Open Access Article
Open Access ArticleTwo Cu(II)-triadimenol complexes as potential fungicides: synergistic actions and DFT calculations†
Jie Lia,
Huiyu Liua,
Zhaoqi Guoa,
Mingyan Yangb,
Jirong Songac and
Haixia Ma *a
*a
aSchool of Chemical Engineering, Northwest University, Xi'an, Shaanxi 710069, China. E-mail: mahx@nwu.edu.cn; Fax: +86-029-88307755; Tel: +86-029-88307755
bDepartment of Environmental Science and Engineering, Chang'an University, Xi'an, Shaanxi 710054, China
cConservation Technology Department, The Palace Museum, Beijing, China
First published on 15th January 2018
Abstract
Two Cu(II) complexes, namely, [CuL4(H2O)2]·2NO3·2CH3OH 1 and [CuL2(CH3COO)2] 2, (L = (1S,2R)-1-(4-chlorophenoxy)-3,3-dimethyl-1-(1,2,4-triazol-1-yl)butan-2-ol, triadimenol, a commercial 1,2,4-triazole pesticide) were synthesized and characterized by elemental analysis, IR spectra, UV-Vis spectra and single crystal X-ray diffraction. Crystal structural analysis shows that the different types of salts (copper acetate is covalent, while copper nitrate is ionic) contribute to different crystal structures: complex 1 consists of one copper cation, four ligands, two coordinated water molecules, two free nitrate anions and two uncoordinated methanol molecules. Complex 2 is composed of one copper cation, two ligands and two acetate anions, without free molecules. The two complexes and the ligand triadimenol were also screened for antifungal activities against four selected fungi. The antifungal results reveal that both the complexes show better bioactivities in comparison with L, and that complex 1 has higher bioactivities than complex 2. To elaborate the reasons of the enhanced bioactivities after complexation, the interaction levels between Cu2+ cation and triadimenol, as well as the density functional theory (DFT) method were carried out. The results indicate that three factors made the antifungal activities stronger after forming Cu(II) complexes: new active site of copper cation, synergic interactions between Cu2+ cation and L, and improved penetration of the metal complexes into the lipid membranes.
1 Introduction
A new generation of 1,2,4-triazole fungicide is urgently needed because of its substantial environmental contamination and a rapid selection of resistant strains.1–6 Metal-based pesticides represent a novel group of fungicides with potential applications for the control of various phytopathogens. Because complexation of pesticides with metals has many advantages including the enhancement of persistence, longer shelflife, reduction of mammalian toxicity and conversion of nonsystemic to systemic pesticides.7–9 Moreover, the pesticides coordinated with transition metals could be utilized as a kind of controlled release formulation which has the capacity of alleviating the toxicity and decreasing the pesticide residue.10,11Triadimenol, chemically named (1S,2R)-1-(4-chlorophenoxy)-3,3-dimethyl-(1,2,4-triazol-1-yl)butan-2-ol, is an important 1H-1,2,4-triazole fungicide with a broad spectrum of activity against mildews and rusts in cereals, fruits and vegetables.12,13 This compound inhibits fungal proliferation by the interference with steroid biosynthesis and fungal cell-membrane formation mediated by cytochrome P450-dependent 14α-sterol demethylase (P450DM), an essential enzyme in ergosterol biosynthesis in fungi and cholesterol synthesis in mammalian cells.14 However, the intensive use and single active site of triadimenol have led to the problem of the antifungal resistance.15–17
Although the complexes of triadimenol have many potential advantages and synthetic accessibility, the related research is still rare.18–20 Zhang reported the synthesis and crystal structure of CuCl2 complex with triadimenol.18 Qian reported the formation, crystal structure and dielectric physical properties of its Cu(CH3COO)2 complex with triadimenol.19 Our group reported the structure, crystal structure and antifungal activities of ZnBr2 complex with triadimenol and found that the Zn(II) complex are more active than triadimenol against Botryosphaeria ribis, Gibberella nicotiancola, Colletotrichum gloeosporioides and Alternaria solani.20 To inject new vitality into triadimenol, we are interested in exploiting the Cu(II) coordination chemistry of triadimenol and the potential application in agricultural application. Even though the structure of the complex of triadimenol and copper acetate [CuL2(CH3COO)2] 2 (L = triadimenol) has been proposed,19 its antifungal activities against the selected phytopathogens such as Elsinoe ampelina, Glomerella cingulata, Physalospora berengeriana and Gibberella saubinetii and its theoretical investigation of the electronic structure have not yet been reported, to the best of our knowledge.
In this paper, two Cu(II) complexes based on triadimenol L, [CuL4(H2O)2]·2NO3·2CH3OH 1 and [CuL2(CH3COO)2] 2 were prepared. Their crystal structures and antifungal activities against four selected plant pathogenic fungi were also reported. Additionally, with the assistance of density functional theoretical (DFT) investigation, a reason analysis of the increased bioactivities was made in this paper.
2 Results and discussion
2.1 Synthesis, IR, UV-Vis, EPR spectroscopy and magnetic properties
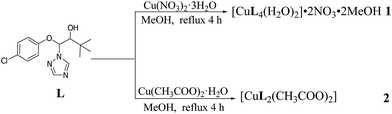
As shown in Scheme 1, reaction of triadimenol with Cu(NO3)2·3H2O led to a mononuclear triazole complex coordinated with four ligands and two water molecules. The nitrate anions did not participate with coordination. While, reaction of triadimefon with the dimer of Cu(CH3COO)2·H2O generated a mononuclear complex coordinated with only two ligands and two acetate anions.Both complexes 1 and 2 display the characteristic bands of hydroxyl groups. The hydroxyl ν(O–H) stretching vibrations in the IR spectrum of 1 and 2 are present at 3362 and 3340 cm−1, respectively. The bands observed in 3137–2867 cm−1 region are assigned to the stretching C–H vibrations of complexes 1 and 2. The IR absorption band at 1386 cm−1 of complex 1 may correspond to the antisymmetric stretching vibration ν(NO) of nitrate. This suggest the nitrate group is in uncoordinated manner. In complex 2, the absorption bands at 1403 cm−1 and 1578 cm−1 are attributed to νs(COO−) and νas(COO−), respectively, which indicates that the coordinated behavior of the acetato group with the central metal ion in bidentate manner.21
The UV-Vis spectra of the complexes 1 and 2 and the ligand L were recorded in CH3OH (Fig. S1†). For L, the absorption bands at 222, 276 nm are attributed to π–π* and n–π*transitions. Upon complexation, the π–π* band shifts from 224 and 223 nm, while the n–π* band moves to 275 nm. Furthermore both the complexes present a relatively broad band in the visible region, with maxima centered at about 535 nm and 528 nm, respectively for complex 1 and 2, indicating a distorted octahedral geometry in each case. The broadness of the band observed in each case may be attributed to Jahn–Teller distortions.22 In Cu(II) octahedral or pseudo-octahedral environments this band is usually composed of a number of components that in all the present cases remain unresolved. These bands originated in transitions from the dxy, dz2 and dxz, dyz pair to the σ antibonding and half-filled dx2 − dy2 level and the relative order of these transitions depend upon the extent of axial metal–ligand interaction and on the overall geometry around the metal center, which indicates that there is electron transfer between metal ions and the ligand.23
The EPR spectra of the two Cu(II) complexes were recorded at room temperature as crystal sample (Fig. S2†). They exhibit a well-resolved anisotropic signal in the parallel and perpendicular Cu2+ region. There are three hyperfine peaks in the parallel region derived from the coupling of the copper nucleus and the unpaired electron. The observed data show that g∥ = 2.226 for complex 1 and 2.223 for complex 2, and the data of g⊥ for complex 1 and complex 2 are 2.054 and 2.048, respectively. The results indicate the oxidation state of copper in both the complexes to be +2. The fact g‖ > g⊥ confirms an elongated octahedral stereochemistry with a dx2 − dy2 ground state in both the complexes,24 which is in good agreement with the determined X-ray structures.
The XMT versus T plot (XMT being the magnetic susceptibility per Cu atom) of the two complexes is given in Fig. S3.† At room temperature the XMT values for complexes 1 and 2 are 0.38 and 0.39 cm3 mol−1 K, respectively, which agrees well with that expected for isolated copper(II) ions,25 clearly demonstrating both the two Cu(II) complexes are mononuclear complexes. The XMT of the two complexes remains almost constant until around 50 K and then sharply decreases to a value 0.35–0.36 cm3 mol−1 K at 2 K. This decrease is due to very weak intermolecular antiferromagnetic interactions.26
2.2 Molecular structures of complexes 1 and 2
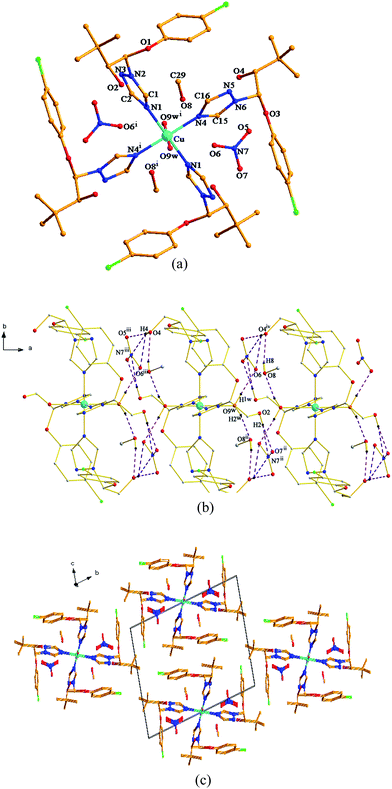
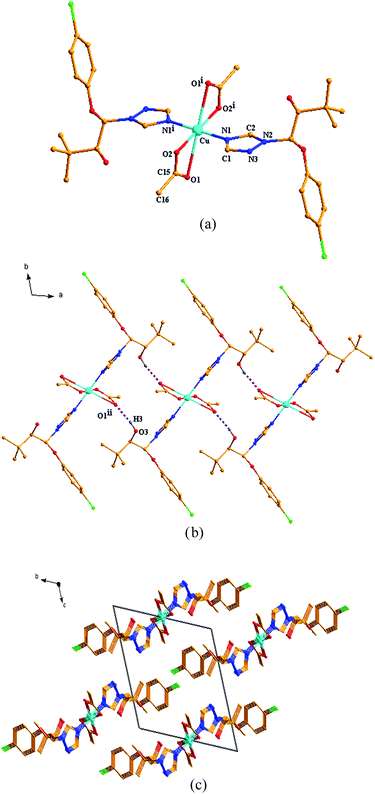
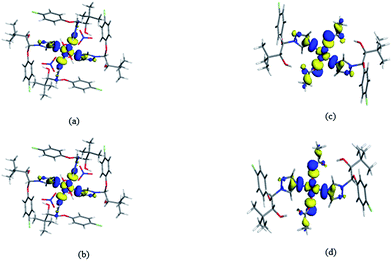
As shown in Fig. 1a and 2a, although both the Cu(II) centers in the two title complexes absorb octahedral geometry, the coordination environments of the Cu(II) centers in the two complexes are different. In complex 1, the Cu(II) atom is coordinated with four L ligands and two water molecules. While two NO3− anions and two methanol molecules are present in the 2nd coordination sphere (Fig. 1a). However, in complex 2, the Cu(II) atom is coordinated with only two L ligands and two CH3COO− anions without free molecules (Fig. 2a). The different types of salts (copper acetate is covalent, while copper nitrate is ionic) should be due to the diversified crystal structures even with the same ligand and metal centers.In complex 1, Cu atom and four coordinated N atoms from four triazole rings form the equatorial plane. The least-squares plane equation is 7.635x + 2.877y + 3.759z = 9.0731, with the mean deviation from the plane 0 Å, indicating that these five atoms form a perfect plane. The axial position is occupied by Cu, O9w and O9wi atoms. The Cu–O9w bond length is 2.392(2) Å, much longer than the Cu–N bond lengths (2.035(2) and 2.153(2) Å), thus forming an elongated octahedron around the Cu atom. The dihedral angles of benzene ring planes and their attached triazole planes are 75.709(109)° and 75.632(87)°, respectively. The triazole ring planes are coplanar with their symmetric planes, and approximately perpendicular to their adjacent triazole ring planes (dihedral angle 77.802(98)°). While in complex 2, Cu atom and four O atoms from two CH3COO− anions define the equatorial plane, and the least-squares plane equation is 3.315x + 5.963y + 2.834z = 10.5179, with the mean deviation from the plane 0 Å. The axial position is occupied by Cu, N1 and N1i atoms. The Cu–N bond length is 1.9950(18) Å, which is slightly shorter than that of complex 1. The bond length of Cu–O1 is 2.6610(27) Å, much longer than that of Cu–O2 (1.9410(16) Å). The triazole ring plane is coplanar with its symmetric plane. The dihedral angle of benzene ring planes and their attached triazole planes is 77.433(81)°, which is close to those of complex 1.
Both of the two complexes form infinite chain structures by hydrogen bonds. As shown in Fig. 1b, in the structure of complex 1, there is one kind of intramolecular hydrogen bond O9w–H1w⋯O6, which is generated by O9w atom of water molecule as hydrogen bond donor to O6 of free nitrate. And there are seven kinds of intermolecular hydrogen bonds: O2 atom takes part in hydrogen bonds of O2–H2⋯O7ii, O2–H2⋯N7ii and O9w also takes part in hydrogen bond of O9–H2w⋯O8ii (symmetry code: (ii) −x + 1, −y + 1, −z); O4 atom acts as hydrogen bond donor to O5iii, O6iii and N7iii of free nitrate forming hydrogen bonds of O4–H4⋯O5iii, O4–H4⋯O6iii and O4–H4⋯⋯N7iii (symmetry code: (iii) x + 1, y, z); meanwhile, O4 atom also acts as hydrogen bond acceptor to O8 from the adjacent molecule, generating O8–H8⋯H4iv (symmetry code: (iv) x − 1, y, z). The intermolecular hydrogen bonds contribute to the formation of a one-dimensional framework along c-axis in the crystal structure of complex 1 (Fig. 1b). In the structure of complex 2, there is only one kind of intermolecular hydrogen bond: the hydroxyl groups of the ligand form hydrogen bond with the coordinated O1 atom from CH3COO− anion on the adjacent [CuL2(CH3COO)2] units of O3–H3⋯O5ii (H3⋯O5ii 1.916 Å), thus generating a 1D chain along c-axis (Fig. 2b). As illustrated in Fig. 1c and 2c, the 1D chains are connected by van der Waals forces to give the 3D framework along the a-axis in both the two complexes.
Structure refinement details of the complexes are summarized in Table 1. Selected bond lengths and angles are listed in Table 2, and hydrogen bonds are shown in Table 3.
| Parameter | 1 | 2 |
|---|---|---|
| Empirical formula | C58H84Cl4CuN14O18 | C32H42Cl2CuN6O8 |
| Formula weight | 1470.73 | 773.15 |
| Cryst system | Triclinic | Triclinic |
| Space group | P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
| a (Å) | 7.8341(16) | 8.5849(16) |
| b (Å) | 15.510(3) | 10.5243(19) |
| c (Å) | 16.076(3) | 12.043(3) |
| α (°) | 68.996(4) | 111.668(4) |
| β (°) | 88.380(4) | 108.999(4) |
| γ (°) | 88.253(4) | 96.088(3) |
| V (Å3) | 1822.4(6) | 923.9(3) |
| Z | 1 | 1 |
| Dc (g cm−3) | 1.340 | 1.390 |
| F(000) | 771 | 403 |
| Reflcns collcd | 8863 | 4768 |
| Unique/observed | 6315/4408 | 3399/3022 |
| Rint | 0.0265 | 0.0184 |
| Goodness-of-fit on F2 | 0.999 | 1.140 |
| R1 (I ≥ 2σ(I)) | 0.0512 | 0.0387 |
| wR2 (I ≥ 2σ(I)) | 0.1276 | 0.1177 |
| Complex 1 | Complex 2 | ||
|---|---|---|---|
| a Symmetry codes: complex 1 (i) −x + 2, −y + 1, −z. Complex 2 (i) −x + 1, −y + 1, −z + 2. | |||
| Cu–N1 | 2.035(2) | Cu–N1 | 1.9950(18) |
| Cu–N1i | 2.035(2) | Cu–N1i | 1.9950(18) |
| Cu–N4 | 2.030(2) | Cu–O1 | 2.6610(27) |
| Cu–N4i | 2.030(2) | Cu–O1i | 2.6610(27) |
| Cu–O9w | 2.392(2) | Cu–O2 | 1.9410(16) |
| Cu–O9wi | 2.392(2) | Cu–O2i | 1.9410(16) |
| N1–Cu–N1i | 180.00(13) | N1–Cu–N1i | 180.00(12) |
| N4–Cu–N4i | 180.00(18) | O2–Cu–O2i | 180.00(13) |
| O9w–Cu–O9wi | 180.00(11) | O1–Cu–O1i | 180.00(1) |
| N1–Cu–N4 | 91.01(9) | N1–Cu–O2 | 89.13(7) |
| N1–Cu–N4i | 88.99(9) | N1–Cu–O2i | 90.87(7) |
| D–H⋯A | D–H | H⋯A | D⋯A | ∠D–H⋯A |
|---|---|---|---|---|
| a Symmetry code: complex 1 (ii) −x + 1, −y + 1, −z; (iii) x + 1, y, z; (iv) x − 1, y, z; complex 2 (ii) −x, −y + 1, −z + 2. | ||||
| Complex 1 | ||||
| O9w–H1⋯O6 | 0.844 | 2.039 | 2.845 | 159.65 |
| O2–H2⋯O7ii | 0.820 | 1.949 | 2.753 | 166.36 |
| O2–H2⋯N7ii | 0.820 | 2.689 | 3.491 | 166.23 |
| O9w–H2⋯O8iii | 0.843 | 1.964 | 2.796 | 169.03 |
| O4–H4⋯O5iii | 0.820 | 2.009 | 2.808 | 164.36 |
| O4–H4⋯O6iii | 0.820 | 2.545 | 3.098 | 125.94 |
| O4–H4⋯N7iii | 0.820 | 2.584 | 3.338 | 153.52 |
| O8–H8⋯O4iv | 0.820 | 1.977 | 2.754 | 157.88 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Complex 2 | ||||
| O3–H3⋯O1ii | 0.820 | 1.977 | 2.754 | 157.88 |
2.3 Biological activities
| Compound | E. ampelina | G. cingulata | P. berengeriana | G. saubinetii |
|---|---|---|---|---|
| 1 | 1.81 | 1.46 | 1.20 | 0.97 |
| 2 | 3.34 | 4.15 | 2.53 | 1.52 |
| L | 8.39 | 7.63 | 4.54 | 6.69 |
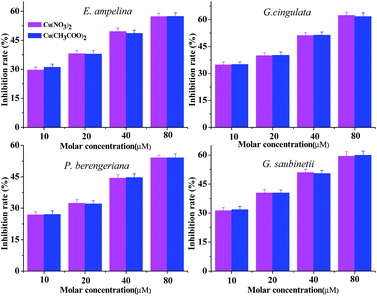
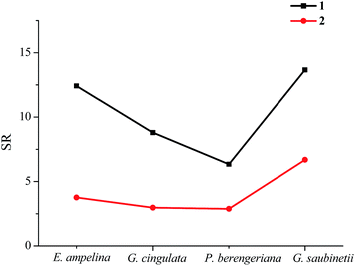
The bioassay of the metal salts (Fig. 3) show that Cu(NO3)2 and Cu(CH3COO)2·H2O have certain inhibitory effects on the four plant pathogenic fungi and the inhibition rate increases with the growth of the concentration of the metal salts. It also can be found that the fungicidal activities of Cu(NO3)2 and Cu(CH3COO)2·H2O against the same selected fungi did not exhibit obvious diversity; this result indicates that the bioactivities of the title complexes were related to only the ligand and metal cation, substantively irrelative to the anions. Therefore, it was the coordination of the ligand and the Cu cation that increased the bioactivities.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 of the molecular-level mixture of copper cation and triadimenol than that for the ratio 1
4 of the molecular-level mixture of copper cation and triadimenol than that for the ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. This also leads to higher bioactivities for complex 1 than complex 2 against the selected fungi; (iii) both the synthesized complexes show different synergy levels to the different tested fungi. The synergy levels concerning the tested fungi are in the sequence of G. saubinetii > E. ampelina > G. cingulata > P. berengeriana, which implies that the antifungal activities against G. saubinetii are improved to the greatest extent, and against P. berengeriana to the smallest extent after complexation.
2. This also leads to higher bioactivities for complex 1 than complex 2 against the selected fungi; (iii) both the synthesized complexes show different synergy levels to the different tested fungi. The synergy levels concerning the tested fungi are in the sequence of G. saubinetii > E. ampelina > G. cingulata > P. berengeriana, which implies that the antifungal activities against G. saubinetii are improved to the greatest extent, and against P. berengeriana to the smallest extent after complexation.
2.4 Theoretical calculations
| Atoms | Atomic charges (e) | fk+ | fk− | fk0 | |
|---|---|---|---|---|---|
| 1 | Cu | 0.613 | 0.073 | 0.077 | 0.075 |
| N1 | −0.415 | 0.019 | 0.019 | 0.019 | |
| C2 | 0.156 | 0.001 | 0.001 | 0.001 | |
| N3 | −0.206 | 0.011 | 0.011 | 0.011 | |
| N2 | −0.140 | 0.003 | 0.002 | 0.003 | |
| C1 | 0.210 | 0.006 | 0.006 | 0.006 | |
| O5 | −0.386 | 0.085 | 0.086 | 0.085 | |
| O6 | −0.472 | 0.061 | 0.062 | 0.061 | |
| O7 | −0.433 | 0.074 | 0.075 | 0.075 | |
| N7 | 0.585 | 0.021 | 0.021 | 0.021 | |
| 2 | Cu | 0.618 | 0.098 | 0.192 | 0.145 |
| N1 | −0.389 | 0.020 | 0.011 | 0.015 | |
| C2 | 0.192 | 0.012 | 0.013 | 0.012 | |
| N3 | −0.389 | 0.039 | 0.039 | 0.039 | |
| N2 | −0.138 | 0.018 | 0.020 | 0.019 | |
| C1 | 0.251 | 0.012 | 0.012 | 0.012 | |
| O1 | −0.539 | 0.042 | 0.035 | 0.038 | |
| O2 | −0.540 | 0.075 | 0.056 | 0.038 | |
| C15 | 0.500 | 0.024 | 0.022 | 0.023 | |
| C16 | −0.346 | 0.027 | 0.022 | 0.024 | |
| L | N1 | −0.330 | 0.036 | 0.035 | 0.035 |
| C2 | 0.139 | 0.025 | 0.030 | 0.028 | |
| N2 | −0.141 | 0.003 | 0.006 | 0.001 | |
| N3 | −0.203 | 0.003 | 0.007 | 0.005 | |
| C1 | 0.179 | 0.007 | 0.022 | 0.015 |
According to the analysis above, it can be observed that the results of HOMO–LUMO, Mulliken atomic charge calculations and Fukui analysis keep consistent, which indicate that the metal cations, coordinated anions and triazole rings may be the active sites of the complexes. However, previous bioactivity results show that the antifungal activities have poor correlation with the coordinated anions,11,27 thus the copper cation and the triazole rings originating from the ligand should be the main active sites inhibiting the growth of fungi. However, it is well known that only the triazole ring is the active site of the ligand L, which is also responsible for a rapid selection of resistance strains of L. Thus we can infer that the increased active site of Cu cations and the synergic interactions between the Cu cation and L should be two factors for the increased bioactivities of the complexes.
Triadimenol belongs to the ergosterol biosynthesis inhibitors, which can impair the pathogenic ability of plant fungi by destroying the structure of the cell membrane. As a result of Mulliken atomic charge calculations, the polarity of the atoms in the complexes is reduced, which is helpful for the enhanced penetration of the metal complexes into the lipid membranes and consequently enhance the bioactivities.
Therefore, the added active site of copper cation, the synergetic interactions between the copper cation and L, as well as the enhanced penetration of the metal complexes into the lipid membranes should be three main reasons for the enhanced antifungal activities after complexation.
3 Experimental section
3.1 Materials and general methods
Triadimenol was purchased from commercial source and repeatedly recrystallized by isopropyl alcohol solvent. All other reagents were of reagent grade and used as received. Elemental analysis was performed on a Vario EL III elemental analyzer. IR spectrum was carried out on EQUINX 55 with KBr presser bit. X-ray diffraction data were collected on a Bruker SMART APEX CCD diffractometer. UV-Vis absorption spectra of the complexes in methanol solution are acquired on Shimadzu UV-78 spectrophotometer. The EPR experiments on Cu2+ (2D5/2) complexes have been performed at room temperature. The field-derivative EPR spectra have been registered by a conventional X-band (ν = 9.77 GHz) Bruker EMX model spectrometer employing. Magnetization and variable-temperature (1.7–300 K) magnetic susceptibility measurements were carried out with a Quantum Design MPMS-XL-7. Experimental susceptibilities were corrected for diamagnetism, temperature-independent paramagnetism.3.2 Synthesis of [CuL4(H2O)2]·2NO3·2CH3OH 1 and [CuL2(CH3COO)2] 2
A solution of copper nitrate trihydrate (0.2416 g, 1 mmol) or copper acetate monohydrate (0.1997 g, 1 mmol) in methanol (5 mL) was added drop wise to a solution of triadimenol (0.5915 g, 2 mmol) in methanol (10 mL). The resulting mixture was refluxed for 4 h to give a clear solution, and then cooled to room temperature. Upon slow evaporation, blue crystals were obtained at room temperature.1: blue crystals, yield: 52%. C58H84Cl4CuN14O18: anal. calc. C 47.37, H 5.76, N 13.33; found: C 47.94, H 5.81, N l3.12. IR (KBr, σ/cm−1): 3362 (m), 3133 (m), 2963 (s), 2867 (w), 1588 (s), 1534 (s), 1467 (s), 1220 (m), 1077 (m), 1002 (m), 819 (m), 730 (m), 668 (m).
2: blue crystals, yield: 68%. C32H42Cl2CuN6O8: anal. calc. C 49.71, H 5.48, N 10.87; found: C 49.16, H 5.68, N l1.03. IR (KBr, σ/cm−1): 3340 (m), 3137 (m), 2963 (m), 2867 (w), 1578 (s), 1491 (s), 1403 (s), 1220 (m), 1020 (m), 811 (m), 752 (m), 659 (m).
3.3 X-ray crystallography
Single-crystal X-ray diffraction (XRD) measurements of 1 and 2 were carried out on a Bruker SMART APEX CCD diffractometer equipped with a graphite monochromator using Mo Kα radiation (0.71073 Å) at 296(2) K in the F–ω scan mode. Unit cell dimensions were obtained with least-squares refinements and semi-empirical absorption corrections were applied using the SADABS program.42 The structures were solved by direct methods and refined by full-matrix least squares techniques based on F2 with the SHELXTL program.43 All non-hydrogen atoms were obtained from the difference Fourier map and refined with atomic anisotropic thermal parameters. The hydrogen atoms were added according to the theoretical models. Crystallographic data of complexes 1 and 2 have been deposited at the Cambridge Crystallographic Data Center with CCDC 1414257 and 1055002.3.4 Antifungal assay
Four important phytopathogens (Elsinoe ampelina, Glomerella cingulata, Physalospora berengeriana and Gibberella saubinetii) were provided by Shaanxi Microbiology Institute, China, and selected for antifungal activity studies. The antifungal activities were carried out by the mycelial growth rate method.11,32 The ligand L and the title complexes were diluted by starch and ground into dust, then added to potato dextrose agar (PDA) medium, respectively, to obtain a range of concentrations (1, 2, 4 and 8 mg L−1) before pouring into the Petri dishes (7.5 cm in diameter). The antifungal activities of the metal salts Cu(NO3)2·3H2O, Cu(CH3COO)2·H2O were also investigated in the range of concentrations of 10, 20, 40 and 80 μmol L−1. Each concentration was tested in triplicate. Parallel controls were maintained with starch mixed with PDA medium. The diameter of fungal colonies on PDA plates was measured after 72 h. Percentage inhibition of mycelial growth was calculated using the formula (1). Because the synthesized complexes can be regarded as molecular-level mixtures of L and inorganic salts, synergy ratios (SR) were calculated to investigate the extent of the interactions between L and inorganic salts according to Wadley approach22 using the formulas (2) and (3).
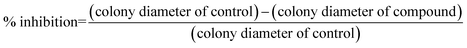 | (1) |
| EC50 (expected) = (a + b)/[(a/EC50A) + (b/EC50B)] | (2) |
| SR = EC50 (expected)/EC50 (observed) | (3) |
3.5 Computational method
The entire calculations are carried out with the DMol3 software,44 which is based on the density functional theory. Symmetry operations were applied for all structures. The generalized gradient approximation (GGA) of Perdew, Burke, and Ernzerhof (PBE) exchange–correlation functional45 is used. Double numerical basis sets including polarization functions (DNP)46,47 are performed to describe the valence orbitals of all the atoms in our calculations. To describe the cores, all-electron relativistic calculations are used.4 Conclusions
Two Cu(II) complexes, [CuL4(H2O)2]·2NO3·2CH3OH (1) and [CuL2 (CH3COO)2] (2), were synthesized using the commercial fungicide triadimenol as ligand. The Cu atoms in both the complexes adopt the same coordination geometry, but have different coordination environment due to the different coordination sphere the counter anions. Both the Cu(II) complexes show more active to inhibit the four selected fungi than the ligand triadimenol, which indicates the potential applications of these complexes in the fields as antifungal agents. Furthermore, complex 1 has stronger antifungal activities than complex 2 since the synergy levels were better for the ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 of copper cation and triadimenol than that for the ratio 1
4 of copper cation and triadimenol than that for the ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. The experimental investigation of the synergistic interactions between Cu2+ and triadimenol, in conjunction with the theoretical investigations of the electronic structures of the complexes reveal that the new active site of Cu cation, the synergic interactions between the Cu cation and triadimenol, as well as the greater penetration through the cell membrane of microorganisms all contribute to the enhanced biocidal properties.
2. The experimental investigation of the synergistic interactions between Cu2+ and triadimenol, in conjunction with the theoretical investigations of the electronic structures of the complexes reveal that the new active site of Cu cation, the synergic interactions between the Cu cation and triadimenol, as well as the greater penetration through the cell membrane of microorganisms all contribute to the enhanced biocidal properties.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is funded by the Provincial Natural Science Foundation of Shaanxi, China (Grant No. 2016JZ003).Notes and references
- R. R. Dalvi and C. D. Howell, Bull. Environ. Contam. Toxicol., 1977, 17, 225–232 CrossRef CAS PubMed.
- I. S. Kim, L. A.Beaudette, J. H. Shim, J. T. Trevors and T. S. Yong, Plant Soil, 2002, 239, 321–331 CrossRef CAS.
- J. A. Zarn, B. J. Brschweiller and J. R. Schlatter, Environ. Health Perspect., 2003, 111, 255–261 CrossRef CAS PubMed.
- S. Pille and M. Koppel, Agric. Food Sci., 2010, 19, 34–42 Search PubMed.
- R. D. Horsley, J. D. Pederson, P. B. Schwarz, K. McKay, M. R. Hochhalter and M. P. McMullen, Agron. J., 2006, 98, 194–197 CrossRef CAS.
- L. Lucini and G. P. Molinari, Pest Manage. Sci., 2009, 65, 440–443 CrossRef CAS PubMed.
- Y. H. Wang, D. Y. Yu, P. Xu, B. Y. Guo, Y. F. Zhang, J. Z. Li and H. L. Wang, Ecotoxicol. Environ. Saf., 2014, 107, 276–283 CrossRef CAS PubMed.
- M. Kamiya and K. Kameyama, Chemosphere, 2001, 45, 231–235 CrossRef CAS PubMed.
- E. Morillo, T. Undabeytia, C. Maqueda and A. Rams, Chemosphere, 2002, 47, 747–752 CrossRef CAS PubMed.
- P. Z. Zhang, Q. Y. Fu, R. X. Chi, C. X. Yang and J. G. Xu, J. Zhejiang Univ. Sci. Technol., 2003, 15, 142–145 Search PubMed.
- X. Chen and C. L. Yang, J. Agric. Food Chem., 2009, 57, 2441–2446 CrossRef CAS PubMed.
- L. G. Yarullina, R. I. Kasimova, B. R. Kuluev, O. B. Surina, L. M. Yarullina and R. I. Ibragimov, Agric. Sci., 2014, 5, 906–912 Search PubMed.
- M. Miazzi and H. R. Hajjeh, J. Plant Pathol., 2011, 93, 729–735 CAS.
- J. C. Kapteyn, J. B. Pillmoor and M. A. De Waard, J. Pestic. Sci., 1992, 34, 37–43 CrossRef CAS.
- S. Hippe and U. Giesen, Ann. Appl. Biol., 2010, 112, 79–90 CrossRef.
- T. L. Peever and M. G. Milgroom, Phytopathology, 1992, 82, 821–828 CrossRef CAS.
- R. A. Wyand and J. K. Brown, Fungal Genet. Biol., 2005, 42, 726–735 CrossRef CAS PubMed.
- P. Z. Zhang, J. Wu, Y. Q. Gong, X. R. Hu and J. M. Gu, Chin. J. Inorg. Chem., 2003, 19, 909–912 CAS.
- K. Qian, Y. B. Jin and Y. X. Li, Chin. J. Inorg. Chem., 2006, 22, 1671–1674 CAS.
- J. Li, T. Xi, B. Yan, M. Y. Yang, H. X. Ma and J. R. Song, Chin. J. Struct. Chem., 2015, 34, 1825–1829 CAS.
- R. Y. Wang, C. C. Shi, Q. Shi, Y. C. Gao and Q. Z. Shi, Chin. J. Inorg. Chem., 2000, 16, 3321–3327 Search PubMed.
- A. B. P. Lever, Inorganic Electronic Spectroscopy, Elsevier, Amsterdam, 2nd edn, 1984 Search PubMed.
- A. P. S. Pannu, P. Kapoor, G. Hundal, R. Kapoor, M. R. Martin, J. B. Rayond and S. H. Maninder, Polyhedron, 2011, 30, 1691–1702 CrossRef CAS.
- S. Chandra and G. K. Lokesh, Spectrochim. Acta, Part A, 2004, 60, 1751–1761 CrossRef PubMed.
- S. R. Choudhury, C. Y. Chen, S. Seth, T. Kar, H. M. Lee, E. Colacio and S. Mukhopadhyay, J. Coord. Chem., 2009, 62, 540–551 CrossRef CAS.
- K. R. Gruenwald, A. M. Kirillov, M. Haukka, J. Sanchiz and A. J. L. Pombeiro, Dalton Trans., 2009, 12, 2109–2120 RSC.
- J. Li, T. Xi, B. Yan, M. Y. Yang, J. R. Song and H. X. Ma, New J. Chem., 2015, 39, 6997–7003 RSC.
- F. M. Wadley, Experimental statistics in entomology, Graduate School Press, Washington State University, Pullman, 1976 Search PubMed.
- H. Saral, Ö. Özdamar, İ. Uçar, Y. Bekdemir and M. Aygün, J. Mol. Struct., 2016, 1103, 9–19 CrossRef CAS.
- J. X. Mu, Z. W. Zhai, M. Y. Yang, Z. H. Sun, H. K. Wu and X. H. Liu, Crystals, 2016, 6, 4 CrossRef.
- M. Larif, A. Adad, R. Hmammouchi, A. I. Taghki, A. Soulaymani, A. Eimidaoui, M. Bouachrine and T. Lakhlifi, Arabian J. Chem., 2017, 10, 946–955 CrossRef.
- J. X. Mu, Y. X. Shi, M. Y. Yan, Z. H. Sun and X. H. Liu, Molecules, 2016, 21, 68 CrossRef PubMed.
- W. Boufas, N. Dupont, M. Berredjem, K. Berrezag, I. Becheker, H. Berredjem and N. E. Aouf, J. Mol. Struct., 2014, 1074, 180–185 CrossRef CAS.
- P. Govindasamy, S. Gunasekaran and S. Srinivasan, Spectrochim. Acta, Part A, 2014, 130, 329–336 CrossRef CAS PubMed.
- K. C. Zheng, J. P. Wang, W. L. Peng, Y. Shen and F. C. Yun, Inorg. Chim. Acta, 2002, 328, 247–253 CrossRef CAS.
- V. Balachandran, S. Rajeswari and S. Lalitha, J. Mol. Struct., 2012, 1007, 63–73 CrossRef CAS.
- G. B. Bagihalli, P. G. Avaji, S. A. Patil and P. S. Badami, Eur. J. Med. Chem., 2008, 43, 2639–2649 CrossRef CAS PubMed.
- C. R. A. María, C. L. S. Estefania, S. Jesús, P. Pelagatti and F. Zani, J. Inorg. Biochem., 2005, 99, 2231–2239 CrossRef PubMed.
- J. Li, Y. Zhang, M. Y. Yang and H. X. Ma, RSC Adv., 2017, 7, 33364–33372 RSC.
- K. Fukui, T. Yonezzawa and H. Shingui, J. Chem. Phys., 1952, 20, 722–725 CrossRef CAS.
- C. J. Brala, I. Fabijanić, A. K. Marković and V. Pilepić, Comput. Theor. Chem., 2014, 1049, 1–6 CrossRef.
- G. M. Sheldrick, SADABS, University of Göttingen, Germany, 2000 Search PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112–122 CrossRef CAS PubMed.
- B. Delley, Phys. Rev. B, 2002, 65, 85403–85409 CrossRef.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865 CrossRef CAS PubMed.
- B. Delley, J. Phys. Chem., 1996, 100, 6107–6110 CrossRef CAS.
- W. J. Hehre, L. Radom, P. V. R. Schlyer and J. A. Pople, Ab Initio Molecular Orbital Theory, Wiley, New York, 1986 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1414257 1 and 1055002 2. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7ra10572j |
| This journal is © The Royal Society of Chemistry 2018 |