal, Tetrahedron, 2012, 68, 6099 CrossRef CAS; (c) T. Geiger, H. Benmansour, B. Fan, R. Hany and F. Nuesch, Macromol. Rapid Commun., 2008, 29, 651 CrossRef CAS.
- (a) M. Shiri, Chem. Rev., 2012, 112, 3508 CrossRef CAS PubMed; (b) S. Cacchi and G. Fabrizi, Chem. Rev., 2011, 111, PR215 CrossRef PubMed; (c) G. R. Humphrey and J. T. Kuethe, Chem. Rev., 2006, 106, 2875 CrossRef CAS PubMed; (d) M. Bandini and A. Eichholzer, Angew. Chem., Int. Ed., 2009, 48, 9608 CrossRef CAS PubMed; (e) R. Dalpozzo, Chem. Soc. Rev., 2015, 44, 742 RSC; (f) Z. Gu, Y. Li, S. Ma, S. Li, G. Zhou, S. Ding, J. Zhang, S. Wang and C. Zhou, RSC Adv., 2017, 7, 41869 RSC.
- C. Blum and A. Hutchison, US Pat, 5606059, 1997 Search PubMed.
- H.-G. Jang, S. K. Jeon, J. Y. Lee and S.-H. Hwang, RSC Adv., 2014, 4, 57679 RSC.
- B. A. J. Clark, J. Parrick and R. J. J. Dorgan, J. Chem. Soc., Perkin Trans. 1, 1976, 1361 RSC.
- J. Bergman and H. Vallberg, Acta Chem. Scand., 1997, 51, 742 CrossRef CAS.
- B. Malapel-Andrieu and J. Y. Mérour, Tetrahedron, 1998, 54, 11095 CrossRef CAS.
- F.-A. Alphonse, S. Routier, G. Coudert and J.-Y. Mérour, Heterocycles, 2001, 55, 925 CrossRef CAS.
- P. Mannes, E. Onyango and G. Gribble, J. Org. Chem., 2016, 81, 12478 CrossRef CAS PubMed.
- (a) E. E. Galenko, A. F. Khlebnikov and M. S. Novikov, Chem. Heterocycl. Compd., 2016, 52, 637 CrossRef CAS; (b) A. F. Khlebnikov and M. S. Novikov, Tetrahedron, 2013, 69, 3363 CrossRef CAS; (c) C.-Y. Huang and A. G. Doyle, Chem. Rev., 2014, 114, 8153 CrossRef CAS PubMed; (d) A. F. Khlebnikov and M. S. Novikov, Top. Heterocycl. Chem., 2016, 41, 143 Search PubMed.
- (a) M. Schläpfer-Dähler and H. Heimgartner, Helv. Chim. Acta, 1993, 76, 2321 CrossRef; (b) J.-P. Obrecht, P. Schönholzer, C. J. Jenny, R. Prewo and H. Heimgartner, Helv. Chim. Acta, 1988, 71, 1319 CrossRef CAS.
- J. Averdung, E. Albrecht, J. Lauterwein, H. Luftmanna, J. Mattay, H. Mohn, W. H. Miiller and H.-U. ter Meer, Chem. Ber., 1994, 127, 787 CrossRef CAS.
- (a) Y. Mei, P. A. Bentley and W. Wang, Tetrahedron Lett., 2006, 47, 2447 CrossRef CAS; (b) X.-D. Jiang, R. Gao, Y. Yue, G.-T. Sun and W. Zhao, Org. Biomol. Chem., 2012, 10, 6861 RSC.
- (a) P. A. Sakharov, N. V. Rostovskii, A. F. Khlebnikov and M. S. Novikov, Tetrahedron, 2017, 73, 4663 CrossRef CAS; (b) N. V. Rostovskii, P. A. Sakharov, M. S. Novikov, A. F. Khlebnikov and G. L. Starova, Org. Lett., 2015, 17, 4148 CrossRef CAS PubMed.
- N. V. Rostovskii, M. S. Novikov, A. F. Khlebnikov, S. M. Korneev and D. S. Yufit, Org. Biomol. Chem., 2013, 11, 5535 CAS.
- D. A. Candito and M. Lautens, Org. Lett., 2010, 12, 3312 CrossRef CAS PubMed.
- B.-C. Hong, A. K. Gupta, M.-F. Wu and J.-H. Liao, Tetrahedron Lett., 2004, 45, 1663 CrossRef CAS.
- (a) M. Drögemüller, R. Jautelat and E. Winterfeldt, Angew. Chem., Int. Ed. Engl., 1996, 35, 1572 CrossRef; (b) E. Haak and E. Winterfeldt, Synlett, 2004, 1414 CrossRef CAS.
- (a) E. E. Galenko, A. V. Galenko, M. S. Novikov, A. F. Khlebnikov, I. V. Kudryavtsev, M. A. Terpilowski, M. K. Serebriakova, A. S. Trulioff and N. V. Goncharov, ChemistrySelect, 2017, 2, 7508 CrossRef CAS; (b) E. E. Galenko, V. A. Bodunov, A. V. Galenko, M. S. Novikov and A. F. Khlebnikov, J. Org. Chem., 2017, 82, 8568 CrossRef CAS PubMed; (c) A. V. Galenko, E. E. Galenko, F. M. Shakirova, M. S. Novikov and A. F. Khlebnikov, J. Org. Chem., 2017, 82, 5367 CrossRef CAS PubMed; (d) E. E. Galenko, O. A. Tomashenko, A. F. Khlebnikov, M. S. Novikov and T. L. Panikorovskii, Beilstein J. Org. Chem., 2015, 11, 1732 CrossRef CAS PubMed.
- (a) K. V. Zavyalov, M. S. Novikov, A. F. Khlebnikov, N. V. Rostovskii and G. L. Starova, Russ. J. Org. Chem., 2017, 53, 1214 CrossRef CAS; (b) L. D. Funt, O. A. Tomashenko, I. P. Mosiagin, M. S. Novikov and A. F. Khlebnikov, J. Org. Chem., 2017, 82, 7583 CrossRef CAS PubMed; (c) O. A. Tomashenko, M. S. Novikov and A. F. Khlebnikov, J. Org. Chem., 2017, 82, 616 CrossRef CAS PubMed; (d) I. A. Smetanin, M. S. Novikov, A. V. Agafonova, N. V. Rostovskii, A. F. Khlebnikov, I. V. Kudryavtsev, M. A. Terpilowski, M. K. Serebriakova, A. S. Trulioff and N. V. Goncharov, Org. Biomol. Chem., 2016, 14, 4479 RSC; (e) L. D. Funt, O. A. Tomashenko, A. F. Khlebnikov, M. S. Novikov and A. Y. Ivanov, J. Org. Chem., 2016, 81, 11210 CrossRef CAS PubMed; (f) E. E. Galenko, A. V. Galenko, A. F. Khlebnikov, M. S. Novikov and J. R. Shakirova, J. Org. Chem., 2016, 81, 8495 CrossRef CAS PubMed.
- N. V. Rostovskii, J. O. Ruvinskaya, M. S. Novikov, A. F. Khlebnikov, I. A. Smetanin and A. V. Agafonova, J. Org. Chem., 2017, 82, 256 CrossRef CAS PubMed.
- X. Lei, L. Li, Y.-P. He and Y. Tang, Org. Lett., 2015, 17, 5224 CrossRef CAS PubMed.
- (a) Y. Jiang, R. Sun, X.-Y. Tang and M. Shi, Chem. – Eur. J., 2016, 22, 17910 CrossRef CAS PubMed; (b) H. M. L. Davies and J. S. Alford, Chem. Soc. Rev., 2014, 43, 5151 RSC.
- (a) H. Ding, S. Hong and N. Zhang, Tetrahedron Lett., 2015, 56, 507 CrossRef CAS; (b) Y. Wang, X. Lei and Y. Tang, Chem. Commun., 2015, 51, 4507 RSC; (c) Y.-Z. Zhao, H.-B. Yang, X.-Y. Tang and M. Shi, Chem. – Eur. J., 2015, 21, 3562 CrossRef CAS PubMed; (d) T. Ryu, Y. Baek and P. H. Lee, J. Org. Chem., 2015, 80, 2376 CrossRef CAS PubMed.
- N. S. Y. Loy, S. Kim and C.-M. Park, Org. Lett., 2015, 17, 395 CrossRef CAS PubMed.
- For selected examples, see: (a) G. R. Sheng, K. Huang, S. C. Ma, J. Qian, P. Lu and Y. G. Wang, Chem. Commun., 2015, 51, 11056 RSC; (b) Y. P. Xing, G. R. Sheng, J. Wang, P. Lu and Y. G. Wang, Org. Lett., 2014, 16, 1244 CrossRef CAS PubMed; (c) C. Wang, H. Zhang, B. Lang, A. Ren, P. Lu and Y. Wang, Org. Lett., 2015, 17, 4412 CrossRef CAS PubMed; (d) J. Qian, G. Sheng, K. Huang, S. Liu, P. Lu and Y. Wang, Org. Lett., 2016, 18, 3682 CrossRef CAS PubMed.
- S. Auricchio, A. Bini, E. Pastormerlo and A. M. Truscello, Tetrahedron, 1997, 53, 10911 CrossRef CAS.
- M. I. Komendantov, R. R. Bekmukhametov and R. R. Kostikov, Chem. Heterocycl. Compd., 1978, 14, 843 CrossRef.
- N. V. Rostovskii, A. V. Agafonova, I. A. Smetanin, M. S. Novikov, A. F. Khlebnikov, J. O. Ruvinskaya and G. L. Starova, Synthesis, 2017, 49, 4478 CrossRef CAS.
- CCDC 1579961† (4j) contains the supplementary crystallographic data for this paper.
- N. S. Y. Loy, A. Singh, X. Xu and C.-M. Park, Angew. Chem., Int. Ed., 2013, 52, 2212 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, compound characterization data and copies of 1H and 13C NMR spectra for new compounds. CCDC 1579961. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7ob02637d |
| This journal is © The Royal Society of Chemistry 2018 |