Single-molecule junctions of π molecules
Y.
Komoto
a,
S.
Fujii
b and
M.
Kiguchi
 *b
*b
aThe Institute of Scientific and Industrial Research, Osaka University, ISIR 2nd Building 8-1 Mihogaoka, Ibaraki, Osaka, 567-0047, Japan
bDepartment of Chemistry, Graduate School of Science and Engineering, Tokyo Institute of Technology, 2-12-1 W4-10 Ookayama, Meguro-ku, Tokyo 152-8551, Japan. E-mail: fujii.s.af@m.titech.ac.jp; kiguti@chem.titech.ac.jp
First published on 1st November 2017
Abstract
A single-molecule junction, where a single molecule is connected to metal electrodes, has been investigated for potential device applications. π molecules have various functions, and thus, they are ideal molecules as active components of electronic devices. A single-molecule junction study can also reveal the intrinsic properties of a molecule without the effect of intermolecular interaction. Meanwhile, the molecule in the single-molecule junction can exhibit unconventional physical and chemical properties which are not observed in other phases (e.g. gases and bulk crystals), when a molecule strongly interacts with metal. Under the strong metal–molecule interaction, the single-molecule junction can be regarded as a new material including metal electrodes, and a double interface material. A single-molecule junction is, thus, a fascinating research target for a wide range of areas from application to fundamental science.
Single-molecule junctions
A single-molecule junction, where a single molecule is connected to metal electrodes, has been extensively investigated for potential device applications.1 π molecules have various functions (e.g. photochromism, magnetism, high conductivity, and large thermopower), and thus, they are ideal molecules as active components of electronic devices. Switching, diode properties and other useful functionalities have been reported for single-molecule junctions with π molecules. In addition to the device application, an investigation of a single-molecule junction can reveal the intrinsic properties of the π molecule when the interaction between the molecule and metal electrodes is weak and also when the molecule–molecule interaction is weak. In a bulk crystal, the distance between molecules is small compared with the single-molecule junction, and thus, the intermolecular interaction should be considered to discuss its properties.A single-molecule junction can be prepared by the break-junction technique.2–4 In the presence of molecules, a metal wire is broken by mechanical stretch. After breaking the metal wire, a nanogap is formed between metal electrodes, and the molecules are captured in the nano gap, which forms the molecular junction. The number of molecules bridging the metal electrodes is controlled by the gap size. We can fabricate the single-molecule junction by tuning the appropriate gap size. Using the break junction technique, various single-molecule junctions have been fabricated, and their electrical conductances have been investigated. In this paper, we discuss the device application, physical and chemical properties, and future perspectives of single-molecule junctions of π molecules.
Device application
Device application is a main topic in the research of single-molecule junctions. Various single molecular devices have been developed with functional π molecules. The basic components of electronic devices are switches, transistors, diodes, memories and conducting leads. They are realized with the single-molecule junctions of π molecules.5–9In the fabrication of a single molecular transistor, the key technology is the preparation of a thin gate dielectric. The distance between the molecule and the gate electrode should be close to the molecular size, in order to apply a sufficiently strong electric field to the molecule. Song et al. succeeded in preparing a 3 nm thick Al2O3 layer on an Al gate electrode by oxidizing an Al film in an oxygen atmosphere. Using the thin dielectric, a single molecular transistor is realized with octanedithiol (ODT) and benzenedithiol (BDT)5 (Fig. 1a). The conductance of the ODT and BDT single-molecule junction increases as the gate voltage becomes negative (∼3 V), indicative of a p-type transistor. The on/off ratio is more than 100 for the BDT single-molecule junction.
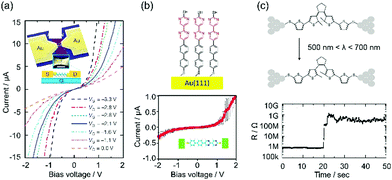 | ||
| Fig. 1 Single molecular device: (a) single molecular transistor,5 (b) single molecular diode using the asymmetric diblock dipyrimidinyl–diphenyl molecules,6 (c) single molecular photoswitch using a photochromic molecule.10 | ||
In the fabrication of a single molecular diode, the key factor is controlling the molecular orientation relative to the polarity of the applied bias voltage. Diez-Perez et al. succeeded in controlling the molecular orientation through a selective deprotection strategy.6 The molecule consists of an electron-deficient bipyrimidinyl unit and an electron-rich biphenyl unit, which features a p–n junction of a semiconductor device. The molecule is terminated with two different protecting groups, trimethylsilylethyl and cyanoethyl. First, the cyanoethyl group is removed from the molecule. The molecules are bound to the bottom Au electrode via a diphenyl end. Second, the trimethylsilylethyl group is removed from the molecule, and the thiol group at the dipyrimidinyl end can bind to the top Au electrode. The molecular orientation is controlled through these processes. The rectification ratio is five at a bias voltage of 1.5 V, and electrons preferentially transfer from the dipyrimidinyl to the diphenyl unit (Fig. 1b).
In the fabrication of single molecular switches, the key is the choice of external perturbation (e.g. light,8,10 electrochemical potential,11 and mechanical force9). The photochromic diarylethene undergoes reversible ring-closing and opening reactions upon alternating irradiation with ultra violet (UV) and visible light. Single molecular photoswitches have been prepared using diarylethene (Fig. 1c).10 Recently, Jia et al. reported a high performance single molecular photoswitch (on/off ratio: 100, stability: >a year, reproducibility: >100 cycles).8 In this paper, we introduce limited examples of single molecular devices. Currently, various single molecular devices have been reported with π molecules.
Investigation of the properties of π molecules revealed by the single-molecule junction study
The single-molecule junction study can reveal the intrinsic properties of the π molecule, when the interaction between the molecule and metal electrodes is weak and also when the molecule–molecule interaction is weak. A quantum behaviour can appear for the single-molecule junction, reflecting its small size. The overlap of π orbitals is investigated by the conductance measurement of biphenyl single-molecule junctions (Fig. 2ab). Venkatramann et al. controlled the twist angle of the benzene rings of the biphenyl by various substitutions.12 As the twist angle (θ) between the two rings increases, the junction conductance follows the equation G ∼ cos2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ. The observed twist angle dependence is consistent with the fact that the overlap of π orbitals between benzene rings is described by cos
θ. The observed twist angle dependence is consistent with the fact that the overlap of π orbitals between benzene rings is described by cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ.
θ.
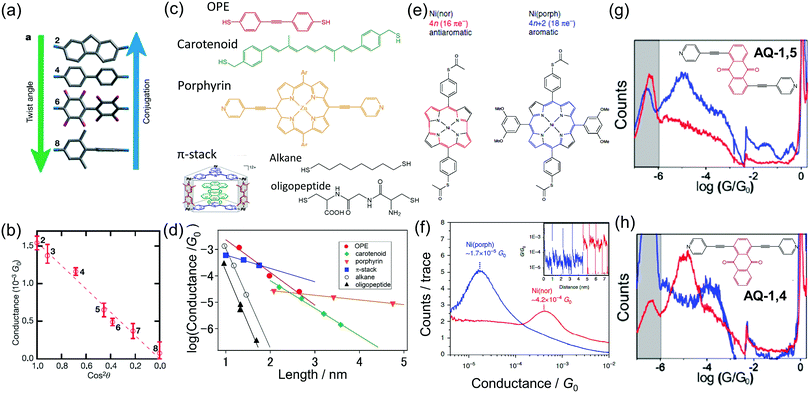 | ||
| Fig. 2 Investigation of the properties on molecules based on single molecular measurement (a and b) conductance of biphenyl single-molecule junction as a function of twist angle,12 (c and d) length dependence of conductance for saturated chains (alkanes20 and peptides22) and conjugated molecules (oligo(phenylene–ethynylene)) (OPE),14 carotenoid,13 porphyrin,16 π-stack molecules,15 (e and f) conductance histograms of single aromatic (porphyrin) and anti-aromatic (norcorrole) molecules,23 (g and h) conductance histograms of single-molecule junctions of anthraquinone having anchoring groups at different positions. The reduced and oxidized states are shown as blue and red curves, respectively.11 | ||
The high electrical conductivity of π molecules has been revealed by the length dependent conductance measurement (Fig. 2c and d).13–18 In the short single-molecule junction, electrons pass through the single-molecule junction via a tunneling mechanism. The conductance (G) is represented by G = A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−βl), where A is the contact conductance, β is the attenuation factor, and l is the molecular length. When A is designed to be high which is achieved using the molecular orbital rule,19β is the final concern for a good electron conductor, and this is accomplished in π-conjugated molecules. β is below 0.5 Å−1 for conjugated π molecules, while β is 1 Å−1 for saturated chains (alkanes and peptides).20–22 In the case of metal oligo-porphyrin molecular wires, β can be as small as 0.04 Å−1.16 The length dependence measurement also reveals that the coherent length of electrons is about 6 nm for the oligothiophene molecular wire.17 The electron transport mechanism changes from the tunneling mechanism to a hopping mechanism around this critical length.
exp(−βl), where A is the contact conductance, β is the attenuation factor, and l is the molecular length. When A is designed to be high which is achieved using the molecular orbital rule,19β is the final concern for a good electron conductor, and this is accomplished in π-conjugated molecules. β is below 0.5 Å−1 for conjugated π molecules, while β is 1 Å−1 for saturated chains (alkanes and peptides).20–22 In the case of metal oligo-porphyrin molecular wires, β can be as small as 0.04 Å−1.16 The length dependence measurement also reveals that the coherent length of electrons is about 6 nm for the oligothiophene molecular wire.17 The electron transport mechanism changes from the tunneling mechanism to a hopping mechanism around this critical length.
The single-molecule junction measurement reveals good electron conductivity for an antiaromatic molecule (Fig. 2e and f).23 The antiaromatic molecule has 4n (n: integer) π electrons in a cyclic system, while the aromatic molecule has 4n + 2π electrons. High chemical reactivity and paramagnetism are expected for the antiaromatic molecule. Fujii et al. demonstrated high electrical conductance for a norcorrole-based nickel complex (antiaromatic molecule) by single-molecule junction measurement.23 The conductance of the single-molecule junction is higher than that of an aromatic complex (porphyrin-based nickel complex) having a similar molecular structure by a factor of 10. The higher conductivity of the antiaromatic molecule can be explained by the smaller HOMO–LUMO gap.19
Since the length of the single-molecule junction can be shorter than the phase coherent length of electrons, a quantum effect can be observed for the single-molecule junction.11,19,24 Baghernejad et al. showed the quantum interference effect for anthraquinone (AQ) single-molecule junctions (Fig. 2g and h).11 They studied the electron transport properties of the single-molecule junction of AQ derivatives with anchoring groups at different points of the AQ core unit (AQ-1,5 and AQ-1,4). The conductance of the reduced state is 10−5.0G0, while the conductance of the oxidized state is below the detection limit (<10−6G0) for AQ-1,5. For AQ-1,4, the conductance is 10−4.0G0 and 10−5.0G0 for the reduced and oxidized states, respectively. The difference in the conductance of the two redox states is smaller for AQ-1,4 than for AQ-1,5. For AQ-1,4, the change in conductance results from a change in the effective electron density of the functional group to the oligo(phenylene–ethynylene) type backbone. In contrast, the electron pathway for AQ-1,5 goes directly via the AQ core unit. The AQ unit is linearly conjugated for the reduced state, while the AQ unit is cross-conjugated for the oxidized state. Therefore, the conductance of the AQ-1,5 single-molecule junction is largely suppressed in the oxidized state.
Future perspectives
Although various investigations have been performed for the single-molecule junction, unconventional physical and chemical properties have not been discovered up to now. It is an open question whether the physical law (e.g. Ohm's law and Wiedemann–Franz law) that governs matter on the bulk scale also governs matter at the single-molecule junction. With respect to the electrical conductance, Ohm's law breaks down. Quantized conductance is observed for the single-molecule junction.25 A recent study on an Au atomic junction confirms the validity of the Wiedemann–Franz law in quantum point contacts.26In the single-molecule junction, the metal–molecule interaction shifts and broadens the discrete molecular levels. The electronic structure of the molecule is different from its original one. Novel properties should appear for this unique double interface material. Currently, few unique phenomena are reported for single-molecule junctions. The electrical conductance of a benzene single-molecule junction is as large as that of an Au atomic junction, although benzene is a bulk insulator.27 The current–voltage curves of the single atomic junction of ferromagnets (Fe, Co, and Ni) unexpectedly show Kondo peaks, which means that the local magnetic moments are screened by the conduction electrons.
In most of the previous studies, electron transport properties have been investigated for single-molecule junctions. Electrons pass through the single-molecule junction, so the electrical measurement is the straight way to study the junction. But, it is interesting to study other properties, such as magnetic, thermal, mechanical, and optical properties, and chemical reactivity, to extract the potential of the single-molecule junction. Theoretical calculation shows that single atomic chains of Pd are ferromagnetic, while bulk Pd is paramagnetic.28 The appearance of ferromagnetism is explained by the decrease in dimensionality. The decrease in the dimensionality also increases the bond strength of metal atomic wires. Mechanically stable atomic wires are formed for Au, Pt, and Ir.29 A similar effect can be expected for the single-molecule junction, which is also a low dimensional material. Among various properties, we pay attention to the chemical reactivity of the single-molecule junction. In the single-molecule junction, a molecule interacts with metal via two interfaces. A molecule can be in a strong electric field and an enhanced field, and mechanical force can be concentrated on a very small area (single molecule). A catalytic reaction proceeds on the metal surface, where a molecule interacts with the metal via one interface. We can expect a unique chemical reaction on this system. Actually, Fujii et al. recently found that the activation barrier for the bowl inversion reaction of a sumanene molecule was suppressed in the case of the single-molecule junction.30
Finally, we comment on single molecular electronics. For device application, the integration of the single-molecule devices is the key technology. Currently, most of the single-molecule junctions are fabricated using the break junction technique. The break junction technique can control only one gap, so only one single-molecule junction can be prepared on one tip. To fabricate electric circuits, multiple single molecular devices should be integrated on one tip. Fixed nanogap electrodes have been fabricated on flat substrates (e.g. Si),31 but the formation probability of the molecular junction is low and it is difficult to control the number of molecules bridging the metal electrodes. So, a break junction technique using an electric or magnetic field without using mechanical force should be developed in order to realize electric circuits with single-molecule junctions.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We much appreciate Prof. Kei Murakoshi and Prof. Jan Ruitenbeek for discussions. This work was financially supported by Grants-in-Aids for Scientific Research (No. 26102013) from MEXT.Notes and references
- J. C. Cuevas and E. Scheer, Molecular electronics: an introduction to theory and experiment, World Scientific, 2017 Search PubMed.
- M. A. Reed, C. Zhou, C. Muller, T. Burgin and J. Tour, Science, 1997, 278, 252–254 CrossRef CAS.
- R. Smit, Y. Noat, C. Untiedt, N. Lang, M. V. van Hemert and J. Van Ruitenbeek, Nature, 2002, 419, 906–909 CrossRef CAS PubMed.
- B. Xu and N. J. Tao, Science, 2003, 301, 1221–1223 CrossRef CAS PubMed.
- H. Song, Y. Kim, Y. H. Jang, H. Jeong, M. A. Reed and T. Lee, Nature, 2009, 462, 1039–1043 CrossRef CAS PubMed.
- I. Diez-Perez, J. Hihath, Y. Lee, L. Yu, L. Adamska, M. A. Kozhushner, I. I. Oleynik and N. Tao, Nat. Chem., 2009, 1, 635–641 CrossRef CAS PubMed.
- S. Fujii, T. Tada, Y. Komoto, T. Osuga, T. Murase, M. Fujita and M. Kiguchi, J. Am. Chem. Soc., 2015, 137, 5939–5947 CrossRef CAS PubMed.
- C. Jia, A. Migliore, N. Xin, S. Huang, J. Wang, Q. Yang, S. Wang, H. Chen, D. Wang and B. Feng, Science, 2016, 352, 1443–1445 CrossRef CAS PubMed.
- M. Kiguchi, T. Ohto, S. Fujii, K. Sugiyasu, S. Nakajima, M. Takeuchi and H. Nakamura, J. Am. Chem. Soc., 2014, 136, 7327–7332 CrossRef CAS PubMed.
- D. Dulic, S. J. van der Molen, T. Kudernac, H. T. Jonkman, J. J. de Jong, T. N. Bowden, J. van Esch, B. L. Feringa and B. J. van Wees, Phys. Rev. Lett., 2003, 91, 207402 CrossRef PubMed.
- M. Baghernejad, X. Zhao, K. Baruel Ornso, M. Fueg, P. Moreno-Garcia, A. V. Rudnev, V. Kaliginedi, S. Vesztergom, C. Huang, W. Hong, P. Broekmann, T. Wandlowski, K. S. Thygesen and M. R. Bryce, J. Am. Chem. Soc., 2014, 136, 17922–17925 CrossRef CAS PubMed.
- L. Venkataraman, J. E. Klare, C. Nuckolls, M. S. Hybertsen and M. L. Steigerwald, Nature, 2006, 442, 904–907 CrossRef CAS PubMed.
- J. He, F. Chen, J. Li, O. F. Sankey, Y. Terazono, C. Herrero, D. Gust, T. A. Moore, A. L. Moore and S. M. Lindsay, J. Am. Chem. Soc., 2005, 127, 1384–1385 CrossRef CAS PubMed.
- V. Kaliginedi, P. Moreno-Garcia, H. Valkenier, W. Hong, V. M. Garcia-Suarez, P. Buiter, J. L. Otten, J. C. Hummelen, C. J. Lambert and T. Wandlowski, J. Am. Chem. Soc., 2012, 134, 5262–5275 CrossRef CAS PubMed.
- M. Kiguchi, T. Takahashi, Y. Takahashi, Y. Yamauchi, T. Murase, M. Fujita, T. Tada and S. Watanabe, Angew. Chem., Int. Ed., 2011, 50, 5708–5711 CrossRef CAS PubMed.
- G. Sedghi, V. M. Garcia-Suarez, L. J. Esdaile, H. L. Anderson, C. J. Lambert, S. Martin, D. Bethell, S. J. Higgins, M. Elliott, N. Bennett, J. E. Macdonald and R. J. Nichols, Nat. Nanotechnol., 2011, 6, 517–523 CrossRef CAS PubMed.
- S. K. Lee, R. Yamada, S. Tanaka, G. S. Chang, Y. Asai and H. Tada, ACS Nano, 2012, 6, 5078–5082 CrossRef CAS PubMed.
- M. Kiguchi, J. Inatomi, Y. Takahashi, R. Tanaka, T. Osuga, T. Murase, M. Fujita, T. Tada and S. Watanabe, Angew. Chem., Int. Ed., 2013, 52, 6202–6205 CrossRef CAS PubMed.
- T. Tada and K. Yoshizawa, ChemPhysChem, 2002, 3, 1035–1037 CrossRef CAS PubMed.
- X. Li, J. He, J. Hihath, B. Xu, S. M. Lindsay and N. Tao, J. Am. Chem. Soc., 2006, 128, 2135–2141 CrossRef CAS PubMed.
- N. Tao, Nat. Nanotechnol., 2006, 1, 173–181 CrossRef CAS PubMed.
- X. Xiao, B. Xu and N. Tao, J. Am. Chem. Soc., 2004, 126, 5370–5371 CrossRef CAS PubMed.
- S. Fujii, S. Marques-Gonzalez, J. Y. Shin, H. Shinokubo, T. Masuda, T. Nishino, N. P. Arasu, H. Vazquez and M. Kiguchi, Nat. Commun., 2017, 8, 15984 CrossRef CAS PubMed.
- T. Tada, in Single-Molecule Electronics, ed. M. Kiguchi, Springer, 2016, ch. 7, pp. 165–190 Search PubMed.
- N. Agraït, Phys. Rep., 2003, 377, 81–279 CrossRef.
- N. Mosso, U. Drechsler, F. Menges, P. Nirmalraj, S. Karg, H. Riel and B. Gotsmann, Nat. Nanotechnol., 2017, 12, 430–433 CrossRef CAS PubMed.
- M. Kiguchi, O. Tal, S. Wohlthat, F. Pauly, M. Krieger, D. Djukic, J. C. Cuevas and J. M. van Ruitenbeek, Phys. Rev. Lett., 2008, 101, 046801 CrossRef CAS PubMed.
- A. Delin, E. Tosatti and R. Weht, Phys. Rev. Lett., 2004, 92, 057201 CrossRef CAS PubMed.
- S. R. Bahn and K. W. Jacobsen, Phys. Rev. Lett., 2001, 87, 266101 CrossRef CAS PubMed.
- S. Fujii, M. Ziatdinov, S. Higashibayashi, H. Sakurai and M. Kiguchi, J. Am. Chem. Soc., 2016, 138, 12142–12149 CrossRef CAS PubMed.
- A. Morpurgo, C. Marcus and D. Robinson, Appl. Phys. Lett., 1999, 74, 2084–2086 CrossRef CAS.
| This journal is © the Partner Organisations 2018 |



