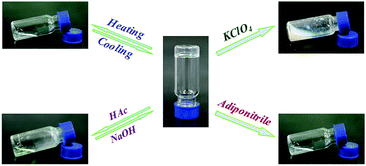
Pillar[5]arene-based multifunctional supramolecular hydrogel: multistimuli responsiveness, self-healing, fluorescence sensing, and conductivity†
Jin-Fa
Chen
,
Qi
Lin
 ,
Hong
Yao
,
You-Ming
Zhang
,
Hong
Yao
,
You-Ming
Zhang
 and
Tai-Bao
Wei
and
Tai-Bao
Wei
 *
*
Key Laboratory of Eco-Environment-Related Polymer Materials, Ministry of Education of China, Key Laboratory of Polymer Materials of Gansu Province, College of Chemistry and Chemical Engineering, Northwest Normal University, Lanzhou, Gansu 730070, P. R. China. E-mail: weitaibao@126.com; Fax: +86 9317973191; Tel: +86 9317973191
First published on 16th March 2018
Abstract
A novel pillar[5]arene-based multifunctional supramolecular hydrogel (HG) has been successfully constructed. The obtained supramolecular hydrogel shows excellent self-healing capacity. Meanwhile, this supramolecular hydrogel exhibited good multi-stimuli responsive behavior including thermal-responsiveness, pH-responsiveness, anion-responsiveness, and competitive agent-responsiveness. In addition, this hydrogel could effectively detect iron ions (Fe3+) by fluorescence. The fluorescence spectra detection limit was 7.86 × 10−10 M, indicating the ultra-sensitivity of the hydrogel to Fe3+. The thin film based on this hydrogel was also prepared, which was confirmed to be a convenient test kit for detecting Fe3+. Furthermore, this supramolecular hydrogel (HG) is also an excellent conductive material.
Introduction
Hydrogels are 3D hydrophilic cross-linked soft materials with physical characteristics similar to soft biological tissues, and can hold a large amount of water via surface tension or capillary effect, making them increasingly important for widespread applications ranging from academic research to industrial fields.1 In view of the types of driving forces for crosslinking, hydrogels can be divided into two major categories: chemical hydrogels and supramolecular hydrogels (physical hydrogel). Generally, chemical hydrogels are formed by permanent chemical crosslinks between the polymer chains via non-reversible covalent bonds.2 Chemical hydrogels based on both natural and synthetic polymers have been of great interest for a wide range of biomedical applications from drug delivery to tissue engineering owing to their hydrophilic character and potential to be biocompatible.3 However, such hydrogels are often brittle, at times opaque and without the ability to self-heal when the cross-linked network is broken, thus greatly limiting their application in various biomedical fields.4Supramolecular hydrogels, which perfectly combine the advantages of chemical hydrogels with reversibility of supramolecular interactions,5 are a novel class of noncovalently cross-linked gel materials.6 The supramolecular cross-linking by various noncovalent interactions such as hydrogen bonding, metal–ligand coordination, π–π stacking, host–guest recognition, and electrostatic interaction remarkably reduces the structural flexibility and alters the macroscopic performance, resulting in the formation of 3D cross-linked networks. In sharp contrast, such noncovalent hydrogels show not only the moderate mechanical properties gained from polymeric building blocks, but also show reversible gel–sol transition behavior in response to a wide variety of bio-related stimuli (e.g., pH, redox agents, enzymes, bioactive molecules)7 and processability inherent to the supramolecular cross-linking units, which can serve as either intelligent carriers for delivering versatile therapeutic agents (e.g., drugs, genes, proteins)8 or promising matrices for repairing and regenerating tissues and organs in the human body. Therefore, supramolecular hydrogels have been attracting increasing attention from scientists.
Pillararenes,9 a new class of macrocycles, are composed of hydroquinone units linked by methylene bridges at the para positions. Pillararenes have been garnering much attention due to their broad applications, such as fluorescent sensing,10 biological imaging,11 transmembrane transport,12 gas adsorption13 and so on.14 Of course, pillararene-based supramolecular gels have also been constructed and prepared via various noncovalent interactions, especially host–guest interactions.15 Many pillararene-based supramolecular gels have been reported, however, due to the poor solubility of pillararenes in aqueous media, most investigations of pillararene-based supramolecular gels have been conducted in organic media, which inhibit the application of pillararenes in soft materials. Therefore, to investigate and expand the applications of pillararenes in water, preparation of the pillararene-based supramolecular hydrogel is very necessary.16
Herein, following our success of pillar[5]arene-based supramolecular chemistry,17 a novel multifunctional fluorescent supramolecular hydrogel (HG) is successfully prepared by a functionalized pillar[5]arene-based self-assembly.18 Compared with the chemical gels, this supramolecular hydrogel shows excellent self-healing capacity because of the dynamic self-assembly. It is worth to notice that the healing process in the supramolecular hydrogel is automatically without external treatment. Meanwhile, this supramolecular hydrogel also exhibited good thermal-responsiveness, pH-responsiveness, anion-responsiveness and competitive agent-responsiveness. In addition, this hydrogel could effectively detect iron ions (Fe3+) by fluorescence. The fluorescence spectra detection limit was 7.86 × 10−10 M, indicating the ultra-sensitivity of the hydrogel to Fe3+. More importantly, this supramolecular hydrogel (HG) is also an excellent conductive material.
Results and discussion
The gelator BTAP5 is shown in Scheme 1 and the synthesis details are presented in Scheme S1 (ESI†). Gelator BTAP5 and its intermediate have been characterized by 1H NMR, 13C NMR, and ESI mass spectrometry (Fig. S1–S9, ESI†).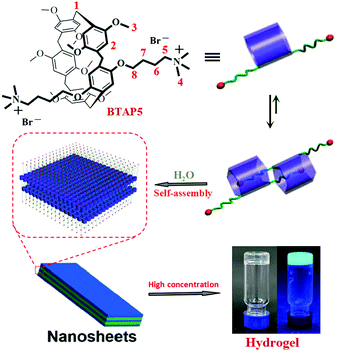 | ||
| Scheme 1 Structure and proton designations of BTAP5, and further self-assembly to form a fluorescent supramolecular hydrogel. | ||
Firstly, we established the self-assembly mode of BTAP5 by 1H NMR and 2D NOESY experiment. In the concentration dependent 1H NMR spectrum of BTAP5 (Fig. S10, ESI†), with the increasing amount of BTAP5, H1, H2 and H3 signals of BTAP5 showed a gradual downfield shift. Simultaneously, the −CH3 (H4) and −CH2 (H5) resonance signals showed obvious downfield shifts, respectively. These indicate that the n-butyltrimethyl ammonium are partially included in the cavity of BTAP5, which was stabilized by C–H⋯O hydrogen bonding interactions and C–H⋯π interactions. The assignment and correlation of the protons were further validated by the NOESY NMR spectrum of BTAP5 (Fig. S11, ESI†). The NOESY spectrum of BTAP5 shows cross peaks A and B representing the correlations between the signal of protons H2 of aromatic protons and those of protons H4 and H5 of n-butyltrimethyl ammonium. Meanwhile, the cross peaks C and D also implied that the n-butyltrimethyl ammonium are included in the cavity of BTAP5. These results suggested that the aggregates of BTAP5 changed from monomers into [c2]daisy chain dimers as the concentration increased (Scheme 1).
Secondly, the gelation properties of the synthesized gelator BTAP5 were investigated. The novel supramolecular hydrogel was prepared by dissolving the monomers BTAP5 in H2O at 75 °C followed by cooling to room temperature, respectively. Upon increasing the concentration of the gelator, formed a hydrogel (HG) at a phase-transition temperature of approximately 28 °C for BTAP5 at the concentration of 5 wt% (10 mg mL−1 = 1 wt%). It is noteworthy that the phase transfer temperature gradually increases with increasing the concentration of the gelator (Fig. S12, ESI†). Moreover, it is interesting to find that the high concentration BTAP5 showed reversible glue–sol phase transitions upon heating and cooling, which could be reversibly achieved many times (Fig. 2). In order to learn the driving forces of the hydrogel formation process, the temperature dependences of BTAP5 were investigated using the UV-Vis spectrum in H2O solution. The absorbance of BTAP5 (0.1 mM) slightly decreased with increasing temperature, implying the occurrence of intermolecular hydrogen bonding interactions because hydrogen bonds are temperature-dependent (Fig. S13, ESI†).19 The X-ray peak (Fig. S14, ESI†) at 2θ = 22.61° (d = 3.93 Å) confirms the π–π stacking interactions. Thus, the gelator BTAP5 self-assembled to hydrogel HG through the hydrogen bonds and π–π stacking interactions. The morphological features of the hydrogel HG were studied using SEM and TEM (Fig. 1), and showed a loose nanosheet structure (Scheme 1).
According to SEM and TEM spectra, we can deduce two possible assembly processes. As shown in Fig. S15 (ESI†), we can easily find that the cavity of BTAP5 forms 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complexes with n-butyltrimethyl ammonium from the assembly process 1. However, in the ESI-MS spectrum (Fig, S16, ESI†), we only found the 1
2 complexes with n-butyltrimethyl ammonium from the assembly process 1. However, in the ESI-MS spectrum (Fig, S16, ESI†), we only found the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 correlation peak between DMP5 and G′, and no 1
1 correlation peak between DMP5 and G′, and no 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 correlation peak can be found. This phenomenon suggested that the nanosheet structure was formed due to stacking of [c2]daisy chain dimers. The [c2]daisy chain dimer could be caused because the 1
2 correlation peak can be found. This phenomenon suggested that the nanosheet structure was formed due to stacking of [c2]daisy chain dimers. The [c2]daisy chain dimer could be caused because the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex was formed between the cavity of BTAP5 and n-butyltrimethyl ammonium. In addition, the X-ray diffraction measurement also show a strong diffraction at 13.89° (Fig. S14, ESI†), which implied the thickness of the sheet-like structures. Hence, the calculated thickness of the sheet-like aggregates was 11.12 nm, which is larger than the extend length of the [c2]daisy chain dimer (Fig. S17, ESI†) and indicates that the nanosheets were multilayers.
1 complex was formed between the cavity of BTAP5 and n-butyltrimethyl ammonium. In addition, the X-ray diffraction measurement also show a strong diffraction at 13.89° (Fig. S14, ESI†), which implied the thickness of the sheet-like structures. Hence, the calculated thickness of the sheet-like aggregates was 11.12 nm, which is larger than the extend length of the [c2]daisy chain dimer (Fig. S17, ESI†) and indicates that the nanosheets were multilayers.
The response to external stimuli was a significant property for smart hydrogels. Therefore, stimuli-responsive behaviors of the supramolecular hydrogel HG were investigated. As the acid has a strong proton-donating ability, the pH value of the solution has a strong effect on the formation of hydrogen bonds. Therefore, the supramolecular hydrogel HG showed the corresponding acid–base responsiveness. As shown in Fig. 2, after the addition of several drops of the CH3COOH solution, intermolecular hydrogen bonds have been destroyed.20 Correspondingly, the supramolecular hydrogel dissociated into a transparent solution within a short time. The fiber-like structure was clearly observed by scanning electron microscopy (Fig. S18a, ESI†). In the 1H NMR spectrum (Fig. S19, ESI†), H1, H2, H3 and H4 signals of BTAP5 showed a gradual downfield shift. This results also suggested that intermolecular hydrogen bonds are destroyed. Subsequent addition of NaOH aqueous solution to the transparent solution led to the recovery of gel state.
Inspired by the applications of dynamic characteristics of ion competitive coordination in the area of self-organization and supramolecular transformations,21 the anion-stimuli-responsive behavior of the resultant supramolecular hydrogels was expected. As shown in Fig. 2, if ClO4− was added to the viscous solution at high temperature, the obtained solution could not form a supramolecular gel on cooling, and the viscous solution ultimately became a turbid solution. As detected by SEM measurement (Fig. S18b, ESI†), the sheet-like structure of hydrogel was also destroyed and turned into a block microstructure after the addition of KClO4. 1H NMR investigation of BTAP5 (Fig. S20, ESI†) evidenced that this stimuli-responsive gel–sol phase transition was essentially a consequence of the ClO4−-induced disassembly. The possible cause of this stimulus response is that ClO4− have a stronger binding capacity than cavity of pillararene with n-butyltrimethyl ammonium.
In addition, if the competitive guest adiponitrile was added to the viscous solution at high temperature, the obtained solution could not form a supramolecular hydrogel on cooling due to adiponitrile having stronger competitive binding with pillar[5]arenes than the n-butyltrimethyl ammonium,22 resulting in destroying the [c2]daisy chain dimers (Fig. 2). The SEM measurement (Fig. S18c, ESI†) also showed the sheet structure of hydrogel were destroyed and turned into a inter-weaved network structure after the addition of adiponitrile.
Owing to dynamic reversibility of hydrogen bonding interactions and π–π stacking interactions, the self-healing property of the supramolecular hydrogel HG was studied. As depicted in Fig. 3, three pieces of hydrogels were prepared, of which two hydrogel pieces were colored with a methyl red dye to render a better contrast, and the other is colorless. Interestingly, three pieces of hydrogel are put together, and they can merge into a single one autonomously in 3 min, and the joint is strong enough to be lifted without breakage of the three parts. After 24 h, it was found that the color pink gradually spread into the colorless part, which again verified the self-healing behavior. It is worth to notice that the healing process in the supramolecular HG hydrogel is automatically without external treatment, which is advantageous comparing with the materials that needs external intervention to heal cracks.
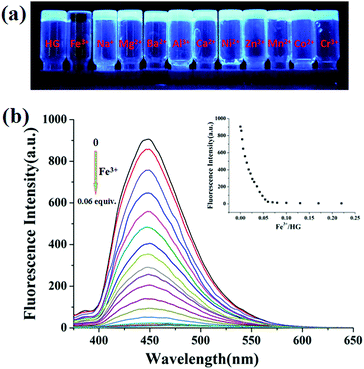
Next, the cation sensing ability of this supramolecular hydrogel was also investigated. The recognition profiles of hydrogel (5 wt%) toward various cations, including Fe3+, Na+, Mg2+, Ba2+, Al3+, Ca2+, Ni2+, Zn2+, Mn2+, Co2+ and Cr3+, were primarily investigated using fluorescence spectroscopy. As shown in Fig. S21 (ESI†), when adding one equivalent of Fe3+ to the supramolecular hydrogel HG, the fluorescence emission band at 449 nm disappeared. Other cations could not induce similar fluorescence changes (Fig. 4a). Therefore, this hydrogel could effectively sense Fe3+ with specific selectivity. Moreover, when gradually adding Fe3+, the emission intensity at 449 nm decreased with the increasing concentration of Fe3+ as shown in Fig. 4b. The detection limit of the fluorescence spectral change was calculated on the basis of 7.86 × 10−10 M (Fig. S22, ESI†), indicating the ultra-sensitivity to Fe3+. Compared with other detection techniques and pillararene-based sensors described in previous reports (Tables S1 and S2, ESI†), this work showed a lower detection limit. Therefore, this supramolecular hydrogel with specific response to Fe3+ ions has great potential in future biological and environmental monitoring. In addition, Fe3+ responsive films based on this supramolecular hydrogel were prepared by pouring the heated water solution of the hydrogel onto a clean glass surface and then drying it in air. These thin films were utilized to sense Fe3+. As shown in Fig. S23 (ESI†), when Fe3+ ions were added onto the thin film, an obvious color change was observed under irradiation at 365 nm using a UV lamp. Therefore, the thin film could be a convenient test kit for detecting Fe3+. In the FT-IR spectra (Fig. S24, ESI†) and the powder XRD patterns (Fig. S25, ESI†), when Fe3+ cations were added to the hydrogel HG, all the peaks did not cause obvious shifts. Therefore, we think the heavy atom effect of Fe3+ ions causes blue fluorescence quenching.
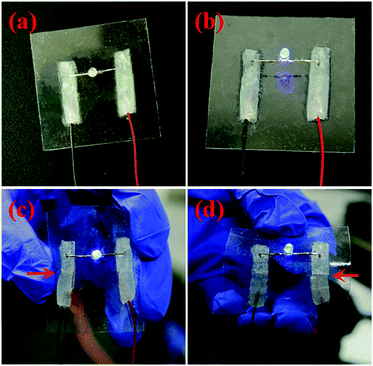
Finally, conductive properties of the supramolecular hydrogel HG have been studied. Because gelator BTAP5 is a typical cationic pillar[5]arene, there are free-moving charged ions (Br−) in water solution. Therefore, we believe that this hydrogel has the corresponding conductivity. In order to demonstrate the conductivity of the hydrogel HG, we designed a complete circuit composed of a LED bulb with driving voltage of 1.5 V as the electrical load, HG hydrogel film as the conductor and one dry batteries (1.5 V) as the power source. As shown in Fig. 5, a circle of HG hydrogel thin film was coated on PDMS substrate, then the LED bulb was involved and the power source was linked into the circuit by two copper wires. The bulb was successfully lighted when the circuit was switched to close status, indicating the good conductivity of our hydrogel HG. Notably, this circuit could work well under bended, even folded states. At last, the self-healing property of the hydrogel HG was also demonstrated on the circuit. As shown in Fig. 5c and d, in a typical test of HG hydrogel film was cut and the circuit then became open and the bulb was extinguished. After about 10 s, the circuit was re-established and the LED bulb could be lighted up again. This self-healing test was successfully conducted for multiple times at the same position and also at other positions. Furthermore, the conductivity of HG hydrogel film was tested by using four-probe method and can reach as high as 5.7 S m−1. These data and phenomena show the great potential of the hydrogel HG for practical applications such as self-healing electronics, biosensors, and artificial skins.
Conclusions
In summary, a functionalized pillar[5]arene-based gelator BTAP5 has been synthesized, which could further self-assemble into a [c2]daisy chain dimer via the intermolecular hydrogen bonding interactions. Furthermore, the [c2]daisy chain dimer could self-assemble to form a fluorescent supramolecular hydrogel (HG) at high concentration by the π–π stacking interactions. Interestingly, the obtained supramolecular hydrogel HG exhibited thermoreversible gel–sol transformation, whereas, adding the acid (CH3COOH) resulted in the gel state dissociated into a transparent solution. More importantly, when KClO4 was added to the viscous solution at high temperature, the obtained solution could not form a supramolecular hydrogel on cooling, and the viscous solution ultimately became a turbid solution. Moreover, the supramolecular hydrogel also exhibited competitive agent-responsiveness. These phenomena showed that this supramolecular hydrogel has excellent stimulus-response behavior. When Fe3+ are added to the supramolecular hydrogel, the strong blue fluorescence of hydrogel is clearly quenched, and this hydrogel could highly selectively and ultra-sensitively (7.86 × 10−10 M) detect Fe3+ cations. Moreover, a thin film of the fluorescent supramolecular hydrogel was prepared, which was confirmed to be a convenient test kit for detecting Fe3+. It is noteworthy that this supramolecular hydrogel is also an excellent self-healing and conductive material. This research not only developed a novel multiple-stimuli responsive supramolecular hydrogels based on pillararene but also expanded the property of pillararene-based gels about self-healing and conductivity. Thus, this good example might stimulate wide interest of scientists for further development of new pillararene-based supramolecular hydrogels.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC) (No. 21662031; 21661028; 21574104) and the Program for Changjiang Scholars and Innovative Research Team in University of Ministry of Education of China (IRT 15R56).Notes and references
- (a) L. A. Estroff and A. D. Hamilton, Chem. Rev., 2004, 104, 1201 CrossRef CAS PubMed; (b) N. M. Sangeetha and U. Maitra, Chem. Soc. Rev., 2005, 34, 821 RSC; (c) Y. Gao, F. Zhao, Q. Wang, Y. Zhang and B. Xu, Chem. Soc. Rev., 2010, 39, 3425 RSC; (d) F. Zhao, M. L. Ma and B. Xu, Chem. Soc. Rev., 2009, 38, 883 RSC.
- O. Wichterle and D. Lim, Nature, 1960, 185, 117 CrossRef.
- (a) H. Zhang, L. Bré, T. Zhao, B. Newland, M. Da Costa and W. Wang, J. Mater. Chem. B, 2014, 2, 4067 RSC; (b) Y. Dong, W. U. Hassan, R. Kennedy, U. Greiser, A. Pandit, Y. Garcia and W. Wang, Acta Biomater., 2014, 10, 2076 CrossRef CAS PubMed.
- J. Li, NPG Asia Mater., 2010, 2, 112 CrossRef.
- (a) T. Aida, E. W. Meijer and S. I. Stupp, Science, 2012, 335, 813 CrossRef CAS PubMed; (b) X. Yan, F. Wang, B. Zheng and F. Huang, Chem. Soc. Rev., 2012, 41, 6042 RSC; (c) R. Dong, Y. Zhou and X. Zhu, Acc. Chem. Res., 2014, 47, 2006 CrossRef CAS PubMed.
- (a) S. S. Babu, V. K. Praveen and A. Ajayaghosh, Chem. Rev., 2014, 114, 1973 CrossRef CAS PubMed; (b) E. A. Appel, J. del Barrio, X. J. Loh and O. A. Scherman, Chem. Soc. Rev., 2012, 41, 6195 RSC.
- (a) M. Ikeda, T. Tanida, T. Yoshii, K. Kurotani, S. Onogi, K. Urayama and I. Hamachi, Nat. Chem., 2014, 6, 511 CrossRef CAS PubMed; (b) T. Yoshii, M. Ikeda and I. Hamachi, Angew. Chem., Int. Ed., 2014, 53, 7264 CrossRef CAS PubMed; (c) J. Zhou, X. Du, Y. Gao, J. Shi and B. Xu, J. Am. Chem. Soc., 2014, 136, 2970 CrossRef CAS PubMed; (d) L. E. R. O’Leary, J. A. Fallas, E. L. Bakota, M. K. Kang and J. D. Hartgerink, Nat. Chem., 2011, 3, 821 CrossRef PubMed.
- (a) E. A. Appel, X. J. Loh, S. T. Jones, F. Biedermann, C. A. Dreiss and O. A. Scherman, J. Am. Chem. Soc., 2012, 134, 11767 CrossRef CAS PubMed; (b) H. Komatsu, S. Matsumoto, S.-i. Tamaru, K. Kaneko, M. Ikeda and I. Hamachi, J. Am. Chem. Soc., 2009, 131, 5580 CrossRef CAS PubMed.
- (a) T. Ogoshi, T. Yamagishi and Y. Nakamoto, Chem. Rev., 2016, 116, 7937 CrossRef CAS PubMed; (b) M. Xue, Y. Yang, X. Chi, Z. Zhang and F. Huang, Acc. Chem. Res., 2012, 45, 1294 CrossRef CAS PubMed; (c) D. Cao, Y. Kou, J. Liang, Z. Chen, L. Wang and H. Meier, Angew. Chem., Int. Ed., 2009, 48, 9721 CrossRef CAS PubMed.
- (a) J.-F. Chen, Q. Lin, Y.-M. Zhang, H. Yao and T.-B. Wei, Chem. Commun., 2017, 53, 13296 RSC; (b) M. Bojtár, J. Kozma, Z. Szakács, D. Hessz, M. Kubinyi and I. Bitter, Sens. Actuators, B, 2017, 248, 305 CrossRef.
- C. Sathiyajith, R. R. Shaikh, Q. Han, Y. Zhang, K. Meguellati and Y.-W. Yang, Chem. Commun., 2017, 53, 677 RSC.
- R. Wang, Y. Sun, F. Zhang, M. Song, D. Tian and H. Li, Angew. Chem., 2017, 129, 5378 CrossRef.
- L.-L. Tan, H. Li, Y. Tao, S. X.-A. Zhang, B. Wang and Y.-W. Yang, Adv. Mater., 2014, 26, 7027 CrossRef CAS PubMed.
- (a) K. Yang, Y. Pei, J. Wen and Z. Pei, Chem. Commun., 2016, 52, 9316 RSC; (b) L.-L. Tan and Y.-W. Yang, J. Inclusion. Phenom., 2015, 81, 13 CrossRef CAS; (c) Y. Wang, G. Ping and C. Li, Chem. Commun., 2016, 52, 9858 RSC; (d) C. Li, Chem. Commun., 2014, 50, 12420 RSC.
- (a) L. Liu, D. Cao, Y. Jin, H. Tao, Y. Kou and H. Meier, Org. Biomol. Chem., 2011, 9, 7007 RSC; (b) W. Cui, H. Tang, L. Xu, L. Wang, H. Meier and D. Cao, Macromol. Rapid Commun., 2017, 38, 1700161 CrossRef PubMed; (c) X. Wu, Y. Yu, L. Gao, X.-Y. Hu and L. Wang, Org. Chem. Front., 2016, 3, 966 RSC; (d) P. Chen, J. H. Mondal, Y. Zhou, H. Zhu and B. Shi, Polym. Chem., 2016, 7, 5221 RSC; (e) Y. Yao, Y. Sun, H. Yu, W. Chen, H. Dai and Y. Shi, Dalton Trans., 2017, 46, 16802 RSC.
- (a) M. Ni, N. Zhang, W. Xia, X. Wu, C. Yao, X. Liu, X.-Y. Hu, C. Lin and L. Wang, J. Am. Chem. Soc., 2016, 138, 6643 CrossRef CAS PubMed; (b) H. Ju, F. Zhu, H. Xing, Z. L. Wu and F. Huang, Macromol. Rapid Commun., 2017, 38, 1700232 CrossRef PubMed.
- (a) T.-B. Wei, J.-F. Chen, X.-B. Cheng, H. Li, B.-B. Han, Y. Yao, Y.-M. Zhang and Q. Lin, Polym. Chem., 2017, 8, 2005 RSC; (b) Q. Lin, K.-P. Zhong, J.-H. Zhu, L. Ding, J.-X. Su, H. Yao, T.-B. Wei and Y.-M. Zhang, Macromolecules, 2017, 50, 7863 CrossRef CAS; (c) Q. Lin, Y.-Q. Fan, P.-P. Mao, L. Liu, J. Liu, Y.-M. Zhang, H. Yao and T.-B. Wei, Chem. – Eur. J., 2018, 24, 777 CrossRef CAS PubMed.
- T.-B. Wei, J.-F. Chen, X.-B. Cheng, H. Li, B.-B. Han, Y.-M. Zhang, Y. Yao and Q. Lin, Org. Chem. Front., 2017, 4, 210 RSC.
- B. Shi, D. Xia and Y. Yao, Chem. Commun., 2014, 50, 1393 Search PubMed.
- L. Fang, Y. Hu, Q. Li, S. Xu, M. K. Dhinakarank, W. Gong and G. Ning, Org. Biomol. Chem., 2016, 14, 4039 CAS.
- (a) C. Han, G. Yu, B. Zheng and F. Huang, Org. Lett., 2012, 14, 1712 CrossRef CAS PubMed; (b) S. Sun, X.-Y. Hu, D. Chen, J. Shi, Y. Dong, C. Lin, Y. Pan and L. Wang, Polym. Chem., 2013, 4, 2224 RSC; (c) Q. Lin, T.-T. Lu, X. Zhu, T.-B. Wei, H. Li and Y.-M. Zhang, Chem. Sci., 2016, 7, 5341 RSC.
- (a) X. Shu, S. Chen, J. Li, Z. Chen, L. Weng, X. Jia and C. Li, Chem. Commun., 2012, 48, 2967 RSC; (b) X. Shu, W. Chen, D. Hou, Q. Meng, R. Zheng and C. Li, Chem. Commun., 2014, 50, 4820 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Characterizations, 1H NMR date and other materials. See DOI: 10.1039/c8qm00065d |
| This journal is © the Partner Organisations 2018 |