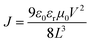Open Access Article
Open Access ArticleEfficient carbazole-based small-molecule organic solar cells with an improved fill factor†
Yongtao Liu,
Yanna Sun,
Miaomiao Li,
Huanran Feng,
Wang Ni ,
Hongtao Zhang*,
Xiangjian Wan
,
Hongtao Zhang*,
Xiangjian Wan and
Yongsheng Chen
and
Yongsheng Chen *
*
State Key Laboratory and Institute of Elemento-Organic Chemistry, The Centre of Nanoscale Science and Technology, Key Laboratory of Functional Polymer Materials, Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), College of Chemistry, Nankai University, Tianjin, 300071, China. E-mail: htzhang@nankai.edu.cn; yschen99@nankai.edu.cn
First published on 26th January 2018
Abstract
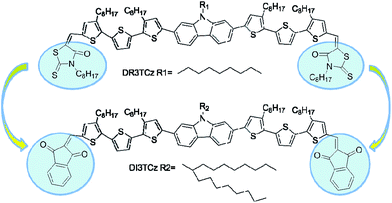
In this study, a new acceptor–donor–acceptor (A–D–A) small molecule, DI3TCz, with carbazole as the central unit and 1,3-indanedione as the end group, was designed and synthesized for application in organic solar cells. In contrast to the molecule DR3TCz with rhodanine as end groups, DI3TCz exhibited deep a LUMO energy level and a nearly unchanged HOMO energy level with a narrow optical band gap of 1.75 eV and red shifted absorption. Compared with DR3TCz, the DI3TCz device showed a PCE of 6.46% with a remaining high Voc value of 0.97 V, improved Jsc of 10.40 mA cm−1 and a notable FF of 0.65, which is the highest PCE value reported to data for carbazole-based small molecules OPVs.
Introduction
Bulk heterojunction (BHJ) organic photovoltaics (OPVs) are promising, sustainable candidates for solar energy conversion and have attracted great attention with their advantages, such as solution processability, lightweight nature, cost-effectiveness, and flexibility.1 In the past decade, extensive efforts have been focused on the performance improvement of BHJ OPVs with PCBM derivatives as acceptor materials. Various polymers and small molecules have been designed and evaluated as donor materials.2 Compared with polymers, small molecules have well-defined chemical structure and less batch-to-batch device variation.3 Thus, a great number of studies have been conducted on small molecule based OPVs (SM-OPVs). Presently, significant progress has been made with power conversion efficiencies (PCEs) over 10% for single junction SM-OPVs devices.4From the equation of PCE = Voc × Jsc × FF/Pin, PCE is directly related to the open-circuit voltage (Voc), short-circuit current density (Jsc), and fill factor (FF). To achieve outstanding PCE, these parameters should be simultaneously optimized.5 Generally, SM-OPVs exhibit high Voc, low Jsc and FF in contrast to their polymer counterpart. Therefore, how to improve Jsc and FF without sacrificing the Voc is an effective strategy for the PCE improvement of SM-OPVs.6
Recent studies have shown that the highest occupied molecular orbital (HOMO) energy level of acceptor–donor–acceptor (A–D–A) small molecules is mainly determined by the central donor unit, and the lowest unoccupied molecular orbital (LUMO) mainly depends on the terminal acceptor group. Recently, our group has reported a series of small-molecule donor materials with carbazole as the central building block.7 The devices based on those carbazole-derived small-molecule donor materials exhibit high Voc owing to the deep HOMO levels of the central carbazole unit. However, the Jsc and FF are not desirable, which is related to the narrow absorption of those molecules in the visible region and unfavourable active layers morphologies. Thus, there is much improved room of the device performance for above carbazole-based small molecules through careful molecules design and device optimizations.
In this study, we report a new small molecule DI3TCz with carbazole unit as the central building block and 1,3-indanedione as the end group. The molecule DI3TCz has the same molecular backbone as one of our previously reported small molecule DR3TCz (Scheme 1). The 1,3-indanedione unit has stronger electron withdrawing ability compared with rhodanine unit.8 Thus, red shifted absorption of DI3TCz in contrast to DR3TCz is expected after introducing the 1,3-indanedione end group. Meanwhile, the much planar structure of the 1,3-indanedione units may facilitate with phase separation in the active layer. On the other hand, in order to improve the solubility of DI3TCz, a large branched alkyl side chain, heptadecan-9-yl, was introduced on the N atom of the carbazole central unit of DI3TCz. As expected, OPV devices based on DI3TCz exhibit an much improved Jsc and FF compared with those of DR3TCz based devices ascribed to the better light absorption and favorable morphology of the active layer. A decent PCE with value of 6.46% was achieved. To our knowledge, it is the highest PCE value reported to date for carbazole-based small molecules OPVs.
Experimental section
Materials and synthesis
All reactions and manipulations were carried out under argon using standard Schlenk techniques. Scheme S1† shows the detailed procedures to synthesize DI3TCz. Compound 1,9-(heptadecan-9-yl)-2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-9H-carbazole, was commercially obtained and used without further purification. 5′′-Bromo-3,3′′-dioctyl-[2,2′:5′,2′′-terthiophene]-5-carbaldehyde and 5′′,5′′-(9-(heptadecan-9-yl)-9H-carbazole-2,7-diyl)bis(3,3′′-dioctyl-[2,2′:5′,2′′-terthiophene]-5-carbaldehyde) were synthesized according to literature.7bSynthetic procedures
DCHO3TCz (140 mg, 0.10 mmol) was dissolved in a solution of dry CHCl3 (50 mL). 1,3-Indanedione (146 mg, 1.00 mmol) and three drops of triethylamine were added, and the resulting solution was stirred for 24 h under argon at room temperature. After solvent removal, the crude product was dissolved in 8 mL chloroform. To this solution, CH3OH (30 mL) was added to precipitate out the product. The above dissolve and precipitate procedure was repeated several times to completely remove the unreacted 1,3-indanedione. Then, the product was separated by silica-gel chromatography using a mixture of dichloromethane and petroleum ether (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as the eluent to afford DI3TCz as a black solid (120 mg, 72% yield). 1H NMR (400 MHz, CDCl3): δ 8.01–8.06 (m, 2H), 7.93–7.97 (m, 4H), 7.87 (s, 2H), 7.81 (s, 2H), 7.74–7.76 (m, 5H), 7.59 (s, 1H), 7.49 (s, 2H), 7.41–7.42 (b, 2H), 7.21–7.26 (b, 4H), 4.63–4.67 (m, 5H), 2.87 (s, 8H), 2.36–2.40 (m, 2H), 1.99–2.03 (m, 2H), 1.70–1.80 (m, 8H), 1.44–1.47 (m, 10H), 1.30–1.35 (m, 40H), 1.16–1.20 (m, 14H), 0.87–0.89 (m, 12H), 0.78–0.81 (m, 6H). 13C NMR (100 MHz, CDCl3): δ 190.42, 189.74, 145.43, 145.40, 144.13, 144.10, 142.05, 141.48, 140.73, 140.52, 139.16, 135.67, 134.94, 134.72, 128.17, 126.32, 126.08, 123.73, 122.93, 122.76, 120.78, 120.55, 117.30, 108.57, 105.84, 56.62, 33.89, 31.96, 31.92, 31.80, 30.66, 30.19, 29.49, 29.38, 26.87, 22.73, 22.63, 14.16. MS (MALDI-TOF): calculated for C105H127NO4S6 [M]+, 1659.53; found 1660.27.
1) as the eluent to afford DI3TCz as a black solid (120 mg, 72% yield). 1H NMR (400 MHz, CDCl3): δ 8.01–8.06 (m, 2H), 7.93–7.97 (m, 4H), 7.87 (s, 2H), 7.81 (s, 2H), 7.74–7.76 (m, 5H), 7.59 (s, 1H), 7.49 (s, 2H), 7.41–7.42 (b, 2H), 7.21–7.26 (b, 4H), 4.63–4.67 (m, 5H), 2.87 (s, 8H), 2.36–2.40 (m, 2H), 1.99–2.03 (m, 2H), 1.70–1.80 (m, 8H), 1.44–1.47 (m, 10H), 1.30–1.35 (m, 40H), 1.16–1.20 (m, 14H), 0.87–0.89 (m, 12H), 0.78–0.81 (m, 6H). 13C NMR (100 MHz, CDCl3): δ 190.42, 189.74, 145.43, 145.40, 144.13, 144.10, 142.05, 141.48, 140.73, 140.52, 139.16, 135.67, 134.94, 134.72, 128.17, 126.32, 126.08, 123.73, 122.93, 122.76, 120.78, 120.55, 117.30, 108.57, 105.84, 56.62, 33.89, 31.96, 31.92, 31.80, 30.66, 30.19, 29.49, 29.38, 26.87, 22.73, 22.63, 14.16. MS (MALDI-TOF): calculated for C105H127NO4S6 [M]+, 1659.53; found 1660.27.
Characterization
The 1H and 13C NMR spectra were recorded on a Bruker AV-400 spectrometer. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) was carried out on a Bruker Autoflex III instrument. Cyclic voltammetry (CV) measurements were carried out on an LK98B II microcomputer-based electrochemical analyzer in dichloromethane solutions at room temperature using a conventional three-electrode configuration, with a glassy carbon electrode, a saturated calomel electrode, and a Pt wire as the working, reference, and counter electrodes. Dichloromethane was distilled from calcium hydride under dry argon immediately before use. Tetrabutylammonium phosphorus hexafluoride (Bu4NPF6, 0.1 M) in dichloromethane was used as the supporting electrolyte; the molecules were dissolved in the above dichloromethane solution before the measurement, and the scan rate was 100 mV s−1. UV-vis spectra were recorded on a JASCO V-570 spectrophotometer. Thermogravimetric analysis (TGA) was carried out on a NETZSCH STA 409PC instrument under a purified nitrogen gas flow at a heating rate of 10 °C min−1. Atomic force microscopy (AFM) measurements were carried out using a Bruker MultiMode 8 instrument in the “tapping” mode. Transmission electron microscopy (TEM) images were recorded on a Philips Technical G2 F20 instrument at 200 kV.The space-charge-limited current (SCLC) mobility was measured using the diode configuration of ITO/PEDOT:PSS/donor:PC71BM/Au for hole mobility and ITO/Al/donor:PC71BM/Al for electron mobility and fitting the results to a space-charge-limited form, where SCLC is described by
The current density–voltage (J–V) characteristics of photovoltaic devices were recorded on a Keithley 2400 source-measure unit. The photocurrent was measured under a simulated illumination of 100 mW cm−2 (AM 1.5G irradiation) using a xenon-lamp-based solar simulator (SAN-EI XES-70S1) in an argon-filled glovebox. Simulator irradiance was characterized using a calibrated spectrometer, and the illumination intensity was set using a certified silicon diode. The external quantum efficiency values (EQEs) of the encapsulated devices were recorded using a halogen tungsten lamp, monochromator, optical chopper, and lock-in amplifier under air, and the photon flux was determined using a calibrated silicon photodiode.
Results and discussion
Synthesis and thermal property
Scheme 1 shows the chemical structure of DI3TCz, which was prepared by the Knoevenagel condensation of DCHO3TCz with 1,3-indanedione in the presence of triethylamine. Purification using conventional silica-gel chromatography, followed by recrystallization from chloroform and hexane, afforded the final product. The structure was verified by NMR and MALDI-TOF-MS. The molecule DI3TCz is rather soluble in common organic solvents and exhibits good thermal stability up to 340 °C under N2 (Fig. S1†).Fig. 1 shows the optical absorption of DI3TCz in solution (chloroform) and solid state (thin film). The detailed absorption data were summarized in Table 1. The absorption data of DR3TCz were also given in Fig. 1 and Table 1 for comparison. As shown in Fig. 1 and Table 1, DI3TCz exhibits redshifted absorption in solution and solid state in contrast to that of DR3TCz. The shoulder peak in the film state, indicative of the effective π–π packing between molecule backbones; this effective packing is beneficial for charge transport.9 Additionally, DI3TCz exhibits a considerably bathochromic shift from solution (539 nm) to solid state (575 nm) with a wide range. This result might be related to its reduced π–π stacking distances between backbones and improved order after introduction of the 1,3-indanedione end group.10 The optical band gap of DI3TCz is 1.75 eV, which is much smaller than that of DR3TCz with value of 1.88 eV. The electrochemical properties of DI3TCz was investigated by cyclic voltammetry (CV). Ferrocene/ferrocenium of the (Fc/Fc+) redox couple (4.8 eV below the vacuum level) was used as the internal calibration. The HOMO and LUMO energy levels are −5.09 and −3.46 eV, respectively. As shown in Table 1, DI3TCz has nearly same HOMO to that of DR3TCz because of the same carbazole central unit. While DI3TCz shows deeper LUMO values than that of DR3TCz ascribed to the stronger electron withdrawing ability of 1,3-indanedione.
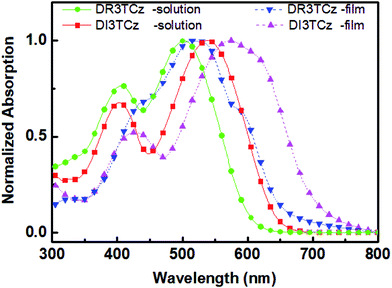 | ||
| Fig. 1 UV-vis absorption spectra of DI3TCz and DR3TCz in chloroform solution and on the solid state. | ||
| Molecule | λsolmax/nm | λfilmmax/nm | Eoptg/eV | HOMO/eV | LUMO/eV |
|---|---|---|---|---|---|
| DI3TCz | 539 | 575 | 1.75 | −5.09 | −3.46 |
| DR3TCz | 502 | 516 | 1.88 | −5.08 | −3.28 |
Photovoltaic performance
BHJ solar cells were fabricated using DI3TCz as electron donor and PC71BM as electron acceptor with a device structure glass/ITO/PEDOT:PSS/donor:PC71BM/PrC60MA/Al and tested under the illumination of AM 1.5G, 100 mW cm−2. PrC60MA is a fullerene derivative for electron transport layer developed by Jen's group (Fig. S8†). The active layer was spin-coated from its chloroform solution. The optimized blend ratio for DI3TCz was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8 (DI3TCz:PC71BM) by weight. Fig. 2a shows the optimized J–V curves of DI3TCz:PC71BM devices. The corresponding photovoltaic parameters are summarized in Table 2. For comparison, the best J–V curve and photovoltaic parameters are given in Fig. 2a and Table 2. The DI3TCz-based device without post-treatment exhibits only a PCE of 3.07%, with a Jsc of 7.96 mA cm−1 and FF of 0.39. The device performance significantly improves after solvent vapour annealing (SVA) with chloroform due to the significant enhancement of Jsc and FF. The optimal device exhibits a Voc of 0.97 V, a Jsc of 10.40 mA cm−1, and an FF of 0.65, resulting in a PCE of 6.46%. To our knowledge, this is the highest PCE value for carbazole-based small molecule OPVs to date. As shown in Table 2, the Voc of DR3TCz:PC71BM device is nearly same as that of DR3TCz:PC71BM device, which is consistent with their similar HOMO levels. The Jsc of DI3TCz based device is much higher than that of DR3TCz ascribed to the broad and red shifted absorption of DI3TCz. Note, DI3TCz based device gives a notable FF of 0.65, which is mainly attributed to the balanced mobility discussed below. Fig. 2b shows the EQE spectra of the optimal devices of DI3TCz. Compared with DR3TCz, DI3TCz based devices exhibits a broad photocurrent response from 300 to 700 nm, which is consistent with the absorption spectra of the blend films and higher Jsc than that of DR3TCz based devices.
0.8 (DI3TCz:PC71BM) by weight. Fig. 2a shows the optimized J–V curves of DI3TCz:PC71BM devices. The corresponding photovoltaic parameters are summarized in Table 2. For comparison, the best J–V curve and photovoltaic parameters are given in Fig. 2a and Table 2. The DI3TCz-based device without post-treatment exhibits only a PCE of 3.07%, with a Jsc of 7.96 mA cm−1 and FF of 0.39. The device performance significantly improves after solvent vapour annealing (SVA) with chloroform due to the significant enhancement of Jsc and FF. The optimal device exhibits a Voc of 0.97 V, a Jsc of 10.40 mA cm−1, and an FF of 0.65, resulting in a PCE of 6.46%. To our knowledge, this is the highest PCE value for carbazole-based small molecule OPVs to date. As shown in Table 2, the Voc of DR3TCz:PC71BM device is nearly same as that of DR3TCz:PC71BM device, which is consistent with their similar HOMO levels. The Jsc of DI3TCz based device is much higher than that of DR3TCz ascribed to the broad and red shifted absorption of DI3TCz. Note, DI3TCz based device gives a notable FF of 0.65, which is mainly attributed to the balanced mobility discussed below. Fig. 2b shows the EQE spectra of the optimal devices of DI3TCz. Compared with DR3TCz, DI3TCz based devices exhibits a broad photocurrent response from 300 to 700 nm, which is consistent with the absorption spectra of the blend films and higher Jsc than that of DR3TCz based devices.
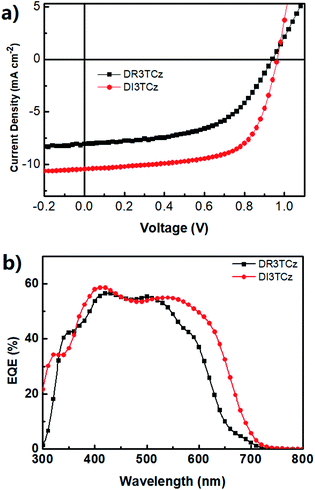 | ||
| Fig. 2 (a) Typical J–V curves of optimal devices based on DR3TCz:PC71BM and DI3TCz:PC71BM. (b) EQE spectra of devices based on DR3TCz:PC71BM and DI3TCz:PC71BM. | ||
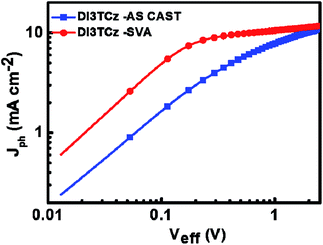
To understand the effect of SVA on photovoltaic performance of the DI3TCz-based devices, the relationship between the photocurrent (Jph) and effective voltage (Veff) is obtained from the J–V data and shown in Fig. 3. Jph (Jph = JL − JD) is dependent on Veff (Veff = V0 − Va), where JL and JD are the current densities under illumination and in the dark, respectively; Va is the applied voltage; and the V0 is the voltage at which Jph = 0.11 As shown in Fig. 3, with increasing Veff, the saturation photocurrent (Jsat) for the DI3TCz-based devices after SVA treatment is attained earlier. And it value is greater than that of the as cast device. This result indicated that the DI3TCz-based device exhibited more efficient charge dissociation and collection after SVA treatment.
Morphology, molecular ordering, and mobility
Fig. 4a and b shows the atomic force microscopy (AFM) images of the active layer morphology. The blend film without post-treatment exhibits a root-mean-square (rms) surface roughness of 0.58 nm (Fig. 4a). After SVA, the rms roughness of the blend films slightly increases to 0.69 nm (Fig. 4b). Additionally, as shown in TEM images (Fig. 4c and d), after SVA treatment, the blend film based on DI3TCz:PC71BM exhibits clear phase separation with interpenetrating networks of donor and acceptor phases. That morphology is beneficial for the dissociation of excitons and charge transport, which is attributed to improvement of device FF and Jsc.12 The mobilities of the blend films were measured by the SCLC method. The hole and electron mobilities of DI3TCz:PC71BM-based devices without post-treatment are 2.17 × 10−5 cm2 V−1 s−1 and 2.35 × 10−5 cm2 V−1 s−1, respectively. These values improved to 6.11 × 10−5 cm2 V−1 s−1 and 4.30 × 10−5 cm2 V−1 s−1, respectively, after SVA treatment. The improvement in hole and electron mobilities is consistent with the interpenetrating donor and acceptor networks morphology and attributed to the good FF of the devices.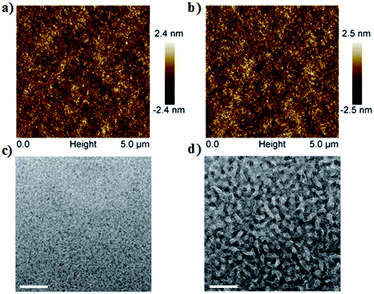 | ||
| Fig. 4 (a and b) Tapping-mode AFM-phase images of the active layers of DI3TCz:PC71BM. (c and d) TEM images of DI3TCz:PC71BM blend films. (a and c) As-cast and (b and d) with SVA post-treatment. | ||
Conclusions
In summary, we have designed and synthesized small molecule donor DI3TCz with 1,3-indanedione as the end group. In contrast to its rhodanine analogy DR3TCz, DI3TCz showed red shifted absorptions and nearly unchanged HOMO level. Compared with that of DR3TCz, the DI3TCz device showed a high Voc value of 0.97 V and improved Jsc of 10.40 mA cm−1. Additionally, a notably FF of 0.65 was achieved due to the interpenetrating donor and acceptor network morphology and balanced hole and electron mobilities. The PCE (6.46%) achieved for optimized DI3TCz:PC71BM device is the highest PCE reported to data for carbazole-based small molecules OPVs. The enhancement in photovoltaic performance is attributed to the tuning of the absorptions and energy levels via the use of the acceptor indandione unit.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge the financial support from MoST (2014CB643502) and NSFC (91433101).Notes and references
- (a) H. J. Zhang, Q. Zhang, M. M. Li, B. Kan, W. Ni, Y. C. Wang, X. Yang, C. X. Du, X. J. Wan and Y. S. Chen, J. Mater. Chem. C, 2015, 3, 12403–12409 RSC; (b) C. Yi, X. W. Hu, H. C. Liu, R. D. Hu, C. H. Hsu, J. Zheng and X. Gong, J. Mater. Chem. C, 2015, 3, 26–32 RSC; (c) K. Sun, Z. Y. Xiao, S. R. Lu, W. Zajaczkowski, W. Pisula, E. Hanssen, J. M. White, R. M. Williamson, J. Subbiah, J. Y. Ouyang, A. B. Holmes, W. W. H. Wong and D. J. Jones, Nat. Commun., 2015, 6, 6013 CrossRef CAS PubMed; (d) Y. Z. Lin, Y. F. Li and X. W. Zhan, Chem. Soc. Rev., 2012, 41, 4245–4272 RSC; (e) Y. J. Cheng, S. H. Yang and C. S. Hsu, Chem. Rev., 2009, 109, 5868–5923 CrossRef CAS PubMed; (f) W. Q. Chen, X. Yang, G. K. Long, X. J. Wan, Y. S. Chen and Q. C. Zhang, J. Mater. Chem. C, 2015, 3, 4698–4705 RSC; (g) L. M. Chen, Z. R. Hong, G. Li and Y. Yang, Adv. Mater., 2009, 21, 1434–1449 CrossRef CAS.
- (a) S. Q. Zhang, L. Ye, W. C. Zhao, B. Yang, Q. Wang and J. H. Hou, Sci. China: Chem., 2015, 58, 248–256 CrossRef CAS; (b) Q. Zhang, B. Kan, F. Liu, G. K. Long, X. J. Wan, X. Q. Chen, Y. Zuo, W. Ni, H. J. Zhang, M. M. Li, Z. C. Hu, F. Huang, Y. Cao, Z. Q. Liang, M. T. Zhang, T. P. Russell and Y. S. Chen, Nat. Photonics, 2015, 9, 35–41 CrossRef CAS; (c) J. B. You, L. T. Dou, K. Yoshimura, T. Kato, K. Ohya, T. Moriarty, K. Emery, C. C. Chen, J. Gao, G. Li and Y. Yang, Nat. Commun., 2013, 4, 1446 CrossRef PubMed; (d) L. Ye, S. Q. Zhang, L. J. Huo, M. J. Zhang and J. H. Hou, Acc. Chem. Res., 2014, 47, 1595–1603 CrossRef CAS PubMed; (e) Y. H. Liu, J. B. Zhao, Z. K. Li, C. Mu, W. Ma, H. W. Hu, K. Jiang, H. R. Lin, H. Ade and H. Yan, Nat. Commun., 2014, 5, 13094 Search PubMed; (f) C. Liu, C. Yi, K. Wang, Y. L. Yang, R. S. Bhatta, M. Tsige, S. Y. Xiao and X. Gong, ACS Appl. Mater. Interfaces, 2015, 7, 4928–4935 CrossRef CAS PubMed; (g) S. H. Liao, H. J. Jhuo, Y. S. Cheng and S. A. Chen, Adv. Mater., 2013, 25, 4766–4771 CrossRef CAS PubMed; (h) B. Kan, Q. Zhang, M. M. Li, X. J. Wan, W. Ni, G. K. Long, Y. C. Wang, X. Yang, H. R. Feng and Y. S. Chen, J. Am. Chem. Soc., 2014, 136, 15529–15532 CrossRef CAS PubMed; (i) F. Huang, Sci. China: Chem., 2015, 58, 190 CrossRef CAS; (j) X. W. Hu, C. Yi, M. Wang, C. H. Hsu, S. J. Liu, K. Zhang, C. M. Zhong, F. Huang, X. Gong and Y. Cao, Adv. Energy Mater., 2014, 4, 1400378 CrossRef; (k) Z. C. He, C. M. Zhong, S. J. Su, M. Xu, H. B. Wu and Y. Cao, Nat. Photonics, 2012, 6, 591–595 CrossRef; (l) J. D. Chen, C. H. Cui, Y. Q. Li, L. Zhou, Q. D. Ou, C. Li, Y. F. Li and J. X. Tang, Adv. Mater., 2015, 27, 1035–1041 CrossRef CAS PubMed.
- (a) H. X. Zhou, L. Q. Yang, A. C. Stuart, S. C. Price, S. B. Liu and W. You, Angew. Chem., Int. Ed., 2011, 50, 2995–2998 CrossRef CAS PubMed; (b) Y. M. Sun, G. C. Welch, W. L. Leong, C. J. Takacs, G. C. Bazan and A. J. Heeger, Nat. Mater., 2012, 11, 44–48 CrossRef CAS PubMed; (c) A. Mishra and P. Bauerle, Angew. Chem., Int. Ed., 2012, 51, 2020–2067 CrossRef CAS PubMed; (d) A. K. K. Kyaw, D. H. Wang, D. Wynands, J. Zhang, T. Q. Nguyen, G. C. Bazan and A. J. Heeger, Nano Lett., 2013, 13, 3796–3801 CrossRef CAS PubMed; (e) G. R. He, X. J. Wan, Z. Li, Q. Zhang, G. K. Long, Y. S. Liu, Y. H. Hou, M. T. Zhang and Y. S. Chen, J. Mater. Chem. C, 2014, 2, 1337–1345 RSC.
- B. Kan, M. M. Li, Q. Zhang, F. Liu, X. J. Wan, Y. C. Wang, W. Ni, G. K. Long, X. Yang, H. R. Feng, Y. Zuo, M. T. Zhang, F. Huang, Y. Cao, T. P. Russell and Y. S. Chen, J. Am. Chem. Soc., 2015, 137, 3886–3893 CrossRef CAS PubMed.
- X. G. Guo, N. J. Zhou, S. J. Lou, J. Smith, D. B. Tice, J. W. Hennek, R. P. Ortiz, J. T. L. Navarrete, S. Y. Li, J. Strzalka, L. X. Chen, R. P. H. Chang, A. Facchetti and T. J. Marks, Nat. Photon, 2013, 7, 825–833 CrossRef CAS.
- (a) T. S. van der Poll, J. A. Love, T. Q. Nguyen and G. C. Bazan, Adv. Mater., 2012, 24, 3646–3649 CrossRef CAS PubMed; (b) S. X. Sun, Y. Huo, M. M. Li, X. W. Hu, H. J. Zhang, Y. W. Zhang, Y. D. Zhang, X. L. Chen, Z. F. Shi, X. Gong, Y. S. Chen and H. L. Zhang, ACS Appl. Mater. Interfaces, 2015, 7, 19914–19922 CrossRef CAS PubMed; (c) W. Ni, M. M. Li, X. J. Wan, H. R. Feng, B. Kan, Y. Zuo and Y. S. Chen, RSC Adv., 2014, 4, 31977–31980 RSC; (d) Y. S. Liu, C. C. Chen, Z. R. Hong, J. Gao, Y. Yang, H. P. Zhou, L. T. Dou, G. Li and Y. Yang, Sci. Rep., 2013, 3, 11547 Search PubMed; (e) Q. D. Li, F. Liu, X. W. Hu, W. Z. Xu, L. P. Wang, X. H. Zhu, X. Gong and Y. Cao, IEEE Photonics J., 2015, 5, 1118–1124 Search PubMed; (f) V. Gupta, A. K. K. Kyaw, D. H. Wang, S. Chand, G. C. Bazan and A. J. Heeger, Sci. Rep., 2013, 3, 1965 CrossRef PubMed.
- (a) W. Ni, M. M. Li, B. Kan, Y. Zuo, Q. Zhang, G. K. Long, H. R. Feng, X. J. Wan and Y. S. Chen, Org. Electron., 2014, 15, 2285–2294 CrossRef CAS; (b) H. R. Feng, M. M. Li, W. Ni, F. Liu, X. J. Wan, B. Kan, Y. C. Wang, Y. M. Zhang, Q. Zhang, Y. Zuo, X. Yang and Y. S. Chen, J. Mater. Chem. A, 2015, 3, 16679–16687 RSC.
- G. K. Long, X. J. Wan, B. Kan, Y. S. Liu, G. R. He, Z. Li, Y. W. Zhang, Y. Zhang, Q. Zhang, M. T. Zhang and Y. S. Chen, Adv. Energy Mater., 2013, 3, 639–646 CrossRef CAS.
- (a) Y. Ruiz-Morales, J. Phys. Chem. A, 2002, 106, 11283–11308 CrossRef CAS; (b) A. Guerrero, S. Loser, G. Garcia-Belmonte, C. J. Bruns, J. Smith, H. Miyauchi, S. I. Stupp, J. Bisquert and T. J. Marks, Phys. Chem. Chem. Phys., 2013, 15, 16456–16462 RSC; (c) J. E. Anthony, Chem. Rev., 2006, 106, 5028–5048 CrossRef CAS PubMed.
- (a) C. Uhrich, R. Schueppel, A. Petrich, M. Pfeiffer, K. Leo, E. Brier, P. Kilickiran and P. Baeuerle, Adv. Funct. Mater., 2007, 17, 2991–2999 CrossRef CAS; (b) M. Turbiez, P. Frere, M. Allain, C. Videlot, J. Ackermann and J. Roncali, Chem.–Eur. J., 2005, 11, 3742–3752 CrossRef CAS PubMed.
- (a) L. Y. Lu, Z. Q. Luo, T. Xu and L. P. Yu, Nano Lett., 2013, 13, 59–64 CrossRef CAS PubMed; (b) P. W. M. Blom, V. D. Mihailetchi, L. J. A. Koster and D. E. Markov, Adv. Mater., 2007, 19, 1551–1566 CrossRef CAS.
- T. L. Nguyen, H. Choi, S. J. Ko, M. A. Uddin, B. Walker, S. Yum, J. E. Jeong, M. H. Yun, T. J. Shin, S. Hwang, J. Y. Kim and H. Y. Woo, Energy Environ. Sci., 2014, 7, 3040–3051 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra10387e |
| This journal is © The Royal Society of Chemistry 2018 |