Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Effect of OH− on chemical mechanical polishing of β-Ga2O3 (100) substrate using an alkaline slurry
Chuanjin Huang ab,
Wenxiang Muc,
Hai Zhoub,
Yongwei Zhu*a,
Xiaoming Xub,
Zhitai Jiac,
Lei Zhengb and
Xutang Tao
ab,
Wenxiang Muc,
Hai Zhoub,
Yongwei Zhu*a,
Xiaoming Xub,
Zhitai Jiac,
Lei Zhengb and
Xutang Tao *c
*c
aCollege of Mechanical and Electrical Engineering, Nanjing University of Aeronautics and Astronautics, Nanjing 210016, China. E-mail: meeywzhu@nuaa.edu.cn
bCollege of Mechanical Engineering, Yancheng Institute of Technology, Yancheng 224051, China
cState Key Laboratory of Crystal Materials, Key Laboratory of Functional Crystal Materials and Device, Shandong University, Jinan, 250100, China. E-mail: txt@sdu.edu.cn
First published on 8th February 2018
Abstract
β-Ga2O3, a semiconductor material, has attracted considerable attention given its potential applications in high-power devices, such as high-performance field-effect transistors. For decades, β-Ga2O3 has been processed through chemical mechanical polishing (CMP). Nevertheless, the understanding of the effect of OH− on β-Ga2O3 processed through CMP with an alkaline slurry remains limited. In this study, β-Ga2O3 substrates were successively subjected to mechanical polishing (MP), CMP and etching. Then, to investigate the changes that occurred on the surfaces of the samples, samples were characterised through atomic force microscopy (AFM), three-dimensional laser scanning confocal microscopy (LSCM), scanning electron microscopy (SEM) and X-ray photoelectron spectroscopy (XPS). LSCM and SEM results showed that β-Ga2O3 is highly vulnerable to brittle fracture during MP. AFM revealed that an ultrasmooth and nondamaged surface with a low Ra of approximately 0.18 nm could be obtained through CMP. XPS results indicated that a metamorphic layer, which mainly contains soluble gallium salt (Ga(OH)4−), formed on the β-Ga2O3 surface through a chemical reaction. A dendritic pattern appeared on the surface of β-Ga2O3 after chemical etching. This phenomenon indicated that the chemical reaction on the β-Ga2O3 surface occurred in a nonuniform and selective manner. The results of this study will aid the optimization of slurry preparation and CMP.
1 Introduction
The application of β-type gallium oxide (β-Ga2O3) as a semiconductor material of high-power devices,1–3 such as high-performance field-effect transistors,4,5 Schottky barrier diodes6,7 and ultraviolet transparent electrodes,8 has attracted considerable attention because β-Ga2O3 has excellent material properties, including a wide band gap of approximately 4.9 eV, and stable chemical and thermal properties.9 Although numerous studies have been conducted on β-Ga2O3 materials,10–14 few have focused on the ultraprecision machining of these materials. Chemical mechanical polishing (CMP), a key step in the ultraprecision machining of crystal materials, is an efficient technique that yields ultrasmooth and undamaged crystal surfaces.15The main cleavage plane of the β-Ga2O3 crystal is along its (100) surface,16 which is the most thermodynamically stable and the most suitable surface for thin-film growth among all surfaces.17 However, cleavage renders the crystal surface fragile and hinders the ultraprecise machining of β-Ga2O3.2 Ga–O bonds in the unstable octahedral of the β-Ga2O3 (100) structure fracture easily.16 The β-Ga2O3 (100) structure is composed of a slightly distorted tetrahedral and a highly distorted octahedral. Ga–O bonds in β-Ga2O3 (100) are highly sensitive to mechanical stress and cause crystal cleavage during processing. Therefore, chemical action may be a feasible method for generating new bonds that can shield Ga–O bonds during CMP.18–20 Slurries with alkali and oxidisers applied in the CMP of GaN and GaAs can be applied in the CMP of β-Ga2O3.18,20,21 However, gallium oxide is in a stable oxidation state, and wet etching experiments on β-Ga2O3 are utilised only as a reference for evaluating the use of sodium hydroxide as a mordant without an oxidant.22,23 The β-Ga2O3 (100) surface needs to be processed through CMP using an alkaline slurry with OH− to obtain an ultrasmooth crystal surface. Moreover, the chemical action mechanism that underlies the CMP of β-Ga2O3 must be investigated.
By evaluating the morphology and chemical composition of the β-Ga2O3 surface after treatment, this study investigated the effect of OH− on β-Ga2O3 processed through mechanical polishing (MP) without chemical action and to CMP with chemical action. Moreover, the chemical action between β-Ga2O3 and sodium hydroxide during wet etching was investigated. The surface morphology of β-Ga2O3 was examined through atomic force microscopy (AFM), laser-scanning confocal microscopy (LSCM) and scanning electron microscopy (SEM). Results indicated that a metamorphic layer that formed on the crystal surface through a chemical reaction was removed during CMP. X-ray photoelectron spectroscopy (XPS) was also used to study the surface chemistry of β-Ga2O3. The different chemical states of the metamorphic layer during different treatment processes were studied. This experimental analysis may be useful for optimising slurry preparation.
2 Material and methods
β-Ga2O3 substrates were cut from a crystal bar along the (100) plane and prepared via the edge-defined film-fed crystal growth method. The β-Ga2O3 (100) substrates had a size of approximately 10 mm × 10 mm × 1 mm (length × width × thickness). Prior to the experiments, the substrates were ground to expose a large flat surface, which served as the foundation of subsequent experiments.This experiment comprised three steps, as follows: firstly, the substrates were treated through MP. β-Ga2O3 was polished using a polishing tester (UNIPOL-1502, MTI-KJ Corp., Ltd.) with silk as a soft pad at a pressure of 15 kPa. Deionised water (DI) and diamond paste (mean diameter 1 μm) were used as chemically inert lapping media. The other process conditions were as follows: plate speed of 35 rpm and polishing time of 4 h. Secondly, the substrates were treated through CMP. β-Ga2O3 was polished with the same polishing tester equipped with an Politex pad (Rohm & Haas Co., Ltd.). Commercial colloidal silica (NS-54, Fuso Chemical Co., Ltd.) mixed with diluted sodium hydroxide was used as the slurry. Slurry pH was adjusted to 11, and slurry concentration was approximately 20 wt%. The slurry was not reused. The other process conditions were as follows: plate rotation speed of 35 rpm, operating pressure of 15 kPa, slurry flow rate of 50 mL min−1 and polishing time of 15 h. Finally, the β-Ga2O3 substrates were etched with 20 wt% sodium hydroxide solution at 55 °C for 2 h. The substrates were successively cleaned with liquid cleaner, DI water and ethyl alcohol and dried by an air spray gun for each measurement.
The surface morphology of β-Ga2O3 after treatment was characterised using Keyence VK-X110K 3D LSCM, FEI Nova NanoSEM 450 and Bruker Dimension Icon AFM. The surface average roughness (Ra) values after MP were determined using LSCM with 100 μm × 100 μm scans. Ra after CMP was determined through AFM with 1 μm × 1 μm scans. The surface chemical composition was analysed with Thermo Scientific Escalab250Xi (XPS). Firstly, samples subjected to MP, CMP and etching were studied. The surfaces of these samples were measured in normal mode with 0° electron emission angle. Secondly, to analyse the chemical composition of the crystal surface subjected to CMP, the crystal surface was measured in angle-resolved mode with an electron emission angle of 0° to 75°. All data were obtained using a monochromatised Al-Kα source (1486.6 eV) and a pass energy of 40 eV. XPS peak assignment was based on an acceptable standard reference database and literature reports.17,24–26
3 Results and discussion
3.1 Surface morphology analysis
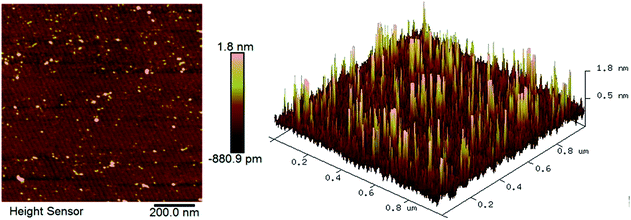
The AFM images of the CMP-treated β-Ga2O3 surface are shown in Fig. 1. The Ra of the sample was approximately 0.18 nm. The surface did not exhibit a regular terraced morphology but instead exhibited negligible roughness similar to that reported in previous studies.27,28 This result indicated that the wafer surface might have been covered with a layer of chemical substances. The ultrasmooth surface of the wafer processed through CMP is shown in Fig. 1. The wafer exhibited surface contamination, which is shown as a series of brightly coloured points.29 The properties of the polished surface with residual chemical products were characterised through SEM and XPS to identify the chemical action mechanism that underlies the CMP of β-Ga2O3.The surface morphology of β-Ga2O3 treated through MP, CMP and etching are shown in Fig. 2. As shown in Fig. 2(a) and (d), after MP treatment, the substrate appeared rough with an Ra of 52 nm and was covered with tongue-patterned cleavage pits. During MP, abrasive diamond grains cut into the crystal plane and resulted in the instantaneous generation of concentrated stress. New cracks were initiated on the crystal plane, and crack expansion accelerated until the plane underwent low-energy brittle fracture, which is very easy to occur because of the cleavage of β-Ga2O3. After CMP, the surface Ra of the β-Ga2O3 substrate decreased from 54 nm to 0.18 nm, and the cleavage pits disappeared. The ultrasmooth mirror-like surface of the substrate obtained after CMP is shown in Fig. 2(b) and (e). Micro cleavage pits caused by local cleavage fracture were difficult to remove during MP, as shown in Fig. 2(a) and (b). By contrast, micro cleavage pits were easily removed during CMP because β-Ga2O3 was chemically corroded in the alkaline environment. Numerous etching pits were present on the surface of β-Ga2O3, as shown in Fig. 2(c) and (f). This pattern indicated that a chemical reaction occurred between Ga2O3 and sodium hydroxide. The surface morphology of the β-Ga2O3 substrate dramatically changed through chemical action.
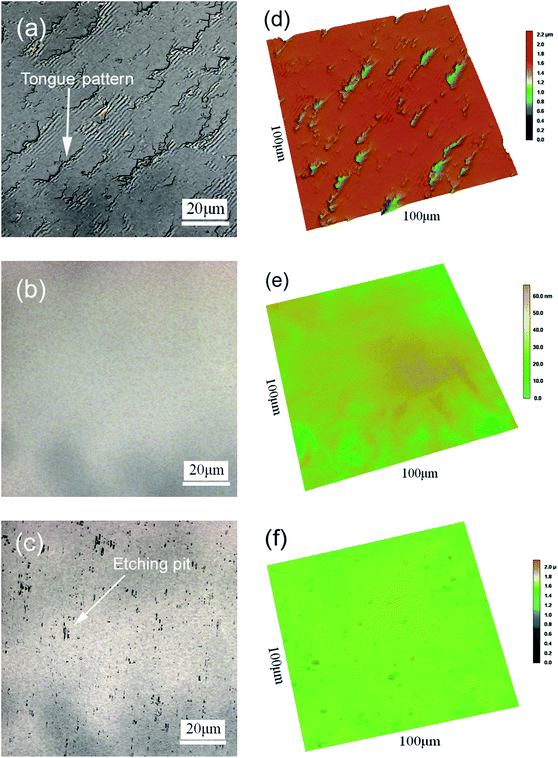 | ||
| Fig. 2 Two-dimensional LSCM images of β-Ga2O3 (100) after (a) MP, (b) CMP and (c) etching. (d), (e) and (f) are the corresponding three-dimensional images of (a), (b) and (c). | ||
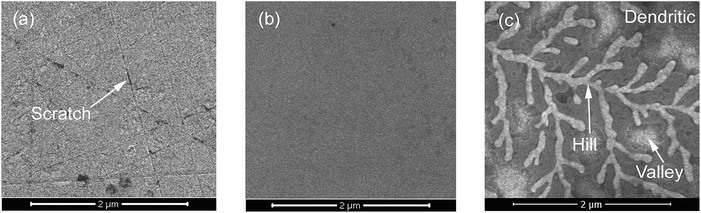
The crystal surfaces obtained through different processes using slurries containing sodium hydroxide were subjected to microscopy observation to describe the chemical mechanism that acts on β-Ga2O3 during CMP. The SEM images of the surfaces treated through MP, CMP and etching are shown in Fig. 3. As shown in Fig. 3(a), numerous scratches formed on the crystal surface after MP. Scratches between cleavage pits appeared flat, as shown in Fig. 2(a). The crisscrossing microscratches indicated that the cleavage properties of β-Ga2O3 caused microscratch defects even when abrasive grains did not produce cleavage pits after the crystal face was cut. An ultrasmooth mirror-like surface was obtained after CMP, as shown in Fig. 3(b). This surface exhibited fewer cleavage defects than the surface shown in Fig. 3(a). Fig. 3(c) shows the β-Ga2O3 surface after etching with sodium hydroxide solution and CMP. The crystal face exhibited a dendritic pattern. The bright section of the dendritic pattern shown in Fig. 3(c) represented a protruding structure similar to a peak, and the remaining grey portion indicated a low-lying area similar to a valley. The presence of dendrites implied that sodium hydroxide selectively corrodes β-Ga2O3. The valleys of the crystal surface indicated areas that are susceptible to corrosion under alkaline conditions, and the peaks indicated areas that are relatively resistant to corrosion. Generally, β-Ga2O3 exhibits uneven corrosion under alkaline conditions during CMP. Therefore, the use of abrasive silica particles to remove the residual metamorphic layer is an essential step in CMP.
The formation and removal of the metamorphic layer on the surface of β-Ga2O3 during CMP are important for obtaining an ultrasmooth surface. The chemical composition of the metamorphic layer was analysed.
3.2 Surface chemical composition analysis
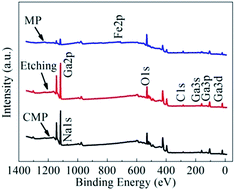
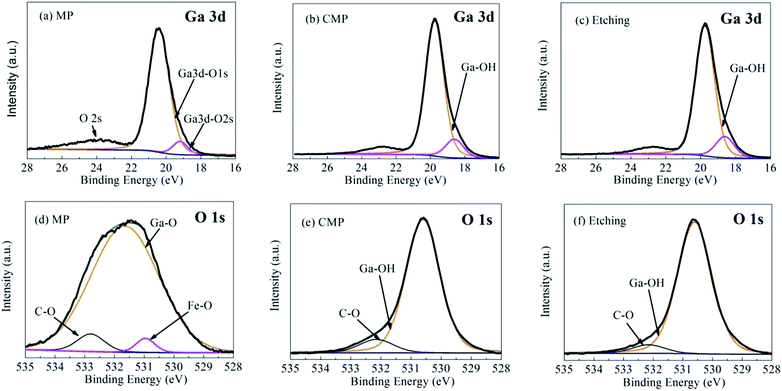
As shown in Fig. 4, peaks that correspond to Ga 3d, O 1s and C 1s photoelectrons are present in the survey scan spectra of the β-Ga2O3 substrates treated through MP, CMP and etching. The prominent C 1s peaks observed in all wide-survey XPS spectra may have originated from the adsorption of adventitious carbon contaminants on the surface; these contaminants could be partly removed by heat treatment.17 The C 1s peak at 284.8 eV could be used to calibrate the peak positions of other elements. Fig. 4 also shows that surface contaminants can be attributed to different processes. For example, Fe was contributed by the iron plate during grinding, and Na was contributed by the slurry used for CMP. These contaminants could be removed through subsequent processes. The spectra of the surfaces would be similar if the effect of Fe and Na contaminants are ignored.Fig. 5 shows the Ga 3d and O 1s XPS spectra of the β-Ga2O3 surface after various treatments. Curve fitting was performed by using the Shirley background, and the symmetric Lorentzian–Gaussian peak shape function value was set as 20%. The evolution of the Ga 3d XPS spectra of the β-Ga2O3 surface after different treatments is illustrated in Fig. 5(a)–(c). Fig. 5(a) shows that the peak representing the Ga–O bond of β-Ga2O3 is located at 20.4 eV after MP, whereas that representing O 2s, which could be attributed to the hybridization of the Ga 3d and O 2s surface states, is located at 24 eV.24 Fig. 5(a)–5(b) show that the Ga 3d peak acquired at normal emission shifted to a low binding energy from MP to CMP. This shift was caused by a chemical reaction. After CMP, the peak position shifted by approximately 0.9 eV from 20.4 eV (Ga–O bond) to 19.5 eV (Ga–OH bond).26 This shift could be attributed to the breakage of Ga–O bonds through a tribochemical action, which allowed surface Ga to bond with hydroxyl from sodium hydroxide. This result suggested that the surface of β-Ga2O3 undergoes tribochemical reactions with sodium hydroxide to form new reaction products, such as soluble gallium salt (Ga(OH)4−), during CMP.18 These salts were then partly absorbed on the polished surface. Chemical reactions during CMP and etching exhibited similar results, as shown in Fig. 5(c). A detailed analysis of the O 1s core level peaks of the substrate is shown in Fig. 5(d)–(f). The O 1s peaks at 531.4, 532.8 and 530.9 eV observed after MP and shown in Fig. 5(d) could be respectively attributed to the main Ga–O bond peak,24 C–O from carbon contaminants17 and Fe–O contributed by cast-iron grinding discs before MP.26 Fig. 5(d)–(f) show that the Ga–O bond of β-Ga2O3 was replaced by Ga–OH after CMP and etching due to chemical action, indicating that the same condition shown by Fig. 5(a)–(c) was observed for CMP. Moreover, the low full width at half maximum (FWHM) of the Ga 3d and O 1s peaks observed from Fig. 5(b) and (e) indicated the excellent crystalline quality of the studied samples.9
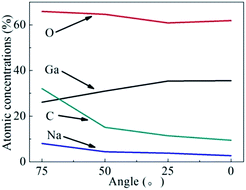
Fig. 6 shows the variation tendency of the atomic composition, including Ga, O, C and Na, of the β-Ga2O3 surface layer after CMP. The atomic composition of the β-Ga2O3 surface layer was identified through angle-resolved XPS (ARXPS). The Ga-to-O ratio of the β-Ga2O3 surface layer significantly increased with surface depth and was close to the stoichiometric ratio of 2/3, indicating that gallium salts and other oxide contaminants may be distributed on the surface. Trace amounts of metal contaminants, such as Na, were present on the surface and were likely contributed by the slurry containing sodium hydroxide. Fig. 4 and 6 suggested that metal ions can penetrate the crystal surface through mechanical abrasion during CMP but not during static etching. To prevent metal pollution, the slurry formula must be optimised by selecting the appropriate organic alkali and a suitable complexing agent.19,29–31 An extremely thin metamorphic layer was adsorbed on the crystal surface treated through CMP. This layer is useful for removing surface crystal materials during CMP.
3.3 Polishing mechanisms
The chemical action mechanism of β-Ga2O3 is summarised as the formation of a metamorphic surface layer. Firstly, XPS data shown in Fig. 5 indicated that the chemical action mechanism underlying CMP can be used to describe the formation of the metamorphic layer. When Ga2O3 was subjected to CMP with alkali hydroxide under basic pH, reaction products that contain gallium salts, such as Ga(OH)4−, may leave a film on the crystal surface.20 The reaction that yields gallium salt is as follows:| Ga2O3(s) + 2OH−(aq) + 3H2O(l) → 2[Ga(OH)4]−(aq) | (1) |
SEM data shown in Fig. 3 revealed that a dendritic pattern appeared on the β-Ga2O3 crystal surface after immersion in slurry containing sodium hydroxide. This pattern indicated that the surface corrosion of β-Ga2O3 is selective and that generation of new metamorphic layers is uneven. Rugged metamorphic layers could be uniformly removed through mechanical abrasion to yield ultrasmooth, nondamaged crystal surfaces.
A metamorphic layer formed on the surface of β-Ga2O3 through a chemical reaction during CMP under alkaline conditions. This layer was mainly composed of gallium salts. The adsorption of these salts on the β-Ga2O3 surface changed the physical and chemical properties of the crystal surface, such as hardness, brittleness, wettability and hydrolysability, thus improving the processing properties of the crystal. Therefore, the generation of a metamorphic layer influences material removal during CMP and mitigates cleavage during polishing.
4 Conclusions
β-Ga2O3 (100) surfaces were treated through MP, CMP and etching. The treated surfaces were then characterised through AFM, LSCM, SEM and XPS to reveal the effect of sodium hydroxide on ultraprecision polishing. The LSCM results showed that the cleavage of β-Ga2O3 hindered the processing of crystals to an ultrasmooth surface, and the apparent fragility of the crystal during MP resulted in the formation of surface micropits. SEM and XPS results proved that the formation of a metamorphic layer on the surface of β-Ga2O3 has a critical role in the removal of materials formed by the chemical reaction between Ga2O3 and sodium hydroxide during CMP. Ga–O bonds on the surface of β-Ga2O3 replaced cleaved Ga–OH bonds, leading to the formation of soluble gallium salts. The metamorphic layer can be easily removed during CMP to prevent surface damage. SEM results showed that dendritic patterns formed on the crystal surface after etching. These patterns indicated that β-Ga2O3 is nonuniformly and selectively corroded during chemical reaction due to. AFM indicated that an ultrasmooth surface with a low Ra of approximately 0.18 nm was obtained through CMP. In summary, the chemical reaction of OH− on β-Ga2O3 may help aid the optimisation of slurry preparation for CMP.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work has been supported by The National Natural Science Foundation of China (No. 51675457 and 51575470), Joint Innovation Fund Project of Jiangsu Province (BY2016065-55).References
- M. Higashiwaki, K. Sasaki, A. Kuramata, T. Masui and S. Yamakoshi, Development of gallium oxide power devices, Phys. Status Solidi A, 2014, 211, 21–26 CrossRef CAS
.
- E. G. Víllora, S. Arjoca, K. Shimamura, D. Inomata and K. Aoki, β-Ga2O3 and single-crystal phosphors for high-brightness white LEDs and LDs, and β-Ga2O3 potential for next generation of power devices, Proc. SPIE-Int. Soc. Opt. Eng., 2014, 89871U Search PubMed
.
- W. Mu, Y. Yin, Z. Jia, L. Wang, J. Sun, M. Wang, C. Tang, Q. Hu, Z. Gao, J. Zhang, N. Lin, S. Veronesi, Z. Wang, X. Zhao and X. Tao, An extended application of β-Ga2O3 single crystals to the laser field: Cr4+:β-Ga2O3 utilized as a new promising saturable absorber, RSC Adv., 2017, 7, 21815–21819 RSC
.
- M. Higashiwaki, K. Sasaki, T. Kamimura, M. Hoi Wong, D. Krishnamurthy, A. Kuramata, T. Masui and S. Yamakoshi, Depletion-mode Ga2O3 metal-oxide-semiconductor field-effect transistors on β-Ga2O3 (010) substrates and temperature dependence of their device characteristics, Appl. Phys. Lett., 2013, 103, 123511 CrossRef
.
- M. Higashiwaki, K. Sasaki, A. Kuramata, T. Masui and S. Yamakoshi, Gallium oxide (Ga2O3) metal-semiconductor field-effect transistors on single-crystal β-Ga2O3 (010) substrates, Appl. Phys. Lett., 2012, 100, 013504 CrossRef
.
- R. Suzuki, S. Nakagomi, Y. Kokubun, N. Arai and S. Ohira, Enhancement of responsivity in solar-blind β-Ga2O3 photodiodes with a Au Schottky contact fabricated on single crystal substrates by annealing, Appl. Phys. Lett., 2009, 94, 222102 CrossRef
.
- M. Higashiwaki, K. Konishi, K. Sasaki, K. Goto, K. Nomura, Q. T. Thieu, R. Togashi, H. Murakami, Y. Kumagai and M. Bo, Temperature-dependent capacitance-voltage and current-voltage characteristics of Pt/Ga2O3 (001) Schottky barrier diodes fabricated on n-Ga2O3 drift layers grown by halide vapor phase epitaxy, Appl. Phys. Lett., 2016, 108, 1759 CrossRef
.
- M. Zhong, Z. Wei, X. Meng, F. Wu and J. Li, High-performance single crystalline UV photodetectors of β-Ga2O3, J. Alloys Compd., 2015, 619, 572–575 CrossRef CAS
.
- W. Mu, Z. Jia, Y. Yin, Q. Hu, Y. Li, B. Wu, J. Zhang and X. Tao, High quality crystal growth and anisotropic physical characterization of β-Ga2O3 single crystals grown by EFG method, J. Alloys Compd., 2017, 714, 453–458 CrossRef CAS
.
- Y. Liu and Z. H. Li, Adsorption and decomposition mechanism of formic acid on the Ga2O3 surface by first principle studies, Surf. Sci., 2017, 656, 86–95 CrossRef CAS
.
- H. Yamaguchi, A. Kuramata and T. Masui, Slip system analysis and X-ray topographic study on β-Ga2O3, Superlattices Microstruct., 2016, 99 CrossRef CAS
.
- K. Hanada, T. Moribayashi, T. Uematsu, S. Masuya, K. Koshi, K. Sasaki, A. Kuramata, O. Ueda and M. Kasu, Observation of nanometer-sized crystalline grooves in as-grown β-Ga2O3 single crystals, Jpn. J. Appl. Phys., 2016, 55, 030303 CrossRef
.
- L. Feng, Y. Li, X. Su, S. Wang, H. Liu, J. Wang, Z. Gong, W. Ding, Y. Zhang and F. Yun, Growth and characterization of spindle-like Ga2O3 nanocrystals by electrochemical reaction in hydrofluoric solution, Appl. Surf. Sci., 2016, 389, 205–210 CrossRef CAS
.
- T. Onuma, S. Fujioka, T. Yamaguchi, Y. Itoh, M. Higashiwaki, K. Sasaki, T. Masui and T. Honda, Polarized Raman spectra in β-Ga2O3 single crystals, J. Cryst. Growth, 2014, 401, 330–333 CrossRef CAS
.
- Y. Chen, A. Chen and J. Qin, Polystyrene core–silica shell composite particles: effect of mesoporous shell structures on oxide CMP and mechanical stability[J], RSC Adv., 2017, 7(11), 6548–6558 RSC
.
- V. M. Bermudez, The structure of low-index surfaces of β-Ga2O3, Chem. Phys., 2006, 323, 193–203 CrossRef CAS
.
- A. Navarro-Quezada, Z. Galazka, S. Alamé, D. Skuridina, P. Vogt and N. Esser, Surface properties of annealed semiconducting β-Ga2O3 (100) single crystals for epitaxy, Appl. Surf. Sci., 2015, 349, 368–373 CrossRef CAS
.
- J. B. Matovu, P. Ong, L. H. A. Leunissen, S. Krishnan and S. V. Babu, Fundamental Investigation of Chemical Mechanical Polishing of GaAs in Silica Dispersions: Material Removal and Arsenic Trihydride Formation Pathways, ECS J. Solid State Sci. Technol., 2013, 2, 432–439 CrossRef
.
- R. R. Nair, A. Gupta, S. N. Victoria and R. Manivannan, Chemical mechanical planarization of germanium using oxone® based silica slurries, Wear, 2017, 376–377, 86–90 CrossRef CAS
.
- J. Wang, T. Wang, G. Pan and X. Lu, Effect of photocatalytic oxidation technology on GaN CMP, Appl. Surf. Sci., 2016, 361, 18–24 CrossRef CAS
.
- H. Aida, T. Doi, H. Takeda, H. Katakura, S. W. Kim, K. Koyama, T. Yamazaki and M. Uneda, Ultraprecision CMP for sapphire, GaN, and SiC for advanced optoelectronics materials, Curr. Appl. Phys., 2012, 12, S41–S46 CrossRef
.
- S. Ohira and N. Arai, Wet chemical etching behavior of β-Ga2O3 single crystal, Phys. Status Solidi C, 2008, 5, 3116–3118 CrossRef CAS
.
- T. Oshima, T. Okuno, N. Arai, Y. Kobayashi and S. Fujita, Wet Etching of β-Ga2O3 Substrates, Jpn. J. Appl. Phys., 2009, 48, 040208 CrossRef
.
- A. Navarro-Quezada, S. Alamé, N. Esser, J. Furthmüller, F. Bechstedt, Z. Galazka, D. Skuridina and P. Vogt, Near valence-band electronic properties of semiconducting β-Ga2O3 (100) single crystals, Phys. Rev. B, 2015, 92, 195306 CrossRef
.
- A. I. Serykh and M. D. Amiridis, In situ X-ray photoelectron spectroscopy study of supported gallium oxide, Surf. Sci., 2010, 604, 1002–1005 CrossRef CAS
.
- NIST X-ray Photoelectron Spectroscopy Database, https://srdata.nist.gov/xps/.
- X. Shi, C. Zou, G. Pan, H. Gong, L. Xu and Y. Zhou, Atomically smooth gallium nitride surface prepared by chemical-mechanical polishing with S2O82−–Fe2+ based slurry, Tribol. Int., 2017, 110, 441–450 CrossRef CAS
.
- Y. Zhou, G. Pan, H. Gong, X. Shi and C. Zou, Characterization of sapphire chemical mechanical polishing performances using silica with different sizes and their removal mechanisms, Colloids Surf., A, 2017, 513, 153–159 CrossRef CAS
.
- X. Shi, G. Pan, Y. Zhou, L. Xu, C. Zou and H. Gong, A study of chemical products formed on sapphire (0001) during chemical–mechanical polishing, Surf. Coat. Technol., 2015, 270, 206–220 CrossRef CAS
.
- K. Yadav, J. C. Bisen, S. N. Victoria and R. Manivannan, Sodium hypochlorite as an oxidizing agent in silica based ruthenium chemical mechanical planarization slurry, Microelectron. Eng., 2017, 180, 96–100 CrossRef CAS
.
- K. Asghar, M. Qasim, D. M. Nelabhotla and D. Das, Effect of surfactant and electrolyte on surface modification of c-plane GaN substrate using chemical mechanical
planarization (CMP) process, Colloids Surf., A, 2016, 497, 133–145 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2018 |