Open Access Article
Open Access ArticleEvaluation and optimization of a new microbial enhancement plug-flow ditch system for the pretreatment of acid mine drainage: semi-pilot test
Yongwei Song *a,
Heru Wanga,
Jun Yanga,
Lixiang Zhoub,
Jingcheng Zhoua and
Yanxiao Caoa
*a,
Heru Wanga,
Jun Yanga,
Lixiang Zhoub,
Jingcheng Zhoua and
Yanxiao Caoa
aDepartment of Environmental Engineering, School of Information and Safety Engineering, Zhongnan University of Economics and Law, Wuhan 430073, China. E-mail: syw1984888@sina.com; Fax: +86 27 88385169; Tel: +86 27 88385169
bDepartment of Environmental Engineering, College of Resources and Environmental Sciences, Nanjing Agricultural University, Nanjing 210095, China
First published on 3rd January 2018
Abstract
Acid mine drainage (AMD) is typically characterized by low pH, a high concentration of sulfate and dissolved heavy metals. Therefore, it is of practical significance to promote the transformation of soluble Fe and SO42− into iron hydroxysulfate minerals by biomineralization of Acidithiobacillus ferrooxidans. This enhances the lime neutralization efficiency of AMD by reducing the production of ferric hydroxide and waste gypsum. In this study, a new microbial enhanced plug-flow ditch reaction system was developed for the pretreatment of AMD on a semi-pilot scale. System stability under different hydraulic retention times (HRTs) was examined and the effects of microbe enhancement-lime neutralization technology and direct lime neutralization technology were compared. The bio-oxidation efficiency of Fe2+ (5 g L−1) reached 100% in some parts of the system when HRT was 3 and 2 days, and the time taken to reach steady state was 6 and 4 days, respectively. When the HRT was 1 day, the reaction system had operated for 4 days before the equilibrium was lost. At the optimum HRT (2 days) and after the system was stable, the average precipitation rate of total Fe was 53.62% and the average removal rate of As(III) was 17.27%. Following microbial enhanced pretreatment, the amount of lime required and waste residues generated for AMD neutralization decreased by 75.00% and 85.25%, respectively. This result supports the application of microbial enhancement-lime neutralization passive treatment technology for AMD.
1. Introduction
Sulfide minerals in spent ore and mine tailings will oxidize when exposed to air and moisture during or after the processes of mining and beneficiation. Therefore, acid mine drainage (AMD) is typically characterized by high acidity (pH < 5.0).1 Because of the sulfide minerals that contain heavy metals (Cr, Cu, Cd, Ni, etc.) or metalloids (As, Hg, etc.) dissolved in acidic solution, AMD typically contains elevated concentrations of Fe (Fe2+ and Fe3+), SO42−, and toxic metals, posing a threat to the environment and human health.2,3 AMD is typically treated using active and passive methods.4 Active treatment methods include lime neutralization, sulfide precipitation, and reduction-oxidation (redox), resulting in the precipitation of toxic metals. Passive treatment methods use natural geochemical and biochemical techniques to remove pollutants from wastewater with minimum investment and maintenance costs. Examples of active methods include aerobic/anaerobic wetlands, anoxic limestone drains, and permeable reactive treatment zones (PRTZs).5 At present, lime neutralization is widely adopted owing to its convenience and speed and it can raise the pH level and remove Fe and SO42− from the AMD system through the formation of Fe(OH)3, Fe(OH)2, and CaSO4.6 However, higher operating costs and the difficulty of dewatering the waste residue are serious disadvantages of their application.7–9 Therefore, developing an effective and feasible AMD pretreatment method prior to lime neutralization has become an increasingly urgent issue.Solubility products show that Fe2+ can only be precipitated as Fe(OH)2 under highly basic conditions, while Fe3+ can form Fe(OH)3 at pH 3–4. Therefore, rapid conversion of Fe2+ into Fe3+ is essential for AMD pretreatment via the lime neutralization method. However, abiotic oxidation of Fe2+ by aeration is very slow at pH below 4.0.10 The abiotic oxidation efficiency of Fe2+ reaches 3% after 72 h in modified 9 K liquid medium with an initial pH of 2.5.11 Therefore, improving Fe2+ oxidation under low pH is a significant problem in AMD treatment. Acidithiobacillus ferrooxidans (A. ferrooxidans), one of the most common acidophilic Fe-oxidizing microorganisms, can efficiently catalyze the conversion of Fe2+ into Fe3+ without the excessive use of an AMD neutralizer and the generation of a highly alkaline effluent.12 Song et al. found that 8.96 g L−1 of Fe2+ could be completely bio-oxidized within 24 h when the density of A. ferrooxidans exceeded 5 × 107 UFC mL−1 in 9 K liquid medium with an initial pH of 2.5–3.0.13 Further, the process of Fe2+ oxidation by A. ferrooxidans in the acidic sulfate environment is often accompanied by the hydrolysis of Fe3+ that generates iron oxyhydroxysulfate minerals, such as schwertmannite and jarosite.14 Eqn (1)–(3) present the schwertmannite and jarosite formation:
| 4Fe2+ + O2 + 4H+ → 4Fe3+ + 2H2O | (1) |
| 8Fe3+ + 14H2O + SO42− → Fe8O8(OH)6SO4 (schwertmannite) + 22H+ | (2) |
| M+ + 3Fe3+ + 2SO42− + 6H2O → MFe3(SO4)2(OH)6 (jarosite) + 6H+ | (3) |
Schwertmannite and jarosite are commonly found in acidic, Fe and SO42−-rich environments.15 Previous studies confirmed that these minerals are ideal adsorbents with relatively strong adsorption or co-precipitation effects on heavy metals.16–18 Liao et al. observed that As(III) adsorption by schwertmannite was enhanced with the increase in the solution pH from 3.0 to 9.0 and the maximum adsorption capacity was 113.9 mg g−1 at pH 7.5.19 Jarosite is also an efficient mineralogical control on aqueous concentrations of As in As-contaminated water.20 In addition, iron oxyhydroxysulfate minerals possess significant settling characteristics compared to Fe(OH)3.21 Therefore, AMD pre-processing using A. ferrooxidans, followed by the treatment of the effluent via the lime neutralization method has a significant potential in the treatment of AMD.
In recent years, many types of reactors operating under both batch and continuous regimes have been studied to improve the bio-oxidation rate of Fe2+.22,23 However, a continuously operating system will dilute and washout the Fe-oxidizing microorganisms, restricting its commercial application. Therefore, using immobilized cells would be a superior approach to improve the rate of oxidation and achieve high cell concentrations inside the reactor.24 Long et al. investigated the kinetics of continuous Fe2+ oxidation by PVA-cryogel-immobilized A. ferrooxidans in a 365 mL packed-bed bioreactor. A maximum Fe2+ oxidation rate of 1.89 g L−1 h−1 was achieved when the dilution rate was greater than 0.38 h−1.25 The feasibility of jarosite as a support for A. ferrooxidans immobilization was also investigated in the present work.26 Wang et al. reported that jarosite as a microbial carrier can maintain a high concentration of A. ferrooxidans cells to improve Fe2+ oxidation and promote the precipitation of Fe3+ by simultaneously acting as a jarosite seed.27 The presence of seed materials can provide a solid surface, favors the jarosite surface creation, and promotes the rate of jarosite precipitation.28,29 Thus, the newly-formed jarosite can be retained or recycled to the reactor system as a new biomass carrier and a seed crystal to strengthen the process, forming a self-reinforcing positive feedback loop, whereas the excess can easily be removed, owing to its favorable settling properties. The rapid oxidation of Fe2+ and precipitation (iron oxyhydroxysulfate mineral formation) of Fe3+ by A. ferrooxidans can play multiple roles in improving the AMD treatment. The optimum amount of lime for Fe2+ oxidation and total Fe precipitation for iron oxyhydroxysulfate mineral formation during Fe2+ oxidation in AMD systems in the presence of A. ferrooxidans have been investigated under laboratory conditions.11 However, a semi-pilot study of AMD bioaugmentation pretreatment prior to lime neutralization in a continuously operating system has not been reported to date.
Mining activities typically take place in mountainous or hilly areas. The use of conventional sewage treatment techniques in these areas will inevitably incur high construction, operation, and management costs, as well as disrupt the original topography and ecological balance. AMD usually flows from the upper reaches of a mine to the downstream along a longer ditch. The plug-flow characteristics of in situ mining wastewater ditches could be integrated with microbial enhancement. Given that A. ferrooxidans is an aerobic bacterium, the continuous supply of O2 is essential for the biosynthesis of iron oxyhydroxysulfate minerals.30 In a plug-flow system, O2 required by A. ferrooxidans can be obtained through the drop aeration of AMD in the ditch. Such conditions are conducive to Fe2+ bio-oxidation and Fe3+ hydrolysis in AMD systems in the presence of A. ferrooxidans. Hydraulic residence time (HRT, determined by flow velocity) plays an important role in the overall activity of microorganisms. A very short HRT may not allow adequate time for bacterial activity in Fe2+ bio-oxidation and Fe3+ hydrolysis or may result in biomass being washed out of the system.31 On the contrary, a very long HRT may reduce the processing capacity and result in high costs.
In the present work, a microbial (A. ferrooxidans) enhanced plug-flow ditch system was designed. The main purpose of this study was to evaluate the operational stability of the system under different HRTs and investigate the Fe2+ bio-oxidation efficiency, total Fe precipitation rate, dissolved O2 concentration, and removal efficiencies of As(III) in AMD. Furthermore, we compared the microbial enhancement-lime neutralization method with the original lime neutralization method to provide evidence to support the application of the new microbial enhancement-lime neutralization passive technique for AMD treatment. The outcomes of this study will provide the critical parameters for the engineering treatment of AMD.
2. Materials and methods
2.1. Preparation of synthetic AMD
Synthetic AMD, containing different concentrations of elements, was prepared as follows: As2O3 was used to prepare 100 mg L−1 stock solutions of As(III). Then, the stock solutions of FeSO4·7H2O and As(III) were added into a 1/5× modified 9 K broth medium to obtain 5 g L−1 Fe2+ and 1 mg L−1 As(III), respectively. Finally, the pH of these final solutions was adjusted to 2.50 using 1 mol L−1 H2SO4.2.2. Preparation of A. ferrooxidans cell suspensions
A. ferrooxidans LX5 (CGMCC no. 0727), obtained from the China General Microbiological Culture Collection Center (CGMCC), was grown in a 9 K medium containing the following analytical grade salts: 3.00 g (NH4)2SO4, 0.10 g KCl, 0.50 g K2HPO4, 0.50 g MgSO4·7H2O, 0.01 g Ca(NO3)2, and 44.48 g FeSO4·7H2O in 1 L deionized water. The pH value was adjusted to 2.50 using H2SO4. The FeSO4 solution was filtered, sterilized, and subsequently added to the remaining autoclaved (121 °C for 15 min) medium components. Cultures of A. ferrooxidans were incubated in 500 mL Erlenmeyer flasks, each containing 225 mL of 9 K medium and 10% (v/v) inoculum at 28 °C, placed on a rotary shaker at 180 rpm. The cells were harvested during the late logarithmic growth phase (72 h after inoculation). The cultures were initially filtered (Whatman no. 4 filter paper) to remove the precipitate. Subsequently, the filtrates were centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000× g for 10 min at 4 °C to precipitate the bacterial cells and the supernatant was discarded. After twofold washing with a dilute H2SO4 solution (pH 1.50), the cells were resuspended in a dilute H2SO4 solution (pH 2.50). The A. ferrooxidans cell numbers were determined as ∼2 × 109 UFC mL−1 by the double-layer plate method.32
000× g for 10 min at 4 °C to precipitate the bacterial cells and the supernatant was discarded. After twofold washing with a dilute H2SO4 solution (pH 1.50), the cells were resuspended in a dilute H2SO4 solution (pH 2.50). The A. ferrooxidans cell numbers were determined as ∼2 × 109 UFC mL−1 by the double-layer plate method.32
2.3. Construction of the microbial enhanced plug-flow ditch system
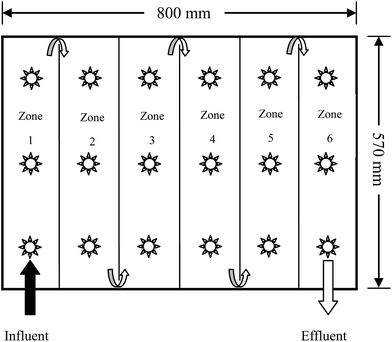
The new plug-flow ditch system was composed of an 800 mm × 570 mm × 505 mm (length × width × height) transit box, consisting of six vertical ditches (zones 1–6) separated by PVC baffles. Three bubble aeration heads were installed at the bottom of each ditch to simulate the oxygenation process of drop aeration in a natural ditch environment. One 10 mm × 10 mm diversion hole was fixed at the bottom of each PVC baffle between two ditches, while a 400 mm high effluent outlet was fixed at the end of zone 6. Hence, the effective volume of the ditch system was approximately 180 L. The plug-flow direction of the AMD is shown in Fig. 1.2.4. Microbial enhanced plug-flow assay of AMD
The concentrated dormant cells of A. ferrooxidans were diluted and evenly mixed by stirring them with deionized water to achieve a cell density of ∼5 × 107 UFC mL−1. The resulting 180 L solution containing diluted bacteria was evenly distributed between the zones of the ditch system. The concentration of dissolved O2 in each zone of the system was adjusted to 8 mg L−1 using an oxygenation and aeration device.The synthetic AMD was pumped continually at a flow velocity of 2.5 L h−1 into zone 1, after which it was transferred to zones 2, 3, 4, 5, and 6, prior to being discharged via the outlet. At that time, the HRT of the AMD was 3 days. The system was operated continually in this pattern for 10 days. Samples were collected daily from each zone to measure pH, Fe2+, and O2 concentrations to assess the continuous stability of the plug-flow system. Moreover, residual concentrations of total Fe and As3+ in the effluent from zone 6 were measured to calculate their respective removal rates. The abovementioned steps were repeated with HRTs of 2 days and 1 day to observe the operational stability of the plug-flow ditch system with varying HRTs and to examine the actual effectiveness of the microbial enhancement-lime neutralization passive technique for AMD treatment. Given that the abiotic oxidation of Fe2+ is hardly initiated below pH 4.0, a control group without the inoculation of A. ferrooxidans was not designed in this study. All experiments were performed in triplicate.
2.5. Analytical procedures
The solution pH was measured using a digital pH meter with a resolution of 0.01 pH unit (pHS-3C, Shanghai INESA Scientific Instrument Co., Ltd). The dissolved O2 concentrations were measured using a Thermo DO analyzer with a resolution of 0.01 mg L−1 (ECDO270042). Fe2+ and total Fe concentrations were determined using the 1,10-phenanthroline method.33 The mineral phase and the morphology of the precipitate were determined by X-ray diffraction (XRD, Bruker D8A25) using CuKα radiation (40 kV, 40 mA) and field emission scanning electron microscopy (SU8010, Hitachi High-Technologies Corporation, Tokyo, Japan).34 The chemical analysis of As species was performed using X-ray photoelectron spectroscopy (XPS, VG-Multilab 2000). The XPS spectra were obtained with a monochromated Al-Kα X-ray source, with an analyzer pass energy of 25 eV and a step size of 0.05 eV.35 Total As concentrations in the solution was analyzed through atomic fluorescence spectroscopy (AFS-9730, Beijing Haiguang Instrument Co., Ltd) with a detection limit of 0.01 μg L−1.36 Experimental data were analyzed using SAS 9.2 software. The data shown in the figures are presented as the mean values with standard deviations to show their reproducibility and reliability.3. Results and discussion
3.1. Variations in pH with HRT in the plug-flow ditch system
Fig. 2 shows the changes in pH with time in each zone of the microbial enhanced plug-flow ditch system under different HRTs. With an adequate supply of nutrients (e.g., N, P, K), A. ferrooxidans utilizes Fe2+ in AMD as an energy source for its metabolism. This reaction is accompanied by the hydrolysis of Fe3+ into iron oxyhydroxysulfate minerals.12–14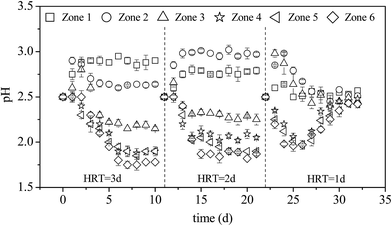 | ||
| Fig. 2 Variations in pH with HRT during the treatment of AMD in the plug-flow ditch system (initial pH was 2.50). | ||
Eqn (1)–(3) clearly show that the oxidation of Fe2+, mediated by A. ferrooxidans to form minerals, involves a two-step acid effect process. The first step is the acid-depleting bio-oxidation of Fe2+, while the second step is the acid-producing hydrolysis of Fe3+ to form minerals. Hence, the status and stability of the system under different HRTs can be determined via pH changes in each zone. When the HRT was 3 days, the pH of zone 1 increased from 2.50 to 2.88 and remained relatively stable after the system had been in operation for 2 days; the pH of zones 2 and 3 increased at the beginning and then decreased gradually over time; the pH of zones 4–6 decreased gradually with the prolongation of microbial enhancement and stabilized on the sixth day at values 2.10, 2.00, and 1.95, respectively. eqn (1) and (2) show that bio-oxidation of Fe2+ is the dominant (acid-depleting) process in zone 1, while in zones 2–6, Fe3+ was continually hydrolyzed to form minerals (acid-producing) with continuous hydraulic action. There were differences in the degree of pH changes in zone 2 between HRTs of 2 and 3 days. When the system HRT was shortened from 3 to 2 days, the main region where A. ferrooxidans oxidation of Fe2+ took place also changed, meaning that the pH in zone 2 was higher than that in zone 1. On the other hand, the plug-flow ditch system was only operated for four days and the pH of each zone began to gradually return to the initial value at the HRT of 1 day. Previous research showed that A. ferrooxidans can be covered with or absorbed by iron oxyhydroxysulfate minerals during the bio-synthesis of iron oxyhydroxysulfate minerals by A. ferrooxidans, which can prevent the washout of A. ferrooxidans cells in a continuously operating system.26,27 However, Song et al. reported that the total Fe precipitation efficiency (iron oxyhydroxysulfate mineral formation) was only ∼9% at 24 h when the density of A. ferrooxidans exceeded 5 × 107 UFC mL−1 in the 9 K liquid medium with the initial pH of 2.50.13 The results (HRT = 1 d) indirectly reflect the gradual decline in the cell density of A. ferrooxidans suspended in the system as a result of the continuous discharge of the effluent that eventually led to the collapse of the system. To confirm this discovery, the density of A. ferrooxidans in the liquid phase of zone 6 was determined after the plug-flow ditch system was in operation for ten days when HRT was one day. The cell number of A. ferrooxidans significantly (P < 0.05) decreased from the initial value of 5 × 107 UFC mL−1 to 3 × 103 UFC mL−1 in the liquid phase.
3.2. Variations in Fe2+ and O2 concentrations with HRT in the plug-flow ditch system
The oxidation of Fe2+ to Fe3+ is required prior to lime neutralization because Fe3+ could precipitate at a much lower pH than Fe2+.27 Therefore, improving the efficiency of Fe2+ oxidation is a key regulatory step in AMD treatment. When HRT was 3 days, Fe2+ concentrations in zones 1–3 gradually increased under continuous AMD influx and stabilized on the sixth day at 4.61, 3.45, and 1.01 g L−1 (equivalent to Fe2+ oxidation rates of 7.80%, 31.0% and 79.8%), respectively (Fig. 3). The results showed that A. ferrooxidans began to exploit the energy source in zone 1 with increasing utilization levels as the influent flowed in continually and 5 g L−1 of Fe2+ in AMD was completely oxidized by A. ferrooxidans when the influent reached zone 4. When HRT was 2 days, the utilization rate of Fe2+ by A. ferrooxidans in the same zone was lower than that under an HRT of 3 days. However, the Fe2+ concentration in each zone showed an increasing trend as HRT was shortened to 1 day, which was consistent with the change in pH, indicating that A. ferrooxidans had an extremely low Fe2+ utilization rate. Thus, the entire system rapidly became imbalanced.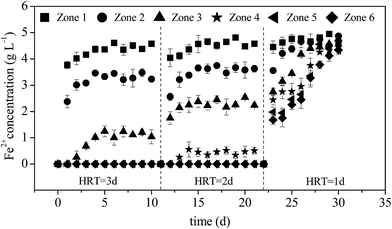 | ||
| Fig. 3 Variations in Fe2+ concentrations with HRT during the treatment of AMD in the plug-flow ditch system (initial Fe2+ concentration was 5 g L−1). | ||
The trends in pH and Fe2+ concentrations show that the system can be easily stabilized under a longer HRT. In practical applications, a shorter HRT is generally expected to achieve a higher treatment capacity. However, the metabolism, growth, and reproductive activity of A. ferrooxidans are the key factors that affect the operational stability of the system and accelerate the oxidation of Fe2+ to form minerals, limiting the possibility of continually shortening the HRT. The system will be irreversibly disrupted and become imbalanced because of the greater hydraulic load if HRT drops below its lowest critical value.37 Given that Fe2+ in the effluent of the system could be completely oxidized when the HRT was 2 days, the recommended optimum HRT for the plug-flow ditch system was 2 days.
A. ferrooxidans is an obligate aerobe that requires an adequate supply of O2 for growth. O2 serves as the only electron acceptor for oxidizing Fe2+.38,39 When the HRT was 3 and 2 days, O2 concentrations in zones 1–6 initially decreased and then increased (Fig. 4). Moreover, zones 3 and 4 had the lowest O2 concentrations (2.95 mg L−1 and 3.34 mg L−1, respectively). The bio-oxidation rate of Fe2+ affected the changes in O2 concentrations in the plug-flow ditch system (Fig. 3). When the system was stable, the oxidation rates of Fe2+ in zones 1–6 were 7.80, 31.0, 79.8, 100, 100, and 100% (the net oxidation rates of Fe2+ were 7.80, 23.2, 48.8, 20.2, 0, and 0%) and the corresponding O2 concentrations were 5.58, 4.86, 2.95, 4.76, 7.08, and 7.46 mg L−1, respectively, at the HRT of 3 days. After Fe2+ was completely oxidized, the O2 concentration in the solution increased rapidly and almost returned to the initial value. Statistical analysis of the results revealed that all the treatment groups significantly (P < 0.05). Therefore, the status of Fe2+ bio-oxidation by A. ferrooxidans can be evaluated using the O2 concentration in each zone of the system. However, because of the decrease in the A. ferrooxidans density at the HRT of 1 day due to the imbalanced system, A. ferrooxidans had a relatively weak capacity for Fe2+ oxidation. Hence, the O2 concentration in each zone was basically maintained at the initial aeration level. Many investigations indicated that O2 supply was only essential in the period of Fe2+ oxidation but was not required during the Fe3+ precipitation reaction.40,41 This study suggests that O2 supply has an important function in the bio-oxidation of Fe2+; however, its function in the formation of iron oxyhydroxysulfate minerals can be neglected because the energy released from the oxidation of Fe2+ can be used for the subsequent hydrolysis reaction of Fe3+.42,43 This advantage would considerably reduce the cost of the biosynthesis of iron oxyhydroxysulfate minerals because the aeration operation can be eliminated in the Fe3+ hydrolysis process.
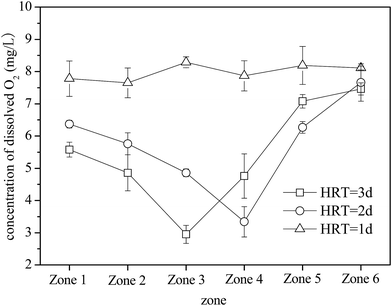 | ||
| Fig. 4 Variations in O2 concentrations with HRT when the plug-flow ditch system is stable (initial O2 concentration was 8 mg L−1). | ||
3.3. Variations in the removal rate of total Fe and heavy metals with HRT when the plug-flow ditch system is stable
During Fe2+ oxidation process by A. ferrooxidans, iron oxyhydroxysulfate minerals are generated because of the facilitation of subsequent Fe3+ hydrolysis.12–14 Fe removed from AMD by the formation of iron oxyhydroxysulfate minerals have certain advantages over Fe(OH)3 because these minerals have significant settling characteristics that aid the dewaterability of neutralized precipitates after AMD treatment by lime neutralization.21 Fig. 5 shows that total Fe precipitation rates in the simulated AMD exceeded 60% in zones 1–6 of the system when HRT was 3 days. The total Fe precipitation rate in the effluent from zone 6 decreased after the HRT was shortened to 2 days, even though Fe2+ had been completely oxidized by A. ferrooxidans in zone 5 (Fig. 3). However, more than 50% of Fe in the liquid phase still entered the solid phase, indicating that the capacity of Fe3+ hydrolysis to form minerals was basically unaffected. Conversely, the system was disrupted by hydraulic load and became imbalanced after shortening the HRT to 1 day, impeding Fe2+ oxidation by A. ferrooxidans and Fe3+ hydrolysis to form minerals. The results were consistent with those in previous reports that suggested that the Fe2+ oxidation rate, to a great extent, had a dominant function in the formation of iron oxyhydroxysulfate minerals.44–46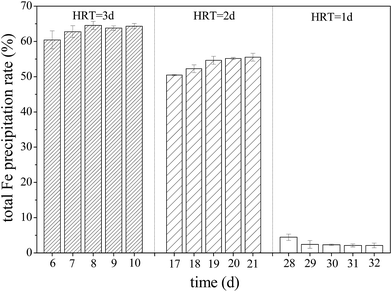 | ||
| Fig. 5 Variations in total Fe precipitation rate with HRT when the plug-flow ditch system is stable (initial total Fe concentration was 5 g L−1). | ||
Previous studies confirmed that iron oxyhydroxysulfate minerals are ideal adsorbents with relatively strong adsorption or co-precipitation effects on heavy metals.16–18 The mechanism of As(III) adsorption on iron oxyhydroxysulfate minerals involves either ligand exchange between As(III) species and a hydroxyl group and sulfate or the formation of As(III)–Fe(III)–SO42− precipitates on the mineral surface.19 Owing to the influence of HRT on the total Fe precipitation rate in the synthetic AMD, a longer system HRT was more conducive to improving the removal rates of As(III) from AMD (Fig. 6). For HRTs of 3 and 2 days, the average removal rates of As(III) from the effluent in zone 6 were 21.58% and 17.27%, respectively. The system showed a significantly lower removal rate of As(III), mainly because of the relatively higher effluent acidity (pH < 2.0). Duquesne et al. noted that at such acidity, As(III) mainly exists in the form of electronically neutral H3AsO3 that rarely replaces HSO4− and SO42− groups in iron oxyhydroxysulfate minerals.47 XPS analysis revealed that As(III) in a solution adsorbed by minerals was in the form of As2O3 (Fig. 7) because the drying of iron oxyhydroxysulfate minerals caused the loss of crystalline water in H3AsO3 and resulted in the formation of As2O3.
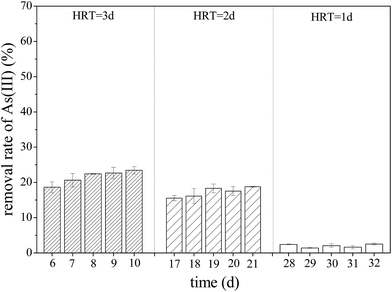 | ||
| Fig. 6 Variations in the removal rate of As(III) with HRT when the plug-flow ditch system is stable (initial As(III) concentration was 1 mg L−1). | ||
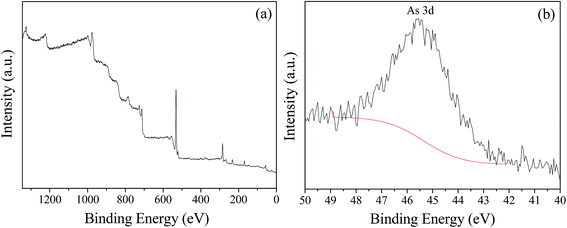 | ||
| Fig. 7 XPS spectra of iron oxyhydroxysulfate minerals collected from zone 6 of the plug-flow ditch system (HRT = 2 d). | ||
3.4. The structural characteristics of iron oxyhydroxysulfate minerals collected from the plug-flow ditch system under an HRT of 2 days
The X-ray diffractogram (XRD) pattern and scanning electron microscopy (SEM) image of iron oxyhydroxysulfate minerals harvested from zone 6 after the system ceased operation when the HRT was 2 days are shown in Fig. 8. Based on the comparisons with the Joint Committee on Power Diffraction Standards data files cards no. 22-0827 and no. 47-1775, the minerals are composed of schwertmannite and K-jarosite.48 Although the synthetic AMD contained NH4+ and Na+, there was no detectable diffraction peak of NH4-jarosite or Na-jarosite. Studies by Gramp et al. and Bai et al. suggested that this is mainly caused by the differences in the ability of monovalent cations to form jarosite-like minerals in the following order: K+ > NH4+ > Na+.49,50 The SEM micrograph shows that the resulting minerals mainly consist of 0.5–2.0 μm spherical particles with sparse aggregation structures in the interparticle space. Additionally, there was a minute amount of irregular fine particulate minerals attached to the surface of the spherical particles and the gaps between them.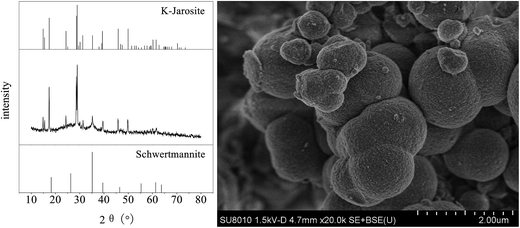 | ||
| Fig. 8 XRD pattern and SEM image of iron oxyhydroxysulfate minerals collected from zone 6 of the plug-flow ditch system (HRT = 2 d). | ||
3.5. Neutralization effect of the system on the effluent under an HRT of 2 days
Previous studies reported that Fe(OH)3, Fe(OH)2, and CaSO4 are the main precipitates formed when lime is added into the Fe and SO42−-rich acidic environment.6 Fig. 9 shows that 2 g L−1 lime is sufficient to completely remove the remaining 2.2 g L−1 Fe3+ from the effluent and the pH of the effluent significantly (P < 0.01) increases from 1.87 to 4.82. An increase in the amount of lime added (from 2 to 3 g L−1) could nearly neutralize the effluent (pH = 6.35), producing 5.23 g L−1 precipitate due to neutralization. The direct neutralization of the influent required up to 12 g L−1 of lime to precipitate 5 g L−1 of Fe2+, generating an effluent with a pH value of up to 10.54 and a precipitate of up to 35.45 g L−1. The pre-processing of synthetic AMD with the microbial enhanced plug-flow ditch system reduced the consumption of lime for neutralization and the amount of waste residues formed by 75.00% and 85.25% compared to that of the influent, respectively. In summary, the microbial enhancement-lime neutralization passive treatment technique can greatly reduce the Fe level in AMD, reduce the consumption of lime, and improve the neutralization efficiency. Further, the iron oxyhydroxysulfate minerals can be used to partially remove the toxic elements, thereby reducing pollution and harmful effects of AMD on the environment.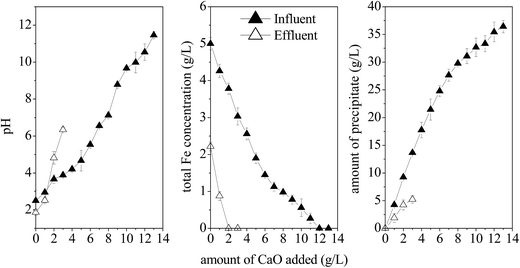 | ||
| Fig. 9 Comparison of the neutralization efficiency between influent and effluent after simulated AMD treatment (HRT = 2 d). | ||
4. Conclusions
The oxidation of Fe2+ facilitated by A. ferrooxidans prior to lime neutralization has a significant potential for the treatment of AMD. A simulated AMD was subjected to the microbial enhanced pretreatment in a plug-flow ditch system with an effective volume of 180 L.(1) HRT had a strong influence on microbial activity during the treatment of AMD. For HRTs of 3 and 2 days, the system required 6 and 4 days, respectively, to stabilize. When HRT was 1 day, the system began to become imbalanced after 4 days of operation.
(2) For HRTs of 3 and 2 days, the average total Fe precipitation rates of the stabilized system were 63.18% and 53.62%, respectively. The average removal rates of As(III) were 21.58% and 17.27%, respectively. The precipitate was a mixture of schwertmannite and K-jarosite, and As(III) in solution adsorbed by minerals was in the form of As2O3. Considering the treatment capacity and the effects together, the selected optimum HRT for the plug-flow ditch system of the AMD is 2 days.
(3) At HRT of 2 days, after the pre-processing of synthetic AMD with the microbial enhanced plug-flow ditch system, the consumption of lime for neutralization and the amount of waste residues reduced by 75.00% and 85.25%, respectively, compared to those of the influent. The outcomes of this study support the promotion and engineering applications of microbial enhancement-lime neutralization passive treatment technology for AMD. Future studies will focus on the selection of microbial immobilization carriers, the sedimentation and dewatering performance of neutralizing products, and the economic cost analysis of this technology.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (21637003), the Natural Science Foundation of Hubei Province, China (2016CFB289), and the Talent Introduction Foundation of Zhongnan University of Economics and Law (31541711302).References
- L. X. Zhou, Earth Sci. Front., 2008, 15, 74–82 CAS.
- M. Vhahangwele, J. Water Process Eng., 2016, 10, 67–77 CrossRef.
- Z. L. Wu, L. C. Zou, J. H. Chen, X. K. Lai and Y. G. Zhu, Int. J. Miner. Process., 2016, 149, 18–24 CrossRef CAS.
- D. B. Johnson and K. B. Hallberg, Sci. Total Environ., 2005, 338, 3–14 CrossRef CAS PubMed.
- P. L. Younger, S. A. Banwart and R. S. Hedin, Mine Water: Hydrology, Pollution, Remediation, Kluwer Academic Press, The Netherlands, 2002 Search PubMed.
- P. Herrera, H. Uchiyama, T. Lgarashi, K. Asakura, Y. Ochi, N. Iyatomi and S. Nagae, Miner. Eng., 2007, 20, 1255–1260 CrossRef CAS.
- Y. W. Song, M. Wang, J. R. Liang and L. X. Zhou, Hydrometallurgy, 2014, 143, 23–27 CrossRef CAS.
- W. C. Lee, S. W. Lee, S. T. Yun and P. K. Lee, J. Hazard. Mater., 2016, 301, 332–341 CrossRef CAS PubMed.
- K. Meschke, V. Herdegen, T. Aubel, E. Janneck and J. U. Repke, J. Environ. Chem. Eng., 2015, 4, 2848–2856 CrossRef.
- Y. Yang, Y. Li and Q. Y. Sun, Trans. Nonferrous Met. Soc. China, 2014, 24, 3332–3342 CrossRef CAS.
- F. W. Liu, J. Zhou, T. J. Jin, S. S. Zhang and L. L. Liu, Water Sci. Technol., 2016, 73, 1442–1453 CrossRef PubMed.
- Y. W. Song, B. W. Zhao, M. B. Huo, C. H. Cui and L. X. Zhou, Environ. Sci., 2013, 34, 3264–3271 CAS.
- Y. W. Song, H. R. Wang, J. R. Liang and L. X. Zhou, Acta Sci. Circumstantiae, 2016, 36, 3683–3690 CAS.
- J. Zhu, M. Gan, D. Zhang and L. Chai, Mater. Sci. Eng., C, 2013, 33, 2679–2685 CrossRef CAS PubMed.
- J. M. Bigham, U. Schwertmann, S. J. Traina, R. L. Winland and M. Wolf, Geochem. Cosmochim. Acta, 1996, 60, 2111–2121 CrossRef CAS.
- S. L. Zhang, S. Y. Jia, B. Yu, Y. Liu and S. H. Wu, Chem. Geol., 2016, 420, 270–279 CrossRef CAS.
- M. Gan, S. G. Sun and Z. H. Zheng, Appl. Surf. Sci., 2015, 356, 986–997 CrossRef CAS.
- K. M. Mihone, F. Hana and R. Sanda, J. Geochem. Explor., 2015, 148, 161–168 CrossRef.
- Y. H. Liao, J. R. Liang and L. X. Zhou, Chemosphere, 2011, 83, 295–301 CrossRef CAS PubMed.
- S. G. Johnston, E. D. Burton, A. F. Keene, B. P. Friedrich, A. Voegelin and M. G. Blackford, Chem. Geol., 2012, 334, 9–24 CrossRef CAS.
- P. Asokan, M. Saxena and S. R. Asolekar, J. Hazard. Mater., 2006, 137, 1589–1599 CrossRef CAS PubMed.
- M. Nemati, S. T. L. Harrison, G. S. Hansford and C. Webb, Biochem. Eng. J., 1998, 1, 171–191 CrossRef CAS.
- Z.-E. Long, Y. H. Huang, Z. L. Cai, W. Cong and F. Ouyang, Hydrometallurgy, 2004, 74, 181–187 CrossRef CAS.
- Z.-E. Long, Y. H. Huang, Z. L. Cai, W. Cong and F. Ouyang, Biotechnol. Lett., 2003, 25, 245–249 CrossRef CAS PubMed.
- Z.-E. Long, Y. H. Huang, Z. L. Cai, W. Cong and F. Ouyang, Process Biochem., 2004, 39, 2129–2133 CrossRef CAS.
- B. Escobar, E. Jedlicki, J. Wiertz and T. Vargas, Hydrometallurgy, 1996, 40, 1–10 CrossRef CAS.
- M. Wang and L. X. Zhou, Hydrometallurgy, 2012, 125–126, 152–156 CrossRef CAS.
- J. E. Dutrizac, Metall. Trans. B, 1983, 14, 531–539 CrossRef.
- J. E. Dutrizac, Hydrometallurgy, 1996, 42, 293–312 CrossRef CAS.
- M. Gleisner, J. R. R. Herbert and P. C. F. Kockum, Chem. Geol., 2006, 225, 16–69 CrossRef CAS.
- C. M. Neculita, G. J. Zagury and B. Bussiere, J. Environ. Qual., 2007, 36, 1–16 CrossRef CAS PubMed.
- Y. H. Liao, L. X. Zhou, J. R. Liang and H. X. Xiong, Mater. Sci. Eng., C, 2009, 29, 211–215 CrossRef CAS.
- F. W. Liu, S. Y. Gao, M. Wang, Y. S. Bu, C. H. Cui and L. X. Zhou, China Environ. Sci., 2014, 34, 713–719 CAS.
- F. W. Liu, S. Y. Gao, M. Wang, H. Y. Yu, C. H. Cui and L. X. Zhou, Acta Sci. Circumstantiae, 2015, 35, 476–483 CAS.
- Q. W. Huang, D. W. Zeng, S. Q. Tian and C. S. Xie, Mater. Lett., 2012, 83, 76–79 CrossRef CAS.
- S. Houngaloune, T. Kawaai, N. Hiroyoshi and M. Ito, Hydrometallurgy, 2014, 147–148, 30–40 CrossRef CAS.
- C. M. Neculita, G. J. Zagury and B. Bussiere, Appl. Geochem., 2008, 23, 3442–3451 CrossRef CAS.
- J. T. Pronk, K. Liem, P. Bos and J. G. Kuenen, Appl. Environ. Microbiol., 1991, 57, 2063–2068 CAS.
- N. Ohmura, K. Sasaki, N. Matsumoto and H. Saiki, J. Bacteriol., 2002, 184, 2081–2087 CrossRef CAS PubMed.
- Y. H. Liao, L. X. Zhou, S. Y. Bai, J. R. Liang and S. M. Wang, Appl. Geochem., 2009, 24, 1739–1746 CrossRef CAS.
- Q. J. Hou, D. Fang, J. R. Liang and L. X. Zhou, PLoS One, 2015, 10, 1–12, DOI:10.1371/journal.pone.0120966.
- J. Majzlan and A. Navrotsky, Geochim. Cosmochim. Acta, 2004, 68, 1049–1059 CrossRef CAS.
- A. Amouric, A. C. Brochier, D. B. Johnson, V. Bonnefoy and K. B. Hallberg, Microbiology, 2011, 157, 111–122, DOI:10.1099/mic.0.0445370.
- S. Huang and L. X. Zhou, Mater. Sci. Eng., C, 2012, 32, 916–921 CrossRef CAS.
- A. Mahiroglu, E. T. Yel and M. F. Sevimli, J. Hazard. Mater., 2009, 166, 782–787, DOI:10.1016/j.jhazmat.2008.11.119.
- S. Y. Bai, J. R. Liang and L. X. Zhou, Acta Mineral. Sin., 2011, 31, 118–125 CAS.
- K. Duquesne, S. Lebrun, C. Casiot and O. Bruneel, Appl. Environ. Microbiol., 2003, 69, 6165–6173 CrossRef CAS PubMed.
- JCPDS, Mineral Powder Diffraction Files, International Center for Diffraction Data, Swarthmore, Pennsylvania, 2002 Search PubMed.
- J. P. Gramp, F. S. Jones, J. M. Bigham and O. H. Tuovinen, Hydrometallurgy, 2008, 94, 29–33 CrossRef CAS.
- S. Y. Bai, Z. H. Xu, M. Wang, Y. H. Liao, J. R. Liang, C. C. Zheng and L. X. Zhou, Mater. Sci. Eng., C, 2012, 32, 2323–2329 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2018 |