Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Fabrication of ultralong perovskite structure nanotubes†
Xiaofeng Wu,
Keke Huang,
Long Yuan and
Shouhua Feng
and
Shouhua Feng *
*
State Key Laboratory of Inorganic Synthesis and Preparative Chemistry, College of Chemistry, Jilin University, Changchun 130012, P. R. China. E-mail: shfeng@jlu.edu.cn; Fax: +86-431-85168624; Tel: +86-431-85168661
First published on 2nd January 2018
Abstract
Fabrication of high quality nanotubes with thin walls, long length, and uniform diameter is challenging, especially in complex structure oxides, such as perovskites. In this paper, we elucidate a facile method for the preparation of perovskite manganite nanotubes by a sacrificial template assisted pulsed laser deposition technique. Morphological analysis reveals a typical hollow tubular nanostructure of the nanotubes with lengths up to tens of micrometers and the average diameter of 160 nm. The nanotubes are composed of perovskite crystallites with a granular size of 5–10 nm, and show superparamagnetism behavior. The kinetic energy of the plume is crucial for the nanotube formation, which could be tuned by the laser energy density, the pressure of deposition and the target–substrate distance. This sacrificial template method illustrated here does not only propose a universal technique for high quality inorganic nanotube material fabrication, but also provides a promising route to new one-dimensional materials and devices.
1. Introduction
The design, fabrication and modification of low dimensional materials can not only provide new materials for practical applications, but also be fundamental to understanding the basic relationship among structure, defects, valence and spin in low dimensional solid science.1 Low dimensional nanomaterials have attracted considerable attention due to their unusual size-dependent properties and promising applications compared to their bulk counterparts.2,3 Nanotubes (NTs) with hollow 1D nanostructure are the smallest dimension that can provide passageways for effective transfer of electrons and large specific area for chemical reactions.4–6 Besides the most famous carbon nanotubes, other widely studied nanotube materials include TiO2,7 MnO2,8 Ta3N5,9 and CuInSe2.10 The hollow inner space makes it easy for matter transportation and energy transfer, which improves the performance in accumulation of photogenerated current,11 water splitting under visible light,9,12 supercapacitor electrodes,13 lithium batteries,8 and biosensors.14 However, only a few perovskite materials have been reported for the formation of nanotube morphology, with superior properties in ferroelectric memory,15 catalysts16 and magnetoelectric sensors.17 Most of these perovskite nanotubes are Ti-based materials, such as BaTiO3,18 PbTiO3 (ref. 19) and PbZr1−xTixO3.20 In contrast, Mn-based perovskite NTs have been rarely reported, regardless of their fascinating physical and chemical properties in the formation of bulks, films and nanoparticles.21,22Several strategies have been developed for the synthesis of perovskite nanotubes, such as hydrothermal growth,23 electrospinning16 and template-assisted sol–gel technique.15,17,24,25 However, only several compounds have been reported as tubular morphology with hydrothermal method, such as PbTiO3,23 SrTiO3 and BaTiO3.26,27 Although the nanotubes prepared via electrospinning method hold a tubular structure, they are usually composed of nanoscale particles without a rigid wall to keep an uniform one-dimensional hollow structure.16,28 Among these fabricating techniques, template-assistant method is the most popular and effective approach. The general procedure of this method is (i) preparing relevant polymeric precursors, and then (ii) filling the precursors into the porous templates, (iii) heat treating for the crystallization of perovskites, (iv) finally etching the template to obtain nanotubes. Although those chemical solution processes show significant advantages in size control and large amount synthesis, complicated operation steps and extremely toxic and unstable organometallic precursors are inevitable.29 Pulsed laser deposition (PLD) is a versatile physical vapor deposition technique for fabricating multi-component oxide films with controllable stoichiometry.30 2-Dimensional mesh-like thin films and high-density nanodot arrays have been directly prepared by PLD utilizing anodic aluminium oxide (AAO) as masks.31–34 Template-assisted PLD has been only reported in PbZrO3 nanotube preparation,35 however, the high quality nanotube fabrication process and growing kinetics still need further investigation.
In previous work, we reported the utilization of PLD for high quality epitaxy films of perovskite structure manganites and ferrites on SrTiO3 substrate.36,37 Recently, free-standing single-crystal perovskite films have been fabricated by etching the sacrificial water-soluble layers via PLD epitaxy technique,38 which indicates the advantages of template sacrificial method in low dimensional materials fabrication. In this paper, La0.75Ca0.25MnO3 (LCMO) is selected as a model compound to illustrate a preparation route for ultrathin and long nanotubes via template-assisted PLD. The morphology analysis shows its tubular feature with the thickness of 20 nm and the average length of 50 μm. The structural and superparamagnetic results indicates that the nanotubes are composed of well crystallized perovskite nanoparticles. The growth kinetics and formation mechanism of those nanotubes is proposed. This method illustrated here can be possibly applied for most of inorganic tubular nanostructure preparations.
2. Experimental section
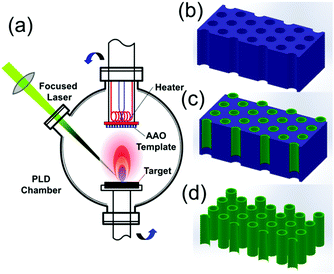
Double-pass AAO membranes (Shangmu Tech., pore size ∼ 300 nm, thickness ∼ 100 μm) were used as the templates for the growth of LCMO nanotubes array by laser induced plasma filling. The AAO template was processed by ultrasonic cleaning in sequence of acetone, ethanol and deionized water, and finally dried in high-purified N2 ambient. Then the template was mounted closely on a metal heater plate by silver paint and was transferred into the PLD chamber. The chamber base pressure is 3 × 10−6 Torr. The oblique incidence laser (KrF, 248 nm) with repetition rate of 5 Hz and energy fluence of 2.0 J cm−2 ablated the LCMO target (KJMTI Co., Ltd) and the plume filled the pores which faced perpendicularly to the target surface. The distance from the target to the substrate was 45 mm. The pulse number was 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. The depositions were carried out in the flowing oxygen of 50 mTorr with the template temperature fixed at 973 K. Finally, the filled template was annealed in situ at 1023 K in 400 Torr oxygen for half an hour to form the crystalline structure before cooling to room temperature with a rate of 10 K min−1.
000. The depositions were carried out in the flowing oxygen of 50 mTorr with the template temperature fixed at 973 K. Finally, the filled template was annealed in situ at 1023 K in 400 Torr oxygen for half an hour to form the crystalline structure before cooling to room temperature with a rate of 10 K min−1.
During the deposition process, the evaporated materials from the target were deposited on the surface boundaries of the porous template and also filled the pores. To obtain pure tubular materials, the surface of the deposited template was polished mechanically by abrasive paper (Starcke, P2500), and then the filled templates with polished surface were immersing in 1 wt% NaOH solution for 12 h to etch the Al2O3 template. At last, the perovskite NTs were separated by carefully rinsing and drying.
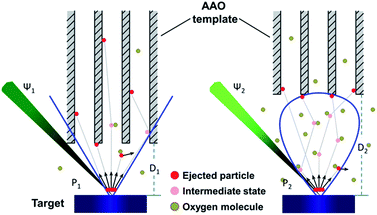
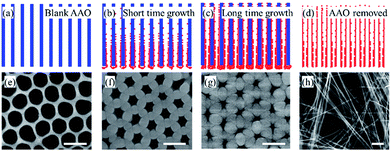
A schematic of the template-assisted pulsed laser deposition set-up used in this work is shown in Fig. 1(a), the AAO template replaces the smooth substrate which is usually used in films deposition. The typical procedure for NTs fabrication can be simply divided into two steps: filling the AAO template by PLD (Fig. 1(b and c)) and the template removal by liquid etching (Fig. 1(c and d)).
The structure was determined by X-ray diffraction (XRD, Rigaku D/Max2550) with Kα radiation from 10° to 90° at a step width of 0.02°. The morphology, composition analysis and cross-sectional lamella sample preparation were taken on an electron/focused ion dual beam system (FIB, FEI Helios 600i) equipped with an energy dispersive X-ray spectrometer (EDS, EDAX Apollo X). The microstructure and element distribution mapping graphs were characterized by transmission electron microscope (TEM, FEI Tecnai F20). The magnetic properties were performed on a superconducting quantum interference device-vibrating sample magnetometer vibrating sample magnetometer (SQUIDS-VSM, Quantum Design).
3. Results and discussion
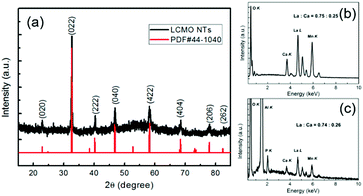
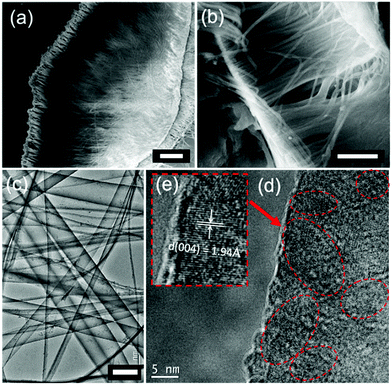
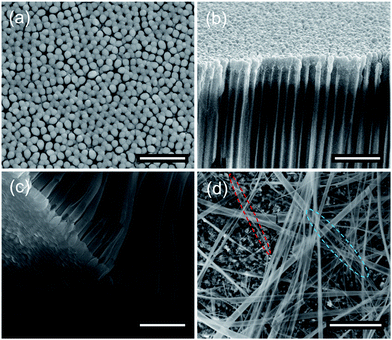
The structure of the NTs was characterized by powder X-ray diffraction, shown in Fig. 2. All the diffraction peaks can be indexed perfectly to the standard monoclinic perovskite structure of bulk La0.8Ca0.2MnO3 both in diffraction angle and intensity (JCPDS no. 44-1040), and there is no detectable second phase, indicating that the well-crystallized perovskite-type phase was successfully transferred from the target to the NTs by the PLD method. The composition of the as-prepared LCMO NTs was evaluated by EDX analysis technique, and the result is well consistent with the target (Fig. 2(b and c)). The element concentrations of Al and P result from the template.Fig. 3 shows the morphological investigation of the as-prepared LCMO NTs by SEM and HRTEM. The panoramic SEM image of LCMO nanotube arrays in Fig. 3(a) reveals an uniform fluffy feature with an average length of 50 μm. A higher magnification SEM image in Fig. 3(b) illustrates that the central part of each nanostructure is distinct in contrast to its edges, indicating the obvious feature of tubular morphology. The average outer diameter of the NTs is about 160 nm, which is smaller than the pore size of the template. The diminution of the diameter is attributed to the perovskite crystallization and densification during the post-annealing and cooling processes.39,40 The wall of the NTs is translucent even the SEM working at 5 kV accelerating voltage, which reflects the thickness is thin enough that the electron beam can pass through easily. Based on this approach, the thickness of the NTs decreases with the increase of the pore depth to the surface, the maximum observed thickness is about 20 nm examined by cross-section TEM (experimental details and results about cross section sample shown in Fig. S1 and S2†). This thin-walled feature determines the poor mechanical strength of the NTs, hence the ultrasonic processing was avoided during the NTs dispersion. This indicates that the length of the NTs can be controlled by the amount of deposition from several to tens of micrometers. It is worthy to mention that the nanowire-like structure with the diameter of ca. 10 nm is observed in TEM graphs, which may originate from either the broken walls of nanotubes or the curl of NTs in annealing process (Fig. 3(c)). HRTEM graphs of LCMO NTs are shown to investigate the morphology and microstructure of the as-prepared NT samples, Fig. 3(c–e). The nanotubes are grown with ultra-thin walls as can be clearly seen in Fig. 3(c). Apparently, the nanotubes are consist of the nanoparticles with the grain size of 5–10 nm, as indicated in Fig. 3(d). And the well crystallized part is mainly in the outer side of the nanotubes, i.e. adjacent to the AAO template inner walls. This is because the relatively higher crystal growth temperature and richest oxide plasma stocks in primary deposition steps at the inner sides of AAO templates. The marked fringes with lattice spacing of 1.94 Å are in consistent with the {004} planes of LCMO with monoclinic perovskite structure (Fig. 3(e)).
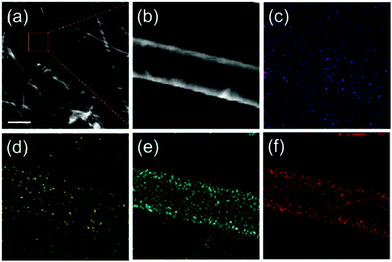
To further investigate the elemental distribution in the nanotubes, a single LCMO nanotube was selected for EDX element mapping by high angle annular dark field scanning TEM (HAADF-STEM). The HAADF image and its corresponding signals of La–L, Ca–K, Mn–L and O–K are shown in Fig. 4. We observe that all elements are uniformly distributed in the wall of this individual nanotube. It is clearly to discriminate that the signal of element distribution is more intense in the side parts (wall) than that in the center, indicating a typical hollow structure of the prepared nanotubes with the wall thickness of about 20 nm (Fig. S2†).
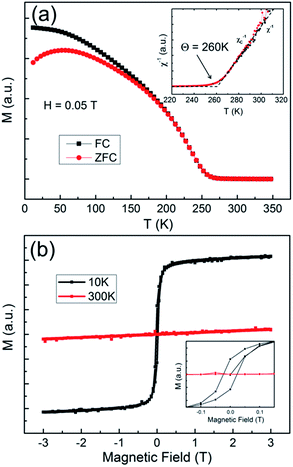
The fabricated LCMO NTs stacked by nanoparticles is also clarified by the magnetism analysis. As shown in Fig. 5(a), temperature dependent magnetization curves was measured by increasing the temperature under a field of 0.05 T in the field-cooling (FC) and zero-field-cooling (ZFC) modes, respectively. The magnetization of the NTs undergoes a wide temperature range decreasing with the rise of the temperature in FC mode. The ZFC magnetization shows the maximum at 55 K and begins to diverge from FC at 210 K. The inverse susceptibility showing a linear relation to temperature can be related to the Curie–Weiss relation with the Curie temperature of 260 K as shown in the inset in Fig. 5(a). Those features reveal that the superparamagnetism (SPM) existing in the NTs is caused by the size reduction of the manganites, which is consistent with the results of La0.8Ca0.2MnO3 nanoparticles with the size of 17 nm prepared by sol–gel method.41 The SPM behavior is an essential feature of most manganites when their particle size is decreased to nanometer-scale,42 and it is also witnessed by the unsaturated hysteresis cycle measured at 10 K with the applied field up to 3 T. The M–H loops and the enlargement of the low field data (inset) are shown in Fig. 5(b), with a sign of pure paramagnetism at 300 K.
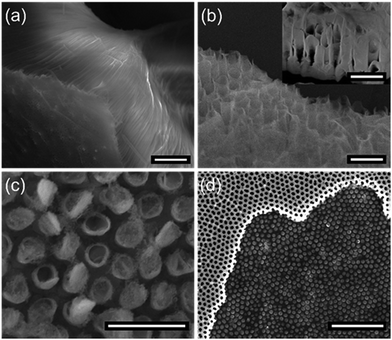
The formation of perovskite nanotube array is dependent on the geometry structure of AAO template. In the honeycomb-like AAO templates, ridge morphology forms on the wall of hexagonal cells at the surface of electrochemically-etched AAO template. Besides, the diameter of the pore at the surface is larger than that at inner parts. Hence, an inclined plane formed at each side of the ridge.43 When the plume particles with random moving path arrived at the surface of the template during the deposition, they will either settle down to the inclined plane or be injected to the sidewall of the pores. The growing speed on the inclined plane of the ridge is much higher than that on the sidewall of AAO pores. With the increase of the deposited substances migrating on the incline plane, particles injected to the pores are getting decreased simultaneously. Fig. 6(a and b) show the top-view and cross-section morphology of the nanotube array in AAO template. The deposited substances remain a tubular structure with spherical hat at the surface nearly sealing the pores. Conversely, the sidewalls are not filled up. To further expound the reason why this preparation method prefers to produce nanotubes instead of nanowires, a thin layer of PMMA was prepared on the deposited template by spin-coating, and then the AAO was removed by chemical etching without polishing. As shown in Fig. 6(c), tubular arrays arrange exactly under the spherical nanostructure. Besides, nanowires are also found at the terminal of the nanotube arrays (Fig. 6(d)), which may result from the curl of NTs (red circle) and the broken walls (blue circle) during the annealing process, which is also found in TEM in Fig. 3(c).
Mishina et al. has reported that the ejected species can not penetrate into the pores by RF magnetron sputtering,44 which may be explained by the kinetic energy (Ek) of particles impinging onto the surface is much lower than that in PLD method. In order to find out whether the particle's kinetic energy is the critical factor of NTs fabrication, a series of comparative experiments were carried out for the growth mechanism exploration. The fabrication of perovskite manganite nanotube arrays depends on four main factors: (i) energy density of the laser acting on the target, (ii) distance from the AAO template to the target, (iii) oxygen pressure of the chamber and (iv) geometrical morphology of AAO template. When the energy density of the laser is lowered to 1.5 J cm−2 (a half value of that in Fig. 3(a)), the length of the nanotube is reduced to ca. 20 μm (Fig. 7(a)). If the distance from target to AAO is fixed at 65 mm, short tubular fluffy manganite arrays are formed (Fig. 7(b)). No nanotube formation is observed when the oxygen pressure higher than 200 mTorr (Fig. 7(c)). In the similar oxygen partial pressure of 2.5 × 10−1 mbar (190 mTorr), Liu et al. lowered the energy density of the laser (1.5 J cm−2) and only lateral mesoscopic patterned morphology was obtained.31 If a thin AAO template (thickness of 300 nm) is used, nanodot array is deposited on the substrate without any tubular structure formation (Fig. 7(d)).
Kinetic energy of the primary ejected particles is the key for ultralong nanotube growth inside of the pore of the template. As we all know, the kinetic energy of the clusters at the moment of reaching the substrate mainly depends on the energy density of the laser, the oxygen pressure in the chamber and the target–substrate distance during the deposition process.45 The primary ejected particles kinetic energy can be systematically enhanced by increasing the laser fluence, and then the energy reduced by scattering with the oxygen molecule and the collision probability will be further improved with the long distance towards the template. With high primary ejected particles kinetic energy, low oxygen pressure and short distance between template and target, the clusters could reach the long depth inside of AAO pores (Fig. 8).
The general growth progress of the NTs is proposed as following. When a focused laser beam with large energy density (1–5 J cm−2) and short duration (25 ns) is applied on the polycrystalline target, the material evaporated in the form of a dense plasma in the normal direction of the target surface. The growth process is illustrated with schematic diagrams and corresponding SEM images, Fig. 9. The plasma plume with high kinetic energy, which contains neutral atoms, molecules, ions, ionic clusters etc., deposits on the sidewalls and the surface of the template simultaneously. The former particles penetrate into the pores and settle down statistically along the radial direction of the AAO template pores. The latter particles adsorb on template surface and grow not only in vertical but also in lateral direction, as shown in Fig. 9(b and c). With the increase of the deposition time, the deposited substances accumulate on the surface and the corresponding pore diameter is shrinked, which prevents the following ingress of plume clusters. After PLD growth, the filled template was annealed to improve the NT's crystallinity. At last, the well-fabricated NTs were obtained after chemical dissolving the AAO template (Fig. 9(d)). The SEM images in Fig. 9(e–h) clearly show the corresponding steps of the aforementioned process. Due to the adsorption priority, the amount of particles occupy on the sidewalls decreases with the increase of pore depth, which leads to an inhomogeneous thickness of the NTs. Above all, this method is very feasible to fabricate high quality nanotubes with ultrathin and long nanotube arrays with uniform diameter. The composition, final length, wall thickness and diameter of the nanotubes could be well tailored by the target, template's pore size and PLD deposition parameters. Besides, the method proposed here builds a basic idea to fabricate various functional and multicomponent oxide nanotubes with desirable one-dimensional materials, which can be used for new concept nano-device research, such as single tube based chemical sensors and magnetoelectrical transistors. Thus, we have performed an effective and facile method for ultralong and ultrathin perovskite structure oxides nanotube fabrication.
4. Conclusions
In summary, we have demonstrated an effective method for fabrication of high quality inorganic nanotubes with ultrathin and long morphology by template-assistant pulsed laser deposition technique. The length, wall thickness and diameter of the nanotube arrays can be well tailored by the deposition conditions and the template. Structure, morphology and magnetism of the La0.75Ca0.25MnO3 nanotubes have been investigated. The kinetic energy of the laser produced plasma plays a pivotal role in the formation of the nanotubes. The method illustrated here shows potentials for other functional inorganic nanotubes fabrication with promising applications.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was supported by the “Thirteenth Five Year” Project of Science and Technology Research in the Education Department of Jilin Province (Grant No. JJKH2017082KJ) and National Natural Science Foundation of China (Grant No. 21427802 & 21671076).Notes and references
- Y. Sun, S. Gao, F. Lei, C. Xiao and Y. Xie, Acc. Chem. Res., 2015, 48, 3–12 CrossRef CAS PubMed.
- B. K. Teo and X. H. Sun, Chem. Rev., 2007, 107, 1454–1532 CrossRef CAS PubMed.
- A. Pakdel, C. Zhi, Y. Bando and D. Golberg, Mater. Today, 2012, 15, 256–265 CrossRef CAS.
- C. N. R. Rao and A. Govindaraj, Adv. Mater., 2009, 21, 4208–4233 CrossRef CAS.
- P. M. Rorvik, T. Grande and M. A. Einarsrud, Adv. Mater., 2011, 23, 4007–4034 CrossRef CAS PubMed.
- X. H. Zhu, Z. G. Liu and N. B. Ming, J. Mater. Chem., 2010, 20, 4015–4030 RSC.
- K. Lee, A. Mazare and P. Schmuki, Chem. Rev., 2014, 114, 9385–9454 CrossRef CAS PubMed.
- A. L. M. Reddy, M. M. Shaijumon, S. R. Gowda and P. M. Ajayan, Nano Lett., 2009, 9(3), 1002–1006 CrossRef CAS PubMed.
- X. Feng, T. J. LaTempa, J. I. Basham, G. K. Mor, O. K. Varghese and C. A. Grimes, Nano Lett., 2010, 10, 948–952 CrossRef CAS PubMed.
- J. Xu, C.-Y. Luan, Y.-B. Tang, X. Chen, J. A. Zapien, W.-J. Zhang, H.-L. Kwong, X.-M. Meng, S.-T. Lee and C.-S. Lee, ACS Nano, 2010, 4(10), 6064–6070 CrossRef CAS PubMed.
- G. K. Mor, K. Shankar, M. Paulose, O. K. Varghese and C. A. Grimes, Nano Lett., 2006, 6(2), 215–218 CrossRef CAS PubMed.
- G. K. Mor, K. Shankar, M. Paulose, O. K. Varghese and C. A. Grimes, Nano Lett., 2005, 5(1), 191–195 CrossRef CAS PubMed.
- M. Salari, S. H. Aboutalebi, K. Konstantinov and H. K. Liu, Phys. Chem. Chem. Phys., 2011, 13, 5038–5041 RSC.
- Y. Zhang, P. Xiao, X. Zhou, D. Liu, B. B. Garcia and G. Cao, J. Mater. Chem., 2009, 19, 948–953 RSC.
- X. L. Liu, M. Y. Li, Z. Q. Hu, Y. D. Zhu, S. X. Dong and X. Z. Zhao, Mater. Lett., 2012, 82, 57–60 CrossRef CAS.
- J. J. Xu, D. Xu, Z. L. Wang, H. G. Wang, L. L. Zhang and X. B. Zhang, Angew. Chem., 2013, 52, 1–5 CrossRef.
- M. I. Dolz, W. Bast, D. Antonio, H. Pastoriza, J. Curiale and R. D. Sánchez, J. Appl. Phys., 2008, 103, 083909 CrossRef.
- J. Liu, Y. Sun and Z. Li, CrystEngComm, 2012, 14, 1473–1478 RSC.
- B. Im, H. Jun, K. H. Lee, S.-H. Lee, I. K. Yang, Y. H. Jeong and J. S. Lee, Chem. Mater., 2010, 22, 4806–4813 CrossRef CAS.
- J. Kim, S. A. Yang, Y. C. Choi, J. K. Han, K. O. Jeong, Y. J. Yun, D. J. Kim, S. M. Yang, D. Yoon, H. Cheong, K.-S. Chang, T. W. Noh and S. D. Bu, Nano Lett., 2008, 8(7), 1813–1818 CrossRef CAS PubMed.
- M. B. Salamon and M. Jaime, Rev. Mod. Phys., 2001, 73, 583–628 CrossRef CAS.
- S. Royer, D. Duprez, F. Can, X. Courtois, C. Batiot-Dupeyrat, S. Laassiri and H. Alamdari, Chem. Rev., 2014, 114, 10292–10368 CrossRef CAS PubMed.
- Y. Yang, X. H. Wang, C. F. Zhong, C. K. Sun, G. F. Yao and L. T. Li, J. Am. Ceram. Soc., 2008, 10, 3388–3390 CrossRef.
- M. Kumaresavanji, C. T. Sousa, A. Pires, A. M. Pereira, A. M. L. Lopes and J. P. Araujo, Appl. Phys. Lett., 2014, 105, 083110 CrossRef.
- F. Wang, J. B. Wang, X. L. Zhang, B. Li and Y. C. Zhou, J. Cryst. Growth, 2009, 311, 4495–4498 CrossRef CAS.
- Y. Mao, S. Banerjee and S. S. Wong, Chem. Commun., 2003, 408–409 RSC.
- Y. Yang, X. Wang, C. Sun and L. Li, Nanotechnology, 2009, 20, 055709 CrossRef PubMed.
- H. W. Park, D. U. Lee, P. Zamani, M. H. Seo, L. F. Nazar and Z. Chen, Nano Energy, 2014, 10, 192–200 CrossRef CAS.
- Y. B. Mao, S. Banerjee and S. S. Wong, J. Am. Chem. Soc., 2003, 125, 15718–15719 CrossRef CAS PubMed.
- M. O'Sullivan, J. Hadermann, M. S. Dyer, S. Turner, J. Alaria, T. D. Manning, A. M. Abakumov, J. B. Claridge and M. J. Rosseinsky, Nat. Chem., 2016, 8, 347–353 CrossRef PubMed.
- B. J. Rodriguez, X. S. Gao, L. F. Liu, W. Lee, I. I. Naumov and A. M. Bratkovsky, Nano Lett., 2009, 9, 1127–1131 CrossRef CAS PubMed.
- S. Hong, T. Choi, J. H. Jeon, Y. Kim, H. Lee and H. Y. Joo, Adv. Mater., 2013, 25, 2339–2343 CrossRef CAS PubMed.
- Z. Li, Y. Wang, G. Tian, P. Li, L. Zhao, F. Zhang, J. Yao, H. Fan, X. Song, D. Chen, Z. Fan, M. Qin, M. Zeng, Z. Zhang, X. Lu, S. Hu, C. Lei, Q. Zhu, J. Li, X. Gao and J. Liu, Sci. Adv., 2017, 3, e1700919 CrossRef PubMed.
- H. J. Liu, S. L. Lim and C. K. Ong, Mater. Lett., 2008, 62, 2006–2008 CrossRef CAS.
- S. Singh and S. B. Krupanidhi, Appl. Phys. A, 2007, 87, 27–31 CrossRef CAS.
- X. Wu, K. Huang, C. Hou, L. Ge, X. Chu, S. Wang and S. Feng, Chem. J. Chin. Univ., 2013, 34, 2063–2067 CAS.
- Y. Sun, X. Wu, L. Yuan, M. Wang, M. Han, L. Luo, B. Zheng, K. Huang and S. Feng, Chem. Commun., 2017, 53, 2499–2502 RSC.
- D. Lu, D. J. Baek, S. S. Hong, L. F. Kourkoutis, Y. Hikita and H. Y. Hwang, Nat. Mater., 2016, 15, 1255–1260 CrossRef CAS PubMed.
- M. Hsu, I. Leu, Y. Sun and M. Hon, J. Solid State Chem., 2006, 179, 1421–1425 CrossRef CAS.
- S. Singh and S. B. Krupanidhi, Phys. Lett. A, 2007, 367, 356–359 CrossRef CAS.
- S. B. Xi, W. J. Lu and Y. P. Sun, J. Appl. Phys., 2012, 111, 063922 CrossRef.
- T. Zhang, G. Li, T. Qian, J. Qu, X. Xiang and X. Li, J. Appl. Phys., 2006, 100, 094324 CrossRef.
- S. Y. Jeong, M. C. An, Y. S. Cho, D. J. Kim, M. C. Paek and K. Y. Kang, Curr. Appl. Phys., 2009, 9, S101–S103 CrossRef.
- E. Mishina, V. Stadnichuk, S. Alexandr, Y. Golovko, V. Mukhorotov, S. Nakabayashi, H. Masuda, D. Hashizume and A. Nakao, Phys. E, 2004, 25, 35–41 CrossRef CAS.
- H. U. Krebs, M. Weisheit, J. Faupel, E. Süske, T. Scharf and C. Fuhse, et al., Advances in Solid State Physics, Springer Berlin Heidelberg, 2003, pp. 505–518 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: SEM and TEM view of the cross section of template perovskite nanotubes and SEM results of poorly grown nanotubes with low kinetic energy of the laser sputtered primary ejected particles. See DOI: 10.1039/c7ra10684j |
| This journal is © The Royal Society of Chemistry 2018 |