Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Influence of substrate on the structure of predominantly anatase TiO2 films grown by reactive sputtering
Iuri S. Brandt *ab,
Cristiani C. Plá Cida,
Carlos G. G. Azevedoc,
André L. J. Pereirad,
Luana C. Benettia,
Andre S. Ferlauto
*ab,
Cristiani C. Plá Cida,
Carlos G. G. Azevedoc,
André L. J. Pereirad,
Luana C. Benettia,
Andre S. Ferlauto e,
José H. Dias da Silva
e,
José H. Dias da Silva *c and
André A. Pasa
*c and
André A. Pasa *a
*a
aLaboratório de Filmes Finos e Superfícies, Universidade Federal de Santa Catarina, Florianópolis, 88040-900, Brazil. E-mail: iuri.brandt@pgfsc.ufsc.br; andre.pasa@ufsc.br
bPrograma de Pós-Graduação em Ciência e Engenharia de Materiais, Universidade Federal de Santa Catarina, Florianópolis, 88040-900, Brazil
cFaculdade de Ciências, Universidade Estadual Paulista UNESP, Bauru, SP, 17033-360, Brazil. E-mail: jhdsilva@fc.unesp.br
dUniversidade Federal da Grande Dourados UFGD, Dourados, MS 79804-970, Brazil
eDepartamento de Física, Universidade Federal de Minas Gerais, Belo Horizonte, 31270-901, Brazil
First published on 14th February 2018
Abstract
TiO2 films are grown on LaAlO3 (001), Si (100) and SiO2 substrates by reactive radio frequency sputtering. X-ray diffraction (XRD) pole figures revealed important characteristics about the texture and phase distribution on those films. Combined with spectroscopic ellipsometry, the pole figures allowed the analysis of the growth characteristics over the whole volume of the layers. Details in the microstructure of the films were probed using transmission electron spectroscopy. Anatase is the dominating phase in the films grown on all substrates. On TiO2/LaAlO3 fims, the mosaicity is very low, so that the pole figure closely resembles that of anatase monocrystals. Detailed inspection of XRD pole figures reveals a small amount of rutile in the TiO2/LaAlO3 films. For the growth of TiO2 onto SiO2, rutile and brookite phases are also detected. Transmission electron microscopy and XRD results show the formation of anatase {112} twins in films grown on the different substrates, suggesting that the anatase {112} twin mediates the growth of rutile and brookite phases. Optical results are in agreement with the XRD observations: the optical properties of TiO2/LaAlO3 films are similar to the ordinary values of bulk anatase crystals, indicating the orientation of the film in the [001] direction, whereas results for TiO2/SiO2 are compatible with lower crystalline ordering.
Introduction
Titanium dioxide (TiO2) is a wide band gap semiconductor that has been intensively studied since the observation of water photolysis on its surface in 1972.1 Nowadays, TiO2 is the most widely used oxide for photocatalysis of organic pollutants.2–4 In past years, new applications in the fields of biocompatibility,5 self-cleaning processes,6 photovoltaics,7 lithium ion batteries8 and high-k gate dielectrics9 have reinforced the needs for research on TiO2 film deposition methods. The performance of these films in such technologies strongly depends on the TiO2 polymorphic phase employed: rutile, anatase or brookite. Anatase exhibits advantages over rutile for catalysis, photocatalysis and solar cell applications.2,10–12 However, in some cases, due to synergistic effects, a phase blend is expected to enhance the photocatalytic activity.13 Moreover, the ability to control the texture and the crystallinity of the TiO2 films allows increases in efficiency of lithium batteries or of catalysis processes.14–19Depending on deposition method and substrate nature, pure20–23 or mixed-phase24,25 TiO2 films can be obtained. Although anatase20–22,26–28 and brookite29 are metastable phases, they can be grown in pure form on single crystal substrates via epitaxial stabilization. It is important to determine the relative concentration of each phase, the presence of defects and the predominant crystalline orientation in TiO2 films, in order to better understand and control their growth as well as to better understand the correlation between growth processes and the optical and electronic properties of the corresponding films.
The best epitaxy of the anatase TiO2 occurs when lanthanum aluminate LaAlO3 (001), labeled simply as LAO, is used as substrate.26,28,30–32 This is due to the very low lattice mismatch (0.2%).26 The pulsed laser deposition (PLD) is by far the most used technique for this kind of growth,28,32 followed by the molecular beam epitaxy (MBE) growth.26,27,33 While these techniques offer excellent control of the growth conditions, they have the limitations of depositing in small areas, and high costs. In an attempt to develop alternatives, e-beam evaporation was tested31 and a few groups have considered the use of reactive sputtering deposition.20,30,34 These techniques are simpler, and present lower cost than PLD and MBE. Besides, the reactive sputtering has the potential to produce uniform growth over very large areas.35 This makes the technique attractive for several technological applications, but it still needs further understanding and development.
In this report, we investigate the growth of TiO2 films by reactive sputtering and the dependence of phase formation and film crystallinity on the nature of the substrate. The TiO2 layers were grown onto the (001) surface of LAO, Si (100) and fused silica substrates. Local details in the structure of the films, including twin formation, are analyzed using transmission electron spectroscopy. X-ray diffraction (XRD) pole figures were used for the first time to analyze this epitaxy. They revealed important characteristics about the texture and phase distribution on those films. The joint use of spectroscopic ellipsometry and pole figures allowed the probe of the growth characteristics over the whole volume of the samples.
Experimental
The films were deposited by radio frequency (RF) magnetron sputtering using a 75 mm diameter metallic Ti target (99.999%) in an Ar + O2 atmosphere (99.9999%). The deposition RF power was 120 W, using a KJL Torus magnetron cathode. The total pressure was 5.0 × 10−3 Torr, using Ar flow of 40.0 sccm, while the oxygen flow was 0.2 sccm. The residual pressure was below 1.0 × 10−6 Torr. Fused silica (a-SiO2), Si (100) and LAO (001) substrates, kept at 450 °C, were used simultaneously in the depositions. As no treatment to remove the native oxide on the top of the Si (100) was performed, in what follows we refer to this as the SiO2/Si(100) substrate. It was observed that XRD results were identical for samples deposited onto SiO2/Si(100) and a-SiO2 substrates, so we have shown here just the ones corresponding to TiO2 films deposited onto SiO2/Si(100).Samples deposited under continuous and interrupted O2 flow (20 interruptions per deposition) were analyzed. Further details about sample preparation can be found elsewhere.24
Structural properties were investigated by XRD with CuKα1 radiation (1.54 Å). The pole figures for in-plane and out-plane orientations were performed by electing a diffraction angle, 2θ, with the tilt angle, χ, varying from 0 to 90° and the azimuthal angle, ϕ, varying from 0 to 360°, for each value of χ. In order to obtain structural information associated to a few grains, a 200 kV-TEM (JEOL JEM-2100) was used in bright and dark field imaging modes. Additionally, selected electron area diffraction (SAED) technique was also employed.
Optical characterizations of the films were performed using transmittance and reflectance measurements and spectroscopic ellipsometry. The transmittance measurements at normal incidence and reflectance at nearly normal (8°) were performed in a Perkin Elmer Lambda 1050 spectrophotomer, with 150 mm integrating sphere apparatus, in the 200–2500 nm range. Spectroscopic ellipsometry measurements were performed using a M-2000 J. A. Woollam spectrometer in the 246–1690 nm range.
Results and discussion
LAO substrate
X-ray diffractograms for the TiO2/LAO sample deposited using continuous oxygen flow are shown in Fig. 1. Results on twin samples prepared under modulated oxygen flow display identical results.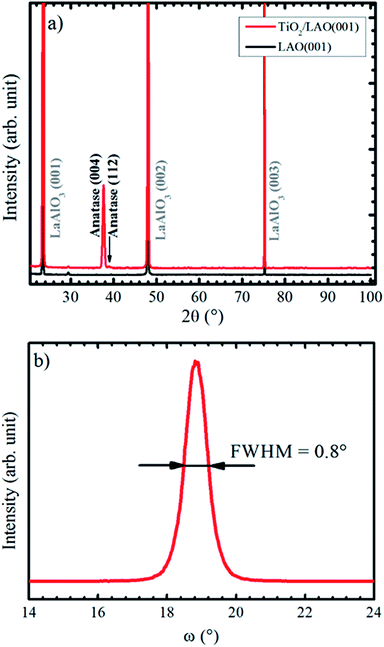 | ||
| Fig. 1 (a) XRD Bragg–Brentano pattern recorded for LAO and TiO2 film grown onto LAO. The arrow indicates the anatase (112) peak position. In (b) it is shown anatase (004) XRD rocking curve. | ||
The large domination of the (004) peak in the Bragg–Brentano diffractogram of Fig. 1a indicates that the TiO2 anatase phase preferentially grows with the [001] direction perpendicular to the LAO substrate surface. Such preferential growth in anatase [001] direction is in agreement with previous reports.36,37 A low intensity peak, close to the anatase (004) and indicated by an arrow in Fig. 1a, is also observed and assigned to anatase [112] direction. All other XRD peaks come from the substrate. Fig. 1b displays a rocking curve of the anatase (004) peak, with full width at half maximum (FWHM) equal to 0.8°. This value is comparable to the ones for thick TiO2 films obtained by pulsed laser deposition,38 which is a well-recognized technique for growing high quality films (see for instance the book by D. B. Chrisey and G. K. Hubler39 and references therein).
Lotnyk and co-workers31 analyze the growth of anatase films onto SrTiO3 and LAO using reactive e-beam evaporation. Substrate temperatures in the 500−1000 °C range were used during the growth (while the substrates were kept at 450 °C in our results). They found that the film–substrate interface is composed by anatase (001) and LAO (001) surfaces, similarly to the present conditions. The full width at half maximum of the rocking curve peaks attained by their growth at 1000 °C on LAO (0.2°) is smaller than the one (0.8°) reported on Fig. 1b. This difference is probably due to the higher substrate temperatures, which promotes ordering of crystallites, as opposed to the bombardment of the substrate by energetic particles during sputtering growth. The incidence of energetic particles from the plasma on the growth surface is likely to produce defects and higher strain effects on the crystallites. The comparison suggests that the sputtering growth at lower power and higher substrate temperatures than the used in the present report should produce higher ordering in the films.
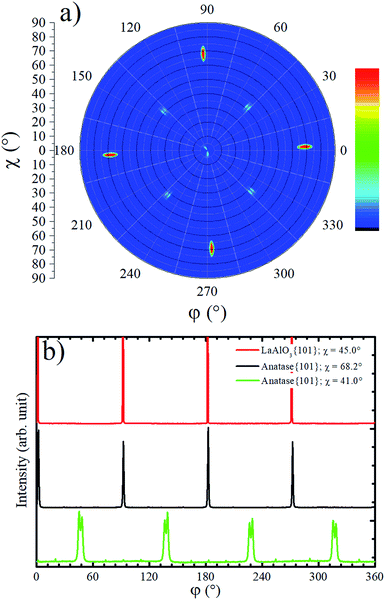
The in-plane texture of the TiO2 film on LAO substrate was investigated performing an anatase {101} pole figure by setting 2θ = 25.224°. In Fig. 2a it is possible to observe four sharp peaks centered at χ = 68.2° that is exactly the interplanar angle between {001} and {101} anatase planes. These four poles make 90° with respect to each other and reveal a single in-plane orientation of the anatase {001} crystals. The weaker shadowed peaks that occur about 41° in Fig. 2a suggest that anatase crystals present tilted domains. This result could not be compared with any report in the literature since the authors did not find previous works showing pole figures of TiO2 films grown on LAO (100). For example, the work of Lotnyk et al. discussed above, only presented pole figures of TiO2 films on LAO (110) and on yttria-stabilized zirconia. Following, these tilted anatase domains are investigated in more detail using specific scans.
Azimuthal scans for χ = 68.2° at 2θ = 25.224° and χ = 45.0° at 2θ = 33.397° are shown in Fig. 2b. The first and second scans select anatase {101} and LAO {101} planes, respectively. As can be observed, there is no offset between {101} peaks of the anatase and LAO, implying that the anatase lattice is not rotated about the [001] substrate normal vector, which is in agreement with an earlier report.36 The ordering of this plane is due to the excellent matching between the base of the anatase conventional unit cell tetragon, and face of the cubic LAO unit cell. The difference of lattice parameters between then is only 0.2%.26 The FWHM of {101} peaks is 0.6° for LAO and 1.1° for anatase. This result implies in a very small difference of the in-plane orientation between anatase crystallites, i.e. a very low mosaicity of the anatase crystals in the film.
In the pole figure of Fig. 2a there are also weak spots at χ = 41.0°, which are explained by the growth of out-of-plane oriented anatase {112} grains already observed by the small peak in Fig. 1a. These spots are rotated by 45° in respect to the anatase {101} ones at χ = 68.2°. The azimuthal curve at χ = 41.0° in Fig. 2b shows that the anatase {112} grains are actually split by 3° in two peaks with a rotation of 43.5° and 46.5° over LAO [001] axis. The presence of anatase grains with such in-plane orientation on TiO2 epitaxial growth over LAO have not been previously reported. Therefore, we were encouraged to propose a possible atomic arrangement of anatase {112} planes in the TiO2 film grown on LAO.
Fig. 3 sketches anatase (112), anatase (001) and LAO (001) planes. In this figure the atomic similarity between anatase (001) and LAO (001), that gives rise to the low lattice mismatch of 0.2%, becomes clear. If anatase (112) is rotated by 45° the square formed by Ti atoms, indicated in the figure, will fit to the anatase (001) plane or likewise to the LAO (001) plane. In anatase [![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 10] direction, the lattice mismatch would be close to zero and, in the direction [
10] direction, the lattice mismatch would be close to zero and, in the direction [![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif)
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 1], it would be approximately 1.6%. Such low values support the 45° rotation of anatase (112) and reasonably agrees with the results in Fig. 2b, where rotations of 43.5° and 46.5° were found. The divergence from 45° is probably related to lattice relaxation processes.
1], it would be approximately 1.6%. Such low values support the 45° rotation of anatase (112) and reasonably agrees with the results in Fig. 2b, where rotations of 43.5° and 46.5° were found. The divergence from 45° is probably related to lattice relaxation processes.
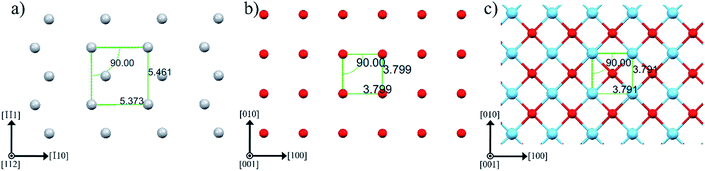 | ||
| Fig. 3 Atomic arrangement of (a) anatase (112), (b) anatase (001) and (c) LAO (001) planes. The atoms Ti, O and La are depicted by gray, red and blue spheres, respectively. These images were created making use of Mercury software.56–58 | ||
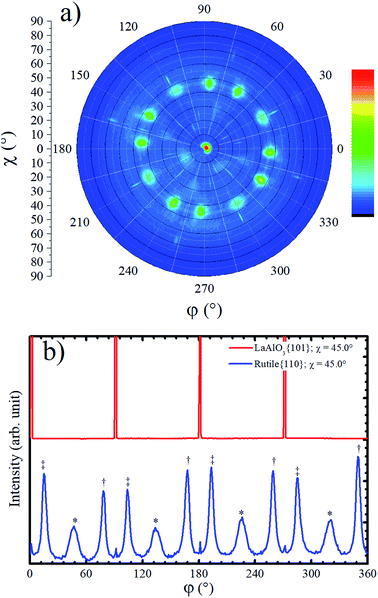
Although the XRD Bragg–Brentano measurement in Fig. 1a has not shown any evidence for the presence of rutile phase in the TiO2/LAO film, a rutile {110} pole figure was measured, and twelve peaks centered at χ = 45.0° were observed, as shown in Fig. 4a. Such diffraction peaks are attributed to rutile crystallites with 〈100〉 out-of-plane growth direction, since rutile {100} planes make 45° with {110} ones. The absence of {h00} peaks in Bragg–Brentano measurements is probably due to the low amount of rutile phase in the TiO2 film combined with extinction effects. The observation of rutile in the pole figure only became possible due to the much higher structural factor of {110} planes in respect to any {h00} plane. For instance, the rutile (200) plane has the highest structural factor among the rutile {h00} planes and it is roughly two and a half times smaller40 than the rutile (110) structural factor.
The fraction of anatase (fA) in the TiO2/LAO sample is estimated using the following equation,41
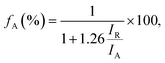 | (1) |
Rutile {110} azimuthal scan for χ = 45.0° is shown in Fig. 4b. The positions of the peaks can be explained on basis of the geometry of the crystal cut interface between LAO and rutile: the first intense peak, relatively narrow, marked with a double cross symbol, corresponds to the diffractions from the rutile (110) planes associated to crystallites that match the diagonal of their rectangular basis a × c (5.46 Å) with cube diagonal a × a (5.36 Å) of LAO lattice. In the unstrained crystal condition, this should correspond to a 12.21° rotation, which is roughly the angle between LAO {101} peaks (see Fig. 4b) and rutile {110} peaks marked with double cross symbol. The clockwise and counterclockwise rotations provide the single and double cross marked peaks. The peaks labelled with an asterisk, correspond to the {110} rutile planes of crystallites grown with a rotation of 45° of the in-plane a axis, [100] direction, relative to the a axis of LAO. The poorer matching of these ones explains the lower intensity and the broader FWHM of these peaks. Then, the fourfold rotation symmetry of the [001] axis of LAO can explain the twelve {110} rutile peaks observed in the pole figure and azimuthal scan of Fig. 4. The small peaks at 0°, 90° and repeated each 90° are not rotated in respect to the substrate and probably correspond to diffraction signal related to the anatase 〈001〉 grains.
Due to the high similarity between atomic arrangements of LAO (001) and anatase (001) planes, the in-plane orientation of rutile grains discussed above is also valid if considered the growth of rutile (100) on top of anatase (001).
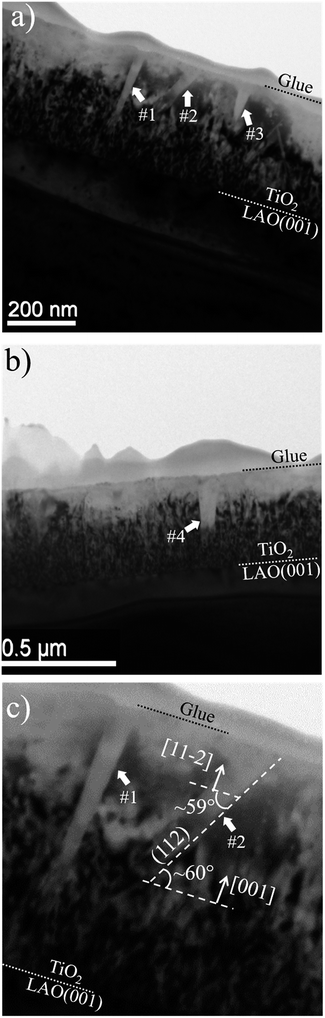
In order to investigate the local structural and morphological aspects of the films, TEM analysis were performed. Fig. 5 presents TEM images of different regions of the TiO2/LAO sample in which two types of columnar structures are observed that are possibly related to twins; (i) almost perpendicular or (ii) tilted with respect to the substrate surface. Similar structures associated to twins were also observed in silver thin films.44 To the best of our knowledge, twin structures were not previously observed in TiO2 films grown on LAO. In the work of H. Xu et al.,36 XRD of TiO2/LAO films did not show the anatase (112) peak and in agreement no twin structure was observed in the TEM images. The structures in Fig. 5 are indicated and numbered as #1, #3 and #4, corresponding to type (i) and #2, corresponding to type (ii). The two first (#1 and #2) are better presented in Fig. 5c, where it is possible to note a ∼60° tilt of #2 in respect to the substrate surface. This angle is very close to the angles between anatase (112) and (001) planes, and between anatase (112) and (11![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) ) planes, where the calculated values are 60.53° and 58.93°,45 respectively. Therefore, we can consider the twin plane being anatase (112), the columnar structure growing in anatase [11
) planes, where the calculated values are 60.53° and 58.93°,45 respectively. Therefore, we can consider the twin plane being anatase (112), the columnar structure growing in anatase [11![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) ] direction and the TiO2 portion surrounding this columnar grain growing in anatase [001] direction, as indicated in Fig. 5c. This consideration is supported by the fact that the major part of the sample presents [001] orientation (see XRD results in Fig. 1a). The other structures (#1, #3 and #4) do not show the ∼60° inclination because the respective columnar grains are ∼90° in-plane rotated in respect to #2. In accordance with azimuthal curves in Fig. 2b that showed four-fold symmetry for anatase (112) grains.
] direction and the TiO2 portion surrounding this columnar grain growing in anatase [001] direction, as indicated in Fig. 5c. This consideration is supported by the fact that the major part of the sample presents [001] orientation (see XRD results in Fig. 1a). The other structures (#1, #3 and #4) do not show the ∼60° inclination because the respective columnar grains are ∼90° in-plane rotated in respect to #2. In accordance with azimuthal curves in Fig. 2b that showed four-fold symmetry for anatase (112) grains.
The formation of anatase {112} twin interfaces in TiO2/LAO sample is probably the source for the growth of the rutile crystallites, which presence was verified by the pole figure in Fig. 4a. It was previously observed in literature that rutile crystallites are likely to nucleate at {112} twin interfaces formed in nanocrystalline titania.46,47
The interpretation that the columnar structures shown in Fig. 5 have a different crystalline orientation than the material surrounding it, is confirmed by dark field imaging of TiO2 grains encompassing a twin. In Fig. 6a it is displayed a SAED pattern took from the region shown in Fig. 5b. The diffraction spots are the expected ones for zone axes (ZA) near to anatase [110] and [111]. Dark field images for the diffracted beams (−101) and (1−12) are displayed in Fig. 6b and c, respectively. Wherein (−101) and (1−12) diffracted beams belong to anatase [110] and [111] ZA, respectively. The dark field images show that the columnar structure is close to the [110] ZA and the TiO2 grains out of this columnar structure are near to the [111] ZA. Therefore, the columnar structure is misoriented in respect to the rest portion of the TiO2 film.
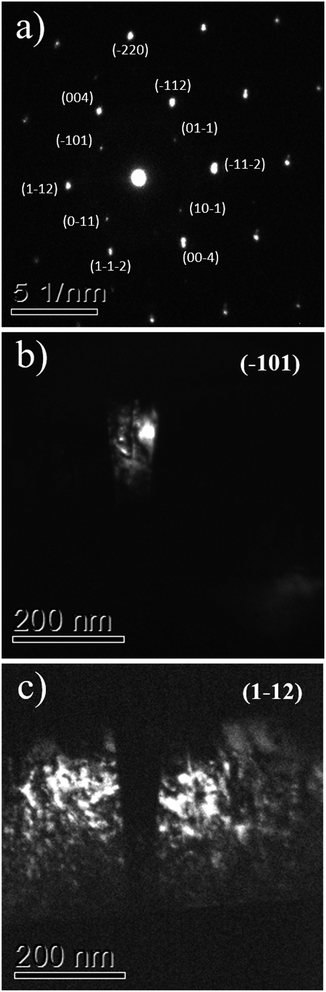 | ||
| Fig. 6 The TEM SAED pattern of the TiO2/LAO film is shown in (a), while (b) and (c) display the dark field images associated to diffractions (−101) and (1−12). | ||
Comparing these results with the literature, it is important to mention that Jeong and co-workers20 were the first to report the growth of single phase anatase onto LAO using reactive sputtering. They claim, that this technique has much better perspective for applications than MBE and PLD. In the growth they have used higher substrate temperatures (750 °C) and deposition power (250 W) than the presently reported. Their analysis is restricted to multiple axis X-ray diffraction and atomic force microscopy measurements. Very narrow peaks in the (004) anatase rocking curves (Δθ = 0.09°) were obtained, revealing very good quality of the anatase crystals. No pole figure, electron microscopy nor optical results were presented. No mention was made to a search for rutile phase or for twin formation.
In a related paper of the same group,30 spectroscopic ellipsometry results of films prepared by reactive sputtering using the same deposition system and under similar conditions were analyzed. In the Optical results section we have compared in detail their results with ours.
Relevant results concerning the anatase growth onto LAO by reactive sputtering were also reported by Wang et al.34 They combined advanced transmission electron microscopy with high-precision first-principles calculation to relate the crystalline structure of TiO2/LAO interface to its electronic properties. They confirm that the rf magnetron can produce high quality anatase crystallites on the top of LAO. Similarly to results of the anatase/LAO (001) epitaxy found in most literature reports, and also in our results, the anatase crystals display the [001] axis perpendicular to the LAO (001) substrate surface, as one could expect by the close match of the surface atomic spacing provided by this geometry. This was already shown in our Fig. 3b and c, and is responsible for the similarity of Fig. 1 in the Wang et al. publication34 and on the present report.
In spite of these similarities, provided by the epitaxy relation between film and substrate, there are important differences in the procedures and results. Differently from the procedure used on this manuscript where a pure metallic Ti target was used, Wang et al. have used a ceramic TiO2 target. Their films were produced at significantly higher temperatures (800 °C), and have been annealed at 1000 °C in air. Even though the FWHM of the diffraction peak is wide in the as grown condition, the annealing produced a significant decrease of the XRD peak width, indicating improvement of the anatase crystal quality with annealing.
XRD pole figures, are not present in this ref. 34. So it is not possible to directly verify if the coherence of the growth occurs in the whole surface or is restricted to the analysis points/axis and if the tilt of the anatase crystallites also occur on their films or not. Besides, the thicknesses of the film grown by them are significantly lower (∼50 nm) than the ones presented here (∼500 nm).
Due to texture effects the presence of small amounts of the rutile phase is difficult to detect in the conventional XRD analysis. The presence of rutile on films grown on LAO could not be detected from our TEM or from the regular XRD analysis. Also the optical results of the films on LAO present no clear indication that this phase is present. This proved the analysis using pole figures to be important.
SiO2/Si(100) substrate
In order to verify the substrate influence on the growth of TiO2 films, this oxide was deposited on SiO2/Si(100). Additionally, we investigated the influence of the O2 atmosphere variation on the structure of TiO2 films grown on SiO2. Fig. 7a shows XRD patterns, obtained in Bragg–Brentano mode, for TiO2 films deposited with continuous (KL33) and discontinuous (KL32) O2 supply. These results are identical to the ones obtained for TiO2 grown on a-SiO2 (results not showed here). In agreement with previous characterization by Raman spectroscopy and XRD, the TiO2 films are formed by the anatase, rutile and brookite phases, with the last two phases in a minor concentration.24 There is a clear reduction in the number of peaks from sample KL33 to KL32, therefore, the sample KL32 displays a less misoriented growth than the KL33 one. This is an evidence of structural modifications caused by the modulation of O2 supply during deposition. Moreover, it demonstrates that the TiO2 growth on SiO2 is kinetically controlled by O2 concentration. On the other hand, the non-dependence on the O2 supply and the high texture presented by TiO2 films grown on LAO are evidences that such growth is less affected by the kinetics of the process and that it is actually thermodynamically governed by the free energy of formation of TiO2 layers on LAO. The kinetically/thermodynamically controlled growth of nanomaterials has been discussed regarding different deposition systems, e.g. chemical synthesis of Rh–Pd alloy nanocrystals48 and electrodeposition of Cu2O films.49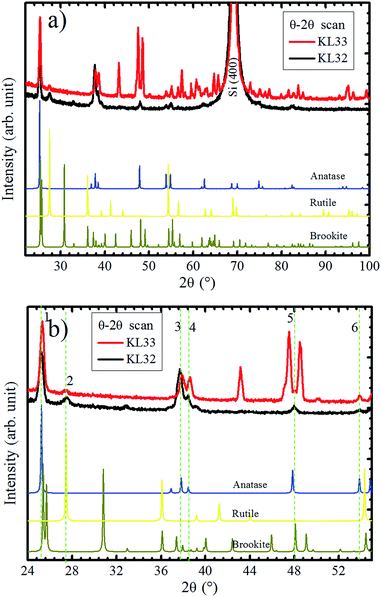 | ||
| Fig. 7 (a) XRD experiments in Bragg–Brentano mode of TiO2/SiO2/Si(100) samples. Samples KL32 (black curve) and KL33 (red curve), correspond to interrupted and continuous deposition O2 flows, respectively. (b) Zoom in of 2θ axis from 24 to 55° with the six XRD coincident peaks for both samples indicated by vertical dashed lines. In the bottom of the figures, reference diffractograms of anatase,45 brookite,45 and rutile40 are given for comparison. | ||
In Fig. 7a, even sample KL32 does not show such a strong out-of-plane preferential orientation as the TiO2 layers grown on LAO, which highlights the role played by the substrate on determining the TiO2 film structure.
A zoom in 2θ axis from 24° to 55° could bring some additional information, as shown in Fig. 7b. The XRD peaks that are observed in both samples are numbered from 1 to 6. The 2θ positions of peaks 1, 3 and 4 for KL33 are displaced from the ones observed for KL32, indicating different lattice parameters for grains grown under continuous and discontinuous O2 supply. Therefore, at least in one of the deposition conditions, part of the TiO2 grains are under lattice strain. On the other hand, peaks 2, 5 and 6 show same positions for both samples, which means equal interplanar spacing. These diffractions are assigned to rutile (110), brookite (321) and anatase (105) planes, respectively. These peaks with equal interplanar spacing for both samples probably emerge due to structural relaxation processes, e.g. crystal twins and phase transformation.50,51
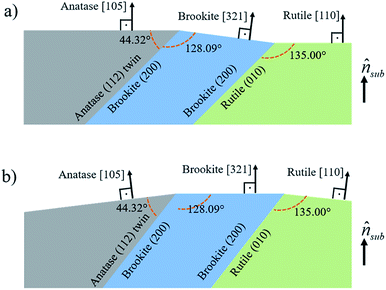
In Fig. 8, it is sketched the growth of grains of the three TiO2 phases, anatase [105], brookite [321] and rutile [110], considering the phase transformation anatase–brookite–rutile already discussed in the literature,46,47,52 which occurs via anatase {112} twin formation followed by brookite (200) and rutile (010) growth. Assuming that the planes anatase (112) twin, brookite (200) and rutile (010) are parallel to each other will imply that the brookite [321] vector in Fig. 8a and anatase [105] and rutile [110] vectors in Fig. 8b not being parallel to the substrate normal (![[n with combining circumflex]](https://www.rsc.org/images/entities/i_char_006e_0302.gif) sub). Intermediary situations in which none of the three directions are parallel to
sub). Intermediary situations in which none of the three directions are parallel to ![[n with combining circumflex]](https://www.rsc.org/images/entities/i_char_006e_0302.gif) sub are also possible. This interpretation is in agreement with XRD rocking curves of brookite (321), rutile (110), and anatase (105) peaks that presented a broad FWHM of ∼14° (results not shown) indicating that those crystals are not all growing parallel to
sub are also possible. This interpretation is in agreement with XRD rocking curves of brookite (321), rutile (110), and anatase (105) peaks that presented a broad FWHM of ∼14° (results not shown) indicating that those crystals are not all growing parallel to ![[n with combining circumflex]](https://www.rsc.org/images/entities/i_char_006e_0302.gif) sub. Therefore, the growth scheme in Fig. 8 supports the hypothesis of a structural relaxation mediated by crystal twining that gives rise to brookite and rutile formation in both conditions of O2 flux. The observation and modeling of the anatase {112} twin formation can be an important step towards understanding the mechanisms responsible for the growth of brookite and rutile phases in predominantly anatase thin films.
sub. Therefore, the growth scheme in Fig. 8 supports the hypothesis of a structural relaxation mediated by crystal twining that gives rise to brookite and rutile formation in both conditions of O2 flux. The observation and modeling of the anatase {112} twin formation can be an important step towards understanding the mechanisms responsible for the growth of brookite and rutile phases in predominantly anatase thin films.
Optical results
With emphasis on the study of the influence of SiO2 and LAO substrates on the growth of TiO2 films using the RF sputtering technique, we analyzed the optical properties of the produced layers by means of optical transmittance and spectroscopic ellipsometry measurements. Fig. 9 displays the refractive index (n) and the extinction coefficient (κ) determined for TiO2 deposited on LAO and a-SiO2. The full symbols, in the weakly absorbing region, represent values obtained via transmittance measurements followed by calculations based on the Cisneros' method.53 The values depicted by open symbols were determined from ellipsometric measurements and using Tauc–Lorentz analytical functions.54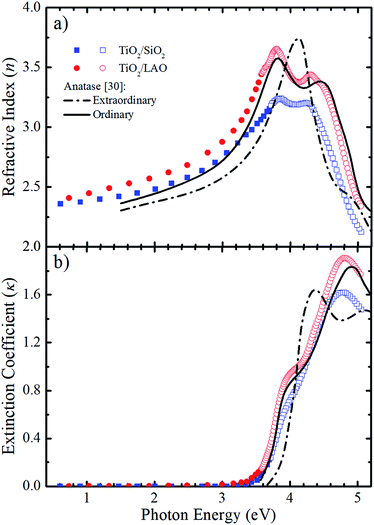 | ||
| Fig. 9 Comparison between the calculated (a) n and (b) κ values for both TiO2 on a-SiO2 (blue) and LAO (red) of samples grown under continuous O2 flow with the values reported by Jellison Jr et al.30 (black lines) for anatase bulk crystals. The calculated values were obtained using transmittance (full symbols) and ellipsometry (open symbols) measurements. | ||
The refractive index and extinction coefficients of bulk anatase crystals obtained by Jellison et al.30 are also plotted in Fig. 9 for comparison. The continuous lines represent the values for light propagation along the anatase c-axis (ordinary), while the dot-dash lines for light propagation perpendicular to the c-axis (extraordinary).
Comparing the curves in Fig. 9a, it can be noticed that the n values for the TiO2/LAO sample behave very similarly to the expected for the ordinary direction of the TiO2 anatase phase. At high photon energies, the two peaks around 3.8 and 4.4 eV, related to the ordinary axis of the anatase phase TiO2 crystal, are reproduced quite well by the TiO2/LAO sample. On the other hand, the TiO2/SiO2 n curve at photon energies higher than 3.5 eV does not fit so well with the n curve for ordinary propagation of light measured in bulk anatase by Jellison Jr et al.30 The results of Fig. 9a, show an agreement between the observations of XRD of Fig. 1, 2 and 7, and the refractive index, which shows a behavior very close to anatase. Nevertheless, the optical data did not reveal the presence of the small amount of the rutile and brookite phases in films deposited in both substrates. In Fig. 9b the shoulder at about 3.9 eV and the maximum around 4.9 eV in the anatase κord curve are observed in both samples. However, the energy position and intensity of the maximum is better reproduced by the TiO2/LAO sample, which reinforces that TiO2 grown on LAO is highly crystalline, the anatase c-axis being perpendicular to the substrate surface.
Therefore, it is clear that the refractive index and extinction coefficient of the samples grown on both substrates are compatible with the strong predominance of the anatase phase. However, due to structural modifications caused by the use of distinct substrates, the electronic properties are different for TiO2 layers grown on LAO and a-SiO2. Analyzing the results of previous investigations and comparing the optical properties of crystalline and polycrystalline anatase by Tanemura and co-workers,55 it becomes evident that the present optical results reinforce the high crystallinity of the TiO2/LAO sample, observed by XRD in Fig. 1 and 2. For the TiO2/SiO2, the optical characteristics are also similar to the ones of anatase crystals, but are also compatible with the more diffuse aspect of the X-ray pole figures24 and high misorientation (Fig. 7), related to a more random orientation of the crystallites.
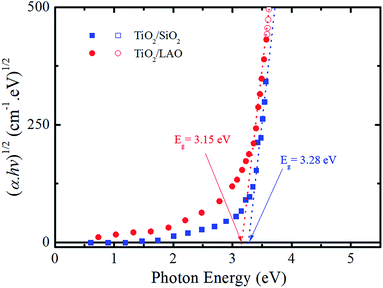
From the extinction coefficient data of Fig. 9b, the absorption coefficient (α) was calculated for each sample and then fitted by the Tauc's relation,54 (αhv)1/2 = A(hv − Eg), that holds for indirect bandgap materials. In this equation hv is the photon energy, A is a constant and Eg is the band gap. The determined Eg values from Fig. 10 are 3.15 and 3.28 eV for TiO2/LAO and TiO2/SiO2 samples, respectively. It is shown that the TiO2 layers on LAO presents a slightly higher absorption in the visible than TiO2 layers deposited onto SiO2.
Conclusions
In summary, the structural properties of TiO2 films grown by reactive sputtering on LAO, SiO2/Si(100) and SiO2 substrates using continuous and modulated oxygen flux were investigated using a combination of techniques. On the analysis presented here, XRD pole figures, and the optical combined T & R and ellipsometry probed large areas, and large volumes, of the films making the conclusions more robust when concerning large area applications. In contrast, the details in the structure were probed using transmission electron spectroscopy. The films are predominantly composed by the anatase phase, especially the ones deposited onto LAO, in which the percentage of anatase was estimated as 96%. On both crystalline and amorphous substrates, the growth of rutile and brookite phases were shown to be mediated by formation of anatase {112} twin interfaces. The TiO2 layers grown on LAO presented high crystallinity with in-plane and out-of-plane orientations, for both the anatase crystals and also for crystallites of the minor rutile phase. The pole figures and azimuthal scans were especially useful in the present analysis, and allowed a detailed investigation of the structure of TiO2 films onto LAO substrate. These techniques have clearly shown the epitaxial growth of the predominant anatase phase and the tilt in orientation produced by twin defect formation. Also, these techniques allowed the detection and an unpreceded detailed texture analysis of the tiny rutile phase. Meanwhile, TiO2 deposited in SiO2 substrates are polycrystalline with some orientation texture. Such structural differences are responsible for distinct optical electronic properties of TiO2 layers grown on LAO and SiO2, as observed from optical transmittance and ellipsometric measurements.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the funding of the Brazilian agencies CAPES, CNPQ, FAPESP (2017/18916-2), FAPESC and FINEP.Notes and references
- A. Fujishima and K. Honda, Nature, 1972, 238, 37–38 CrossRef CAS PubMed.
- A. L. Linsebigler, A. L. Linsebigler, J. T. Yates Jr, G. Lu, G. Lu and J. T. Yates, Chem. Rev., 1995, 95, 735–758 CrossRef CAS.
- M. a. Henderson, Surf. Sci. Rep., 2011, 66, 185–297 CrossRef CAS.
- K. Hashimoto, H. Irie and A. Fujishima, Jpn. J. Appl. Phys., 2005, 44, 8269–8285 CrossRef CAS.
- L.-H. Li, Y.-M. Kong, H.-W. Kim, Y.-W. Kim, H.-E. Kim, S.-J. Heo and J.-Y. Koak, Biomaterials, 2004, 25, 2867–2875 CrossRef CAS PubMed.
- Y. Paz, Z. Luo, L. Rabenberg and a. Heller, J. Mater. Res., 1995, 10, 2842–2848 CrossRef CAS.
- X. Feng, K. Shankar, O. K. Varghese, M. Paulose, T. J. Latempa and C. a. Grimes, Nano Lett., 2008, 8, 3781–3786 CrossRef CAS PubMed.
- D. Wang, D. Choi, J. Li, Z. Yang, Z. Nie, R. Kou, D. Hu, C. Wang, L. V. Saraf, J. Zhang, I. a. Aksay and J. Liu, ACS Nano, 2009, 3, 907–914 CrossRef CAS PubMed.
- M. K. Bera, C. Mahata, a. K. Chakraborty, S. K. Nandi, J. N. Tiwari, J.-Y. Hung and C. K. Maiti, Semicond. Sci. Technol., 2007, 22, 1352–1361 CrossRef CAS.
- L. Liu, H. Zhao, J. M. Andino and Y. Li, ACS Catal., 2012, 2, 1817–1828 CrossRef CAS.
- B. O'Regan and M. Grätzel, Nature, 1991, 353, 737–740 CrossRef.
- K. I. Hadjiivanov and D. G. Klissurski, Chem. Soc. Rev., 1996, 25, 61 RSC.
- D. O. Scanlon, C. W. Dunnill, J. Buckeridge, S. A. Shevlin, A. J. Logsdail, S. M. Woodley, C. R. A. Catlow, M. J. Powell, R. G. Palgrave, I. P. Parkin, G. W. Watson, T. W. Keal, P. Sherwood, A. Walsh and A. A. Sokol, Nat. Mater., 2013, 12, 798–801 CrossRef CAS PubMed.
- C. Arrouvel, M. Digne, M. Breysse, H. Toulhoat and P. Raybaud, J. Catal., 2004, 222, 152–166 CrossRef CAS.
- J. S. Chen, Y. L. Tan, C. M. Li, Y. L. Cheah, D. Luan, S. Madhavi, F. Y. C. Boey, L. a. Archer and X. W. Lou, J. Am. Chem. Soc., 2010, 132, 6124–6130 CrossRef CAS PubMed.
- R. Hengerer, L. Kavan, P. Krtil and M. Gratzel, J. Electrochem. Soc., 2000, 147, 1467–1472 CrossRef CAS.
- T. Luttrell, S. Halpegamage, J. Tao, A. Kramer, E. Sutter and M. Batzill, Sci. Rep., 2014, 4, 1–8 Search PubMed.
- C. L. Olson, J. Nelson and M. S. Islam, J. Phys. Chem. B, 2006, 110, 9995–10001 CrossRef CAS PubMed.
- H. Bin Wu, J. S. Chen, H. H. Hng, X. Wen and D. Lou, Nanoscale, 2012, 4, 2526 RSC.
- B. S. Jeong, J. D. Budai and D. P. Norton, Thin Solid Films, 2002, 422, 166–169 CrossRef CAS.
- B.-S. Jeong, D. P. Norton and J. D. Budai, Solid-State Electron., 2003, 47, 2275–2278 CrossRef CAS.
- B. Jeong, D. P. Norton, J. D. Budai and G. E. Jellison, Thin Solid Films, 2004, 446, 18–22 CrossRef CAS.
- G.-H. Lee and M.-S. Kim, Electron. Mater. Lett., 2010, 6, 77–80 CAS.
- A. L. J. Pereira, P. N. Lisboa Filho, J. Acuña, I. S. Brandt, A. a. Pasa, A. R. Zanatta, J. Vilcarromero, A. Beltrán and J. H. Dias da Silva, J. Appl. Phys., 2012, 111, 113513 CrossRef.
- A. L. J. Pereira, L. Gracia, A. Beltrán, P. N. Lisboa-Filho, J. H. D. Silva and J. Andrés, J. Phys. Chem. C, 2012, 116, 8753–8762 CAS.
- M. Murakami, Y. Matsumoto, K. Nakajima, T. Makino, Y. Segawa, T. Chikyow, P. Ahmet, M. Kawasaki and H. Koinuma, Appl. Phys. Lett., 2001, 78, 2664–2666 CrossRef CAS.
- D. A. Schmidt, T. Ohta, Q. Yu and M. A. Olmstead, J. Appl. Phys., 2006, 99, 113521 CrossRef.
- B. H. Park, J. Y. Huang, L. S. Li and Q. X. Jia, Appl. Phys. Lett., 2002, 80, 1174–1176 CrossRef CAS.
- D. Kim, W.-S. Kim, S. Kim and S.-H. Hong, ACS Appl. Mater. Interfaces, 2014, 6, 11817–11822 CAS.
- G. E. Jellison, L. A. Boatner, J. D. Budai, B.-S. Jeong and D. P. Norton, J. Appl. Phys., 2003, 93, 9537 CrossRef CAS.
- A. Lotnyk, S. Senz and D. Hesse, Thin Solid Films, 2007, 515, 3439–3447 CrossRef CAS.
- V. F. Silva, V. Bouquet, S. Deputier, R. Lebullenger, M. Guilloux-Viry, V. L. Silva, I. M. G. Santos, A. Perrin and I. T. Weber, J. Nanosci. Nanotechnol., 2017, 17, 4326–4334 CrossRef CAS.
- X. Weng, P. Fisher, M. Skowronski, P. A. Salvador and O. Maksimov, J. Cryst. Growth, 2008, 310, 545–550 CrossRef CAS.
- Z. Wang, W. Zeng, L. Gu, M. Saito, S. Tsukimoto and Y. Ikuhara, J. Appl. Phys., 2010, 108, 113701 CrossRef.
- I. Baía, M. Quintela, L. Mendes, P. Nunes and R. Martins, Thin, 1999, 337, 171–175 CrossRef.
- H. Xu, X. Feng, C. Luan and J. Ma, Scr. Mater., 2016, 124, 76–80 CrossRef CAS.
- K. Krupski, A. M. Sanchez, A. Krupski and C. F. McConville, Appl. Surf. Sci., 2016, 388, 684–690 CrossRef CAS.
- R. J. Kennedy and P. A. Stampe, J. Cryst. Growth, 2003, 252, 333–342 CrossRef CAS.
- Pulsed Laser Deposition of Thin Films, ed. D. B. Chrisey and G. K. Hubler, Wiley, New York, 1994 Search PubMed.
- W. H. Baur, Acta Crystallogr., 1956, 9, 515–520 CrossRef CAS.
- R. A. Spurr and H. Myers, Anal. Chem., 1957, 29, 760–762 CrossRef CAS.
- R. Ciancio, A. Vittadini, A. Selloni, R. Arpaia, C. Aruta, F. M. Granozio, U. S. di Uccio, G. Rossi and E. Carlino, J. Nanopart. Res., 2013, 15, 1–8 CrossRef.
- A. C. Breeson, G. Sankar, G. K. L. Goh and R. G. Palgrave, Phys. Chem. Chem. Phys., 2016, 18, 24722–24728 RSC.
- I. Tomov, M. Adamik and P. B. Barna, Thin Solid Films, 2000, 371, 17–27 CrossRef CAS.
- I. Djerdj and A. M. Tonejc, J. Alloys Compd., 2006, 413, 159–174 CrossRef CAS.
- R. L. Penn and J. F. Banfield, Am. Mineral., 1999, 84, 871–876 CrossRef CAS.
- Y. Zhou and K. a. Fichthorn, J. Phys. Chem. C, 2012, 116, 8314–8321 CAS.
- Y. Yan, F. Zhan, J. Du, Y. Jiang, C. Jin, M. Fu, H. Zhang and D. Yang, Nanoscale, 2015, 7, 301–307 RSC.
- S. Pelegrini, I. S. Brandt, C. C. Plá Cid, E. A. Isoppo, A. D. C. Viegas and A. A. Pasa, ECS J. Solid State Sci. Technol., 2015, 4, P181–P185 CrossRef CAS.
- L. A. Clevenger, A. Mutscheller, J. M. E. Harper, C. Cabral and K. Barmak, J. Appl. Phys., 1992, 72, 4918–4924 CrossRef CAS.
- B. Clausen, C. N. Tomé, D. W. Brown and S. R. Agnew, Acta Mater., 2008, 56, 2456–2468 CrossRef CAS.
- R. L. Penn and J. F. Banfield, Am. Mineral., 1998, 83, 1077–1082 CrossRef CAS.
- J. I. Cisneros, Appl. Opt., 1998, 37, 5262–5270 CrossRef CAS PubMed.
- J. Tauc, R. Grigorovici and A. Vancu, Phys. Status Solidi, 1966, 15, 627–637 CrossRef CAS.
- S. Tanemura, L. Miao, P. Jin, K. K, A. Terai and N. Nabatova-Gabain, Appl. Surf. Sci., 2003, 212–213, 654–660 CrossRef CAS.
- I. J. Bruno, J. C. Cole, P. R. Edgington, C. F. Macrae, J. Pearson and R. Taylor, Acta Crystallogr., Sect. B: Struct. Sci., 2002, 58, 389–397 CrossRef.
- C. F. Macrae, P. R. Edgington, P. McCabe, E. Pidcock, G. P. Shields, R. Taylor, M. Towler and J. van de Streek, J. Appl. Crystallogr., 2006, 39, 453–457 CrossRef CAS.
- C. F. Macrae, I. J. Bruno, J. A. Chisholm, P. R. Edgington, P. McCabe, E. Pidcock, L. Rodriguez-Monge, R. Taylor, J. van de Streek and P. A. Wood, J. Appl. Crystallogr., 2008, 41, 466–470 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2018 |