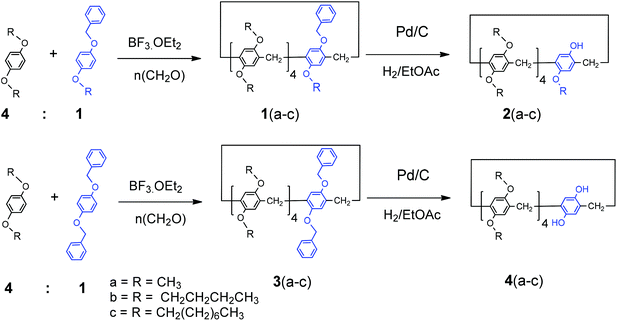
A new approach for the synthesis of mono- and A1/A2-dihydroxy-substituted pillar[5]arenes and their complexation with alkyl alcohols in solution and in the solid state†
Talal F.
Al-Azemi
 *,
Abdirahman A.
Mohamod
,
Mickey
Vinodh
and
Fatemeh H.
Alipour
*,
Abdirahman A.
Mohamod
,
Mickey
Vinodh
and
Fatemeh H.
Alipour
Chemistry Department, Kuwait University, P.O. Box 5969, Safat 13060, Kuwait. E-mail: t.alazemi@ku.edu.kw; Fax: +965-2481-6482; Tel: +965-2498-554
First published on 11th October 2017
Abstract
A new approach was employed for the synthesis of mono- and A1/A2-dihydroxy-functionalized pillar[5]arenes by the removal of the pillar[5]arene-bearing benzyl group(s) using catalytic hydrogenation. Host–guest complexation between a mono-hydroxy-pillar[5]arene with long-chain alkyl alcohol guests was studied. The encapsulation characteristics of the pillar[5]arene was affected by the presence of a hydroxy group, resulting in the formation of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complex with long-chain alkyl alcohols in solution and in the solid state. For comparison, analog experiments were conducted with permethylated pillar[5]arene (DMP5) and long-chain alkyl alcohol guests. The complexation experiments revealed that the absence of a hydroxyl group on the pillar[5]arene frame resulted in the formation of a 1
2 complex with long-chain alkyl alcohols in solution and in the solid state. For comparison, analog experiments were conducted with permethylated pillar[5]arene (DMP5) and long-chain alkyl alcohol guests. The complexation experiments revealed that the absence of a hydroxyl group on the pillar[5]arene frame resulted in the formation of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex. The formed complexes were confirmed by proton nuclear magnetic resonance spectroscopy and single-crystal X-ray analysis.
1 complex. The formed complexes were confirmed by proton nuclear magnetic resonance spectroscopy and single-crystal X-ray analysis.
Introduction
Macrocyclic host molecules include crown ethers,1 calixarenes,2 cucurbiturils,3 cyclodextrins,4 cyclophanes,5 and their structurally related scaffolds.6 Pillararenes have been the focus of considerable attention because of their interesting conformational, physicochemical, and host–guest properties. Pillararenes are a relatively new class of macrocyclic compounds, first reported by Ogoshi et al.7a in 2008; they are the product of the condensation of 1,4-dimethoxybenzene and formaldehyde in the presence of the Lewis acid BF3·OEt2. The most prevalent pillararenes include pillar[5]arenes7a,b pillar[6]arenes,7c and pillar[7]arenes.7d,e All pillararenes are formed from methylene (–CH2–) bridges that link the para-positions of 2,5-dialkoxybenzene rings to form a unique, rigid, pillar architecture, which differs from the basket-shaped structure of meta-bridged calixarenes.Pillar[5]arenes exhibit interesting host–guest properties if the electrostatic potentials of the cavity are significantly negative, favoring the binding of positively charged guests.7f Pillar[5]arene and its derivatives have been shown to act as good hosts for a variety of organic compounds such as viologens,8a alkanediamines,8b dinitrobenzene8c and azobenzene derivatives,8d and neutral molecules.8e–g
Pillararenes provide a useful platform for the construction of various interesting supramolecular systems, and enable properties such as solubility, optical response, and crystallinity to be tuned. The availability of strategically placed functional groups on macrocyclic hosts is of high interest for both organic and supramolecular chemists. There have been several reports on the synthesis of functionalized pillar[5]arenes bearing bromo,9a amino,9b alkyne,9c and hydroxy groups.10–12 Several synthetic methods have been developed to obtain mono-, di-, and tetrahydroxy-functionalized pillar[5]arene derivatives. For example, a mono-hydroxy pillar[5]arene has been synthesized by controlling the de-O-methylation of permethylated pillar[5]arene using 0.9 eq. of BBr3.10a An oxidation-followed-by-reduction strategy was used to synthesize di- and tetrahydroxy pillar[5]arenes.11 Another approach was developed to synthesize mono- and A1/A2-dihydroxy-functionlized pillar[5]arenes through the co-cyclization of 1,4-dimethoxybenzene and 1,4-bis(3-bromopropoxy)benzene, which gave the diallyl ether after an elimination reaction followed by deprotection.12
Herein we report on the syntheses of mono- and A1/A2-dihydroxy-substituted-pillar[5]arenes via the selective removal of benzyl groups by catalytic hydrogenation. The complexation with long-chain-alkyl alcohols was investigated by 1H nuclear magnetic resonance (NMR) titration and X-ray-single-crystal diffraction techniques. To the best of our knowledge, no reports on the use of benzyl groups to synthesize hydroxy-functionalized pillar[5]arenes exist, nor has their complexation with alkyl alcohols been reported.
Results and discussion
Synthesis of mono- and A1/A2-dihydroxy-functionalized pillar[5]arenes
Although different strategies have been employed in the synthesis of mono-, di-, and tetra-hydroxy-pillar[5]arenes, permethylated pillar[5]arene is typically used as the starting material. Introducing a group that can be selectively and easily removed after the formation of the macrocycle host enables the introduction of various types and sizes of substituents to the pillar rim and allows structural modifications to be made to the receptor to tune its guest encapsulation characteristics and reactivity. Pillar[5]arenes bearing benzyl group(s) 1(a–c) were synthesized by the condensation of hydroquinone derivatives of 1-(benzyloxy)-4-alkoxybenzene or 1,4-bis(benzyloxy)benzene and 1,4-dialkoxybenzene in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio with paraformaldehyde in the presence of BF3·OEt2, as shown in Scheme 1.
4 ratio with paraformaldehyde in the presence of BF3·OEt2, as shown in Scheme 1.
The monobenzylpillar[5]arenes 1(a–c) were subjected to catalytic hydrogenation under mild reaction conditions using palladium on charcoal in anhydrous ethyl acetate to remove the benzyl protecting groups. The complete removal of the benzyl groups afforded the corresponding mono-hydroxy pillar[5]arenes, which were isolated as white solids in quantitative yields (Scheme 1). The absence of signals in the 1H NMR (CDCl3) spectra corresponds to the benzyl methylene (Ph-C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2) and phenyl (Ph) groups between 4.5–4.9 and 7.2–7.4 ppm, respectively, indicated the complete removal of the benzyl groups. A broad resonance centered at 8.4 ppm was also indicative of the newly formed free phenolic hydroxy groups (ArOH). Methoxy protons (OC
2) and phenyl (Ph) groups between 4.5–4.9 and 7.2–7.4 ppm, respectively, indicated the complete removal of the benzyl groups. A broad resonance centered at 8.4 ppm was also indicative of the newly formed free phenolic hydroxy groups (ArOH). Methoxy protons (OC![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3) and methylene bridge protons (–C
3) and methylene bridge protons (–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–) were observed at 4.25 and 1.20 ppm, respectively. The resonances in the 13C NMR spectra corresponding to the aromatic carbons of the benzyl groups at 128 ppm were greatly reduced, and the absence of a benzyl methylene signal (Ph-
2–) were observed at 4.25 and 1.20 ppm, respectively. The resonances in the 13C NMR spectra corresponding to the aromatic carbons of the benzyl groups at 128 ppm were greatly reduced, and the absence of a benzyl methylene signal (Ph-![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) H2) at around 70 ppm further confirmed the complete removal of the benzyl group. Similarly, A1/A2-dihydroxypillar[5]arenes were synthesized with different dialkoxy groups by co-cyclization with 1,4-dibenzoxybenzene derivatives, as shown in Scheme 1.
H2) at around 70 ppm further confirmed the complete removal of the benzyl group. Similarly, A1/A2-dihydroxypillar[5]arenes were synthesized with different dialkoxy groups by co-cyclization with 1,4-dibenzoxybenzene derivatives, as shown in Scheme 1.
Binding studies
A mono-hydroxy-pillar[5]arene, Pillar-2a, was selected as a model host for the investigation of the encapsulation ability of long-chain alcohols (hexanol G1, heptanol G2, and octanol G3). 1H NMR titration experiments were performed in CDCl3 at 25 °C at a fixed concentration of the host 2a (11 mM) and at various concentrations of the guest (G1, G2, or G3). For comparison, analogous 1H NMR titration experiments with permethylated-pillar[5]arene (DMP5) were carried out by fixing the concentration of the guest (0.75 mM) and varying the concentration of the host.From the titration experiments, the 1H NMR spectra of an equimolar amount of the host (Pillar-2a) and guests (G1, G2, and G3) in chloroform-d show only one set of peaks, indicating fast-exchange complexation on the 1H NMR time scale at 25 °C. After complexation, a downfield shift was observed for phenyl protons, bridging methylene protons and methyl protons on the Pillar-2a. Fig. 1 show representative examples of the complexation of receptors Pillar-2a and DMP5 with a heptanol guest, G2. The peak assignments are based on the 1H–1H COSY and 1H–13C HSQC experiments of the complexes. Fig. 1c shows the complexation of Pillar-2a with heptanol (G2), the signals of guest methylene protons H3, H4 and H5 overlapped together and showed upfield chemical shift changes due to their presence in the shielding region of the cyclic pillar structure. A similar effect was observed for the methylene protons H2 and H6. However, the guest methyl protons show no significant chemical shift changes, which indicate that these protons are not affected by the cyclic pillar[5]arene structure. At a high concentration of the host, a significant upfield shift for the methylene protons of the guest molecule was observed (see the ESI†). These results indicate that methylene protons were threaded through the cavity of the cyclic host. Analogue 1H NMR titration of DMP5 with a heptanol guest (G2) showed similar results with lower chemical shift changes (Fig. 1b).
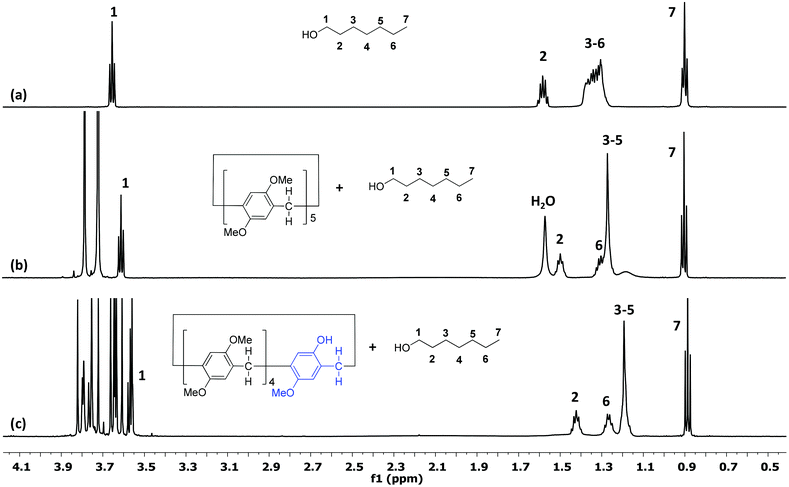 | ||
| Fig. 1 1H NMR spectra (600 MHz, CDCl3, 25 °C) of (a) a heptanol guest (G2); (b) 7.5 mM of G2 and 7.5 mM of DMP5; (c) 10 mM of Pillar-2a and 10 mM of G2. | ||
The stoichiometry of the host–guest was established using Job's plots between the mole fraction of the host (Xhost) and the chemical shift change of phenyl protons on Pillar-2a in 1H NMR multiplied by the mole fraction (Xhost). For example, Job's plots of host Pillar-2a with octanol, G3, in CDCl3 showed maxima at a mole fraction of 0.33, as shown in Fig. 2a. These results indicate a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 host-to-guest stoichiometric ratio of complexation. Similar results were obtained when hexanol and heptanol are used as guests. The absence of a hydroxy functional group in the permethylated pillar[5]arene DMP5, with an alkyl alcohol guest, showed a 1
2 host-to-guest stoichiometric ratio of complexation. Similar results were obtained when hexanol and heptanol are used as guests. The absence of a hydroxy functional group in the permethylated pillar[5]arene DMP5, with an alkyl alcohol guest, showed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host-to-guest stoichiometric ratio of complexation (Fig. 2b). The encapsulation characteristic of the host was affected by the presence of a hydroxy functional group on the pillar[5]arene frame.
1 host-to-guest stoichiometric ratio of complexation (Fig. 2b). The encapsulation characteristic of the host was affected by the presence of a hydroxy functional group on the pillar[5]arene frame.
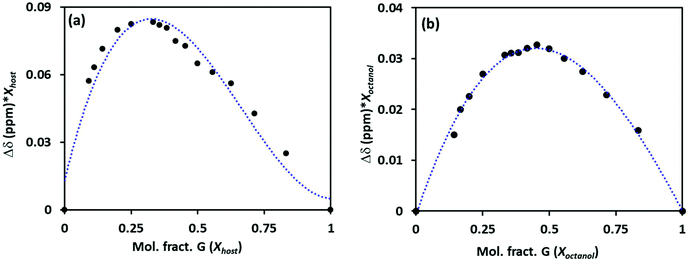 | ||
| Fig. 2 Job's plot for the complexation of hosts Pillar-2a (a) and DMP5 (b) with an octanol guest G3, determined from 1H NMR titration in CDCl3 at 25 °C. | ||
The association constant for complexation was determined to form the nonlinear least-squares treatment of 1H NMR titration based on the chemical shift changes of phenyl protons for host Pillar-2a (see the ESI†). The data fitted well to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 binding isotherm and the association constant K12 was determined to be 8.9 ± 0.2 × 102, 1.3 ± 0.1 × 103 and 1.7 ± 0.1 × 103 M−1 for guests G1, G2, and G3, respectively.13 For the premethylated analog DMP5, the binding constant was determined from chemical shift changes of the methylene protons attached to the hydroxy group of the guest alkyl alcohol at 3.62 ppm and the data fitted to a 1
2 binding isotherm and the association constant K12 was determined to be 8.9 ± 0.2 × 102, 1.3 ± 0.1 × 103 and 1.7 ± 0.1 × 103 M−1 for guests G1, G2, and G3, respectively.13 For the premethylated analog DMP5, the binding constant was determined from chemical shift changes of the methylene protons attached to the hydroxy group of the guest alkyl alcohol at 3.62 ppm and the data fitted to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding isotherm.
1 binding isotherm.
The association constant K11 was calculated to be 124 ± 12, 218 ± 11, and 306 ± 24 M−1 for guests G1, G2, and G3, respectively. The high binding affinity of Pillar-2a with alkyl alcohols was attributed to hydrogen bonding interactions between the hydroxy group on the rigid pillar[5]arene host and the hydroxy group of the alkyl alcohols. For both receptors, increasing the length of the alkyl chain improved binding because of the greater numbers of C–H⋯π interactions and H-bonds between the alkyl chain and the host, as shown by the calculated association constants. This effect is well documented for host–guest pillararene systems.14
Crystallography
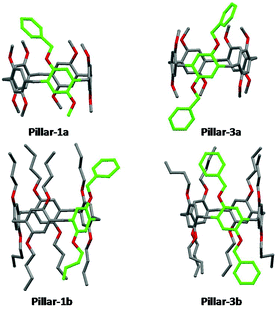
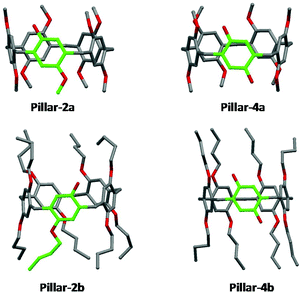
X-ray single crystal structures of benzylated and hydroxylated pillar[5]arene derivatives are shown in Fig. 3 and 4. No suitable crystals of the octoxy-pillar[5]arene derivatives (Pillar 1c–4c) could be obtained for X-ray analysis.The crystal structure of mono-hydroxy Pillar-2b shows the formation of a supramolecular double-threaded dimer where one of the butyl chains is threaded inside the cavity of the adjacent pillar[5]arene (Fig. 5). The formation of the dimer is induced by both intermolecular hydrogen bonds between the two pillararenes and by C–H⋯π interactions between the alkyl chain and the host. For A1/A2-dihydroxy-pillar[5]arene (Pillar-4b), intramolecular hydrogen bonding prevented the formation of a supramolecular dimer. A supramolecular double-threated dimer has been reported in the solid state for a co-pillar[5]arene based on 1,4-dimethoxybenzene and 1-((10-bromodecyl)oxy)-4-methoxybenzene.14
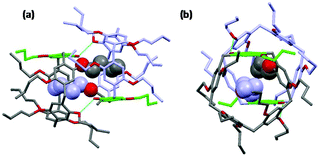
The solutions were slowly cooled and evaporated, allowing the inclusion compounds to crystallize. The structures of the inclusion complexes of a mono-hydroxypillar[5]arene (Pillar-2a) with alkyl alcohols G1–3, derived from XRD data, are shown in Fig. 6. While the physical shape and size of the structures in the crystalline state were different, the crystallographic space group of all inclusion complexes was the same, and the unit cell parameters were similar. Each unit cell contains two alkyl alcohol molecules inside the cavity of two hydroxypillar[5]arenes (see the ESI†). The inclusion complexes of Pillar-2 with alkyl alcohol guests packed to form dimeric head-to-head supramolecular assemblies, whose formation was induced by hydrogen bonding interactions. The supramolecular assembly is shown in the ESI.†
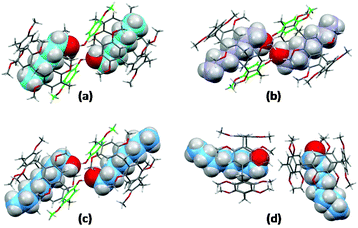 | ||
| Fig. 6 Crystal structures of inclusion complexes of (a) Pillar-2a and hexanol (G1); (b) Pillar-2a and heptanol (G2); (d) Pillar-2a and octanol (G3); (d) DMP5 and octanol (G3). | ||
A close inspection of the crystal structures of the inclusion complexes revealed that the hydroxy group on the pillar[5]arene frame is bonded to two alcohol molecules, one of which is encapsulated inside the host cavity. Single-crystal X-ray diffraction studies agree with the 1H NMR titration results, indicating the formation of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complexation systems when the concentration of the host was kept constant. The data suggest that one alkyl alcohol is encapsulated inside the cavity and that, during the titration, a second molecule binds to the hydroxy group outside the cavity of the pillararene host as the concentration of the guest increases. When the order of the titration is reversed, a 2
2 complexation systems when the concentration of the host was kept constant. The data suggest that one alkyl alcohol is encapsulated inside the cavity and that, during the titration, a second molecule binds to the hydroxy group outside the cavity of the pillararene host as the concentration of the guest increases. When the order of the titration is reversed, a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex was observed (data not shown).
1 complex was observed (data not shown).
For DMP5, a crystal of the inclusion compound obtained by co-crystallizing a saturated solution of the host with an octanol guest in dichloromethane is shown in Fig. 6(d).
Similarly, the unit cell contained two octanol molecules inside the cavity of two permethylated-pillar[5]arene hosts. However, in the absence of a hydroxy group on the host, the two pillararenes are aligned in a face-to-edge manner preventing cooperative binding between the inclusion complexes. The encapsulation characteristics demonstrated in the solid state by the single-crystal X-ray diffraction technique agree with the results of 1H NMR titration experiments, indicating the formation of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex.
1 complex.
Conclusions
In conclusion, mono- and A1/A2-di-hydroxy-functionalized pillar[5]arenes were synthesized by the co-cyclization of hydroquinone derivatives bearing mono- or di-benzyl group(s), followed by catalytic hydrogenation over palladium on charcoal in anhydrous ethyl acetate. Host–guest complexation between mono-hydroxy- and permethylated-pillar[5]arenes with long-chain alcohols was studied by 1H NMR titration. The encapsulation characteristics were affected by the type of functionality present on the receptor. Binding studies demonstrated that the presence of the hydroxy group in mono-hydroxy-pillar[5]arene Pillar-2a promoted the formation of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complex both in solution and in the solid state, as hydrogen-bonding interactions induced a dimeric head-to-head assembly. Analog binding studies conducted using permethylated pillar[5]arene DMP5 indicated that a 1
2 complex both in solution and in the solid state, as hydrogen-bonding interactions induced a dimeric head-to-head assembly. Analog binding studies conducted using permethylated pillar[5]arene DMP5 indicated that a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexation is formed both in solution and in the solid state. Currently, further studies and modifications of mono- and A1/A2-di-hydroxy-functionalized pillar[5]arenes to develop new supramolecular systems and functional materials are underway in our laboratories.
1 complexation is formed both in solution and in the solid state. Currently, further studies and modifications of mono- and A1/A2-di-hydroxy-functionalized pillar[5]arenes to develop new supramolecular systems and functional materials are underway in our laboratories.
Experimental section
Materials and methods
Nuclear magnetic resonance (NMR) spectra were recorded using two spectrometers (Avance II 600 MHz, Bruker, Germany and DPX 400, Bruker, Germany). Electron impact ionization (EI) and high-resolution electrospray ionization mass spectrometry were performed using DFS High-Resolution GC/MS (Thermo Scientific, Germany) and LC MS/MS (Waters Xevo G2-S Qtof, Germany) mass spectrometers, respectively. Single crystal X-ray diffraction spectra were recorded using an R-AXIS RAPID II diffractometer (Rigaku, Japan). The spectra were collected at −123 °C (Oxford Cryosystems, UK). Liquid chromatography (LC) experiments used a PDA detector (LC MS/MS, Thermo Scientific, Germany). Flash column chromatography was performed using silica gel (Silica gel 60, 230–400 mesh ASTM, EMD Millipore, Merck KGaA, Germany). DMF, dichloromethane, and ethyl acetate used for hydrogenation were distilled before use. All other reagents and solvents were of reagent grade and were used without further purification. Permethylated pillar[5]arene (DMP5) was synthesized according to a literature procedure.6a The copillar[5]arenes were synthesized according to a modified procedure reported in the literature.15Synthesis of pillar[5]arene precursors
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 v/v). (Yield 9.45 g, 71%). 1H NMR (400 MHz, CDCl3), δ: 0.91 (t, J = 6.8 Hz, 3H), 1.33 (m, 8H), 1.45 (m, 2H), 1.77 (m, 2H), 3.92 (t, J = 6.8 Hz, 2H), 6.79 (m, 4H).13C NMR (100 MHz, CDCl3), δ: 14.1, 22.7, 26.0, 29.2, 29.4, 31.8, 68.7, 115.6, 116.0, 149.3, 153.3.
20 v/v). (Yield 9.45 g, 71%). 1H NMR (400 MHz, CDCl3), δ: 0.91 (t, J = 6.8 Hz, 3H), 1.33 (m, 8H), 1.45 (m, 2H), 1.77 (m, 2H), 3.92 (t, J = 6.8 Hz, 2H), 6.79 (m, 4H).13C NMR (100 MHz, CDCl3), δ: 14.1, 22.7, 26.0, 29.2, 29.4, 31.8, 68.7, 115.6, 116.0, 149.3, 153.3.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 v/v). (Yield 10.35 g, 83%). 1H NMR (400 MHz, CDCl3), δ: 0.92 (t, J = 7.2 Hz, 3H), 1.33 (m, 8H), 1.46 (m, 2H), 1.78 (m, 2H), 3.92(t, J = 6.4 Hz, 2H), 5.04 (s, 2H), 6.88 (m, 4H), 7.40 (m, 5H). 13C NMR (100 MHz, CDCl3), δ: 14.1, 22.7, 26.1, 29.2, 29.4, 31.8, 68.6, 70.1, 115.4, 115.8, 127.5, 127.8, 128.5, 137.3, 152.8, 153.5.
60 v/v). (Yield 10.35 g, 83%). 1H NMR (400 MHz, CDCl3), δ: 0.92 (t, J = 7.2 Hz, 3H), 1.33 (m, 8H), 1.46 (m, 2H), 1.78 (m, 2H), 3.92(t, J = 6.4 Hz, 2H), 5.04 (s, 2H), 6.88 (m, 4H), 7.40 (m, 5H). 13C NMR (100 MHz, CDCl3), δ: 14.1, 22.7, 26.1, 29.2, 29.4, 31.8, 68.6, 70.1, 115.4, 115.8, 127.5, 127.8, 128.5, 137.3, 152.8, 153.5.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v). (Yield 7.80 g, 90%). 1H NMR (400 MHz, CDCl3), δ: 3.79 (s, 3H), 5.04 (s, 2H), 6.90 (m, 4H), 7.35 (m, 5H). 13C NMR (150 MHz, CDCl3), δ: 55.7, 70.7, 114.7, 115.9, 127.5, 127.9, 128.6, 137.3, 153.0, 154.0. HRMS: (m/z): calcd for C14H14O2: 214.0994; found 214.0988.
40 v/v). (Yield 7.80 g, 90%). 1H NMR (400 MHz, CDCl3), δ: 3.79 (s, 3H), 5.04 (s, 2H), 6.90 (m, 4H), 7.35 (m, 5H). 13C NMR (150 MHz, CDCl3), δ: 55.7, 70.7, 114.7, 115.9, 127.5, 127.9, 128.6, 137.3, 153.0, 154.0. HRMS: (m/z): calcd for C14H14O2: 214.0994; found 214.0988.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 v/v) and collected as a white solid. (Yield 12 g, 90%). 1H NMR (600 MHz, CDCl3), δ: 0.98–1.00 (t, J = 7.8 Hz, 6H), 1.48–1.52 (m, 4H), 1.74–1.78 (m, 4H), 3.92–3.94 (t, J = 6.6 Hz, 4H), 6.84 (s, 4H). 13C NMR (150 MHz, CDCl3), δ: 14.0, 19.5, 31.7, 68.5, 115.6, 153.4. HRMS: (m/z): calcd for C14H22O2: 222.1620; found 222.1614.
60 v/v) and collected as a white solid. (Yield 12 g, 90%). 1H NMR (600 MHz, CDCl3), δ: 0.98–1.00 (t, J = 7.8 Hz, 6H), 1.48–1.52 (m, 4H), 1.74–1.78 (m, 4H), 3.92–3.94 (t, J = 6.6 Hz, 4H), 6.84 (s, 4H). 13C NMR (150 MHz, CDCl3), δ: 14.0, 19.5, 31.7, 68.5, 115.6, 153.4. HRMS: (m/z): calcd for C14H22O2: 222.1620; found 222.1614.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 v/v) and collected as a white solid. (Yield 17.5 g, 87.3%). 1H NMR (600 MHz, CDCl3), δ: 0.89–0.92 (t, J = 6.6 Hz, 6H), 1.29–1.38 (m, 16H), 1.44–1.48 (m, 4H), 1.75–1.79 (m, 4H), 3.91–3.93 (t, J = 6.6 Hz, 4H), 6.84 (s, 4H). 13C NMR (150 MHz, CDCl3), δ: 14.3, 22.8, 26.3, 29.5, 29.6, 29.7, 32.0, 68.9, 115.6, 153.4.
70 v/v) and collected as a white solid. (Yield 17.5 g, 87.3%). 1H NMR (600 MHz, CDCl3), δ: 0.89–0.92 (t, J = 6.6 Hz, 6H), 1.29–1.38 (m, 16H), 1.44–1.48 (m, 4H), 1.75–1.79 (m, 4H), 3.91–3.93 (t, J = 6.6 Hz, 4H), 6.84 (s, 4H). 13C NMR (150 MHz, CDCl3), δ: 14.3, 22.8, 26.3, 29.5, 29.6, 29.7, 32.0, 68.9, 115.6, 153.4.
Synthesis of monobenzyloxy-pillar[5]arenes 1(a–c)
Pillar-1(a): Paraformaldehyde (1.45 g, 48 mmol) was added to a solution of 1,4-dimethoxybenzene (2.21 g, 16 mmol) and 1-benzyloxy-4-methoxy benzene (0.21 g, 1 mmol) in dry dichloromethane (100 mL) under a nitrogen atmosphere. Boron trifluoride diethyl etherate [(BF3·OEt2), (2.0 mL, 16 mmol)] was then added to the solution and the mixture was stirred at room temperature for 1 h. MeOH (100 mL) was poured into the reaction mixture and the solution was concentrated and dissolved in CH2Cl2 (100 mL). The solution was then washed with aqueous NaHCO3 (2 × 50 mL) and H2O (50 mL). The organic layer was dried using Na2SO4, concentrated under vacuum, and subjected to silica gel chromatography (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 v/v, hexane/CH2Cl2) to give Pillar-1(a) as a yellowish solid (405 mg, 49%). Mp: 161–162 °C. 1H NMR (600 MHz, CDCl3), δ: 3.36 (s, 3H), 3.63–3.89 (m, 34H), 4.95–4.97 (d, J = 9.6 Hz, 2H), 6.73–6.89 (m, 10H), 7.31–7.43 (m, 5H). 13C NMR (150 MHz, CDCl3), δ: 29.4, 29.7, 30.1, 53.2, 55.3, 55.7, 70.4, 113.9, 114.3, 114.9, 127.2, 127.5, 127.9, 128.1, 128.4, 138.0, 150.0, 150.7, 150.8, 150.9. HRMS: (m/z): calcd for [M + NH4]+: 844.4061 (for C51H54O10); found 844.4073.
70 v/v, hexane/CH2Cl2) to give Pillar-1(a) as a yellowish solid (405 mg, 49%). Mp: 161–162 °C. 1H NMR (600 MHz, CDCl3), δ: 3.36 (s, 3H), 3.63–3.89 (m, 34H), 4.95–4.97 (d, J = 9.6 Hz, 2H), 6.73–6.89 (m, 10H), 7.31–7.43 (m, 5H). 13C NMR (150 MHz, CDCl3), δ: 29.4, 29.7, 30.1, 53.2, 55.3, 55.7, 70.4, 113.9, 114.3, 114.9, 127.2, 127.5, 127.9, 128.1, 128.4, 138.0, 150.0, 150.7, 150.8, 150.9. HRMS: (m/z): calcd for [M + NH4]+: 844.4061 (for C51H54O10); found 844.4073.
Pillar-1(b): Paraformaldehyde (1.49 g, 38 mmol) was added to a solution of 1,4-dibutoxybenzene (2.84 g, 13 mmol) and 1-benzyloxy-4-butoxy benzene (0.21 g, 0.8 mmol) in dry dichloromethane (100 mL) under a nitrogen atmosphere. Boron trifluoride diethyl etherate [(BF3·OEt2), (1.6 mL, 13 mmol)] was then added to the solution and the mixture was stirred at room temperature for 1 h. MeOH (100 mL) was poured into the reaction mixture and the solution was concentrated and dissolved in CH2Cl2 (100 mL). The solution was then washed with aqueous NaHCO3 (2 × 50 mL) and H2O (50 mL). The organic layer was dried using Na2SO4, concentrated under vacuum, and subjected to silica gel chromatography (60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 v/v, hexane/CH2Cl2) to give Pillar-1(b) as a white solid (345 mg, 36%). Mp: 101–102 °C.1H NMR (400 MHz, CDCl3), δ: 0.9–0.94 (t, J = 7.2 Hz, 3H), 0.99–1.04 (m, 24H), 1.38–1.44 (m, 2H), 1.53–1.62 (m, 18H), 1.79–1.85 (m, 16H), 3.40–3.44 (t, J = 6.4 Hz, 2H), 3.77–3.94 (m, 26H), 4.99 (s, 2H), 6.77–6.98 (m, 10H), 7.34–7.49 (m, 5H). 13C NMR (100 MHz, CDCl3), δ: 14.3, 19.7, 22.7, 29.2, 29.6, 30.1, 31.6, 32.1, 67.5, 68.1, 70.1, 114.9, 127.4, 127.6, 127.8, 128.3, 138.3, 149.8, 150.2. HRMS: (m/z): calcd for [M + NH4]+: 1222.8286 (for C78H108O10); found 1222.8230.
40 v/v, hexane/CH2Cl2) to give Pillar-1(b) as a white solid (345 mg, 36%). Mp: 101–102 °C.1H NMR (400 MHz, CDCl3), δ: 0.9–0.94 (t, J = 7.2 Hz, 3H), 0.99–1.04 (m, 24H), 1.38–1.44 (m, 2H), 1.53–1.62 (m, 18H), 1.79–1.85 (m, 16H), 3.40–3.44 (t, J = 6.4 Hz, 2H), 3.77–3.94 (m, 26H), 4.99 (s, 2H), 6.77–6.98 (m, 10H), 7.34–7.49 (m, 5H). 13C NMR (100 MHz, CDCl3), δ: 14.3, 19.7, 22.7, 29.2, 29.6, 30.1, 31.6, 32.1, 67.5, 68.1, 70.1, 114.9, 127.4, 127.6, 127.8, 128.3, 138.3, 149.8, 150.2. HRMS: (m/z): calcd for [M + NH4]+: 1222.8286 (for C78H108O10); found 1222.8230.
Pillar-1(c): Paraformaldehyde (1.19 g, 38 mmol) was added to a solution of 1,4-dioctoxybenzene (4.26 g, 13 mmol) and 1-benzyloxy-4-octoxy benzene (0.25 g, 0.8 mmol) in dry dichloromethane (100 mL) under a nitrogen atmosphere. Boron trifluoride diethyl etherate [(BF3·OEt2), (1.6 mL, 13 mmol)] was then added to the solution and the mixture was stirred at room temperature for 1 h. MeOH (100 mL) was poured into the reaction mixture and the solution was concentrated and dissolved in CH2Cl2 (100 mL). The solution was then washed with aqueous NaHCO3 (2 × 50 mL) and H2O (50 mL). The organic layer was dried using Na2SO4, concentrated under vacuum, and subjected to silica gel chromatography (90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 v/v, hexane/CH2Cl2) to give Pillar-1(c) as a white solid (465 mg, 34%). Mp: 107–108 °C. 1H NMR (600 MHz, CDCl3), δ: 0.85–0.91 (m, 27H), 1.19–1.23 (m, 57H), 1.35–1.39 (m, 18H), 1.54–1.56 (m, 18H), 1.85 (s, 15H), 3.40–3.42 (t, J = 6 Hz, 2H), 3.76–3.90 (m, 26H), 4.99 (s, 2H), 6.79–7.01 (m, 10H), 7.34–7.49 (m, 5H). 13C NMR (150 MHz, CDCl3), δ: 14.3, 22.8, 26.5, 26.6, 29.3, 29.5, 29.8, 30.0, 31.9, 67.9, 68.5, 70.1, 114.9, 127.4, 127.6, 127.9, 128.3, 138.4, 149.8, 150.2. HRMS: (m/z): calcd for [M + NH4]+: 1727.3920 (for C114H180O10); found 1727.3875.
10 v/v, hexane/CH2Cl2) to give Pillar-1(c) as a white solid (465 mg, 34%). Mp: 107–108 °C. 1H NMR (600 MHz, CDCl3), δ: 0.85–0.91 (m, 27H), 1.19–1.23 (m, 57H), 1.35–1.39 (m, 18H), 1.54–1.56 (m, 18H), 1.85 (s, 15H), 3.40–3.42 (t, J = 6 Hz, 2H), 3.76–3.90 (m, 26H), 4.99 (s, 2H), 6.79–7.01 (m, 10H), 7.34–7.49 (m, 5H). 13C NMR (150 MHz, CDCl3), δ: 14.3, 22.8, 26.5, 26.6, 29.3, 29.5, 29.8, 30.0, 31.9, 67.9, 68.5, 70.1, 114.9, 127.4, 127.6, 127.9, 128.3, 138.4, 149.8, 150.2. HRMS: (m/z): calcd for [M + NH4]+: 1727.3920 (for C114H180O10); found 1727.3875.
Synthesis of monohydroxy-pillar[a]arenes 2(a–c)
Pillar-2(a): To a solution of the starting material Pillar-1(a) (130 mg, 0.16 mmol) in dry ethyl acetate (30 mL) was added 10 wt% Pd(OH)2 on carbon (20 mg). The reaction mixture was stirred at room temperature under an atmosphere of hydrogen for 14 h in the hydrogenation chamber. The catalyst was filtered through Celite. The Celite pad was washed with ethyl acetate (30 mL × 2). The combined filtrate was concentrated under reduced pressure. The residue was purified by silica gel column chromatography (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 v/v, hexane/CH2Cl2) to afford the desired product Pillar-2(a) as a yellow solid (yield 110 mg, 93%). 1H NMR (600 MHz, CDCl3), δ: 3.52 (s, 3H), 3.58 (s, 3H), 3.61–3.64 (m, 12H), 3.71 (s, 4H), 3.75–3.82 (m, 15H), 6.61–6.62 (m, 3H), 6.65–6.67 (m, 3H), 6.70–6.75 (m, 3H), 6.89 (s, 1H). 13C NMR (150 MHz, CDCl3), δ: 29.1, 29.8, 30.2, 30.3, 31.2, 55.9, 56.1, 56.2, 56.6, 113.1, 114.1, 114.3, 114.6, 114.7, 119.1, 125.2, 127.0, 127.9, 128.3, 128.5, 128.9, 129.6, 130.2, 147.8, 148.8, 151.0, 151.1, 151.3, 152.1. HRMS: (m/z): calcd for [M + Cl]: 771.2936 (for C44H48O10); found 771.2910.
80 v/v, hexane/CH2Cl2) to afford the desired product Pillar-2(a) as a yellow solid (yield 110 mg, 93%). 1H NMR (600 MHz, CDCl3), δ: 3.52 (s, 3H), 3.58 (s, 3H), 3.61–3.64 (m, 12H), 3.71 (s, 4H), 3.75–3.82 (m, 15H), 6.61–6.62 (m, 3H), 6.65–6.67 (m, 3H), 6.70–6.75 (m, 3H), 6.89 (s, 1H). 13C NMR (150 MHz, CDCl3), δ: 29.1, 29.8, 30.2, 30.3, 31.2, 55.9, 56.1, 56.2, 56.6, 113.1, 114.1, 114.3, 114.6, 114.7, 119.1, 125.2, 127.0, 127.9, 128.3, 128.5, 128.9, 129.6, 130.2, 147.8, 148.8, 151.0, 151.1, 151.3, 152.1. HRMS: (m/z): calcd for [M + Cl]: 771.2936 (for C44H48O10); found 771.2910.
Pillar-2(b): To a solution of the starting material Pillar-1(b) (100 mg, 0.083 mmol) in dry ethyl acetate (30 mL) was added 10 wt% Pd(OH)2 on carbon (20 mg). The reaction mixture was stirred at room temperature under an atmosphere of hydrogen for 14 h in the hydrogenation chamber. The catalyst was filtered through Celite. The Celite pad was washed with ethyl acetate (30 mL × 2). The combined filtrate was concentrated under reduced pressure. The residue was purified by silica gel column chromatography (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 v/v, hexane/CH2Cl2) to afford the desired product Pillar-2(b) as a white solid (yield 90 mg, 97%). Mp: 166–167 °C. 1H NMR (400 MHz, CDCl3), δ: 0.88–1.01 (m, 25H), 1.37–1.57 (m, 21H), 1.62–1.83 (m, 17H), 3.71–3.88 (m, 26H), 3.95–3.98 (t, J = 6 Hz, 2H), 6.58–6.95 (m, 10H). 13C NMR (150 MHz, CDCl3), δ: 14.1, 19.5, 19.7, 28.7, 29.4, 29.8, 30.5, 31.3, 31.6, 31.9, 32.1, 68.3, 68.6, 69.6, 114.2, 114.9, 115.1, 115.4, 115.5, 115.8, 116.2, 119.2, 125.3, 127.3, 127.9, 128.3, 128.7, 129.7, 130.3, 147.6, 148.3, 150.1, 150.5, 151.3. HRMS: (m/z): calcd for [M + H]+: 1115.7551 (for C71H102O10); found 1115.7496.
60 v/v, hexane/CH2Cl2) to afford the desired product Pillar-2(b) as a white solid (yield 90 mg, 97%). Mp: 166–167 °C. 1H NMR (400 MHz, CDCl3), δ: 0.88–1.01 (m, 25H), 1.37–1.57 (m, 21H), 1.62–1.83 (m, 17H), 3.71–3.88 (m, 26H), 3.95–3.98 (t, J = 6 Hz, 2H), 6.58–6.95 (m, 10H). 13C NMR (150 MHz, CDCl3), δ: 14.1, 19.5, 19.7, 28.7, 29.4, 29.8, 30.5, 31.3, 31.6, 31.9, 32.1, 68.3, 68.6, 69.6, 114.2, 114.9, 115.1, 115.4, 115.5, 115.8, 116.2, 119.2, 125.3, 127.3, 127.9, 128.3, 128.7, 129.7, 130.3, 147.6, 148.3, 150.1, 150.5, 151.3. HRMS: (m/z): calcd for [M + H]+: 1115.7551 (for C71H102O10); found 1115.7496.
Pillar-2(c): To a solution of the starting material Pillar-2(c) (150 mg, 0.088 mmol) in dry ethyl acetate (30 mL) was added 10 wt% Pd(OH)2 on carbon (20 mg). The reaction mixture was stirred at room temperature under an atmosphere of hydrogen for 14 h in the hydrogenation chamber. The catalyst was filtered through Celite. The Celite pad was washed with ethyl acetate (30 mL × 2). The combined filtrate was concentrated under reduced pressure. The residue was purified by silica gel column chromatography (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 v/v, hexane/CH2Cl2) to afford the desired product Pillar-2(c) as a white solid (yield 140 mg, 98%). Mp: 126–127 °C. 1H NMR (600 MHz, CDCl3), δ: 0.83–0.91 (m, 29H), 1.29–1.45 (m, 76H), 1.52–1.54 (m, 15H), 1.83 (m, 15H), 3.11–3.13 (m, 2H), 3.77–3.87 (m, 26H), 6.62–6.96 (m, 10H). 13C NMR (150 MHz, CDCl3), δ: 14.0, 22.5, 25.6, 26.1, 26.3, 27.8, 29.1, 29.9, 31.7, 32.8, 68.2, 114.4, 123.1, 127.9, 129.7, 133.4, 146.3, 149.7, 150.3. HRMS: (m/z): calcd for [M + NH4]+: 1637.3451 (for C107H174O10); found 1637.3486.
70 v/v, hexane/CH2Cl2) to afford the desired product Pillar-2(c) as a white solid (yield 140 mg, 98%). Mp: 126–127 °C. 1H NMR (600 MHz, CDCl3), δ: 0.83–0.91 (m, 29H), 1.29–1.45 (m, 76H), 1.52–1.54 (m, 15H), 1.83 (m, 15H), 3.11–3.13 (m, 2H), 3.77–3.87 (m, 26H), 6.62–6.96 (m, 10H). 13C NMR (150 MHz, CDCl3), δ: 14.0, 22.5, 25.6, 26.1, 26.3, 27.8, 29.1, 29.9, 31.7, 32.8, 68.2, 114.4, 123.1, 127.9, 129.7, 133.4, 146.3, 149.7, 150.3. HRMS: (m/z): calcd for [M + NH4]+: 1637.3451 (for C107H174O10); found 1637.3486.
Synthesis of dibenzyloxy-pillar[5]arenes 3(a–c)
Pillar-3(a): Paraformaldehyde (1.49 g, 48 mmol) was added to a solution of 1,4-dimethoxybenzene (2.21 g, 16 mmol) and 1,4-dibenzyloxybenzene (0.29 g, 1 mmol) in dry dichloromethane (100 mL) under a nitrogen atmosphere. Boron trifluoride diethyl etherate [(BF3·OEt2), (1 mL, 16 mmol)] was then added to the solution and the mixture was stirred at room temperature for 1 h. MeOH (100 mL) was poured into the reaction mixture and the solution was concentrated and dissolved in CH2Cl2 (100 mL). The solution was then washed with aqueous NaHCO3 (2 × 50 mL) and H2O (50 mL). The organic layer was dried using Na2SO4, concentrated under vacuum, and subjected to silica gel chromatography (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 v/v, hexane/CH2Cl2) to give Pillar-3(a) as a yellowish solid (505 mg, 56%). Mp: 114–115 °C. 1H NMR (600 MHz, CDCl3), δ: 3.34 (s, 6H), 3.57 (s, 6H), 3.67–3.69 (d, J = 12 Hz, 12H), 3.77–3.80 (d, J = 20.4 Hz, 8H), 3.93 (m, 2H), 4.85 (br, 4H), 6.71 (s, 2H), 6.75–6.76 (d, J = 9.6 Hz, 4H), 6.81 (s, 2H), 6.93 (s, 2H), 7.32–7.37 (m, 6H), 7.42–7.46 (d, J = 6.6 Hz, 4H). 13C NMR (150 MHz, CDCl3), δ: 29.6, 29.9, 55.5, 55.8, 55.9, 70.6, 114.2, 115.4, 127.4, 127.8, 128.2, 128.3, 128.5, 138.2, 150.3, 150.9. HRMS: (m/z): calcd for [M + NH4]+: 920.4374 (for C57H58O10); found 920.4366.
70 v/v, hexane/CH2Cl2) to give Pillar-3(a) as a yellowish solid (505 mg, 56%). Mp: 114–115 °C. 1H NMR (600 MHz, CDCl3), δ: 3.34 (s, 6H), 3.57 (s, 6H), 3.67–3.69 (d, J = 12 Hz, 12H), 3.77–3.80 (d, J = 20.4 Hz, 8H), 3.93 (m, 2H), 4.85 (br, 4H), 6.71 (s, 2H), 6.75–6.76 (d, J = 9.6 Hz, 4H), 6.81 (s, 2H), 6.93 (s, 2H), 7.32–7.37 (m, 6H), 7.42–7.46 (d, J = 6.6 Hz, 4H). 13C NMR (150 MHz, CDCl3), δ: 29.6, 29.9, 55.5, 55.8, 55.9, 70.6, 114.2, 115.4, 127.4, 127.8, 128.2, 128.3, 128.5, 138.2, 150.3, 150.9. HRMS: (m/z): calcd for [M + NH4]+: 920.4374 (for C57H58O10); found 920.4366.
Pillar-3(b): Paraformaldehyde (1.19 g, 38 mmol) was added to a solution of 1,4-dibutoxybenzene (2.84 g, 12.8 mmol) and 1,4-dibenzyloxybenzene (0.23 g, 0.8 mmol) in dry dichloromethane (100 mL) under a nitrogen atmosphere. Boron trifluoride diethyl etherate [(BF3·OEt2), (1.6 mL, 12.8 mmol)] was then added to the solution and the mixture was stirred at room temperature for 1 h. MeOH (100 mL) was poured into the reaction mixture and the solution was concentrated and dissolved in CH2Cl2 (100 mL). The solution was then washed with aqueous NaHCO3 (2 × 50 mL) and H2O (50 mL). The organic layer was dried using Na2SO4, concentrated under vacuum, and subjected to silica gel chromatography (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 v/v, hexane/CH2Cl2) to give Pillar-3(b) as a white solid (298 mg, 30%). Mp: 83–84 °C. 1H NMR (600 MHz, CDCl3), δ: 0.92 (t, J = 7.2 Hz, 6H), 0.97–1.02 (m, 18H), 1.39–1.45 (m, 2H), 1.53–1.61 (m, 17H), 1.79–1.81 (m, 13H), 3.44 (t, J = 6 Hz, 4H), 3.76–3.97 (m, 22H), 5.00–5.03 (m, 4H), 6.77–6.89 (m, 8H), 7.01 (s, 2H), 7.33–7.38 (m, 6H), 7.48–7.49 (m, 4H). 13C NMR (150 MHz, CDCl3), δ: 14.3, 19.7, 29.3, 29.9, 32.2, 32.3, 67.7, 68.2, 70.2, 114.9, 115.1, 115.2, 127.4, 127.7, 127.9, 128.3, 128.4, 128.5, 128.7, 138.3, 149.9, 150.1. HRMS: (m/z): calcd for [M + Na]+: 1261.7684 (for C81H106O10); found 1261.7701.
70 v/v, hexane/CH2Cl2) to give Pillar-3(b) as a white solid (298 mg, 30%). Mp: 83–84 °C. 1H NMR (600 MHz, CDCl3), δ: 0.92 (t, J = 7.2 Hz, 6H), 0.97–1.02 (m, 18H), 1.39–1.45 (m, 2H), 1.53–1.61 (m, 17H), 1.79–1.81 (m, 13H), 3.44 (t, J = 6 Hz, 4H), 3.76–3.97 (m, 22H), 5.00–5.03 (m, 4H), 6.77–6.89 (m, 8H), 7.01 (s, 2H), 7.33–7.38 (m, 6H), 7.48–7.49 (m, 4H). 13C NMR (150 MHz, CDCl3), δ: 14.3, 19.7, 29.3, 29.9, 32.2, 32.3, 67.7, 68.2, 70.2, 114.9, 115.1, 115.2, 127.4, 127.7, 127.9, 128.3, 128.4, 128.5, 128.7, 138.3, 149.9, 150.1. HRMS: (m/z): calcd for [M + Na]+: 1261.7684 (for C81H106O10); found 1261.7701.
Pillar-3(c): Paraformaldehyde (1.19 g, 38 mmol) was added to a solution of 1,4-dioctyloxybenzene (4.26 g, 12.8 mmol) and 1,4-dibenzyloxybenzene (0.23 g, 0.8 mmol) in dry dichloromethane (100 mL) under a nitrogen atmosphere. Boron trifluoride diethyl etherate [(BF3·OEt2), (1.25 mL, 10 mmol)] was then added to the solution and the mixture was stirred at room temperature for 1 h. MeOH (100 mL) was poured into the reaction mixture and the solution was concentrated and dissolved in CH2Cl2 (100 mL). The solution was then washed with aqueous NaHCO3 (2 × 50 mL) and H2O (50 mL). The organic layer was dried using Na2SO4, concentrated under vacuum, and subjected to silica gel chromatography (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 v/v, hexane/CH2Cl2) to give Pillar-3(c) as a white solid (345 mg, 26%). Mp: 87–88 °C. 1H NMR (600 MHz, CDCl3), δ: 0.82–0.91 (m, 26H), 1.15–1.39 (m, 78H), 1.54–1.85 (m, 16H), 3.42 (t, J = 6.6 Hz, 4H), 3.76–3.92 (m, 22H), 5.01 (d, J = 8.4 Hz, 4H), 6.78 (s, 2H), 6.82 (s, 2H), 6.86 (s, 2H), 6.90 (s, 2H), 7.02 (s, 2H), 7.33–7.37 (m, 6H), 7.48–7.49 (m, 4H). 13C NMR (150 MHz, CDCl3), δ: 14.3, 22.8, 22.9, 26.5, 26.6, 29.3, 29.5, 29.8, 30.0, 30.2, 31.9, 67.9, 68.5, 70.2, 114.8, 114.9, 127.5, 127.7, 127.9, 128.2, 128.4, 128.5, 128.7, 138.3, 149.9, 150.1. HRMS: (m/z): calcd for [M + NH4]+: 1705.3138 (for C113H170O10); found 1705.3176.
20 v/v, hexane/CH2Cl2) to give Pillar-3(c) as a white solid (345 mg, 26%). Mp: 87–88 °C. 1H NMR (600 MHz, CDCl3), δ: 0.82–0.91 (m, 26H), 1.15–1.39 (m, 78H), 1.54–1.85 (m, 16H), 3.42 (t, J = 6.6 Hz, 4H), 3.76–3.92 (m, 22H), 5.01 (d, J = 8.4 Hz, 4H), 6.78 (s, 2H), 6.82 (s, 2H), 6.86 (s, 2H), 6.90 (s, 2H), 7.02 (s, 2H), 7.33–7.37 (m, 6H), 7.48–7.49 (m, 4H). 13C NMR (150 MHz, CDCl3), δ: 14.3, 22.8, 22.9, 26.5, 26.6, 29.3, 29.5, 29.8, 30.0, 30.2, 31.9, 67.9, 68.5, 70.2, 114.8, 114.9, 127.5, 127.7, 127.9, 128.2, 128.4, 128.5, 128.7, 138.3, 149.9, 150.1. HRMS: (m/z): calcd for [M + NH4]+: 1705.3138 (for C113H170O10); found 1705.3176.
Synthesis of A1/A2-dihydroxy-pillar[5]arenes 4(a–c)
Pillar-4(a): To a solution of Pillar-3(a) (100 mg, 0.11 mmol) in dry ethyl acetate (30 mL) was added 10 wt% Pd(OH)2 on carbon (20 mg). The reaction mixture was stirred at room temperature under an atmosphere of hydrogen for 14 h in the hydrogenation chamber. The catalyst was filtered through Celite. The Celite pad was washed with ethyl acetate (30 mL × 2). The combined filtrate was concentrated under reduced pressure. The residue was purified by silica gel column chromatography (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 v/v, hexane/ethyl acetate) to afford the desired product Pillar-4(a) as a yellow solid (yield 75 mg, 95%). 1H NMR (600 MHz, CDCl3), δ: 3.64 (m, 8H), 3.69–3.76 (m, 8H), 379–3.81 (m, 12H), 3.86 (s, 6H), 6.61–6.63 (d, J = 10.8 Hz, 4H), 6.83 (s, 2H), 6.86 (s, 2H), 6.92 (s, 2H). 13C NMR (150 MHz, CDCl3), δ: 29.3, 30.0, 30.9, 55.6, 56.6, 113.2, 113.9, 114.3, 114.8, 118.4, 126.8, 127.2, 127.6, 128.5, 129.3, 147.6, 148.5, 150.9, 152.2. HRMS: (m/z): calcd for [M + NH4]+: 740.3435 (for C43H46O10); found 740.3408.
50 v/v, hexane/ethyl acetate) to afford the desired product Pillar-4(a) as a yellow solid (yield 75 mg, 95%). 1H NMR (600 MHz, CDCl3), δ: 3.64 (m, 8H), 3.69–3.76 (m, 8H), 379–3.81 (m, 12H), 3.86 (s, 6H), 6.61–6.63 (d, J = 10.8 Hz, 4H), 6.83 (s, 2H), 6.86 (s, 2H), 6.92 (s, 2H). 13C NMR (150 MHz, CDCl3), δ: 29.3, 30.0, 30.9, 55.6, 56.6, 113.2, 113.9, 114.3, 114.8, 118.4, 126.8, 127.2, 127.6, 128.5, 129.3, 147.6, 148.5, 150.9, 152.2. HRMS: (m/z): calcd for [M + NH4]+: 740.3435 (for C43H46O10); found 740.3408.
Pillar-4(b): To a solution of Pillar-3(b) (100 mg, 0.08 mmol) in dry ethyl acetate (30 mL) was added 10 wt% Pd(OH)2 on carbon (20 mg). The reaction mixture was stirred at room temperature under an atmosphere of hydrogen for 14 h in the hydrogenation chamber. The catalyst was filtered through Celite. The Celite pad was washed with ethyl acetate (30 mL × 2). The combined filtrate was concentrated under reduced pressure. The residue was purified by silica gel column chromatography (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 v/v, hexane/ethyl acetate) to afford the desired product Pillar-4(b) as a white solid (yield 80 mg, 95%). Mp: 156–157 °C. 1H NMR (600 MHz, CDCl3), δ: 0.98–1.03 (m, 24H), 1049–1.61 (m, 16H), 1.74–1.87 (m, 16H), 3.59 (s, 1H), 3.77–3.79 (m, 7H), 3.85–3.91 (m, 14H), 4.02 (s, 4H), 6.59–6.61 (d, J = 8.4 Hz, 4H), 6.86–6.87 (d, J = 3.6 Hz, 4H), 6.97 (s, 2H). 13C NMR (150 MHz, CDCl3), δ: 14.1, 19.4, 19.6, 19.7, 29.4, 29.9, 31.1, 31.3, 31.8, 32.2, 68.1, 68.4, 69.9, 114.1, 114.6, 115.1, 115.3, 116.2, 118.4, 123.4, 127.1, 127.5, 127.7, 128.1, 128.6, 129.6, 129.8, 133.7, 146.5, 147.4, 147.7, 149.8, 150.1, 150.5, 151.5. HRMS: (m/z): calcd for [M + H]+: 1059.6925 (for C67H94O10); found 1059.6920.
60 v/v, hexane/ethyl acetate) to afford the desired product Pillar-4(b) as a white solid (yield 80 mg, 95%). Mp: 156–157 °C. 1H NMR (600 MHz, CDCl3), δ: 0.98–1.03 (m, 24H), 1049–1.61 (m, 16H), 1.74–1.87 (m, 16H), 3.59 (s, 1H), 3.77–3.79 (m, 7H), 3.85–3.91 (m, 14H), 4.02 (s, 4H), 6.59–6.61 (d, J = 8.4 Hz, 4H), 6.86–6.87 (d, J = 3.6 Hz, 4H), 6.97 (s, 2H). 13C NMR (150 MHz, CDCl3), δ: 14.1, 19.4, 19.6, 19.7, 29.4, 29.9, 31.1, 31.3, 31.8, 32.2, 68.1, 68.4, 69.9, 114.1, 114.6, 115.1, 115.3, 116.2, 118.4, 123.4, 127.1, 127.5, 127.7, 128.1, 128.6, 129.6, 129.8, 133.7, 146.5, 147.4, 147.7, 149.8, 150.1, 150.5, 151.5. HRMS: (m/z): calcd for [M + H]+: 1059.6925 (for C67H94O10); found 1059.6920.
Pillar-4(c): To a solution of Pillar-4(c) (100 mg, 0.059 mmol) in dry ethyl acetate (30 mL) was added 10 wt% Pd(OH)2 on carbon (20 mg). The reaction mixture was stirred at room temperature under an atmosphere of hydrogen for 14 h in the hydrogenation chamber. The catalyst was filtered through Celite. The Celite pad was washed with ethyl acetate (30 mL × 2). The combined filtrate was concentrated under reduced pressure. The residue was purified by silica gel column chromatography (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 v/v, hexane/ethyl acetate) to afford the desired product Pillar-4(c) as a white solid (yield 85 mg, 96%). Mp: 128–129 °C. 1H NMR (600 MHz, CDCl3), δ: 0.91–0.92 (m, 24H), 1.14–1.41 (m, 79H), 1.50–1.56 (m, 17 H), 3.78 (s, 8H), 3.87–3.89 (m, 18H), 6.61–6.62 (d, J = 3.6 Hz, 4H), 6.87–6.89 (d, J = 8.4 Hz, 4H), 6.98 (s, 2H). 13C NMR (150 MHz, CDCl3), δ: 14.3, 22.8, 26.1, 26.5, 29.3, 31.1, 31.9, 68.4, 68.7, 113.9, 114.4, 114.9, 115.9, 118.3, 127.1, 127.4, 127.7, 128.5, 129.6, 147.4, 147.7, 149.8, 150.1, 151.4. HRMS: (m/z): calcd for [M + H]+: 1508.1933 (for C99H158O10); found 1508.1925.
60 v/v, hexane/ethyl acetate) to afford the desired product Pillar-4(c) as a white solid (yield 85 mg, 96%). Mp: 128–129 °C. 1H NMR (600 MHz, CDCl3), δ: 0.91–0.92 (m, 24H), 1.14–1.41 (m, 79H), 1.50–1.56 (m, 17 H), 3.78 (s, 8H), 3.87–3.89 (m, 18H), 6.61–6.62 (d, J = 3.6 Hz, 4H), 6.87–6.89 (d, J = 8.4 Hz, 4H), 6.98 (s, 2H). 13C NMR (150 MHz, CDCl3), δ: 14.3, 22.8, 26.1, 26.5, 29.3, 31.1, 31.9, 68.4, 68.7, 113.9, 114.4, 114.9, 115.9, 118.3, 127.1, 127.4, 127.7, 128.5, 129.6, 147.4, 147.7, 149.8, 150.1, 151.4. HRMS: (m/z): calcd for [M + H]+: 1508.1933 (for C99H158O10); found 1508.1925.
Single-crystal X-ray diffraction analysis
Singles crystals of synthesized pillar[5]arenes and their inclusion complexes that were suitable for single crystal X-ray diffraction were grown by slow solvent evaporation or by a diffusion method using dichloromethane and hexane. Single-crystal X-ray diffractograms were collected on a diffractometer (R-AXIS RAPID, Rigaku, Japan) using the Rigaku's Crystal clear software package at −123 °C. The structure was solved and refined using the Bruker SHELXTL Software package (structure solution program: SHELXS-97; refinement program: SHELXL-97). The crystallographic data of the structures reported in this paper have been deposited at the Cambridge Crystallographic Data Centre as supplementary publications (CCDC 1556922–1556931†).1H NMR titrations
A 0.5 mL sample of the Pillar-2(a) and DMP5 solutions were prepared at a concentration of 10.0 mM and 7.5 mM, respectively, in chloroform-d. A sample of the guest solutions (2 mL) was prepared at a concentration of 0.1 M in a chloroform-d solvent. All titration experiments were carried out in an NMR tube at 298 K, and 1H-NMR spectra were recorded upon successive addition of aliquots of the stock solution of the appropriate guests with a microsyringe. The 1H-NMR spectra changes were fitted to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 binding isotherms by nonlinear least-squares treatment using Microsoft Excel to determine the association constant, Ka.13
2 binding isotherms by nonlinear least-squares treatment using Microsoft Excel to determine the association constant, Ka.13
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The support received from the University of Kuwait, made available through research grant no. SC04/16, the College of Graduate Studies and the facilities of RSP4 (grant no. GS01/01, GS03/01, GS01/03, GS01/10, and GS03/08), is gratefully acknowledged.References
- (a) K. E. Krakowiak, J. S. Bradshaw and D. J. Zamecka-Krakowiak, Chem. Rev., 1989, 89, 929–972 CrossRef CAS; (b) J. S. Bradshaw and R. M. Izatt, Acc. Chem. Res., 1997, 30, 338–345 CrossRef CAS; (c) G. W. Gokel, W. M. Levy and M. E. Weber, Chem. Rev., 2004, 104, 2723–2750 CrossRef CAS PubMed.
- (a) A. Harada, A. Hashidzume, H. Yamaguchi and Y. Takashima, Chem. Rev., 2009, 109, 5974–6023 CrossRef CAS PubMed; (b) C. D. Gutsche, Calixarenes Revisited, Royal Society of Chemistry, Cambridge, 2nd edn, 2000 Search PubMed; (c) C. D. Gutsche, Calixarenes, An Introduction, Royal Society of Chemistry, Cambridge, UK, 2008 Search PubMed; (d) E. Botana, E. Da Silva, J. Benet-Buchholz, P. Ballester and J. de Mendoza, Angew. Chem., Int. Ed., 2007, 46, 198–201 CrossRef CAS PubMed.
- (a) K. Kim, Chem. Soc. Rev., 2002, 31, 96 RSC; (b) A. E. Kaifer, W. Li, S. Silvi and V. Sindelar, Chem. Commun., 2012, 48, 6693 RSC.
- (a) A. Harada, Acc. Chem. Res., 2001, 34, 456 CrossRef CAS PubMed; (b) L. Zhu, D. Zhang, D. Qu, Q. Wang, X. Ma and H. Tian, Chem. Commun., 2010, 46, 2587 RSC; (c) K.-R. Wang, D.-S. Guo, B.-P. Jiang and Y. Liu, Chem. Commun., 2012, 48, 3644 RSC.
- (a) F. Diederich, Cyclophanes, The Royal Society of Chemistry, Cambridge, 1991 Search PubMed; (b) J. C. Barnes, M. Juríček, N. L. Strutt, M. Frasconi, S. Sampath, M. A. Giesener, P. L. McGrier, C. J. Bruns, C. L. Stern, A. A. Sarjeant and J. F. Stoddart, J. Am. Chem. Soc., 2013, 135, 183 CrossRef CAS PubMed; (c) H.-Y. Gong, B. M. Rambo, E. Karnas, V. M. Lynch and J. L. Sessler, Nat. Chem., 2010, 2, 406 CrossRef CAS PubMed.
- (a) N. Morohashi, F. Narumi, N. Iki, T. Hattori and S. Miyano, Chem. Rev., 2006, 106, 5291–5316 CrossRef CAS PubMed; W. Maes and W. Dehaen, Chem. Soc. Rev., 2008, 37, 2393–2402 Search PubMed; (b) M.-X. Wang, Acc. Chem. Res., 2012, 45, 182–195 CrossRef CAS PubMed.
- (a) T. Ogoshi, S. Kanai, S. Fujinami, T. A. Yamagishi and Y. Nakamoto, J. Am. Chem. Soc., 2008, 130, 5022–5023 CrossRef CAS PubMed; (b) C. Han, F. Ma, Z. Zhang, B. Xia, Y. Yu and F. Huang, Org. Lett., 2010, 12, 4360–4363 CrossRef CAS PubMed; (c) Z. Li, J. Yang, J. He, Z. Abliz and F. Huang, Org. Lett., 2014, 16, 2065–2069 Search PubMed; (d) T. Ogoshi, K. Masaki, R. Shiga, K. Kitajima and T.-a. Yamagishi, Org. Lett., 2011, 13, 1264–1266 CrossRef CAS PubMed; (e) Y. Chen, H. Q. Tao, Y. H. Kou, H. Meier, J. L. Fu and R. Cao, Chin. Chem. Lett., 2012, 23, 509–511 CrossRef CAS; (f) T. Ogoshi, Y. Nishida, T.-a. Yamagishi and Y. Nakamoto, Macromolecules, 2010, 43, 3145–3147 CrossRef CAS.
- (a) N. L. Strutt, R. S. Forgan, J. M. Spruell, Y. Y. Botros and J. F. Stoddart, J. Am. Chem. Soc., 2011, 133, 5668–5671 CrossRef CAS PubMed; (b) N. Sturrt, D. Fairen-Jimenez, J. Iehl, M. Lalonde, R. Snurr, O. Farha, J. Hupp and F. Stoddart, J. Am. Chem. Soc., 2012, 134, 17436–17439 CrossRef PubMed; (c) T. Ogoshi, K. Kida and T. Yamagishi, J. Am. Chem. Soc., 2012, 134, 20146–20150 CrossRef CAS PubMed; (d) C. Li, S. Chen, J. Li, K. Han, M. Xu, B. Hu, Y. Yu and X. Jia, Chem. Commun., 2011, 47, 11294–11296 RSC; (e) C. Li, K. Han, J. Li, H. Zhang, J. Ma, X. Shu, Z. Chen, L. Weng and X. Jia, Org. Lett., 2012, 14, 42–45 CrossRef CAS PubMed; (f) T. Ogoshi, K. Demachi, K. Kitajima and T.-a. Yamagishi, Chem. Commun., 2011, 47, 10290–10292 RSC; (g) T. Ogoshi, K. Masaki, R. Shiga, K. Kitajima and T. Yamagishi, Org. Lett., 2011, 13, 1264–1266 CrossRef CAS PubMed.
- (a) T. Ogoshi, R. Shiga, M. Hashizume and T. Yamagishi, Chem. Commun., 2011, 47, 6927–6929 RSC; (b) W. B. Amino Hu, W. J. Hu, X. L. Zhao, Y. A. Liu, J. S. Li, B. Jiang and K. Wen, J. Org. Chem., 2016, 81, 3877–3881 CrossRef PubMed; (c) Y. Guocan, M. Yingjie, H. Chengyou, Y. Yong, T. Guping, M. Zhengwei, G. Changyou and H. Feihe, J. Am. Chem. Soc., 2013, 135, 10310–10313 CrossRef PubMed.
- (a) T. Ogoshi, T. Aoki, K. Kitajima, S. Fujinama, T. A. Yamagishi and Y. Nakamoto, J. Org. Chem., 2011, 76, 328–331 CrossRef CAS PubMed; (b) Y. Chen, M. He, B. Li, L. Wang, H. Meier and D. Cao, RSC Adv., 2013, 3, 21405–21408 RSC; (c) J. Han, X. Hou, C. Ke, H. Zhang, N. L. Strutt, C. L. Stern and J. F. Stoddart, Org. Lett., 2015, 17, 3260–3263 CrossRef CAS PubMed.
- (a) C. Han, Z. Zhang, G. Yu and F. Huang, Chem. Commun., 2012, 48, 9876–9878 RSC; (b) T. Ogoshi, D. Yamafuji, D. Kotera, T. Aoki, S. Fujinami and T.-A. Yamagishi, J. Org. Chem., 2012, 77, 11146–11152 CrossRef CAS PubMed; (c) M. Pan and M. Xue, Eur. J. Org. Chem., 2013, 4787–4793 CrossRef CAS; (d) D. Cao, Y. Kou, J. Liang, Z. Chen, L. Wang and H. Meier, Angew. Chem., Int. Ed., 2009, 48, 9721–9723 CrossRef CAS PubMed.
- (a) N. L. Strutt, R. S. Forgan, J. M. Spruell, Y. Y. Botros and J. F. Stoddart, J. Am. Chem. Soc., 2011, 133, 5668 CrossRef CAS PubMed; (b) N. L. Strutt, D. Fairen-Jimenez, J. Iehl, M. B. Lalonde, R. Q. Snurr, O. K. Farha, J. T. Hupp and J. F. Stoddart, J. Am. Chem. Soc., 2012, 134, 17436 CrossRef CAS PubMed.
- P. Thordarson, Chem. Soc. Rev., 2011, 40, 1305 RSC.
- G. Yu, B. Hua and C. Han, Org. Lett., 2014, 16, 2486 CrossRef CAS PubMed.
- L. Liu, D. Cao, Y. Jin, Y. Kou and H. Meier, Org. Biomol. Chem., 2011, 9, 7007–7010 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1556922–1556931. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7qo00641a |
| This journal is © the Partner Organisations 2018 |