Metal (M = Co, Ni) phosphate based materials for high-performance supercapacitors
Xinran
Li
 ,
Xiao
Xiao
,
Xiao
Xiao
 ,
Qing
Li
,
Qing
Li
 ,
Jilei
Wei
,
Huaiguo
Xue
and
Huan
Pang
,
Jilei
Wei
,
Huaiguo
Xue
and
Huan
Pang
 *
*
School of Chemistry and Chemical Engineering, Institute for Innovative Materials and Energy, Yangzhou University, Yangzhou, 225009, Jiangsu, P. R. China. E-mail: huanpangchem@hotmail.com; panghuan@yzu.edu.cn; Web: http://huanpangchem.wix.com/advanced-material
First published on 13th October 2017
Abstract
With the ever increasing demand for clean, sustainable energy, electrochemical supercapacitors with the advantages of high power density, high efficiency and long life expectancy have become one of the major devices for energy storage and power supply, and have found wide application in hybrid power sources, backup power sources, starting power for fuel cells and burst-power generation in electronic devices. Metal phosphates with the advantages of abundance, environmental friendliness and low cost are emerging as a novel class of promising electrode materials for supercapacitors. This review summarizes recent progress with respect to cobalt and nickel phosphate based micro/nanomaterials applied as supercapacitors, covering ammonium/bimetallic phosphates, cobalt phosphates, nickel phosphates and cobalt/nickel phosphates. Much progress has been made using metal phosphate materials as supercapacitors; however, there is still much room for further improvement.
1. Introduction
Environmental and energy problems have become increasingly severe in modern society.1,2 In recent years, the development of new high-performance energy storage and conversion devices has become a hot research area.3–5 Compared with solar, wind, hydraulic and geothermal energies, electric energy has attracted a great deal of attention due to its prolonged lifespan and intense power density.6–9 Being novel energy storage devices, the energy densities of supercapacitors (SCs) are between those of conventional batteries and traditional capacitors, exhibiting a higher specific capacity as well as a higher specific energy than traditional capacitors.10–12 Meanwhile, SCs show better power efficiency and much faster charge/discharge characteristics than batteries.13–21 SCs also have a large power density, long cycle life and relatively high energy density.22,23 With the development of hybrid cars and electric vehicles, SCs have been receiving more and more attention.24,25 It is necessary to improve the energy density of supercapacitors.26,27 The main approaches include the use of pseudocapacitive materials28,29 and high-voltage electrolytes (molten and organic electrolytes, lithium salts, and ionic liquids)30–35 as well as designing different materials for specific needs.36,37 Recent research studies showed that combining the battery-type and capacitor-type electrodes can improve the capacitance and voltage of SCs.38–42SCs can be categorized into pseudocapacitors (PCs) and electrochemical double-layer capacitors (EDLCs). The differences mainly lie in the charge storage mechanisms; EDLCs use a carbon-based electrode material, which is not electrochemically active;43 that is, no electrochemical reactions occur on the electrode materials while charging, but rather only the pure physical charges build up at the electrode/electrolyte interfaces.44–46 Meanwhile, the electrode materials in pseudocapacitors are electrochemically active.47–49 Typical pseudocapacitive materials include transition metal oxides50–52 and conductive polymers which display a much higher specific capacitance than carbon materials for EDLCs.53–56 As mentioned above, both rechargeable batteries and pseudocapacitors store charges based on redox reactions in the electrode-active materials.57–59 It is common knowledge that the energy density of a SC is decided by the particular capacitance of the electrode materials as well as the overall cell voltage. Accordingly, one efficient method is to develop porous and nano-sized electrode materials for improving capacitance. Another approach is to build hybrid/asymmetric SCs, which can efficiently utilize the potential gap between the two types of electrodes to increase the overall cell voltage; for example, the negative electrode is made of a porous active carbon material, meanwhile, the positive electrode material is made of a pseudocapacitive material.60,61
A nanoporous carbon electrode is an ideal EDLC electrode material, owing to its high conductivity, large surface area, and controlled pore structure.62–64 Despite possessing large specific surface areas, the charges are mostly stored physically on the surface.65 This results in a porous carbon electrode having a lower specific capacitance and energy density. Compared to carbon, pseudocapacitive electrode materials, like conducting polymers and transition metal oxides, have been much more widely investigated recently.66 Among the transition metal oxides, cobalt oxide (Co3O4), ruthenium oxide (RuO2), and nickel oxide (NiO) have traditionally been widely studied.67,68 A RuO2-type electrode involves fast reactions of Faradaic charge-transfer. Meanwhile, its cyclic voltammetry (CV) shape is very broad, showing the form of quasi-rectangular.69,70 These reversible and fast Faradaic reactions with its large surface area, fast proton transport, and intense conductivity result in high capacitance.71 However, the high cost of RuO2 hinders its large-scale application. Hence, important efforts have been made to find promising alternatives to RuO2. For example, cobalt oxide and nickel oxide are considered ideal competitors to RuO2. Moreover, such materials could withstand great current charge/discharge. Meanwhile, the redox actions of cobalt and nickel contribute to the energy storage.72,73
Ni and Co are widely distributed as well as earth-abundant (ranked fifteenth and nineteenth on the earth as shown in Fig. 1a). In the total reserve of nickel resources in the world, laterite nickel ore accounts for about 55% while nickel sulfide ore and ferromanganese nodules account for 28% and 17%, respectively. Moreover, there are hundreds of kinds of minerals containing cobalt existing in nature; most of them exist in the form of linnaeitet (Co3S4), carrolite (CuCo2S4), cobalt glance (CoAsS), smaltite (CoAs2), erythrite (3CoO·As2O5·8H2O) etc.74–80 Although Ni and Co are abundant compared with other metals, their exploitation and utilization by humans are very limited, especially for Co, as shown in Fig. 1b. The main reason is that with the evolution of the Earth, gaseous elements were lost gradually, and the fusible alkali metals and aluminum silicate were enriched on the Earth’s surface.81 Co, Ni, Fe, Cu and other metal elements are abundant in argillaceous sediments and deep sea sediments.82–84 The average content level of Co in the crust is 0.001% (quality), while the total amount of cobalt in the ocean is about 2.3 billion tons. Above all, Co and Ni are promising materials for high performance energy storage and conversion devices; more importantly, they have huge reserves yet to be systematically exploited and rationally utilized.85–88 At present, Co/Ni based hydroxides, metal oxides as well as their composites act as substitutes for those rarely available noble metals (Ru, Pt) for energy storage18–23 and have received extensive research attention.28–32 It becomes apparent that Co/Ni based materials have great prospects for deep study and exploration.
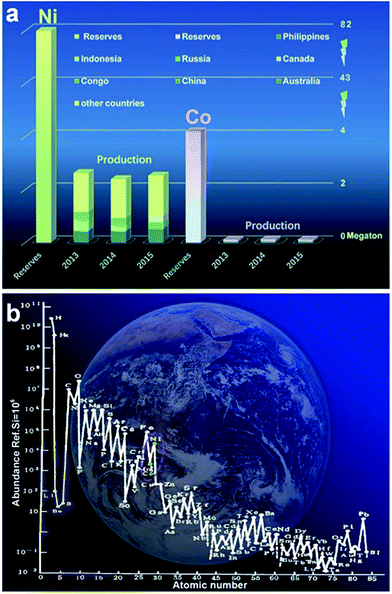 | ||
| Fig. 1 (a) The reserves of Ni and Co and their production in 2013–2015; (b) the content with abundance ref. Si = 106 atoms of atomic numbers 1–85.62 (Ref. 73https://www.usgs.gov/) | ||
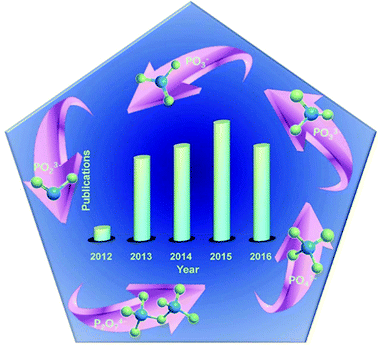
Meanwhile, metal phosphates, particularly the layered metal phosphonates and phosphates, have gained much attention from the research community from 1987 to 1990,89 and show outstanding performance in catalysis, ion exchange, proton conductivity, interface chemistry, photochemistry, and materials science.90–100 Carling and Yuan et al. obtained NH4MIIPO4·H2O (MII = Mn, Fe, Co, and Ni) by precipitation from aqueous solution.101,102 A successful preparation of metal ammonium phosphate microspheres was done by Zhang et al.103 Li et al. prepared amorphous colloidal spheres based on transition-metal phosphates.92 Besides, transition metal phosphates like nickel/cobalt phosphates can act as potential anodes for sorbents, magnetism materials, rechargeable batteries, ion exchangers and heterogeneous catalysts. Meanwhile, PO4 polyhedra with a zeolite structure have outstanding electronic and magnetic properties.104–108 Hence, these materials have attracted great attention. Fig. 2 illustrates that phosphates have been employed as SCs since 2012.109 It is clear that increasing attention has been paid to metal phosphate in SCs since 2012.110–113
In this review, progress with respect to various morphologies of ammonium cobalt (nickel) phosphates, cobalt phosphates, cobalt/nickel phosphates and nickel phosphates is discussed mainly based on previously published papers (2011–2017). Furthermore, SC performances of metal phosphate materials have been tested in a three-electrode system, a two-electrode and asymmetry solid flexible electrode are introduced and compared. The disadvantages of SCs based on phosphate materials are also discussed. Due to their high electrochemical performance, high natural abundance, environment friendliness and low cost, metal phosphate materials are highly promising for SC applications.
2. Cobalt/nickel based phosphate materials
2.1. Ammonium cobalt/nickel phosphates
In recent decades, ammonium metal phosphates have been investigated extensively and employed as pigment protection for paint finishes of metals, fertilizers, and plastics’ or paints’ fire retardants.114 The NH4+ cation inserts between the sheets and interconnects the layers by hydrogen bonds. Such material systems are quite appropriate for discovering the effect of framing, refining mixed transition metals as well as determining their synergetic effects. Nickel(II) phosphates possess numerous varied structures that can increase their ionic conductivity and ion exchange rate.115,116 Nonetheless, there are barely any reports on the electrochemical properties of these materials, not to mention those for electrochemical capacitors. Generally, the pseudocapacitance of the battery type material results from the surface redox reaction, which depends heavily on the electrochemical active surface area of electrode materials. Therefore, ultrathin layered electrode materials with multiple functional atoms exposed to the electrolyte can create sufficient electroactive sites, which unfortunately suffer from poor crystallinity with sacrificed electric conductivity and chemical stability.For the past few years, our group has been devoted to developing nanomaterials for energy storage and conversion devices such as SCs, oxygen evolution reaction catalysts, Li-ion batteries, oxygen reduction reactions, etc. We were the first to study metal phosphate based materials for high-performance supercapacitors. Various NH4CoPO4·H2O nano/microstructures were synthesized by means of facile chemical precipitation without the use of templates and surfactants.109 Varied sources of ammonium solvents were used to synthesize various NH4CoPO4·H2O nano/micromaterials and the obtained samples show different morphologies such as microflowers, microplates, hierarchical architectures, and oblong plates. When pure water was used as a solvent, we obtained hierarchically architectured NH4CoPO4·H2O with the size of 20–30 mm (denoted M5), as shown in the insert of Fig. 3f. Moreover, the supercapacitive performances of NH4CoPO4·H2O nano/microstructures were also examined via CV curves, electrochemical impedance spectroscopy methods as well as galvanostatic charge–discharge measurements in 3.0 M KOH aqueous solution. It was found that NH4CoPO4·H2O hierarchical architecture (M5) electrodes show outstanding supercapacitive characteristics: the specific capacitance of the NH4CoPO4·H2O electrode (M1–M5) as measured from CV curves (Fig. 3f) at a scan rate of 5 mV s−1 achieved 550 F g−1, 418.2 F g−1, 650 F g−1, 365.7 F g−1 and 1142.9 F g−1, respectively. Moreover, the M5 electrode kept a capacitance of 711.6 F g−1 when the scan rate reached 100 mV s−1. The measurement of electrochemical properties of nano/microstructured NH4CoPO4·H2O demonstrates that NH4CoPO4·H2O materials can be utilized as excellent electroactive materials for SCs.
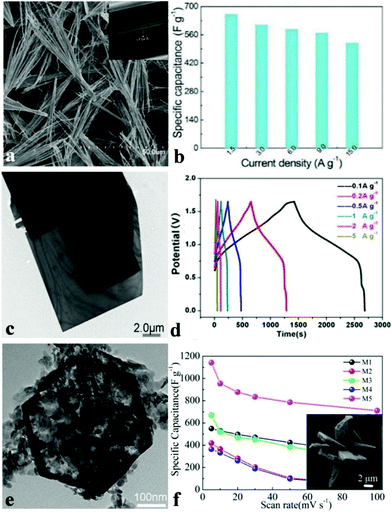 | ||
| Fig. 3 (a) SEM and TEM images of layered NH4CoPO4·H2O microbundles; (b) specific capacitances of layered NH4CoPO4·H2O microbundle electrodes derived from the discharging curves at a current density of 1.5–15.0 A g−1 in 3.0 M KOH electrolytes; (c) TEM images of large rectangular platelets; (d) galvanostatic charge/discharge (GCD) plots at different current densities of NH4CoNiP//HPC asymmetric SC; (e) TEM images of the hexagonal structure; (f) SEM images of the M5 sample, and specific capacitances derived from CV tests. Adapted with permission from (a, b) ref. 100, © 2014 The Royal Society of Chemistry; (c–e) ref. 117, © 2016 Nature; (f) ref. 109, © 2012 Royal Society of Chemistry. | ||
Other than 3D metal phosphate, 1D metal phosphates have also been studied as electrode materials for SCs. Wang et al. synthesized layered NH4CoPO4·H2O microbundles consisting of 1D layered microrods through a facile hydrothermal method.100 The novel layered structures (Fig. 3a) possess channels intended for the diffusion of electrolytes and ions. Besides, electrolyte access and ion intercalation/extraction into/out can also be promoted. The preliminary SC investigation shown in Fig. 3b indicates that the specific capacitance of the layered NH4CoPO4·H2O microbundle electrodes is 662 F g−1 at 1.5 A g−1 and 520 F g−1 even at 15.0 A g−1. The layered NH4CoPO4·H2O microbundle electrodes also exhibited an excellent cycling performance (92.7% of the initial specific capacitance after 3000 cycles). This is an excellent example showing that the definite control of the morphology of nanomaterials influences the electrochemical performance.
Zhao et al. prepared a novel hybridized phosphate via a mild hydrothermal method in order to fabricate asymmetric SCs.117 Single layered (Ni,Co)3(PO4)2·8H2O nanoslices (Fig. 3e) and single crystal (NH4)(Ni,Co)PO4·0.67H2O microplatelets (Fig. 3c) were formed through a sacrificial template approach and a dissolution recrystallization method, respectively. When employed as asymmetric SCs with hierarchical porous carbon, excellent SC performances were obtained. Fig. 3d shows the GCD curves of the asymmetric SC cell at varying densities of current in a voltage window which ranged from 0 to 1.65 V with a closely symmetrical shape, indicating good electrochemical SC characteristics. The specific capacitance could be up to 1128 F g−1 at a current density of 0.5 A g−1. The asymmetric hybrid SC showed a high energy density of 35.3 W h kg−1 at relatively low power density, but still it kept up to 30.9 W h kg−1 at 4400 W kg−1. Such an excellent electrochemical performance is explained by the synergistic effect of the surface redox reaction from ion intercalation and ultrathin nanoslices obtained from the single crystal bulk structure.
Wang et al. synthesized self-organized NH4CoPO4·H2O microflowers through a mild chemical precipitation method.111 The supercapacitive performances of NH4CoPO4·H2O microflowers (Fig. 4a and b) and porous Co2P2O7 (Fig. 4c–e) were studied schematically in 1.0 M KOH, NaOH and LiOH electrolytes. Results show that the crystallinity of these materials significantly affects the electrochemical activity of SCs in various electrolytes. Supercapacitive characteristics of NH4CoPO4·H2O microflowers in 1.0 M KOH electrolyte were better than others (Fig. 4f and g). The specific capacitance of NH4CoPO4·H2O microflower electrodes at a current density of 0.625 A g−1 was about 525 F g−1. It also showed a prolonged cycle life which remained 99.4% after 400 cycles in 1.0 M KOH.
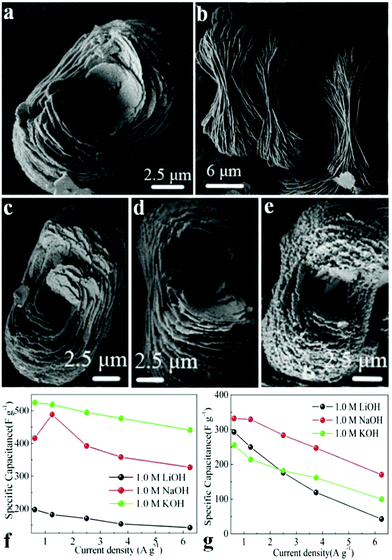 | ||
| Fig. 4 (a, b) SEM images of NH4CoPO4·H2O microflowers (M); (c–e) SEM images of Co2P2O7 samples: (c) P1; (d) P2; (e) P3. Specific capacitances of electrode materials at different scan rates derived from GCD in 1.0 M LiOH, NaOH and KOH aqueous solutions: (f) M; (g) P1; adapted with permission from ref. 111, © 2013 Springer. | ||
Pang et al. obtained porous cobalt pyrophosphate (Co2P2O7) nano/microstructures (microflower, oblong plates, hierarchical architectures, and microplates) via calcination of NH4CoPO4·H2O nano/microstructures (Fig. 5a).118 The NH4CoPO4·H2O nano/microstructures were well preserved after the thermal treatment where NH4CoPO4·H2O was transformed into Co2P2O7 (Fig. 5b). Besides, the supercapacitive performances of porous Co2P2O7 nano/microstructures were studied in 3.0 M KOH solution as shown in Fig. 5c. Different nano/micro structured Co2P2O7 samples have the possibility to have different surface-interface conditions, which assumes a pivotal function in ion intercalation/extraction into/out as well as electrolyte access. The charge–discharge curves of these electrodes with different morphologies at 0.625 Ag−1 are shown in Fig. 5c. The symmetry of charging/discharging cycling curves shows that the Co2P2O7 electrode material possesses perfect electrochemical capacitive characteristics indicative of reversible redox reactions. Compared to others, Co2P2O7 hierarchical architectured electrodes (denoted P5) at 0.625 A g−1 have longer charge–discharge times. P5 exhibited a high specific capacitance of 367 F g−1 at a current density of 0.625 A g−1 in aqueous KOH solution with good cycling stability.
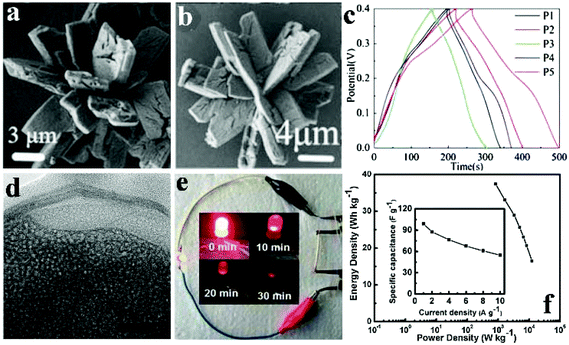 | ||
Fig. 5 (a) SEM images of NH4CoPO4·H2O nano/micromaterials (M1); (b) SEM images of the Co2P2O7 samples (P5); (c) GCD curves of Co2P2O7 electrode materials (P1–P5) at 0.625 Ag−1; (d) TEM image of the sample Co/Ni 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3; (e) a photograph of a red LED lighted up by two cells; (f) corresponding Ragone plot, inset showing the specific capacitance dependent on current densities. Adapted with permission from (a–c) ref. 118, © 2013 Springer; (d–f) ref. 112, © 2016 Springer. 3; (e) a photograph of a red LED lighted up by two cells; (f) corresponding Ragone plot, inset showing the specific capacitance dependent on current densities. Adapted with permission from (a–c) ref. 118, © 2013 Springer; (d–f) ref. 112, © 2016 Springer. | ||
The synergetic effects between mixed transition metal compounds have been proved to have great potential in improving the electrochemical performance of electrode materials. Xiao's group synthesized ammonium/bimetallic phosphates (NH4CoxNi1−xPO4·H2O) (Fig. 5d) with layered nanostructures by a facile one-step solvothermal method.112 They found that the synergistic effect between cobalt and nickel can significantly influence the crystal structure and morphology of NH4CoxNi1−xPO4·H2O, compared with the single-component ammonium phosphates. As shown in Fig. 5f, the asymmetric device based on it presented a high energy density of 37.4 W h kg−1 at a power density of 826 W kg−1. Moreover, the insert of Fig. 5f indicated that the device also exhibited a good long-term cycling stability with 91.2% capacity retention even after 5000 cycles. Moreover, after charging each cell for 15 s, a light-emitting diode (LED, 2.0 V, 20 mA) can be illuminated for nearly 30 min, as shown in Fig. 5e.
As opposed to the conventional all-carbon supercapacitors (EDLC or electric double layer capacitors) which possess high power but lack energy density, pseudocapacitors embank on the high energy density innate to reversible redox reactions and simultaneously offer a facile way to improve the supercapacitors’ energy rate. Raju et al. reported novel NH4NiPO4·H2O with unique morphologies (microplatelets in Fig. 6a denoted ANPmp, microrods in Fig. 6b denoted ANPmr, and microdendrites in Fig. 6c denoted ANPmd) with different supercapacitive properties.119 The ammonium nickel phosphate hydrate with 1D microrod structures sized about 36 nm gave extraordinarily high specific capacitance, power and energy densities in half-cell and full-cell configurations (i.e., symmetric and asymmetric cells, including all-solid-state flexible pseudocapacitors) in different electrolytes. The versatile all-solid-state ANPmr-based pseudocapacitor exhibited high power densities (12.7 mW cm−2) and areal capacitance up to 66 mF cm−2 with excellent energy (21.2 mW h cm−2) as shown in Fig. 6d. The study proves that rod-like morphology (with ∼36 nm width) provides a significant and promising direction for novel 1-D and 2-D materials to obtain high-performance pseudocapacitors, especially for flexible and wearable electronics.
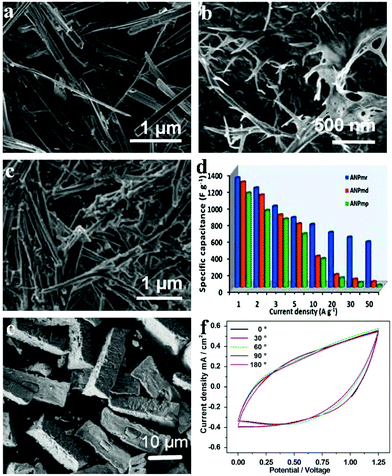 | ||
| Fig. 6 SEM micrograph of as-synthesized various morphologies of (a) ANPmp (microplatelets), (b) ANPmr (microrods) and (c) ANPmd (microdendrites), respectively; (d) specific capacitance vs. current density, i.e., rate capability test; (e) SEM images of as-prepared samples P1; (f) CV curves at a scan rate of 30 mV s−1 with various bending angles (ranging from 0° to 180°) of the P1//graphene device; adapted with permission from (a–d) ref. 119, © 2015 Nature; (e, f) ref. 120 © 2015 Elsevier Ltd. | ||
Other than traditional SCs that are bulky, sometimes environment-unfriendly (mainly owing to the organic electrolytes) and rigid, flexible solid-state electrochemical energy storage devices based on phosphates have also been built. Our group built an asymmetric SC based on amorphous nickel pyrophosphate (Ni2P2O7) microstructures and graphene nanosheets as positive and negative electrode materials, respectively.120 The amorphous Ni2P2O7 microstructures (as shown in Fig. 6e) were obtained by calcination of the ammonium nickel phosphate hydrate microstructures in air at 350 °C. The flexible all-solid-state electrochemical energy storage device showed a maximum volumetric energy density of 0.78 mW h cm−3 as well as good mechanical flexibility under a bending angle of 0–180°. The electrochemical properties of the as-prepared device were measured when it was bent with different angles (0° to 180°) to test its flexibility (Fig. 6f). The shapes of the CV curves under different bending angles matched well with each other for extended bending cycles. Due to the advantages of the high energy density and flexibility originating from both the device architecture and the materials, various applications are anticipated by exploiting it in this work.
Zhao et al. fabricated uniform mesoporous ammonium nickel phosphate hydrate (Fig. 7a) nanostructures via a one-pot hydrothermal method.121 Owing to the distinct characteristics of a large surface area (418 m2 g−1) and controllable mesostructures, such a material showed an extraordinary performance-specific capacitance of 1072 F g−1 (at 1.50 A g−1) in 3.0 M KOH. As shown in the insert of Fig. 7b, the material exhibited a long lifespan, which hardly decayed after 3000 cycles with a retention of 95.0% of the initial specific capacitance.
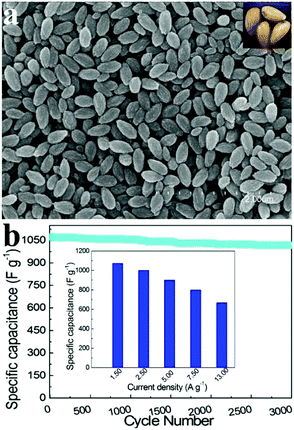 | ||
| Fig. 7 (a) SEM images of the as-prepared samples; in the inset: the optical photo of almonds; (b) specific capacitances of mesoporous NH4NiPO4·H2O nanostructure electrodes derived from the discharging curves at a current density of 1.5–13.0 A g−1 in 3.0 M KOH electrolytes. Adapted with permission from ref. 121 © 2013 The Royal Society of Chemistry. | ||
2.2. Cobalt phosphate
Co based materials have gained interest and attention because of the lower cost; however, certain characteristics of Co-based materials such as the small surface area and poor conductivity limit the cycling performances and rate capability of Co-based SCs. Some porous/nanostructured Co-based materials have been designed and synthesized in such a way that they enhance the electrocatalytic performances, supply effective ion diffusion channels, and increase both electron migration and diffusion. Xie's group successfully synthesized hierarchical 3D cobalt phosphate hydrate (Co3(PO4)2·8H2O) architectures with flower-like morphologies built from 2-dimensional microsheets ranging from 5 to 12 μm at room temperature via a green precipitate process (free of surfactant).122 The SEM images of the as-prepared products (denoted P2) are shown in Fig. 8a. TEM images show the detailed and comprehensive structure of Co3(PO4)2·8H2O (Fig. 8b), indicating that the 3D architecture was assembled from plenty of interconnecting 2D microsheets. The architecture of as-prepared Co3(PO4)2·8H2O provided large nanoscale pore channels to improve ion-transportation. Moreover, excellent rate capability (227 F g−1 at 10 A g−1) and cycling stability (please see Fig. 8c for the capacitance increase to a retention of 102% after 1000 cycles) were observed. This material holds great promise for commercial applications due to the high yield, low cost, and outstanding capacitive behavior. Meanwhile, this synthetic method could be applied in the preparation of other metal phosphates.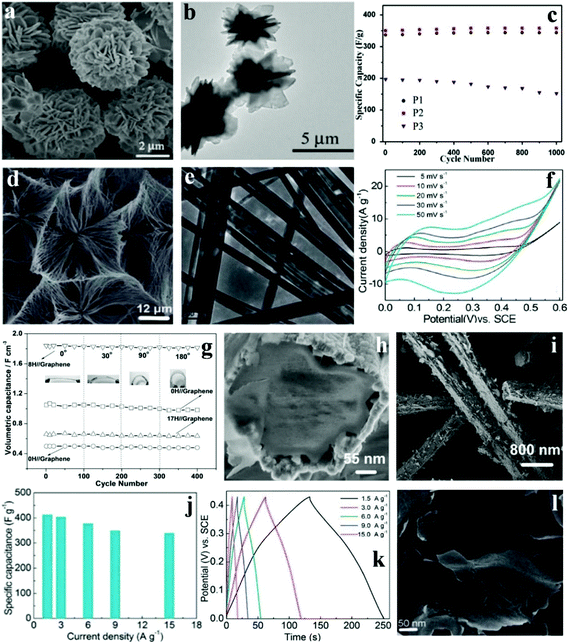 | ||
| Fig. 8 SEM (a) and TEM (b) images of Co3(PO4)2·8H2O (P2); (c) cycling stability of the Co3(PO4)2·8H2O materials at 1 A g−1; SEM (d) and TEM (e) images of Co11(HPO3)8(OH)6; (f) CV curves of Co11(HPO3)8(OH)6 microarchitecture electrodes at different scan rates; (g) specific capacitance over 400 cycles under the curvature of 0°, 30°, 90°, and 180° at a current density of 0.5 mA cm−2; (h, i) SEM images of the as-prepared core–shell Co11(HPO3)8(OH)6–Co3O4 nanohybrids obtained after 8 h under hydrothermal conditions; (j) specific capacitances of CoHPO4·3H2O ultrathin nanosheet electrodes derived from the discharging curves; (k) at the current density of 1.5–15.0 A g−1 in 3.0 M KOH electrolytes; (l) SEM image of the as-prepared CoHPO4·3H2O; Adapted with permission from (a–c) ref. 122, © 2015 Elsevier; (d–f) ref. 108 (g–i) ref. 114, © 2013 The Royal Society of Chemistry; (j–l) ref. 123 © 2015 Elsevier. | ||
Pang et al. synthesized cobalt phosphate (Co11(HPO3)8(OH)6) microarchitectures built from super long nanoribbons under mild hydrothermal conditions without additives.108 The uniform ultralong nanoribbon was sized around 100 nm in width and about 20–30 mm in length, as shown in Fig. 8d and e. Fig. 8f shows the CV curves of the Co11(HPO3)8(OH)6 microarchitecture at varying scan rates. The range is 0 to 0.60 V (vs. SCE) in 3.0 M KOH aqueous solution. It can be clearly seen that the Faradaic pseudocapacitive property of the Co11(HPO3)8(OH)6 microarchitecture is based on the surface redox mechanism of Co2+ to Co3+. In addition, the as-prepared materials were also successfully applied as a novel electrochemical SC possessing excellent specific capacitance (312 F g−1 at 1.25 A g−1), rate capability as well as cycling property (maintaining about 89.4% at 1.25 A g−1 after 3000 cycles). Nonetheless, unlike other cobalt based SC electrode materials, the specific capacitance and rate performance of this Co11(HPO3)8(OH)6 microarchitecture need improving.
Zhang et al. fabricated a flexible solid-state asymmetric SC with graphene nanosheets as the cathode, KOH-polyvinyl alcohol (PVA) gel as the solid state electrolyte and core–shell structured cobalt phosphate–cobalt tetroxide [Co11(HPO3)8(OH)6–Co3O4] hybrids as the anode.123 They developed a facile hydrothermal technique to fabricate core–shell structured Co11(HPO3)8(OH)6–Co3O4 hybrids, with the microporous Co11(HPO3)8(OH)6 microrods as the precursor. As shown in Fig. 8h and i, the 1D structure had a diameter of 380 nm. Moreover, the shell was made of the oxidation layers and the core was made of Co11(HPO3)8(OH)6 microrods. The shell was not close to the core so that efficient nanochannels were formed for electrolytes and ion diffusion. These as-prepared core–shell Co11(HPO3)8(OH)6–Co3O4 hybrids were further used for flexible solid-state asymmetric SCs. In order to test the influence of mechanical action on electrochemical performance, the device was bent with different angles (0°, 30°, 90°, and 180°, Fig. 8g) with the corresponding electrochemical tests conducted at the same time. The result showed that the specific capacitance changed slightly at different bending angles. Conspicuously, the specific capacitances of all devices remained during the bending processes. However, after 3000 cycles, the devices’ stable cycling performance was relatively different. It is worth noting that the specific capacitance of the 8H//graphene devices remained at 98.7% of the initial value after 3000 cycles.
Since the discovery of graphene, 2D layered materials (e.g., 2D compounds, metal chalcogenides, and transition metal oxides) have received renewed attention. Such materials exhibit interesting properties including thermoelectricity, topological insulator effects, and superconductivity; they even cover a relatively wide spectrum of electronic structures, from metals to insulators. Pang et al. synthesized ultrathin cobalt phosphate CoHPO4·3H2O nanosheets through a one-step hydrothermal process without any exfoliation.114 The CoHPO4·3H2O ultrathin nanosheets display a novel layered surface–interface structure (Fig. 8l), which is vital for electrolyte-access and ion-intercalation/extraction into/out. More importantly, novel CoHPO4·3H2O super-thin nanosheets were effectively gathered and integrated to construct the electrodes for SCs. The super thin CoHPO4·3H2O nanosheet electrodes possess a huge specific capacitance (Fig. 8j), which reached up to 413 F g−1 at a current density of 1.5 A g−1, and 338 F g−1 even at 15.0 A g−1. What's more, there was no apparent degradation even after 3000 charge–discharge cycles. The measurement of the electrochemical properties of CoHPO4·3H2O ultrathin nanosheets successfully illustrates that it was a promising SC electrode material; furthermore, the nano/microstructured materials’ chemical and physical properties as well as electrochemical performance can be tuned via accurate control of the morphology of materials.
Traditionally, micro-supercapacitors are fabricated via an expensive and extensive lithographic approach. Alternatively, additive fabrication approaches such as inkjet printing offer a simple and versatile route to make complicated planar electrode structures that is crucial for their mass production and integration into larger systems of micro-supercapacitors. Compared with other printing methods such as screen printing, inkjet printing has advantages in versatility and cost and can be easily integrated into a roll-to-roll mass production system. Pang et al. fabricated a flexible all-solid-state asymmetric micro-SC based on lamellar potassium cobalt phosphate hydrate (K2Co3(P2O7)2·2H2O) nanocrystal whiskers (anode electrode) and graphene nanosheets (cathode electrode) using a solid state electrolyte [KOH-polyvinyl alcohol (PVA) gel].113 They provided a facile method to synthesize lamellar K2Co3(P2O7)2·2H2O nanocrystal whiskers under mild hydrothermal conditions. A low-magnification SEM image in Fig. 9c reflects the morphology of as-prepared samples: nanowhiskers which are 250–400 nm in length. The flexible solid-state asymmetric micro-SC (denoted a micro-device) was fabricated via an inkjet printing method, and the structure scheme of the device is illustrated in Fig. 9a. Silver ink was printed on the PET substrate, functioning as the current collector for the active materials (K2Co3(P2O7)2·2H2O and graphene). Due to the highly inter-connected structure of the lamellar K2Co3(P2O7)2·2H2O nanocrystal whiskers, these nanomaterials exhibited outstanding mechanical and electrical robustness, which enabled the device to function well under highly bent conditions. Fig. 9b shows the cycling performances of the lamellar K2Co3(P2O7)2·2H2O nanocrystal whisker electrode at disparate current densities (5.0, 3.0, 2.0, 1.5 and 1.0 mA cm−2). It is seen that the capacity changed from 1100 mF cm−2 to 810 mF cm−2 as the current density increased from 1.0 mA cm−2 to 5.0 mA cm−2. What's more, the cycle remained stable at each current density.
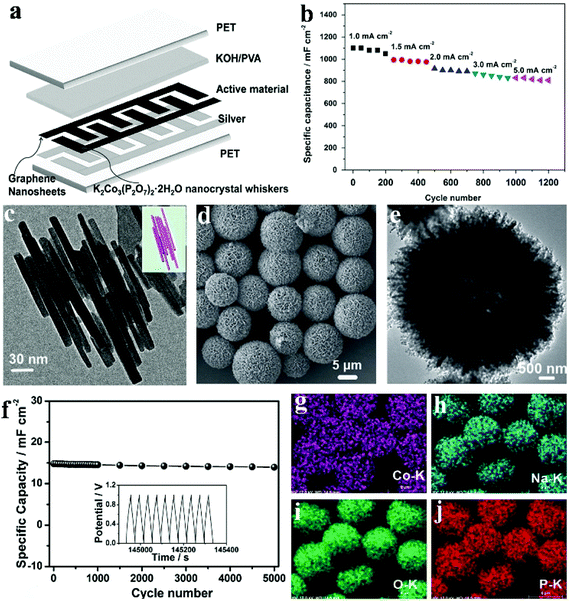 | ||
| Fig. 9 (a) Schematic view of the printed flexible micro-device; (b) in a conventional three-electrode system, a charge/discharge cycling test at different current densities from 1.0 to 5.0 mA cm−2; (c) TEM image, and the model image of overlapping K2Co3(P2O7)2·2H2O nanocrystal whiskers (inset); (d, e) FESEM and TEM images of the as-prepared hierarchically porous NaCoPO4–Co3O4 hollow microspheres; (f) cycling performances of the device at a current density of 1.0 mA cm−2 for 5000 cycles (the insert picture shows the last ten cycles); EDX-elemental mapping images (g–j) of the hierarchically porous NaCoPO4–Co3O4 hollow microspheres. Adapted with permission from (a–c) ref. 113, © 2015 Elsevier; (d–j) ref. 124, © 2015 WILEY-VCH. | ||
Considerable effort has been devoted to developing methods for the preparation of hollow structured materials. Template-based methods are the most popular. Wei et al. developed a facile hydrothermal process to fabricate hierarchically hollow precursors with nanoplates acting as building blocks.124 After a calcination process, hierarchically porous NaCoPO4–Co3O4 hollow microsphere materials were obtained. Fig. 9d and e show the morphology of the as-obtained NaCoPO4–Co3O4; the SEM images demonstrate that the product was made of microsphere particles sized from 5 to 8 μm, indicating that it inherited the morphology of its precursor. The hollow structure can be confirmed by the TEM image in Fig. 9e. The distinct contrast between the black and white ligaments also exhibits nonporous characteristics. The elemental mapping results (Fig. 9g–j) further affirm the even distribution of elements Co, Na, O and P within the hierarchical microspheres. More importantly, the cycling stability of the device was examined by means of a cycling charge/discharge process with a current density of 1.0 mA cm−2 (Fig. 9f). Following 5000 cycles (the insert of Fig. 9f shows the last ten cycles), it retains 94.5% of its initial capacitance, revealing excellent cycling performance. The 5.5% capacitance decrease of the device can be attributed to the consumption of the gel electrolyte via an irreversible electrolyte–electrode material reaction. It is believed that these active NaCoPO4–Co3O4 materials with unique structures can possibly act as potential alternatives for many other applications like lithium-ion batteries, catalysis, and sensors.
2.3. Nickel phosphate
Different calcination temperatures can affect the structural, morphological and capacitance characteristics of materials as electrode materials for SC applications.125 Omar et al. synthesized Ni3(PO4)2 particles using a chemical method to agitate the precursor solution avoiding particle aggregation during the chemical precipitation and followed by calcination at different temperatures.126 The synthesized Ni3(PO4)2 at different calcination temperatures (300, 600 and 900 °C) are represented as N300, N600 and N900, respectively. The SEM images (Fig. 10a) revealed that before the calcination, the particles displayed irregular-shape. However, the particles were not well separated with a high degree of agglomeration. The surface morphologies showed that the particle size increased with increasing the calcination temperatures. The electrochemical performances of N300, N600 and N900 were investigated using CV and GCD in a 1 M KOH electrolyte. It was found in Fig. 10b that N300 exhibited a maximum specific capacity of 620 C g−1 at 0.4 A g−1, which was significantly higher than N600 (46 C g−1) and N900 (14 C g−1). Additionally, the fabricated N300//activated carbon based asymmetric SC can be cycled reversibly at a cell voltage of 1.45 V. The device exhibited 76 W h kg−1 energy density and 599 W kg−1 power density with 88.5% capacitance retention after 3000 cycles.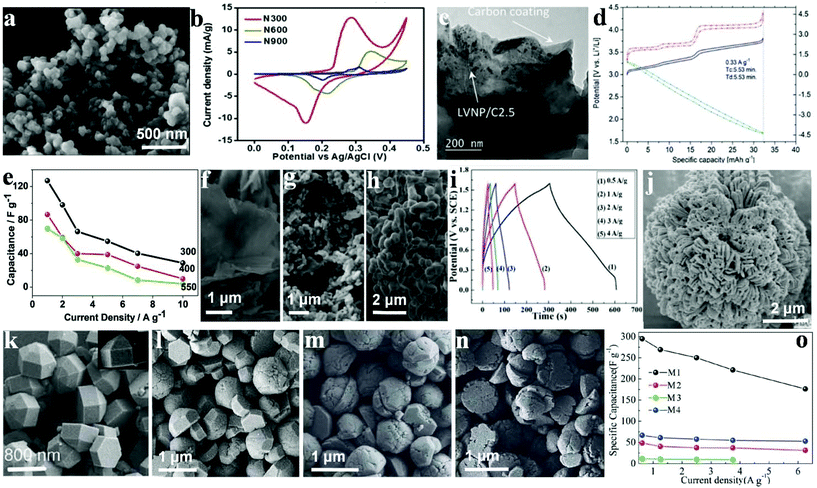 | ||
| Fig. 10 (a) FESEM image of N300; (b) cyclic voltammogram of N300, N600 and N900 at a scan rate of 1 mV s−1; (c) TEM images of LVNP/C2.5; (d) cathode, anode and cell profiles at 0.33 A g−1 (Tc ¼ charge time and Td ¼ discharge time; (e) specific capacitance as a function of current density; field emission scanning electron microscope images of NaNiPO4 powders (f–h) synthesized at 300 400 and 550 °C, respectively; (i) charge–discharge curves of the Fe3P2O8·8H2O//Ni3P2O8 asymmetric cell; (j) SEM images of Ni3P2O8; (k–n) SEM images of M1, M2, M3, and M4, respectively; (o) specific capacitances derived from CV tests. Adapted with permission from (a, b) ref. 126, © 2016 The Royal Society of Chemistry; (c, d) ref. 127, © 2015 The Royal Society of Chemistry; (e–h) ref. 128, ©; (i, j) ref. 129, © 2016 The Royal Society of Chemistry; (k–o) ref. 130, © 2013 Wiley-VCH. | ||
Nonetheless, LVP is quite a promising material for batteries for SC applications, and this is largely because of its high cycling stability and rate capability. The electrochemical performance of pure LVP/C has been effectively improved by Ni-doping. The advantages of introducing Ni in the LVP crystal structure include the following: a higher Li-ion diffusion capacity, a lower charge-transfer resistance on the electrolyte/electrode interface, and a decreased inorganic particle dimension. Secchiaroli et al. successfully synthesized Li3V2−xNix(PO4)3/C (x = 0, 0.05, and 0.1) nanomaterial, and the Li3V2−xNix(PO4)3 nanoparticle, both of which were well spread and integrated into the as-prepared carbon matrix with a mean particle dimension between 63 and 200 nm.127 The carbon matrix was fabricated by decomposition of poly(acrylic acid) (PAA) and glucose, Fig. 10c shows the TEM image of the LVNP/C2.5, agglomerates of merged particles form the powder. The average particle dimension of LVP/C2.5 is about 300 nm. Ni metal doping has the ability to enhance the energy density of the pure LVP/C as shown for LVNP/C2.5, thus demonstrating the highest specific capacity among the three cathodes at the current density range from 0.033 A g−1 to 0.33 A g−1 (Fig. 10d). The system retained 99% of the initial specific capacity measured at 0.033 A g−1 with 93 mA h g−1 at 100 °C as well as a capacity retention of 97% after 1000 cycles at 100 °C.
Transition metal phosphates such as lithium and oxides have been studied for more than two decades. Minakshi et al. synthesized sodium nickel phosphate (NaNiPO4) and measured the difference of products synthesized at different temperatures.128 Both the morphology and structures of the polymorphs were characterized (Fig. 10f–h). According to XRD, NaNiPO4 was in two varied forms (maricite and triphylite), which was determined by the synthetic temperature (300–500 °C). The triphylite form of NaNiPO4 and the as-prepared form together with activated carbon in 2 M NaOH demonstrate a maximum specific capacitance of 85 F g−1 at 1 A g−1 and 125 F g−1, respectively, as shown in Fig. 10e. Moreover, they both reveal remarkable cycling stability with a retention of about 99% of the initial capacity for more than 2000 cycles. Nonetheless, the blocky and coarse maricite form exhibited a lower capacitance of 70 F g−1 with a 40% decrease in capacity after 50 cycles. Such a low performance can be attributed to a reduction in the accessible surface area, which hindered the penetration of the electrolyte. These findings indicate that the as-prepared triphylite revealed a 44 W h kg−1 energy density which is considered a high performance and could be attributed to the hierarchical structure of the porous NaNiPO4 nanosheets. The electrochemical performance of this new promising NaNiPO4-based capacitor is better than that of an oxide or lithium-based phosphate electrode supercapaictor.
Liu et al. synthesized amorphous Ni3P2O8via a facile chemical precipitation technique which acted as an anode electrode material for SCs; they also synthesized Fe3P2O8·8H2O using the same method to replace active carbon material.129 The SEM images of Ni3P2O8 and electrode materials are illustrated in Fig. 10j. It can be seen that these nanoflowers, which are sized about 10 μm in length, were composed of dozens of small individual petals with some degree of agglomeration. The flower-like structure was able to increase the surface area of Ni3P2O8, which brought about a large Cm and excellent rate capability. The novel Ni3P2O8 and Fe3P2O8·8H2O exhibited high specific capacitance as electrode materials for asymmetric SC with Ni3P2O8 as the positive electrode and Fe3P2O8·8H2O as the negative one (see Fig. 10i). A specific capacitance of up to 94 F g−1 was obtained in a high-voltage region of 0–1.6 V. Besides, a large energy density (32.6 W h kg−1) was delivered at 420 W kg−1 power density. The results indicate promising prospects of metal phosphate-based materials for SCs. Such an impressive Ni3P2O8/Fe3P2O8·8H2O-based hybrid SC as well as electrode materials were expected to show good prospects for high performance energy storage systems.
Nickel phosphite (Ni11(PO3)8(OH)6) hexagonal polyhedrons were effectively synthesized by Pang's group under hydrothermal conditions.130 It was found that different nano/microstructured Ni11(PO3)8(OH)6 samples possessed varied surface/interface conditions that assume pivotal functions in electrolyte access and ion intercalation/extraction. As shown in Fig. 10k, the hexagonal polyhedrons (M1) have a smooth surface, sized approximately 1200 nm individually. M2 have the same structure and size as M1, but take the form of a hexagonal prism and a hemisphere as reflected in Fig. 10l. In Fig. 10m, it is observed that some hemispheres have created several gaps and even become slightly decomposed. As is shown in Fig. 10n, the height of the as-prepared hexagonal prism structure has decreased greatly (less than 200 nm) and is not as smooth as M1 or M2. The Ni11(PO3)8(OH)6 hexagonal polyhedrons were prepared as SC electrode materials, as shown in Fig. 10o. M1–M4 achieved a specific capacitance of 356 F g−1, 80 F g−1, 25 F g−1 and 141 F g−1 at 5.0 mV s−1, respectively. Moreover, when the scan rate was increased to 100.0 mV s−1, the capacitance of the M1 electrode remained at 177 F g−1 unlike the others, which underwent sharp decreases.
Another likely substitute for the traditional structured SCs are asymmetric supercapacitors (ASCs) that utilize a battery-type Faradaic electrode with higher power and energy density as the source of power. Microporous nickel phosphite [Ni11(HPO3)8(OH)6] nanocrystals were prepared through a hydrothermal process and then successfully used as an anode electrode in a versatile all-solid-state ASC by Gao et al.131 The magnified SEM images of a simple nanocrystal (Fig. 11b) demonstrated that the size of the as-prepared nanocrystal is 300 nm in length and its form is that of a hexagonal pyramid. Moreover, the big end of this pyramid has a diameter of approximately 195 nm, further confirming a hexagonal structure. Due to its specific micro/nanostructure, the flexible solid-state asymmetric SC, microporous Ni11(HPO3)8(OH)6 nanocrystals were successfully fabricated through a hydrothermal technique. The Ni11(HPO3)8(OH)6//graphene ASC device is for the first time reported as being applied for SCs and it exhibited excellent performance. The symmetrical characteristics of the charging/discharging curves are remarkable, indicating that the Ni11(HPO3)8(OH)6 nanocrystal electrode has a fantastic electrochemical ability, and the redox process is reversible, as shown in Fig. 11a. Because of the simple device fabrication and synthesis processes, this versatile solid-state ASC can have several applications in fields like solar energy harvesters, roll-up display panels, and power-on-chip systems.
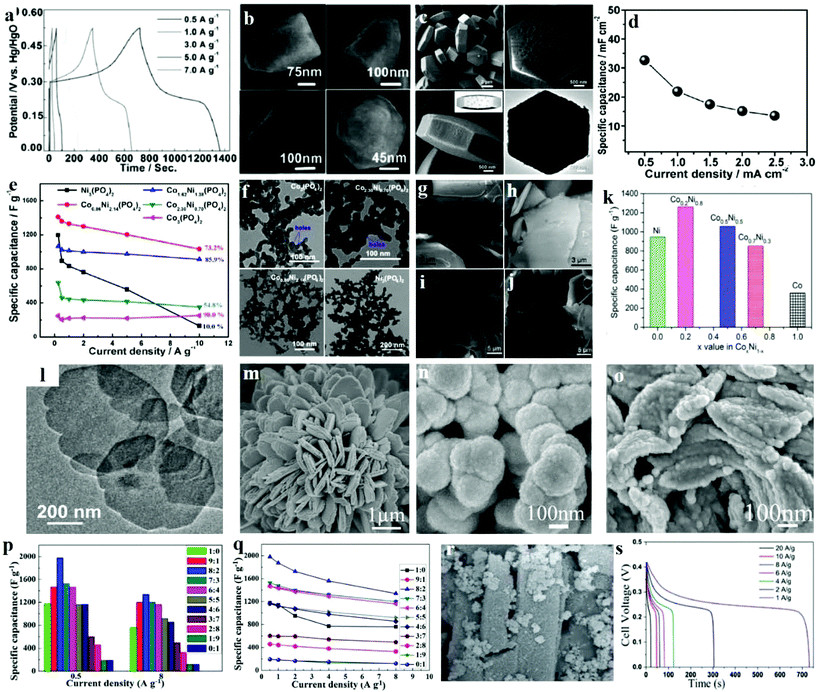 | ||
| Fig. 11 (a) The galvanostatic charge–discharge curves for current densities between 0.5 and 7.0 A g−1 of Ni11(HPO3)8(OH)6; (b) SEM images of Ni11(HPO3)8(OH)6; (c) SEM images of the porous Na-doped Ni2P2O7 hexagonal tablets; (d) specific capacitance at various discharge current densities of Na-doped Ni2P2O7 hexagonal tablets; (e) specific capacitances of the cobalt/nickel phosphate electrodes calculated using discharge curves; (f) TEM images of Co3(PO4)2, Co2.30Ni0.70(PO4)2, Co0.86Ni2.14(PO4)2 and Ni3(PO4)2 samples before pyrolysis; SEM images of the Co0.2Ni0.8 pyrophosphates prepared at calcination temperatures of (g) 300 °C, (h) 400 °C, (i) 500 °C and (j) 600 °C; (k) calculated specific capacitance from the GCD curves for the products CoxNi1−x pyrophosphates at a calcination temperature of 400 °C; (l) TEM images of nickel–cobalt phosphate nanosheets; SEM images of (m) Ni3P2O8, (n) Co3P2O8·8H2O and (o) Ni3P2O8–Co3P2O8·8H2O; (p) specific capacitances of Ni3P2O8–Co3P2O8·8H2O with various Co/Ni molar ratios at a current density of 0.5 A g−1; (q) the dependence of the specific capacitances on Ni/Co molar ratios of Ni3P2O8–Co3P2O8·8H2O; (r) SEM images of MOF-derived NixPyOz; (s) charge–discharge curves of pristine MOF and MOF-derived NixPyOz at different current densities. Adapted with permission from (a, b) ref. 131, © 2014 The Royal Society of Chemistry; (c, d) ref. 132, © 2015 Wiley-VCH; (e, f) ref. 12 © 2016 Elsevier; (g–l) ref. 134, © 2016 American Chemical Society; (m–q) ref. 135 © 2016 Elsevier; (r, s) ref. 133, © 2016 Wiley-VCH. | ||
In recent years, inorganic materials treated with metal ions (e.g., Ni2+, Cu2+, Zn2+, Mn4+) have received much interest, which can positively modify the properties of the materials through a change in electrical conductivity and electron density. Our group prepared hexagonal tablet precursors though a hydrothermal method; then after calcination treatment, it was transformed into porous sodium-doped Ni2P2O7 hexagonal tablets.132 It can be seen in Fig. 11c that the overall morphology and size of the Na-doped Ni2P2O7 hexagonal tablet decreases slightly after annealing. The higher magnification images of individual particles indicate that the Na-doped Ni2P2O7 hexagonal tablet has a porous structure after CO2 and H2O release during the thermal decomposition process. The obtained samples were applied as SC electrode materials, with the sample as the anode and graphene as the cathode. Galvanostatic charge/discharge measurements were tested at a current density of 0.5, 1.0, 1.5, 2.0, and 2.5 mA cm−2. And the equivalent specific capacitances of the as-prepared solid-state hybrid SC were consequently measured (Fig. 11d). At 2.5 mA cm−2, the device's specific capacitances may possibly extend up to 32.6 mF cm−2. The Ni2P2O7//graphene solid-state hybrid SC achieved an excellent energy density of 23.4 W h kg−1 as well as good cycling stability, which proves that the porous sodium-doped Ni2P2O7 hexagonal tablets are novel promising materials for flexible electrodes of SCs.
Metal–organic frameworks (MOFs) with controlled pore size distribution, high porosity, and abundant electroactive constituents have received great attention for SC applications. Bendi et al. reported a new technique for synthesizing Ni-MOF-derived hollow porous nickel phosphate (NixPyOz) for high performance SCs.133 They developed a new strategy to prepare an efficient and original MOF-templated hollow porous NixPyOz composite (as shown in Fig. 11r) electrode for SC. The synthesized NixPyOz was a possible electrode material for electrochemical SCs under three-electrode configuration in the solution of 2 M KOH aqueous electrolyte. Fig. 11s demonstrates the galvanostatic charge/discharge curves of MOF-derived NixPyOz, within a possible voltage window of 0–0.45 V at different current densities. The discharge curve exhibits the properties of pseudocapacitance. The specific capacitance of the MOF-derived NixPyOz at 1 A g−1 was 1627 F g−1. It is also found that phosphate ions can enhance the conductivity of MOFs by providing more Faradaic reactions throughout the interface between the electrolyte and the electrode. The increased redox reactions at metal centers may be due to the resultant microstructures, which have loosely adsorbed structures on microrods with intense conductivity and huge surface area of the MOF-derived NixPyOz. Thus, such a hybrid-structured electrode inherits the strengths of the MOF template (high capacitances drawn from the internal surface areas) and metal phosphates (effective pseudocapacitance).
2.4. Cobalt/nickel phosphate
A uniform three dimensional (3D) mesoporous structure (2–10 nm) can shorten diffusion paths and increase the surface areas for electrolyte ions, but is not frequently reported in phosphate based electrode materials. Tang et al. synthesized cobalt/nickel phosphate nanospheres with honeycomb-like mesopores (CoxNi(3−x)(PO4)2) through a facile hydrothermal process integrated with low temperature pyrolysis.12 The formation of honey-comb like mesopores may result from the dehydration process of the hydrated cobalt/nickel phosphates. The TEM images of Ni3(PO4)2, Co2.30Ni0.70(PO4)2, Co0.86Ni2.14(PO4)2 and Co3(PO4)2 samples prior to the pyrolysis are shown in Fig. 11f. Commonly observed in Co-rich Co2.30Ni0.70(PO4)2 and Co3(PO4)2 samples are typically nanospheres with clear honeycomb-like pores (∼5 nm). Furthermore, the honeycomb-like mesoporous structure offers more active sites for Faradaic reactions. The synergistic effect between cobalt and nickel also occurred in the as-prepared porous cobalt/nickel phosphate electrodes. Fig. 11e shows the capacitance retentions of the electrodes: Co3(PO4)2 at 90.0%, Co2.30Ni0.70(PO4)2 at 54.8%, Co1.62Ni1.38(PO4)2 at 85.9%, Co0.86Ni2.14(PO4)2 at 73.2% and Ni3(PO4)2 at 10.0%, respectively. A home-made activated carbon was used as the cathode material for an asymmetric SC along with the as-prepared cobalt/nickel phosphates. High energy densities of 45.8 W h kg−1 at 42.4 W kg−1 power density and 2.8 kW kg−1 at 30.7 W h kg−1 have been obtained.The synergistic effect of nickel–cobalt species plays crucial roles in the high electrochemical performances of cobalt/nickel binary oxides/sulfides. The cobalt/nickel binary phosphates are thus predicted to give higher supercapacitive performance compared to either cobalt- or nickel-phosphate. Chen et al. incorporated two transition metals by an effective method to enhance the electrochemical performance in SCs for transition metal compound based electrodes.134 Here, amorphous phase Co–Ni pyrophosphates are fabricated as electrodes in SCs. They adjusted the ratios of Co and Ni via changing the calcination temperature, and thereupon the electrochemical performance was also tuned. The morphology of the product was characterized by SEM. The Co0.2Ni0.8NH4PO4·H2O precursor was composed of a plate-like structure. These microplates have nonuniform sizes from 15 to 1 μm. After calcination at 300 and 400 °C, a microplate-like structure was obtained as illustrated in SEM images in Fig. 11g and h. The SEM image in Fig. 11h shows that the product prepared at 400 °C has a smooth surface with a thickness of about 500 nm. Such a morphology was maintained when further increasing the temperature to 500 and 600 °C as shown in Fig. 10i and j. An optimized condition produced the amorphous phase of Co0.2Ni0.8 pyrophosphate at a calcination temperature of 400 °C. The specific capacitances were calculated by using GCD curves as shown in Fig. 11k which are 358, 849, 1056, 1259 and 945 F g−1 at 1.5 A g−1 for Co, Co0.7Ni0.3, Co0.5Ni0.5, Co0.2Ni0.8, and Ni polyphosphates, respectively. Obviously, Co0.2Ni0.8 polyphosphates exhibited the highest specific capacitance, which could be attributed to the electro-active sites involved in the redox reaction from either the valence interchange or the alternate charge between nickel and cobalt cations. This study provides a facile method to construct inexpensive bimetallic transition metal pyrophosphate as efficient electrodes in asymmetric electrochemical capacitors. Furthermore, Pang's group reported recent research studies about ultrathin nickel–cobalt phosphate nanosheets (Fig. 11l), which presented an outstanding pseudocapacitive performance for electrochemical supercapacitors under a solid-state electrolyte.21 The nickel–cobalt phosphate nanosheets were synthesized by a facile hydrothermal process, and exhibited a maximum specific capacitance of 1132.5 F g−1. What's more, this solid-state device based on nickel–cobalt phosphate 2D nanosheets showed an energy density of 35.8 W h kg−1 at 0.7 kW kg−1, and an energy density of 32.5 W h kg−1 at 0.6 kW kg−1.
It is worth noting that the electrochemical performance of binary metallic–nonmetallic compounds is normally better than that of single metallic compounds; however, the ratio of metals also influences the performance. Liu et al. came up with a very basic and general synthetic method for the Ni–Co phosphates with varied cobalt and nickel components with no additional structure-directing agent or surfactant, not even an extra alkali source.136 The SEM images of the samples – Ni3P2O8, Co3P2O8·8H2O, Co3P2O8·8H2O, and Ni3P2O8 – are shown in Fig. 11m, n, and o. Fig. 11m shows flower-like Ni3P2O8 where the flower is composed of bundles of separate single flakes with pores. Meanwhile, Co3P2O8·8H2O shows a flake-like structure consisting of numerous nanoparticles with a certain degree of agglomeration. Similarly, several pores were observed between Co3P2O8·8H2O (Fig. 11n). Neither a flake-like nor flower-like morphology was formed as Ni3P2O8–Co3P2O8·8H2O, which exhibited a particle-like form assembled out of several randomly-distributed particles of Ni3P2O8–Co3P2O8·8H2O (Fig. 11o). Fig. 11p and q show the specific capacitances of Ni3P2O8–Co3P2O8·8H2O with different cobalt–nickel mole ratios. Apparently, the cobalt–nickel mole ratios have a large influence on the electrochemical performance of Ni3P2O8–Co3P2O8·8H2O. The Ni3P2O8 electrode exhibits much higher specific capacitance than Co3P2O8·8H2O. Meanwhile, compared to Ni3P2O8, the Co3P2O8·8H2O electrode shows a more stable rate capacity. Moreover, the specific capacitance of Ni3P2O8–Co3P2O8·8H2O increased at higher cobalt–nickel mole ratios, indicating that Ni3P2O8 with its higher specific capacitance is able to remarkably increase the specific capacitances of the Ni3P2O8–Co3P2O8·8H2O electrode. Ni3P2O8–Co3P2O8·8H2O with a cobalt–nickel mole ratio of 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 exhibited the best electrochemical performance, with outstanding cycling stability and rate capacity. In addition, its specific capacitance is much greater than that of pure Ni3P2O8 and Ni3P2O8–Co3P2O8·8H2O, which indicates a synergism in the given mole ratio. Interestingly, the further increase of the Ni/Co ratio to 9
2 exhibited the best electrochemical performance, with outstanding cycling stability and rate capacity. In addition, its specific capacitance is much greater than that of pure Ni3P2O8 and Ni3P2O8–Co3P2O8·8H2O, which indicates a synergism in the given mole ratio. Interestingly, the further increase of the Ni/Co ratio to 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 resulted in a decrease in the specific capacitance of the electrodes because of the relatively small quantity of Ni3P2O8–Co3P2O8·8H2O, which prompted the synergistic effect to be less obvious. Therefore, the 8
1 resulted in a decrease in the specific capacitance of the electrodes because of the relatively small quantity of Ni3P2O8–Co3P2O8·8H2O, which prompted the synergistic effect to be less obvious. Therefore, the 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 Ni/Co ratio is believed to be the most desirable ratio in such materials. Such a compound is expected to be one of the emerging novel materials to be applied as electrodes in high-performance energy storage systems.
2 Ni/Co ratio is believed to be the most desirable ratio in such materials. Such a compound is expected to be one of the emerging novel materials to be applied as electrodes in high-performance energy storage systems.
Fig. 12 enumerates the list of the morphologies as well as the highest specific capacitance rates taken from the reviewed literature to present more vividly the SC performance of each selected material. Moreover, their electrolytes and cycling stabilities are also included. Phosphates of different nanostructures have been used for SCs, such as nanoflowers, nanotubes/rods, nanoplates, nanospheres, etc.
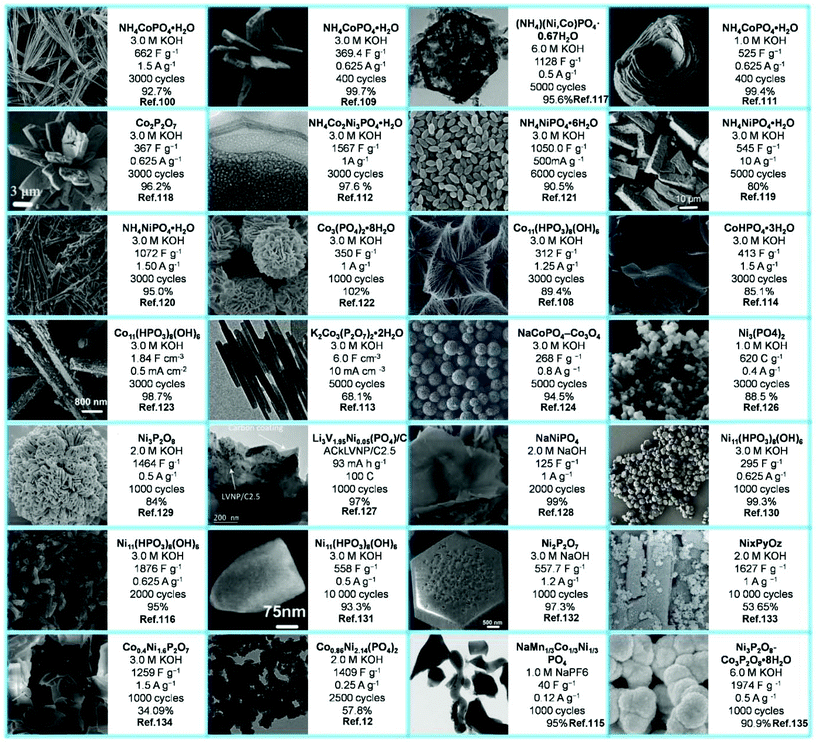 | ||
| Fig. 12 Different morphologies of metal phosphate SCs materials and their SCs performances in different test environments. | ||
3. Conclusions and outlook
This review summarizes some progress with respect to cobalt and nickel phosphate based micro/nanomaterials applied for SCs, covering ammonium/bimetallic phosphates, cobalt phosphates, nickel phosphates and cobalt/nickel phosphates. It is clear that much progress has been made in this direction. Moreover, the morphology of the material is relevant to the diffusion of ions in electrodes, and the surface area of the electrode materials. Furthermore, nanostructured phosphate materials are promising for SC applications due to their larger specific surface area and a reduction in diffusion pathways. Although phosphates as one of the newly-developed materials show great promise for SCs, with the advantages of abundance, environment friendliness and low cost, phosphate SC materials still show weaknesses including poor electrical conductivity and relatively low capacitance. Researchers could improve the performance of phosphate-based SC materials by making nano-sized porous structures and compositing them with highly conductive materials such as activated carbon nanotubes, carbon, graphene and conductive polymer (polyaniline, polypyrrole, and so on).SCs with their high efficiency, prolonged lifespan, and great power density have become key emerging energy storage devices. The biggest challenge for SCs, however, is to increase the energy density. Thus a major focus of SCs studies is to develop novel electrode materials with high capacitance and to develop hybrid SCs with both a battery-type electrode and a SC-type electrode as the energy density is significantly increased due to both a high cell working voltage and a large capacitance in the hybrid system. As far as the design of phosphate based SC electrode materials is concerned, some desirable characteristics are: (1) low internal resistance; (2) better electrochemical and mechanical stability for better cycle performance; (3) a large specific surface area, implying more active sites; and (4) an appropriate pore network, length and size distribution to promote ion diffusion at high rates. Phosphate based materials hold great promise for achieving both high conductivity and high electrochemical activity, promising for high-performance SC electrodes.137–142
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Program for New Century Excellent Talents of the University in China (NCET-13-0645) and the National Natural Science Foundation of China (NSFC-21201010, 21671170 and 21673203), Graduate Student Scientific Research Innovation Projects in Jiangsu Province (SJLX16-0588), Innovation Scientists and Technicians Troop Construction Projects of Henan Province (164200510018), Top-notch Academic Programs Project of Jiangsu Higher Education Institutions (PPZY2015B112), Program for Innovative Research Team (in Science and Technology) in University of Henan Province (14IRTSTHN004), the Six Talent Plan (2015-XCL-030), and a Qinglan Project, Training Program of Innovation and Entrepreneurship for Undergraduates (201711117011Z). We also acknowledge the Priority Academic Program Development of Jiangsu Higher Education Institutions and the technical support we received at the Testing Center of Yangzhou University.References
- L. Liu, Z. Niu, L. Zhang, W. Zhou, X. Chen and S. Xie, Adv. Mater., 2014, 26, 4855 CrossRef CAS PubMed
.
- Z. Wang, Y. Han, Y. Zeng, Y. Qie, Y. Wang, D. Zheng, X. Lu and Y. Tong, J. Mater. Chem. A, 2016, 4, 5828 CAS
.
- J. Xu, Q. Wang, X. Wang, Q. Xiang, B. Liang, D. Chen and G. Shen, ACS Nano, 2013, 7, 5453 CrossRef CAS PubMed
.
- X. Hu, W. Shao, X. Hang, X. Zhang, W. Zhu and Y. Xie, Angew. Chem., Int. Ed., 2016, 55, 5733 CrossRef CAS PubMed
.
- Z. Niu, H. Dong, B. Zhu, J. Li, H. H. Hng, W. Zhou, X. Chen and S. Xie, Adv. Mater., 2013, 25, 1058 CrossRef CAS PubMed
.
- Z. Li, W. Lv, C. Zhang, B. Li, F. Kang and Q. H. Yang, Carbon, 2015, 92, 11 CrossRef CAS
.
- V. Palomares, P. Serras, I. Villaluenga, K. B. Hueso, J. Carretero-González and T. Rojo, Energy Environ. Sci., 2012, 5, 5884 CAS
.
- Y. Xu, Y. Tao, X. Zheng, H. Ma, J. Luo, F. Kang and Q. H. Yang, Adv. Mater., 2015, 27, 8082 CrossRef CAS PubMed
.
- C. Yuan, H. Bin Wu, Y. Xie and X. W. D. Lou, Angew. Chem., Int. Ed., 2014, 53, 1488 CrossRef CAS PubMed
.
- J. Xie, X. Sun, N. Zhang, K. Xu, M. Zhou and Y. Xie, Nano Energy, 2013, 2, 65 CrossRef CAS
.
- C. Yuan, J. Li, L. Hou, X. Zhang, L. Shen and X. W. Lou, Adv. Funct. Mater., 2012, 22, 4592 CrossRef CAS
.
- Y. Tang, Z. Liu, W. Guo, T. Chen, Y. Qiao, S. Mu, Y. Zhao and F. Gao, Electrochim. Acta, 2016, 190, 118 CrossRef CAS
.
- T. Zhai, X. Lu, F. Wang, H. Xia and Y. Tong, Nanoscale Horiz., 2016, 1, 109 RSC
.
- X. R. Li, H. G. Xue and H. Pang, Nanoscale, 2017, 9, 216 RSC
.
- F. Lei, W. Liu, Y. Sun, J. Xu, K. Liu, L. Liang, T. Yao, B. Pan, S. Wei and Y. Xie, Nat. Commun., 2016, 7, 12697 CrossRef CAS PubMed
.
- T. Y. Wang, S. Q. Chen, H. Pang, H. G. Xue and Y. Yu, Adv. Sci., 2017, 1600289 CrossRef PubMed
.
- Z. Niu, W. Zhou, J. Chen, G. Feng, H. Li, W. Ma, J. Li, H. Dong, Y. Ren, D. Zhao and S. Xie, Energy Environ. Sci., 2011, 4, 1440 CAS
.
- M. Winter and R. Brodd, Chem. Rev., 2004, 104, 4245 CrossRef CAS PubMed
.
- P. Simon and Y. Gogotsi, Nat. Mater., 2008, 7, 845 CrossRef CAS PubMed
.
- B. E. Conway, J. Electrochem. Soc., 1991, 138, 1539 CrossRef CAS
.
- H. Xia, C. Y. Hong, X. Q. Shi, B. Li, G. L. Yuan, Q. F. Yao and J. P. Xie, J. Mater. Chem. A, 2015, 3, 1216 CAS
.
-
B. E. Conway, Electrochemical Supercapacitors: Scientific Fundamentals and Technological Applications, Kluwer Aca., New York, 1999 Search PubMed
.
- A. Burke, J. Power Sources, 2000, 91, 37 CrossRef CAS
.
- Z. Y. Teng, H. Y. Lv, C. Y. Wang, H. G. Xue, H. Pang and G. X. Wang, Carbon, 2017, 113, 63 CrossRef CAS
.
- Q. X. Zhao, M. M. Zhao, J. Q. Qiu, H. Pang, W. Y. Lai and W. Huang, Inorg. Chem. Front., 2017, 4, 442 RSC
.
- R. Díaz, M. G. Orcajo, J. A. Botas, G. Calleja and J. Palma, Mater. Lett., 2012, 68, 126 CrossRef
.
- D. O. Miles, D. Jiang, A. D. Burrows, J. E. Halls and F. Marken, Electrochem. Commun., 2013, 27, 9 CrossRef CAS
.
- B. E. Conway, V. Birss and J. Wojtowicz, J. Power Sources, 1997, 66, 1 CrossRef CAS
.
- Y. Zhou, Z. Mao, W. Wang, Z. Yang and X. Liu, ACS Appl. Mater. Interfaces, 2016, 8, 28904 CAS
.
- B. Li, P. Gu, Y. C. Feng, G. X. Zhang, K. S. Huang, H. G. Xue and H. Pang, Adv. Funct. Mater., 2017, 27, 1605784 CrossRef
.
- Q. Liu, C. Y. Chen, J. J. Zheng, L. Wang, Z. B. Yang and W. Y. Yang, J. Mater. Chem. A, 2017, 5, 1421 CAS
.
- J. Theerthagiri, K. Thiagarajan, B. Senthilkumar, Z. Khan, R. A. Senthil, P. Arunachalam, J. Madhavan and M. Ashokkumar, ChemistrySelect, 2017, 2, 201 CrossRef CAS
.
- X. R. Li, S. Y. Ding, X. Xiao, J. Y. Shao, J. L. Wei, H. Pang and Y. Yu, J. Mater. Chem. A, 2017, 5, 12774 CAS
.
- D. Wei and T. W. Ng, Electrochem. Commun., 2009, 11, 1996 CrossRef CAS
.
- Y. Wang, G. Ye, H. Chen, X. Hu, Z. Niu and S. Ma, J. Mater. Chem. A, 2015, 3, 15292 CAS
.
- F. B. V. Khomenko and E. Raymundo Pinero, J. Power Sources, 2010, 195, 4234 CrossRef
.
- M. Wang, F. D. Jin, X. J. Zhang, J. Wang, S. F. Huang, X. Y. Zhang, S. C. Mu, Y. P. Zhao and Y. F. Zhao, ACS Sustainable Chem. Eng., 2017, 5, 5679 CrossRef CAS
.
- J. P. Zheng, J. Electrochem. Soc., 2009, 156, A500 CrossRef CAS
.
- G. G. Amatucci, F. Badway, A. Du Pasquier and T. Zheng, J. Electrochem. Soc., 2011, 148, A930 CrossRef
.
- S. Roldán, C. Blanco, M. Granda, R. Menéndez and R. Santamaría, Angew. Chem., Int. Ed., 2011, 123, 1699 CrossRef PubMed
.
- N. Katsuhiko, S. Ishimoto, J. I. Miyamoto and W. Naoi, Energy Environ. Sci., 2012, 5, 9363 Search PubMed
.
- K. Tan, N. Nijem, P. Canepa, Q. Gong, J. Li, T. Thonhauser and Y. J. Chabal, Chem. Mater., 2012, 24, 3153 CrossRef CAS
.
- Y. Wang, Y. Song and Y. Xia, Chem. Soc. Rev., 2016, 45, 5925 RSC
.
- X. Ji, K. Xu, C. Chen, B. Zhang, H. Wan, Y. Ruan, L. Miao and J. Jiang, J. Mater. Chem. A, 2015, 3, 9909 CAS
.
- M. Jana, S. Saha, P. Khanra, P. Samanta, H. Koo, N. Chandra Murmu and T. Kuila, J. Mater. Chem. A, 2015, 3, 7323 CAS
.
- S. Roldán, D. Barreda, M. Granda, R. Menéndez, R. Santamaría and C. Blanco, Phys. Chem. Chem. Phys., 2015, 17, 1084 RSC
.
- Y. Z. Zhang, T. Cheng, Y. Wang, W. Y. Lai, H. Pang and W. Huang, Adv. Mater., 2016, 28, 5242 CrossRef CAS PubMed
.
- X. Xiao, X. R. Li, S. S. Zheng, H. G. Xue and H. Pang, Adv. Mater. Interfaces, 2017, 1600798 CrossRef
.
- N. Jabeen, Q. Y. Xia, S. V. Savilov, S. M. Aldoshin, Y. Yu and H. Xia, ACS Appl. Mater. Interfaces, 2016, 8, 33732 CAS
.
- S. G. Kandalkar, J. L. Gunjakar and C. D. Lokhande, J. Alloys Compd., 2009, 478, 594 CrossRef CAS
.
- B. Senthilkumar, R. K. Selvan, L. Vasylechko and M. Minakshi, Solid State Sci., 2014, 35, 18 CrossRef CAS
.
- M. G. M. Zukalová, M. Kalbác, L. Kavan and I. Exnar, Chem. Mater., 2005, 17, 1248 CrossRef
.
- L. Zhang, S. S. Zheng, L. Wang, H. Tang, H. G. Xue, G. X. Wang and H. Pang, Small, 2017, 1700917 CrossRef PubMed
.
- S. S. Zheng, X. R. Li, B. Y. Yan, Q. Hu, Y. X. Xu, X. Xiao, H. G. Xue and H. Pang, Adv. Energy Mater., 2017, 1602733 CrossRef
.
- M. Zhou, F. Lu, X. Shen, W. Xia, H. He and X. Zeng, J. Mater. Chem. A, 2015, 3, 21201 CAS
.
- W. Zhang, B. Quan, C. Lee, S. K. Park, X. Li, E. Choi, G. Diao and Y. Piao, ACS Appl. Mater. Interfaces, 2015, 7, 2404 CAS
.
- S. Pan, J. Ren, X. Fang and H. Peng, Adv. Energy Mater., 2016, 6, 1501867 CrossRef
.
- R. Kotz and M. Carlen, Electrochim. Acta, 2000, 45, 2483 CrossRef CAS
.
- X. Zeng, J. Li and L. Liu, Renewable Sustainable Energy Rev., 2015, 52, 1759 CrossRef CAS
.
- Y. Lu, T. Y. Wang, X. R. Li, H. G. Xue and H. Pang, RSC Adv., 2016, 6, 87188 RSC
.
- D. Galizzioli, F. Tantardini and S. Trasatti, J. Appl. Electrochem., 1974, 4, 57 CrossRef CAS
.
- P. Salunkhe, J. Lin, V. Malgras, S. Dou, J. Kim and Y. Yamauchi, Nano Energy, 2015, 11, 211 CrossRef
.
- Y. Y. J. Tan, R. Salunkhe, J. Liu, N. Torad, M. Imura and S. Furukawa, J. Am. Chem. Soc., 2015, 137, 1572 CrossRef PubMed
.
- J. Tan, R. Salunkhe, J. Liu, N. Torad, M. Imura, S. Furukawa and Y. Yamauchi, ACS Nano, 2015, 9, 6288 CrossRef PubMed
.
- J. Tong, H. H. Zhang, J. N. Gu, L. Li and C. Ma, J. Mater. Sci., 2016, 51, 1966 CrossRef CAS
.
- J. Tarascon and M. Armand, Nature, 2001, 414, 359 CrossRef CAS PubMed
.
- T. Brezesinski, J. Wang, S. Tolbert and B. Dunn, Nat. Mater., 2010, 9, 146 CrossRef CAS PubMed
.
- A. Aricò, P. Bruce, B. Scrosati, J. Tarasco and W. Schalkwijk, Nat. Mater., 2005, 4, 366 CrossRef PubMed
.
- V. Augustyn, P. Simon and B. Dunn, Energy Environ. Sci., 2014, 7, 1597 CAS
.
- J. Zheng, P. Cygan and T. Jow, J. Electrochem. Soc., 1995, 142, 2699 CrossRef CAS
.
- H. Shao, N. Padmanathan, D. McNulty, C. Odwyer and K. M. Razeeb, ACS Appl. Mater. Interfaces, 2016, 8, 28592 CAS
.
- J. Du, G. Zhou, H. Zhang, C. Cheng, J. Ma, W. Wei, L. Chen and T. Wang, ACS Appl. Mater. Interfaces, 2013, 5, 7405 CAS
.
- https://www.usgs.gov/ .
- J. A. Koza, Z. He, A. S. Miller and J. A. Switzer, Chem. Mater., 2012, 24, 3567 CrossRef CAS
.
- Y. J. Sa, K. Kwon, J. Y. Cheon, F. Kleitz and S. H. Joo, J. Mater. Chem. A, 2013, 1, 9992 CAS
.
- Y. Zhang, B. Cui, Z. T. Qin, H. Lin and J. B. Li, Nanoscale, 2013, 5, 6826 RSC
.
- J. Liu, F. P. Du, H. J. Zhang, C. Lin, P. Gao, Y. Y. Chen, Z. F. Shi, X. P. Li, T. J. Zhao and Y. H. Sun, RSC Adv., 2015, 5, 39075 RSC
.
- T. Yao, J. F. He, S. Jiang, Z. H. Sun, Q. H. Liu, W. R. Cheng, F. C. Hu, Y. Jiang, Z. Y. Pan and S. Q. Wei, Angew. Chem., Int. Ed., 2015, 54, 8722 CrossRef PubMed
.
- S. K. Singh, V. M. Dhavale and S. Kurungot, ACS Appl. Mater. Interfaces, 2015, 7, 442 CAS
.
- Y. Y. Liang, Y. G. Li, H. L. Wang, J. G. Zhou, J. Wang, T. Regier and H. J. Dai, Nat. Mater., 2011, 10, 780 CrossRef CAS PubMed
.
- X. Y. Lu and C. Zhao, J. Mater. Chem. A, 2013, 1, 12053 CAS
.
- Z. B. Zhuang, W. C. Sheng and Y. S. Yan, Adv. Mater., 2014, 26, 3950 CrossRef CAS PubMed
.
- M. W. Kanan, J. Yano, Y. Surendranath, M. Dinca, V. K. Yachandra and D. G. Nocera, J. Am. Chem. Soc., 2010, 132, 13692 CrossRef CAS PubMed
.
- P. Y. Feng, X. H. Bu and G. D. Stucky, Nature, 1997, 388, 735 CrossRef CAS
.
- Y. Zhao, L. Yang, S. Chen, X. Wang, Y. Ma, Q. Wu, Y. Jiang, W. Qian and Z. Hu, J. Am. Chem. Soc., 2013, 135, 1201 CrossRef CAS PubMed
.
- S. Liu, Q. Zhang, Y. Li, M. Han, L. Gu, C. Nan, J. Bao and Z. Dai, J. Am. Chem. Soc., 2015, 137, 2820 CrossRef CAS PubMed
.
- O. Diazmorales, I. Ledezmayanez, M. T. M. Koper and F. Callevallejo, ACS Catal., 2015, 5, 5380 CrossRef CAS
.
- B. Li, M. B. Zheng, H. G. Xue and H. Pang, Inorg. Chem. Front., 2016, 3, 175 RSC
.
- G. R. Carr and D. Whittaker, J. Chem. Soc., 1987, 1877 CAS
.
- H. Jiang, T. Zhao and C. Li, J. Mater. Chem., 2011, 21, 3818 RSC
.
- S. S. Zheng, H. G. Xue and H. Pang, Coord. Chem. Rev. DOI:10.1016/j.ccr.2017.07.002
.
- C. Chen, W. Chen, J. Lu, D. Chu, Z. Huo, Q. Peng and Y. Li, Angew. Chem., Int. Ed., 2009, 48, 4816 CrossRef CAS PubMed
.
- X. Xiao, S. S. Zheng, X. R. Li, G. X. Zhang, X. T. Guo, H. G. Xue and H. Pang, J. Mater. Chem. B, 2017, 5, 5234 RSC
.
- D. Xu and D. Xue, Phys. B, 2005, 370, 84 CrossRef CAS
.
- D. Xu and D. Xue, Cryst. Growth, 2006, 286, 108 CrossRef CAS
.
- D. Kong, Y. Li, X. Ouyang, A. V. Prosvirin, H. Zhao, J. H. Ross Jr., K. R. Dunbar and A. Clearfield, Chem. Mater., 2004, 16, 3020 CrossRef CAS
.
- R. L. Frost, J. T. Kloprogge, P. A. Williams, W. N. Martens, T. E. Johnson and P. Leverett, Spectrochim. Acta, Part A, 2002, 58, 2861 CrossRef
.
- J. Yang, L. Qi, C. Lu, J. Ma and H. Cheng, Angew. Chem., Int. Ed., 2005, 44, 598 CrossRef CAS PubMed
.
- A. A. Belik, H. J. Koo, M. H. Whangbo, N. Tsujii, P. Naumov and E. T. Muromachi, Inorg. Chem., 2007, 46, 8684 CrossRef CAS PubMed
.
- S. Wang, H. Pang, S. Zhao, W. Shao, N. Zhang, J. Zhang, J. Chen and S. Li, RSC Adv., 2014, 4, 340 RSC
.
- S. G. Carling, P. Day and D. Visser, Inorg. Chem., 1995, 34, 3917 CrossRef CAS
.
- A. Q. Yuan, J. L. Wu, J. Bai, S. M. Ma, Z. Y. Huang and Z. F. Tong, J. Chem. Eng. Data, 2008, 53, 1066 CrossRef CAS
.
- C. F. Zeng, W. W. Ji and L. X. Zhang, CrystEngComm, 2012, 14, 3008 RSC
.
- B. P. Pedersen, H. Kumar, A. B. Waight, A. J. Risenmay, Z. Roe-Zurz, B. H. Chau, A. Schlessinger, M. Bonomi, W. Harries, A. Sali, A. K. Johri and R. M. Stroud, Nature, 2013, 496, 533 CrossRef CAS PubMed
.
- C. Masquelier and L. Croguennec, Chem. Rev., 2013, 113, 6552 CrossRef CAS PubMed
.
- P. Feng, X. Bu and G. D. Stucky, J. Solid State Chem., 1977, 131, 160 CrossRef
.
- A. M. Chippindale, A. R. Cowley, J. C. Chen, Q. Gao and R. Xu, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1999, 55, 845 Search PubMed
.
- H. Pang, Y. Liu, J. Li, Y. Ma, G. Li, Y. Ai, J. Chen, J. Zhang and H. Zheng, Nanoscale, 2013, 5, 503 RSC
.
- H. Pang, Z. Yan, W. Wang, J. Chen, J. Zhang and H. Zheng, Nanoscale, 2012, 4, 5946 RSC
.
- H. Shao, N. Padmanathan, D. McNulty, C. O′Dwyer and K. M. Razeeb, ACS Appl. Mater. Interfaces, 2016, 8, 28592 CAS
.
- X. Wang, Z. Yan, H. Pang, W. Wang, G. Li, Y. Ma, H. Zhang, X. Li and J. Chen, Int. J. Electrochem. Sci., 2013, 8, 3768 CAS
.
- Q. Li, Y. Li, H. Peng, X. Cui, M. Zhou, K. Feng and P. Xiao, J. Mater. Sci., 2016, 51, 9946 CrossRef CAS
.
- H. Pang, Y. Zhang, W. Y. Lai, Z. Hu and W. Huang, Nano Energy, 2015, 15, 303 CrossRef CAS
.
- H. Pang, S. Wang, W. Shao, S. Zhao, B. Yan, X. Li, S. Li, J. Chen and W. Du, Nanoscale, 2013, 5, 5752 RSC
.
- M. M. Sundaram, T. Watcharatharapong, S. Chakraborty, R. Ahuja, S. Duraisamy, P. T. Rao and N. Munichandraiah, Dalton Trans., 2015, 44, 20108 RSC
.
- H. Pang, C. Wei, Y. Ma, S. Zhao, G. Li, J. Zhang, J. Chen and S. Li, ChemPlusChem, 2013, 78, 546 CrossRef CAS
.
- Y. Zhao, Z. Chen, D. B. Xiong, Y. Qiao, Y. Tang and F. Gao, Sci. Rep., 2016, 6, 17613 CrossRef CAS PubMed
.
- H. Pang, Z. Yan, Y. Ma, G. Li, J. Chen, J. Zhang, W. Du and S. Li, J. Solid State Electrochem., 2013, 17, 1383 CrossRef CAS
.
- K. Raju and K. I. Ozoemena, Sci. Rep., 2015, 5, 17629 CrossRef CAS PubMed
.
- H. Pang, Y. Z. Zhang, Z. Run, W.-Y. Lai and W. Huang, Nano Energy, 2015, 17, 339 CrossRef CAS
.
- J. Zhao, H. Pang, J. Deng, Y. Ma, B. Yan, X. Li, S. Li, J. Chen and W. Wang, CrystEngComm, 2013, 15, 5950 RSC
.
- H. Li, H. Yu, J. Zhai, L. Sun, H. Yang and S. Xie, Mater. Lett., 2015, 152, 25 CrossRef CAS
.
- Y. Zhang, M. Zheng, M. Qu, M. Sun and H. Pang, J. Alloys Compd., 2015, 651, 214 CrossRef CAS
.
- C. Wei, C. Cheng, B. Zhou, X. Yuan, T. Cui, S. Wang, M. Zheng and H. Pang, Part. Part. Syst. Charact., 2015, 32, 831 CrossRef CAS
.
- Y. Xu, L. Zheng, C. Z. Wu, F. Qi and Y. Xie, Chem. – Eur. J., 2011, 17, 384 CrossRef CAS PubMed
.
- F. S. Omar, A. Numan, N. Duraisamy, S. Bashir, K. Ramesh and S. Ramesh, RSC Adv., 2016, 6, 76298 RSC
.
- M. Secchiaroli, G. Giuli, B. Fuchs, R. Marassi, M. Wohlfahrt-Mehrens and S. Dsoke, J. Mater. Chem. A, 2015, 3, 11807 CAS
.
- M. Minakshi, D. Mitchell, R. Jones, F. Alenazey, T. Watcharatharapong, S. Chakraborty and R. Ahuja, Nanoscale, 2016, 8, 11291 RSC
.
- M. Liu, J. Li, W. Han and L. Kang, J. Energy Chem., 2016, 25, 601 CrossRef
.
- H. Pang, Z. Yan, Y. Wei, X. Li, J. Li, L. Zhang, J. Chen, J. Zhang and H. Zheng, Part. Part. Syst. Charact., 2013, 30, 287 CrossRef CAS
.
- Y. Gao, J. Zhao, Z. Run, G. Zhang and H. Pang, Dalton Trans., 2014, 43, 17000 RSC
.
- C. Z. Wei, C. Cheng, S. S. Wang, Y. Z. Xu, J. D. Wang and H. Pang, Chem. – Asian J., 2015, 10, 1731 CrossRef CAS PubMed
.
- R. Bendi, V. Kumar, V. Bhavanasi, K. Parida and P. S. Lee, Adv. Energy Mater., 2016, 6, 1501833 CrossRef
.
- C. Chen, N. Zhang, Y. He, B. Liang, R. Ma and X. Liu, ACS Appl. Mater. Interfaces, 2016, 8, 23114 CAS
.
- G. X. Zhang, X. Xiao, B. Li, P. Gu, H. G. Xue and H. Pang, J. Mater. Chem. A, 2017, 5, 8155 CAS
.
- M. C. Liu, J. J. Li, Y. X. Hu, Q. Q. Yang and L. Kang, Electrochim. Acta, 2016, 201, 142 CrossRef CAS
.
- M. Chen, W. Li, X. Shen and G. W. Diao, ACS Appl. Mater. Interfaces, 2014, 6, 4514 CAS
.
- M. Y. Zhu, C. J. Wang, D. H. Meng and G. W. Diao, J. Mater. Chem. A, 2013, 1, 2118 CAS
.
- J. Han, S. Lu, C. J. Jin, M. G. Wang and R. Guo, J. Mater. Chem. A, 2014, 2, 13016 CAS
.
- M. G. Wang, Y. M. Hu, J. Han, R. Guo, H. X. Xiong and Y. D. Yin, J. Mater. Chem. A, 2015, 3, 20727 CAS
.
- L. Fan, L. Tang, H. F. Gong, Z. H. Yao and R. Guo, J. Mater. Chem., 2012, 22, 16376 RSC
.
- M. Y. Zhu, C. J. Wang, D. H. Meng and G. W. Diao, J. Mater. Chem. A, 2013, 1, 2118 CAS
.
| This journal is © the Partner Organisations 2018 |