 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Polymers from sugars and CS2: synthesis and ring-opening polymerisation of sulfur-containing monomers derived from 2-deoxy-D-ribose and D-xylose†
Eva M.
López-Vidal
a,
Georgina L.
Gregory
ab,
Gabriele
Kociok-Köhn
c and
Antoine
Buchard
 *a
*a
aDepartment of Chemistry, University of Bath, Claverton Down, Bath BA2 7AY, UK. E-mail: a.buchard@bath.ac.uk; Fax: +44 (0)1225 386231; Tel: +44 (0)1225 386122
bCentre for Doctoral Training in Sustainable Chemical Technologies, University of Bath, Bath BA2 7AY, UK
cChemical Characterisation and Analysis Facilities, University of Bath, Bath BA2 7AY, UK
First published on 27th February 2018
Abstract
Thionocarbonate (–O–CS–O–) and xanthate (–S–CS–O–) cyclic monomers were synthesised by cyclothiocarbonation of 2-deoxy-D-ribose- and D-xylose-derived diols with carbon disulfide, then polymerised using organocatalytic ring-opening methods. Regular polymer linkages were obtained, with the sugar backbone influencing the regioselectivity of monomer opening. Thermal analysis revealed lower glass transition temperatures compared to carbonate analogues and a low onset of thermal degradation.
Natural monosaccharides are a pool of readily available and functional building blocks that are cheap, non-toxic, stereochemically diverse, and offer a renewable alternative to petroleum-based resources for the synthesis of monomers and polymers.1 Sugars have been used in polymer synthesis for example as pendant groups2 or incorporated into main polymer chains via step-growth methods from aldaric esters and alditols.1b Driven by the versatility and accurate control offered by ring-opening polymerisation methods (ROP),3 combined with the ability of sugars to be functionalised to adjust the properties of the resulting polymers, recent efforts have been devoted to the synthesis and subsequent ROP of sugar-based cyclic monomers.1a These include lactams,4 phosphoesters5 and cyclic carbonates.6 Some of our own work in this field has involved using CO2 to produce sugar-based cyclic carbonates without the need for phosgene derivatives.6a–c
Substitution of some oxygen atoms with sulfur in polymer backbones can result in enhanced physical (e.g. increased crystallinity), thermal, mechanical, electrical and optical properties, as well as advanced characteristics such as adhesion to metals, biological and chemical resistance, and biocompatibility.7 Therefore, we set out to utilise carbon disulfide (CS2), the sulfur analogue of CO2, to make novel sugar-based materials. Sulfur-containing analogues of sugar-based cyclic carbonates have been reported,8 but these have been synthesised using CSCl2 or Im2CS (Im = imidazole) reagents, and investigated mainly for their tendency to undergo O–S rearrangements.6i,8b–e,9 No polymerisation studies have been reported. Furthermore, while being used in the viscose process, CS2 has only been explored in polymer synthesis as a monomer for homopolymerisation or copolymerisation with epoxides and oxetanes.7b,10 Herein, we report the synthesis and polymerisation of novel cyclic xanthate and thionocarbonate monomers from sugar diols and carbon disulfide.
First, the synthesis of a 6-membered cyclic thionocarbonate trans-fused to a sugar furanose ring was targeted. Our hypothesis was that CS2, conversely to CO2,6b,c would allow the cyclocarbonation of the trans 1,3-diol motif of ribofuranose sugars, because of the longer C–S bond (155.3 pm) in CS2 compared to C–O (116.3 pm) in CO2. The resulting monomer would also be highly strained, and therefore prone to ROP. Using an analogous procedure to the one reported previously in our group for the synthesis of cyclic carbonates from diols and CO2,11 CS2 was added to a solution of 1-O-methyl-2-deoxy-D-ribofuranose in acetonitrile, in the presence of 1,8-diazabicyclo[5.4.0]-undec-7-ene (DBU). The reaction mixture changed rapidly from colourless to yellow, and 1H NMR analysis revealed insertion of CS2 into the sugar hydroxy groups (Fig. S1 and S2 in the ESI†). Furthermore, the C3 and C5 atoms of the sugar moiety remained deshielded (signals around 75 ppm), so no O–S rearrangement is believed to take place at this stage. Subsequent addition of mesyl chloride in the presence of triethylamine then led to the formation of cyclic xanthate 1, which was isolated by column chromatography in a 10% yield (Scheme 1). Integration of the anomeric protons in the 1H NMR spectrum confirmed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of the two anomers (Fig. S3†). To the best of our knowledge, 1 is the first cyclic xanthate (and in fact the first cyclic carbonate analogue), trans-fused to a sugar furanose ring. The nature of 1 was determined by NMR and FTIR spectroscopies, as well as electrospray ionisation mass spectrometry and elemental analysis (see ESI†). In particular, the 13C{1H} NMR signal for the C
1 mixture of the two anomers (Fig. S3†). To the best of our knowledge, 1 is the first cyclic xanthate (and in fact the first cyclic carbonate analogue), trans-fused to a sugar furanose ring. The nature of 1 was determined by NMR and FTIR spectroscopies, as well as electrospray ionisation mass spectrometry and elemental analysis (see ESI†). In particular, the 13C{1H} NMR signal for the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S bond is observed around 208 ppm, characteristic of the C(S)SO environment in cyclic xanthates.9e Because of an adjacent, more electropositive sulfur atom, the C5 signal in 1 (Fig. S4†) also appears at significantly lower chemical shift (37–38 ppm) compared to the related cyclic carbonates (67 ppm).6b The 3JH3H4 coupling constants (8.7 and 8.8 Hz) are also larger than for the cis-configured cyclic monomers we reported previously (4.9 and 5.7 Hz),6b and consistent with a trans-fused cyclic monomer. The structure was further corroborated by X-ray diffraction of a co-crystal of both anomers, obtained by recrystallization from hexanes (Fig. S40†). For the α anomer, the furanose ring adopts a 4-exo-3-endo twist conformation (3T4), whereas for the β anomer, the furanose ring has a 3-endo (3E) conformation. From previous mechanistic understanding of the analogous reaction with CO2,111 is not the expected thionocarbonate product, which would form from insertion of CS2 into a hydroxy group, mesylation of the resulting xanthate, then cyclisation via a nucleophilic addition–elimination pathway. As cyclo-thiocarbonation attempts using Im2CS proved unsuccessful, we suspect that the trans-configuration of the diol prevents cyclisation, or that the product is highly unstable. Formation of 1 could however be explained by a putative minor pathway (hence the low yields obtained): insertion of CS2 into the secondary hydroxy group, followed by mesylation of the 5-OH, then cyclisation via intramolecular nucleophilic substitution. 1 could also result from various S–O rearrangements.
S bond is observed around 208 ppm, characteristic of the C(S)SO environment in cyclic xanthates.9e Because of an adjacent, more electropositive sulfur atom, the C5 signal in 1 (Fig. S4†) also appears at significantly lower chemical shift (37–38 ppm) compared to the related cyclic carbonates (67 ppm).6b The 3JH3H4 coupling constants (8.7 and 8.8 Hz) are also larger than for the cis-configured cyclic monomers we reported previously (4.9 and 5.7 Hz),6b and consistent with a trans-fused cyclic monomer. The structure was further corroborated by X-ray diffraction of a co-crystal of both anomers, obtained by recrystallization from hexanes (Fig. S40†). For the α anomer, the furanose ring adopts a 4-exo-3-endo twist conformation (3T4), whereas for the β anomer, the furanose ring has a 3-endo (3E) conformation. From previous mechanistic understanding of the analogous reaction with CO2,111 is not the expected thionocarbonate product, which would form from insertion of CS2 into a hydroxy group, mesylation of the resulting xanthate, then cyclisation via a nucleophilic addition–elimination pathway. As cyclo-thiocarbonation attempts using Im2CS proved unsuccessful, we suspect that the trans-configuration of the diol prevents cyclisation, or that the product is highly unstable. Formation of 1 could however be explained by a putative minor pathway (hence the low yields obtained): insertion of CS2 into the secondary hydroxy group, followed by mesylation of the 5-OH, then cyclisation via intramolecular nucleophilic substitution. 1 could also result from various S–O rearrangements.
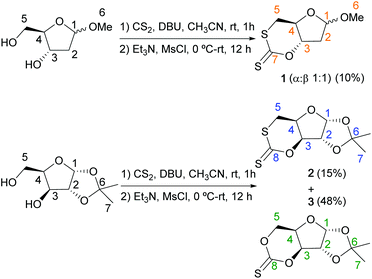 | ||
| Scheme 1 Synthesis of monomers 1–3 (see ESI† for detailed procedures). | ||
Using 1,2-O-isopropylidene-α-D-xylofuranose as a susbstrate was then considered, as in this case the cis-configuration of the 1,3-diol motif should facilitate cyclisation and give the expected thionocarbonate. Using the same procedure, two products were isolated after purification by column chromatography: xanthate 2 and thionocarbonate 3, in 15% and 48% yield respectively (Scheme 1). 2 displayed a xanthate signal in the 13C{1H} NMR signal at 208.6 ppm, while a signal at 187.4 ppm was observed for 3, consistent with a thionocarbonate species (Fig. S15 and S24† respectively).9e Confirmation of the structure of 2 and 3 by NMR and FTIR spectroscopies, as well as electrospray ionisation mass spectrometry and elemental analysis, was further corroborated by single-crystal X-ray diffraction (Fig. S41 and S42†). As expected, using a cis 1,3-diol motif yielded thionocarbonate 3, likely via an addition–elimination mechanism for the ring-closing step.11 However, 2 was still formed in small quantity. The possibility that 2 could result from an alternative SN2-type mechanism was therefore verified experimentally. 1,2-O-isopropylidene-α-D-xylofuranose was tosylated at the 5-position into 4 (Scheme S5†), which, upon addition of CS2 and DBU, should only lead to the substitution of the tosyl group by the xanthate salt (Scheme S6†). Formation of 2 was indeed observed with approximately 50% conversion after 30 minutes (Fig. S39†). While 3 has been previously synthesised using Im2CS,8c to the best of our knowledge 2 is the first cyclic xanthate cis-fused to a sugar furanose ring. These two compounds are the sulfur analogues of the carbonate monomer reported by Gross and coworkers.6i
Ring-opening polymerisation of monomers 1–3 were next studied at room temperature in dichloromethane, using 1,5,7-triazabicyclo[4.4.0]dec-5-ene (TBD) organocatalyst, 4-methylbenzyl alcohol initiator, and 1 mol L−1 initial monomer concentration (Table 1). TBD is one of the most active organocatalysts for the ROP of cyclic carbonates,12 which usually gives good polymerisation control and limits the amount of cyclic species formed by direct nucleophilic initiation, as with diazabicyclo[5.4.0]undec-7-ene (DBU).13 Early trials with DBU gave indeed poor control and decreased activity. Monomer conversion was determined by 1H NMR spectroscopy. Conformational changes brought about by the release of ring strain upon opening led for all monomers to a downfield shift of H-3 and coalescing of the signals assigned to H-5, as well as a general broadening of the resonances (Fig. S43, S54 and S65† for 1–3, respectively). Polymerisation of 1 proceeded rapidly, reaching >99% conversion in less than 15 min at various monomer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) initiator
initiator![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) catalyst feed ratios (Table 1, entries 1–3). Polymerisation of 2 was slightly slower, consistent with the less strained nature of this xanthate compared to 1 (Table 1, entries 5–8), with around 86% monomer conversion after 15 min and >99% after 2 h for a monomer
catalyst feed ratios (Table 1, entries 1–3). Polymerisation of 2 was slightly slower, consistent with the less strained nature of this xanthate compared to 1 (Table 1, entries 5–8), with around 86% monomer conversion after 15 min and >99% after 2 h for a monomer![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) initiator
initiator![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) catalyst ratio of 50
catalyst ratio of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Table 1, entries 5 and 6). Polymerisation of 3 (entries 8–14 in Table 1) proceeded even slower and reached a monomer conversion plateau (66% after 2 hours), indicating a concentration dependent equilibrium polymerisation. A slightly higher initial concentration of 3 (1.58 mol L−1) did not lead to a significant increase in monomer conversion (Table 1, entries 8 and 11). Overall, as expected from the trans configuration on its fused furanose ring and the resulting high strain of its xanthate ring, monomer 1 is more reactive towards ROP than monomers 2 and 3, which feature a cis-fused furanose ring and are less strained cyclic monomers. Xanthate 2 also appears more reactive than analogous thionocarbonate 3, and we suggest that this is because C–S bonds are easier to break than C–O bonds.
1 (Table 1, entries 5 and 6). Polymerisation of 3 (entries 8–14 in Table 1) proceeded even slower and reached a monomer conversion plateau (66% after 2 hours), indicating a concentration dependent equilibrium polymerisation. A slightly higher initial concentration of 3 (1.58 mol L−1) did not lead to a significant increase in monomer conversion (Table 1, entries 8 and 11). Overall, as expected from the trans configuration on its fused furanose ring and the resulting high strain of its xanthate ring, monomer 1 is more reactive towards ROP than monomers 2 and 3, which feature a cis-fused furanose ring and are less strained cyclic monomers. Xanthate 2 also appears more reactive than analogous thionocarbonate 3, and we suggest that this is because C–S bonds are easier to break than C–O bonds.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a
a
| Entry | M | [M]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :[ :[![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C] C]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [I]b [I]b |
Time (h) | Conv.c (%) | M n (calc) | M n (SEC) | Đ |
|---|---|---|---|---|---|---|---|
| a Polymerisations carried out at room temperature in anhydrous CH2Cl2 solvent with initial [M]0 = 1 mol L−1 (M = monomer), unless stated otherwise; all entries correspond to separate experiments. b C is the catalyst, TBD, and I is the initiator, 4-MeBnOH. c Conversion measured by 1H NMR determined by relative integration of the anomeric proton in the 1H NMR spectrum in CDCl3. d In 103 g mol−1, calculated as Mr(I) + (Mr(monomer) × [monomer]0/[I]0 × conv/100%). e In 103 g mol−1, estimated by SEC (RI detector) versus polystyrene standards with THF eluent. f Đ = Mn/Mw. g [M]0 = 1.58 mol L−1. | |||||||
| 1 | 1 | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.25 | >99 | 5.3 | 5.1 | 1.6 |
| 2 | 1 | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.25 | >99 | 10.4 | 5.2 | 1.8 |
| 3 | 1 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.25 | >99 | 20.7 | 11.3 | 2.2 |
| 4 | 2 | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.25 | 86 | 5.5 | 6.0 | 1.5 |
| 5 | 2 | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.25 | 87 | 10.9 | 5.3 | 2.2 |
| 6 | 2 | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2 | >99 | 12.5 | 12.6 | 1.5 |
| 7 | 2 | 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.25 | 86 | 21.5 | 15.7 | 1.7 |
| 8 | 3 | 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.25 | 42 | 2.6 | 5.2 | 1.5 |
| 9 | 3 | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.25 | 40 | 4.8 | 7.4 | 1.3 |
| 10 | 3 | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2 | 66 | 7.8 | 6.7 | 1.2 |
| 11 | 3 | 50g![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.25 | 52 | 6.2 | 7.5 | 1.5 |
| 12 | 3 | 50g![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.5 | 61 | 7.2 | 10.0 | 1.3 |
| 13 | 3 | 50g![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
3 | 62 | 7.3 | 10.3 | 1.3 |
| 14 | 3 | 100g![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.25 | 44 | 10.3 | 10.6 | 1.5 |
Size exclusion chromatography (SEC) in THF confirmed the polymeric nature of the products, and was used to estimate number-average molecular weights (Mn) and dispersities (Đ) versus polystyrene standards. Polymers of up to 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 g mol−1 (Đ 2.2) and 15
300 g mol−1 (Đ 2.2) and 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 g mol−1 (Đ 1.7) could be achieved from 1 and 2, respectively, but limited agreement between predicted and SEC Mn, as well as broad and varying distributions, were observed. The ROP of 3 was more controlled, with better agreement between theoretical molecular weights and those determined by SEC, as well as narrower dispersities. Polymers of up to 10
700 g mol−1 (Đ 1.7) could be achieved from 1 and 2, respectively, but limited agreement between predicted and SEC Mn, as well as broad and varying distributions, were observed. The ROP of 3 was more controlled, with better agreement between theoretical molecular weights and those determined by SEC, as well as narrower dispersities. Polymers of up to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 g mol−1 (Đ 1.5) could be obtained from 3. However, for most polymers, inconsistencies and discrepancies between theoretical Mn, SEC Mn and those estimated by NMR (via the relative integration of the 4-methylbenzyl alcohol end-group, which only accounts for linear polymers) highlight the limited control of the ROP of 1–3 under these organocatalytic conditions. This suggests the formation of cyclic species by back-biting or sensitivity to traces of chain-transfer agent like adventitious moisture (leading to smaller Mn than expected), as well as trans chain exchange phenomena, as sometimes seen in the ROP of cyclic carbonates, including in the polymerisation of sugar-based monomers.6b,c,i
600 g mol−1 (Đ 1.5) could be obtained from 3. However, for most polymers, inconsistencies and discrepancies between theoretical Mn, SEC Mn and those estimated by NMR (via the relative integration of the 4-methylbenzyl alcohol end-group, which only accounts for linear polymers) highlight the limited control of the ROP of 1–3 under these organocatalytic conditions. This suggests the formation of cyclic species by back-biting or sensitivity to traces of chain-transfer agent like adventitious moisture (leading to smaller Mn than expected), as well as trans chain exchange phenomena, as sometimes seen in the ROP of cyclic carbonates, including in the polymerisation of sugar-based monomers.6b,c,i
The FTIR spectra of all polymers were characterised by several strong absorption bands in the 1290–1020 cm−1 region and none at 1757 cm−1, indicative of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S8d,14 but no C
S8d,14 but no C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds (Fig S53, S64 and S75†). Analysis by 13C{1H} NMR also supported the absence of O–S rearrangement during polymerisation as no carbonyl resonance was observed. 13C{1H} NMR was used to investigate further the microstructure of polymers (Fig. 1). For poly(3), three distinct thionocarbonate environments (differing by 0.3–1 ppm) were detected around 193.8 ppm (compared to 187.4 ppm for the monomer), and assigned to tail–tail (or head–head), head–tail, and head–head (or tail–tail) thionocarbonate linkages (Fig. S66†). Their 1
O bonds (Fig S53, S64 and S75†). Analysis by 13C{1H} NMR also supported the absence of O–S rearrangement during polymerisation as no carbonyl resonance was observed. 13C{1H} NMR was used to investigate further the microstructure of polymers (Fig. 1). For poly(3), three distinct thionocarbonate environments (differing by 0.3–1 ppm) were detected around 193.8 ppm (compared to 187.4 ppm for the monomer), and assigned to tail–tail (or head–head), head–tail, and head–head (or tail–tail) thionocarbonate linkages (Fig. S66†). Their 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio suggests random cleavage of the thioacyl–oxygen bond at either side of the thionocarbonate carbonyl and subsequent nonselective propagation of the chain to yield regiorandom polymers. For poly(1), one distinct xanthate resonance was observed at 213.0 ppm (compared to the monomer signals at 208.1 and 207.8 for both anomers) (Fig. S44†). Thus, the thiocarbonyl region suggests a preference for regioregular opening of 1 (likely to liberate a more acidic primary thiol) and subsequent selective propagation of the chain to yield a poly(xanthate). In stark contrast, the polymer resulting from xanthate 2 displayed mainly two distinct thiocarbonyl resonances of similar intensities. Based on the literature,9b,d the resonance at 222.5 ppm is assigned to a trithiocarbonate environment (C(S)S2), and the one at 193.1 ppm is assigned to a thionocarbonate (C(S)O2) (Fig. S55†). This suggests an alternating opening of the monomer at either side of the xanthate thiocarbonyl, and subsequent selective propagation of the chain to yield regioregular polymers with alternating C(S)S2 and C(S)O2 linkages. The origin of this regioregularity is so far unknown.
1 ratio suggests random cleavage of the thioacyl–oxygen bond at either side of the thionocarbonate carbonyl and subsequent nonselective propagation of the chain to yield regiorandom polymers. For poly(1), one distinct xanthate resonance was observed at 213.0 ppm (compared to the monomer signals at 208.1 and 207.8 for both anomers) (Fig. S44†). Thus, the thiocarbonyl region suggests a preference for regioregular opening of 1 (likely to liberate a more acidic primary thiol) and subsequent selective propagation of the chain to yield a poly(xanthate). In stark contrast, the polymer resulting from xanthate 2 displayed mainly two distinct thiocarbonyl resonances of similar intensities. Based on the literature,9b,d the resonance at 222.5 ppm is assigned to a trithiocarbonate environment (C(S)S2), and the one at 193.1 ppm is assigned to a thionocarbonate (C(S)O2) (Fig. S55†). This suggests an alternating opening of the monomer at either side of the xanthate thiocarbonyl, and subsequent selective propagation of the chain to yield regioregular polymers with alternating C(S)S2 and C(S)O2 linkages. The origin of this regioregularity is so far unknown.
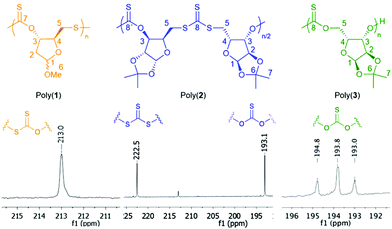 | ||
| Fig. 1 Ring-opening polymerisation of monomers 1–3 and linkages observed by 13C NMR for the respective polymers. | ||
Analysis of the polymers by Matrix-assisted laser desorption/ionisation time-of-flight (MALDI-ToF) mass spectrometry was conducted to confirm the microstructures deduced by NMR, but proved extremely challenging. However, for poly(3) a major cyclic polymer series, with no end-groups and an integer number of sugar thionocarbonate repeat units (m/z ∼232.25) was observed, likely due to backbiting of the polymer chain. A minor linear polymer series with 4-MePhCH2O and OH end groups was also present (Fig. S71†). Poly(1) yielded poor data, although two different polymer series (cyclic and linear) with sugar xanthate repeat units (m/z ∼206.27) were detected, in agreement with NMR analysis (Fig. S49†). However, results from poly(2) (Fig. S60†) disagreed with the major alternating sequence inferred by NMR, revealing poly(xanthate) series (cyclic and linear) with repeating unit m/z ∼248.31, which could come from the selective ionisation of side-products. Such poly(xanthate) traces can actually be detected by NMR in the 13C NMR spectrum (trace signal at 213.0 ppm in Fig. 1).
Thermal properties of the polymers were evaluated by thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) of representative samples. Analysis of poly(1) with Mn 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 g mol−1 (Table 1, entry 3) showed two degradation steps with onsets at ∼120 °C and ∼182 °C, with associated mass losses of 58 and 10% respectively (Fig. S50 and S51†). A glass transition temperature (Tg) of 55 °C was measured (Fig. S52†). Although direct comparison with the analogous polycarbonate is not possible for synthetic reasons, poly(1) displays thermal properties which are very similar to the reported values for a related polycarbonate with a cis-fused motif (Tg ∼ 58 °C and Td (onset) ∼ 125 °C for Mn 25
300 g mol−1 (Table 1, entry 3) showed two degradation steps with onsets at ∼120 °C and ∼182 °C, with associated mass losses of 58 and 10% respectively (Fig. S50 and S51†). A glass transition temperature (Tg) of 55 °C was measured (Fig. S52†). Although direct comparison with the analogous polycarbonate is not possible for synthetic reasons, poly(1) displays thermal properties which are very similar to the reported values for a related polycarbonate with a cis-fused motif (Tg ∼ 58 °C and Td (onset) ∼ 125 °C for Mn 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 g mol−1).6b Xylose-derived polymers were slightly more thermally stable. Poly(2) with Mn 12
600 g mol−1).6b Xylose-derived polymers were slightly more thermally stable. Poly(2) with Mn 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 g mol−1 (Table 1, entry 6) featured two thermal degradation steps (Fig. S61 and S62†), with onsets at ∼77 °C and ∼135 °C, corresponding to mass losses of 6% and 72%, respectively. TGA analysis of poly(3) with Mn 10
500 g mol−1 (Table 1, entry 6) featured two thermal degradation steps (Fig. S61 and S62†), with onsets at ∼77 °C and ∼135 °C, corresponding to mass losses of 6% and 72%, respectively. TGA analysis of poly(3) with Mn 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 g mol−1 (Table 1, entry 14) showed three degradation steps, with onsets at ∼77 °C (10% mass loss), ∼161 °C then ∼298 °C (combined 79% mass loss) (Fig. S72 and S73†). Similar Tg values were measured for poly(2) (∼46 °C, Fig. S63†) and poly(3) (∼48 °C, Fig. S74†). These Tg values are significantly lower than the value for the analogous xylose carbonate, reported by Gross and coworkers (Tg 128 °C), for which no thermal degradation was reported.6i
600 g mol−1 (Table 1, entry 14) showed three degradation steps, with onsets at ∼77 °C (10% mass loss), ∼161 °C then ∼298 °C (combined 79% mass loss) (Fig. S72 and S73†). Similar Tg values were measured for poly(2) (∼46 °C, Fig. S63†) and poly(3) (∼48 °C, Fig. S74†). These Tg values are significantly lower than the value for the analogous xylose carbonate, reported by Gross and coworkers (Tg 128 °C), for which no thermal degradation was reported.6i
This decrease in glass transition temperatures when replacing O by S is consistent with the literature, in particular for aromatic polycarbonates.7i,15 For aliphatic polymers, data is rare, but poly(trimethylene monothiocarbonate) (PTMMTC) has been shown to have a Tg between −17 and −25 °C,7o similar to the one reported for poly(trimethylene carbonate) (PTMC) of −25 °C.6b However, Darensbourg, Zhang and coworkers further noted that when S/O rearrangements occured (yielding for example thionocarbonate linkages), Tg fell to −41 °C. PTMMTC is also semi-crystalline (up to 71% crystallinity while PTMC is amorphous) and displays better thermal stability with onset of degradation occurring at 228.5 °C vs. 197 °C for PTMC. Another example by Endo and coworkers is the comparison done between a norbornene-substituted poly(trimethylene carbonate) and the analogous polythionocarbonate, for which the sulfur aliphatic polymer shows a decrease in Tg (82 °C vs. 108 °C) but an increase in the temperature of degradation onset (Td10% 258 °C vs. 207 °C).16 A possible explanation for the decrease in Tg is the difference in the bond lengths of C–O (1.43 Å) and C–S (1.815 Å) as well as the van der Waals radii of O (1.52 Å) and S (1.85 Å) atoms, which increase the free volume in the polymer. Asymmetrical linkages have also been invoked to explain the enhanced intermolecular interactions between poly(thiocarbonate) chains, leading to crystallinity and higher thermal stability.
Conclusions
In summary, in our attempt to use CS2 in the direct cyclothiocarbonation of the trans 1,3-diol motif of a ribofuranose, we discovered that cyclic xanthate structures could be accessed. We thus isolated the first two examples of 6-membered cyclic xanthate monomers, trans and cis-fused to sugar furanose rings, derived from natural sugars 2-deoxy-D-ribose and D-xylose, respectively. Polymers from these two monomers and from a xylose-derived thionocarbonate, also made using CS2, were obtained by ROP under mild reaction conditions, with organocatalyst TBD and 4-MeBnOH alcohol initiator. MALDI-ToF MS revealed both linear and cyclic topologies, in agreement with the limited control over the polymerisation observed. 13C{1H} NMR analysis suggests that no O–S rearrangement occurs during polymerisation, and that both the nature of the monomer and that of the sugar used influence the regioselectivity of ring-opening. While the deoxyribose xanthate monomer yields a poly(xanthate) species, the xylose cyclic xanthate produces a polymer with alternating trithiocarbonate and thionocarbonate linkages, and the xylose-derived poly(thionocarbonate) shows regiorandom linkages. Thermal analysis revealed lower glass transition temperatures compared to carbonate analogues and a low onset of thermal degradation. Building from this communication, current efforts are focusing on controlling the polymerisation of these novel sulfur-containing monomers and extending our methodology to other sugar feedstocks, in order to accurately investigate the effect of replacing O by S in the linkages of sugar-based polycarbonates, and gain a better understanding of the structure–property relationships of the resulting polymers. Because of their sugar backbone and the high refractive index and Abbe's number of sulphur analogues of polycarbonates,17 these materials could be used in optical and biomedical applications where degradability and biocompatibility is required.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The EPSRC (EP/N022793/1; EP/L016354/1/CDT in Sustainable Chemical Technologies), Roger and Sue Whorrod (fellowship to AB) and the Royal Society (RG/150538; UF/160021 fellowship to AB) are acknowledged for research funding. We thank the University of Bath HPC for computing resources. Analytical facilities were provided through the Chemical Characterisation and Analysis Facility (CCAF) at the University of Bath (http://www.bath.ac.uk/ccaf), as well as the EPSRC UK National Mass Spectrometry Facility at Swansea University for MALDI analysis, with special thanks to Dr Mark F. Wyatt for his help.Notes and references
- (a) G. L. Gregory, E. M. Lopez-Vidal and A. Buchard, Chem. Commun., 2017, 53, 2198–2217 RSC; (b) J. A. Galbis, M. d. G. García-Martín, M. V. de Paz and E. Galbis, Chem. Rev., 2016, 116, 1600–1636 CrossRef CAS PubMed.
- (a) A. J. Varma, J. F. Kennedy and P. Galgali, Carbohydr. Polym., 2004, 56, 429–445 CrossRef CAS; (b) V. Ladmiral, E. Melia and D. M. Haddleton, Eur. Polym. J., 2004, 40, 431–449 CrossRef CAS; (c) R. Narain, D. Jhurry and G. Wulff, Eur. Polym. J., 2002, 38, 273–280 CrossRef CAS; (d) M. Okada, Prog. Polym. Sci., 2001, 26, 67–104 CrossRef CAS; (e) J. A. Galbis and M. d. G. García-Martín, in Carbohydrates in Sustainable Development II, ed. A. P. Rauter, P. Vogel and Y. Queneau, Springer Berlin, Heidelberg, 2010, pp. 147–176 Search PubMed; (f) X. Feng, A. J. East, W. B. Hammond, Y. Zhang and M. Jaffe, Polym. Adv. Technol., 2011, 22, 139–150 CrossRef CAS; (g) J. A. Galbis and M. d. G. García-Martín, in Monomers, Polymers and Composites from Renewable Resources, ed. A. Gandini, Elsevier, Amsterdam, 2008, pp. 89–114 Search PubMed.
- T. Endo, in Handbook of Ring-Opening Polymerization, Wiley-VCH Verlag GmbH & Co. KGaA, 2009, pp. 53–63 Search PubMed.
- (a) E. L. Dane and M. W. Grinstaff, J. Am. Chem. Soc., 2012, 134, 16255–16264 CrossRef CAS PubMed; (b) E. L. Dane, S. L. Chin and M. W. Grinstaff, ACS Macro Lett., 2013, 2, 887–890 CrossRef CAS PubMed; (c) C. Ghobril, B. Heinrich, E. L. Dane and M. W. Grinstaff, ACS Macro Lett., 2014, 3, 359–363 CrossRef CAS PubMed; (d) S. E. Stidham, S. L. Chin, E. L. Dane and M. W. Grinstaff, J. Am. Chem. Soc., 2014, 136, 9544–9547 CrossRef CAS PubMed; (e) S. L. Chin, Q. Lu, E. L. Dane, L. Dominguez, C. J. McKnight, J. E. Straub and M. W. Grinstaff, J. Am. Chem. Soc., 2016, 138, 6532–6540 CrossRef CAS PubMed; (f) R. Xiao, E. L. Dane, J. Zeng, C. J. McKnight and M. W. Grinstaff, J. Am. Chem. Soc., 2017, 139, 14217–14223 CrossRef CAS PubMed; (g) M. Bueno, J. A. Galbis, M. G. García-Martín, M. V. De Paz, F. Zamora and S. Muñoz-Guerra, J. Polym. Sci., Part A: Polym. Chem., 1995, 33, 299–305 CrossRef CAS; (h) M. d. G. García-Martín, M. V. de Paz Báñez and J. A. Galbis, J. Carbohydr. Chem., 2000, 19, 805–815 CrossRef; (i) M. d. G. García-Martín, M. V. de Paz Báñez and J. A. G. Pérez, Carbohydr. Res., 1993, 240, 301–305 CrossRef.
- Y.-Y. T. Tsao and K. L. Wooley, J. Am. Chem. Soc., 2017, 139, 5467–5473 CrossRef CAS PubMed.
- (a) G. L. Gregory, L. M. Jenisch, B. Charles, G. Kociok-Köhn and A. Buchard, Macromolecules, 2016, 49, 7165–7169 CrossRef CAS; (b) G. L. Gregory, G. Kociok-Köhn and A. Buchard, Polym. Chem., 2017, 8, 2093–2104 RSC; (c) G. L. Gregory, E. M. Hierons, G. Kociok-Kohn, R. I. Sharma and A. Buchard, Polym. Chem., 2017, 8, 1714–1721 RSC; (d) K. Tezuka, K. Koda, H. Katagiri and O. Haba, Polym. Bull., 2015, 72, 615–626 CrossRef CAS; (e) K. Mikami, A. T. Lonnecker, T. P. Gustafson, N. F. Zinnel, P.-J. Pai, D. H. Russell and K. L. Wooley, J. Am. Chem. Soc., 2013, 135, 6826–6829 CrossRef CAS PubMed; (f) O. Haba, H. Tomizuka and T. Endo, Macromolecules, 2005, 38, 3562–3563 CrossRef CAS; (g) M. Azechi, K. Matsumoto and T. Endo, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 1651–1655 CrossRef CAS; (h) R. Kumar, W. Gao and R. A. Gross, Macromolecules, 2002, 35, 6835–6844 CrossRef CAS; (i) Y. Shen, X. Chen and R. A. Gross, Macromolecules, 1999, 32, 2799–2802 CrossRef CAS.
- (a) A. Kultys, Sulfur-Containing Polymers in Encyclopedia of Polymer Science and Technology, John Wiley and Sons, Inc., 2010 Search PubMed; (b) M. Luo, Y. Li, Y.-Y. Zhang and X.-H. Zhang, Polymer, 2016, 82, 406–431 CrossRef CAS; (c) E. Marianucci, C. Berti, F. Pilati, P. Manaresi, M. Guaita and O. Chiantore, Polymer, 1994, 35, 1564–1566 CrossRef CAS; (d) J. C. Seferis, in Polymer handbook, ed. J. Brandrup, E. H. Immergut and E. A. Grulke, Wiley, New York, 4th edn, 1999, pp. VI-571–VI-582 Search PubMed; (e) R. K. Sadhir and K. F. Schoch, Chem. Mater., 1996, 8, 1281–1286 CrossRef CAS; (f) B. Ochiai and T. Endo, Prog. Polym. Sci., 2005, 30, 183–215 CrossRef CAS; (g) W. Kuran, Prog. Polym. Sci., 1998, 23, 919–992 CrossRef CAS; (h) G. Montaudo, C. Puglisi, C. Berti, E. Marianucci and F. Pilati, J. Polym. Sci., Part A: Polym. Chem., 1989, 27, 2277–2290 CrossRef CAS; (i) M. F. Rey-Stolle, F. Fernández-Martín, L. H. Tagle and I. Hernández-Fuentes, Polym. Int., 1998, 46, 93–98 CrossRef CAS; (j) H. R. Kricheldorf and G. Schwarz, J. Macromol. Sci., Pure Appl. Chem., 2007, 44, 625–649 CrossRef CAS; (k) J.-L. Yang, H.-L. Wu, Y. Li, X.-H. Zhang and D. J. Darensbourg, Angew. Chem., Int. Ed., 2017, 56, 5774–5779 CrossRef CAS PubMed; (l) C. G. Overberger and J. Weise, J. Polym. Sci., Part B: Polym. Lett., 1964, 2, 329–331 CrossRef CAS; (m) T. J. Bannin and M. K. Kiesewetter, Macromolecules, 2015, 48, 5481–5486 CrossRef CAS PubMed; (n) C. G. Overberger and J. K. Weise, J. Am. Chem. Soc., 1968, 90, 3533–3537 CrossRef CAS; (o) H.-L. Wu, J.-L. Yang, M. Luo, R.-Y. Wang, J.-T. Xu, B.-Y. Du, X.-H. Zhang and D. J. Darensbourg, Macromolecules, 2016, 49, 8863–8868 CrossRef CAS; (p) M. Luo, X.-H. Zhang and D. J. Darensbourg, Acc. Chem. Res., 2016, 49, 2209–2219 CrossRef CAS PubMed.
- (a) M. Benazza, R. Kanso and G. Demailly, Carbohydr. Res., 2010, 345, 346–351 CrossRef CAS PubMed; (b) Y. Tsuda, S. Noguchi and H. Niino, Chem. Pharm. Bull., 2001, 49, 1210–1213 CrossRef CAS PubMed; (c) M. Adiyaman, S. P. Khanapure, S. W. Hwang and J. Rokach, Tetrahedron Lett., 1995, 36, 7367–7370 CrossRef CAS; (d) D. Trimnell, W. N. Doane, C. R. Russell and C. E. Rist, Carbohydr. Res., 1971, 17, 319–326 CrossRef CAS PubMed; (e) D. Trimnell, W. M. Doane and C. R. Russell, Carbohydr. Res., 1972, 22, 351–359 CrossRef CAS.
- (a) X.-H. Zhang, F. Liu, X.-K. Sun, S. Chen, B.-Y. Du, G.-R. Qi and K. M. Wan, Macromolecules, 2008, 41, 1587–1590 CrossRef CAS; (b) D. J. Darensbourg, J. R. Andreatta, M. J. Jungman and J. H. Reibenspies, Dalton Trans., 2009, 8891–8899 RSC; (c) D. J. Darensbourg, S. J. Wilson and A. D. Yeung, Macromolecules, 2013, 46, 8102–8110 CrossRef CAS; (d) J. Diebler, H. Komber, L. Häußler, A. Lederer and T. Werner, Macromolecules, 2016, 49, 4723–4731 CrossRef CAS; (e) M. Luo, X.-H. Zhang and D. J. Darensbourg, Macromolecules, 2015, 48, 5526–5532 CrossRef CAS.
- M. Luo, X.-H. Zhang and D. J. Darensbourg, Polym. Chem., 2015, 6, 6955–6958 RSC.
- G. L. Gregory, M. Ulmann and A. Buchard, RSC Adv., 2015, 5, 39404–39408 RSC.
- (a) F. Nederberg, B. G. G. Lohmeijer, F. Leibfarth, R. C. Pratt, J. Choi, A. P. Dove, R. M. Waymouth and J. L. Hedrick, Biomacromolecules, 2007, 8, 153–160 CrossRef CAS PubMed; (b) M. K. Kiesewetter, E. J. Shin, J. L. Hedrick and R. M. Waymouth, Macromolecules, 2010, 43, 2093–2107 CrossRef CAS.
- D. Pati, X. Feng, N. Hadjichristidis and Y. Gnanou, Macromolecules, 2017, 50, 1362–1370 CrossRef CAS.
- (a) R. M. Silverstein, F. X. Webster, D. J. Kiemle and D. L. Bryce, in Spectrometric Identification of Organic Compounds, John Wiley and Sons, New York, 8th edn, 2014, ch. 2 Search PubMed; (b) B. Tamami and A. R. Kiasar, J. Chem. Res., Synop., 1998, 454–455 RSC.
- M. Yazdani-Pedram, E. Soto, L. H. Tagle, F. R. Diaz, L. Gargallo and D. Radić, Thermochim. Acta, 1986, 105, 149–160 CrossRef CAS.
- K. Kakimoto, N. Nemoto, F. Sanda and T. Endo, Chem. Lett., 2002, 31, 156–157 CrossRef.
- M. Luo, X.-H. Zhang, B.-Y. Du, Q. Wang and Z.-Q. Fan, Polym. Chem., 2015, 6, 4978–4983 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, NMR spectra of monomers and polymers. Single-crystal X-ray diffraction data for 1, 2 and 3. Images of SEC traces, TGA-MS, MALDI-ToF MS and DSC traces. CCDC 1583233–1583235. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c8py00119g |
| This journal is © The Royal Society of Chemistry 2018 |
